Potentially Postbiotic-Containing Preservative to Extend the Use-By Date of Raw Chicken Sausages and Semifinished Chicken Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method Design
2.2. Microbial Collection
2.3. PPCP Production
2.4. Determination of Kinetic Fermentation Parameters
2.5. In Vitro Trial
Spoilage Microbial Obtention and Inoculum Preparation
2.6. Microbial Susceptibility to PPCP
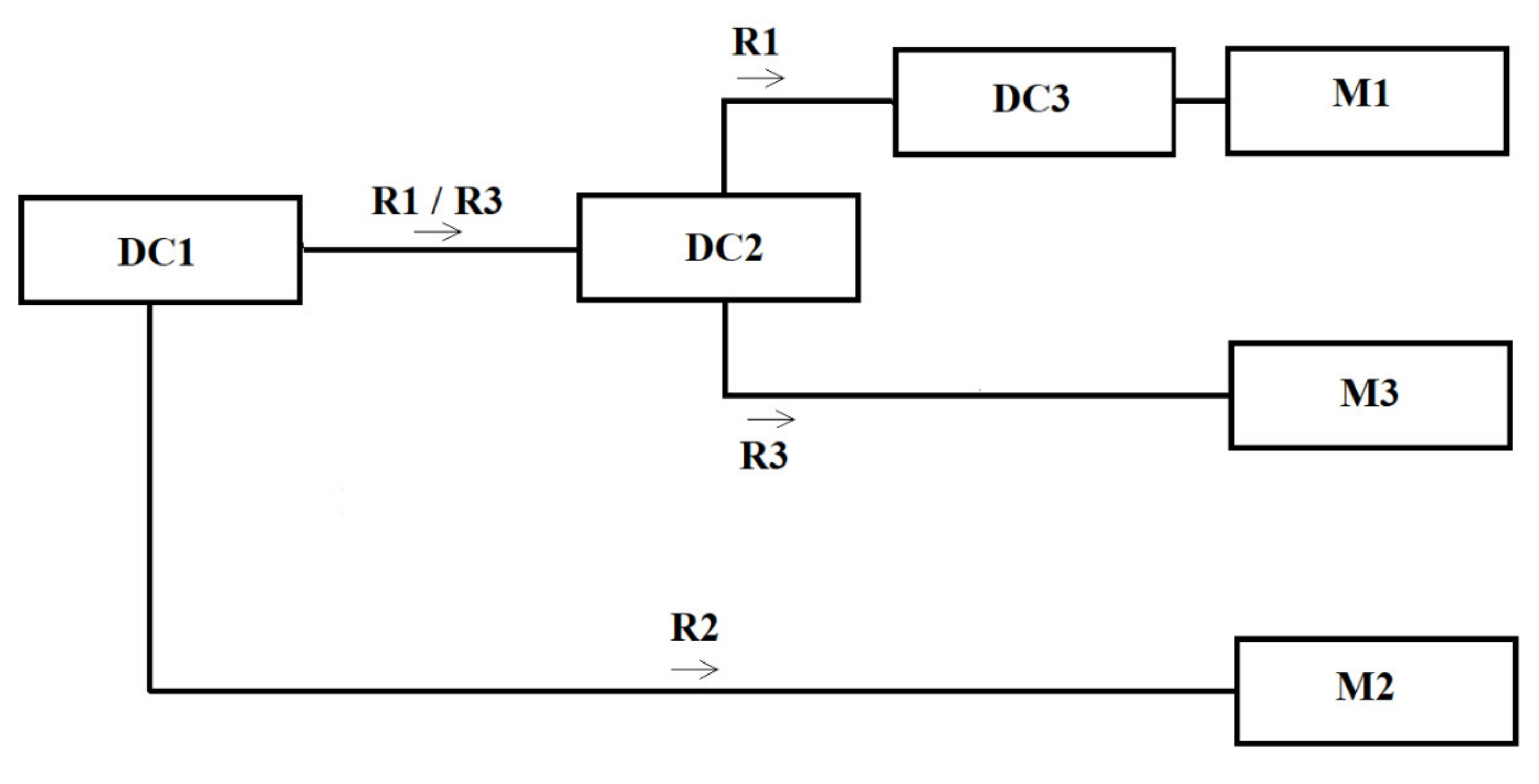
2.7. In Situ Trial
Poultry Products Processing
2.8. Durability Study
2.9. Temperature Profile of the Test
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Growth Phase Modeling
Appendix A.2. Deceleration Phase Modeling
References
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef]
- Yusuf, M. Chapter 12—Natural Antimicrobial Agents for Food Biopreservation. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 409–438. ISBN 978-0-12-811516-9. [Google Scholar]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic acid: A green compound for food biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Mun, S.Y.; Kim, S.K.; Woo, E.R.; Chang, H.C. Purification and characterization of an antimicrobial compound produced by Lactobacillus plantarum EM showing both antifungal and antibacterial activities. LWT 2019, 114, 108403. [Google Scholar] [CrossRef]
- Bouju-Albert, A.; Pilet, M.-F.; Guillou, S. Influence of lactate and acetate removal on the microbiota of French fresh pork sausages. Food Microbiol. 2018, 76, 328–336. [Google Scholar] [CrossRef]
- Liu, X.; Basu, U.; Miller, P.; McMullen, L.M. Differential gene expression and filamentation of Listeria monocytogenes 08-5923 exposed to sodium lactate and sodium diacetate. Food Microbiol. 2017, 63, 153–158. [Google Scholar] [CrossRef]
- Argyri, A.A.; Panagou, E.Z.; Nychas, G.-J.E. 7—Advances in vacuum and modified atmosphere packaging of poultry products. In Advances in Mea, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 205–247. ISBN 978-1-84569-751-8. [Google Scholar]
- EFSA. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, 336, 16–33. [Google Scholar]
- ANVISA. Instrução normativa n° 60, de 23 de dezembro de 2019. Diário Oficial da União 2019, 41, 133. [Google Scholar]
- ANVISA. Resolução de Deretoria Colegiada no 331, de 23 de dezembro de 2019. Diário Oficial da União 2019, 4, 96. [Google Scholar]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef]
- Catania, J.; Pandit, N.G.; Ehrlich, J.M.; Zaman, M.; Stone, E.; Franceschi, C.; Smith, A.; Tanner-Smith, E.; Zackular, J.P.; Bhutta, Z.A.; et al. Probiotic supplementation for promotion of growth in children: A systematic review and meta-analysis. Nutrients 2022, 14, 83. [Google Scholar]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; FAO: Rome, Italy, 2002; p. 11. [Google Scholar]
- Taverniti, V.; Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept). Genes Nutr. 2011, 6, 261–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, G.; Athalye-Jape, G.; Patole, S. Para-probiotics for preterm neonates—The next frontier. Nutrients 2018, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A new perspective for functional foods and nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of lactic acid bacteria postbiotics, evaluation in-vitro antibacterial effect, microbial and chemical quality on chicken drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef]
- Requena, T.; Pérez Martínez, G. 3.14—Probiotics, Prebiotics, Synbiotics, Postbiotics and Other Biotics. What’s Next? In A Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Oxford, UK, 2022; pp. 197–210. ISBN 978-0-12-822036-8. [Google Scholar]
- Anhê, F.F.; Bhatwa, A.; Schertzer, J.D. Determining the metabolic impact of postbiotics in mice. STAR Protoc. 2022, 3, 101098. [Google Scholar] [CrossRef]
- ANVISA. Resolução de Diretoria Colegiada n° 272 de 14 de março de 2019. Diário Oficial da União 2019, 35, 194. [Google Scholar]
- Lemos Junior, W.J.F.; Guerra, A.F.; Tarrah, A.; Duarte, V.S.; Giacomini, A.; Luchese, R.H.; Corich, V. Safety and stability of two potentially probiotic Lactobacillus strains after in vitro gastrointestinal transit. Probiotics Antimicrob. Proteins 2020, 12, 657–666. [Google Scholar] [CrossRef]
- Guerra, A.F.; Lemos Junior, W.J.F.; Santos, G.O.; Andrighetto, C.; Giacomini, A.; Corich, V.; Luchese, R.H. Lactobacillus paracasei probiotic properties and survivability under stress-induced by processing and storage of ice cream bar or ice-lolly. Ciência Rural 2018, 48, 1–9. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Guerra, A.F.; Duarte, V.S.; Treu, L.; Tarrah, A.; Campanaro, S.; Luchese, R.H.; Giacomini, A.; Corich, V. Draft genome sequence data of Lactobacillus paracasei strain DTA 83 isolated from infant stools. Data Br. 2019, 22, 1064–1067. [Google Scholar] [CrossRef]
- Laureano-Melo, R.; Caldeira, R.F.; Guerra, A.F.; Conceição, R.R.D.; Souza, J.S.D.; Giannocco, G.; Marinho, B.G.; Luchese, R.H.; Côrtes, W.S. Maternal supplementation with Lactobacillus paracasei DTA 83 alters emotional behavior in Swiss mice offspring. PharmaNutrition 2019, 8, 100148. [Google Scholar] [CrossRef]
- Tarrah, A.; Duarte, V.S.; Castilhos, J.; Pakroo, S.; Lemos Junior, W.J.F.; Luchese, R.H.; Guerra, A.F.; Rossi, R.C.; Righetto Ziegler, D.; Corich, V.; et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 2019, 54, 489–497. [Google Scholar] [CrossRef]
- Silva, L.C.; Schmidt, G.B.; Alves, L.G.O.; Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.; Martins, J.F.P.; Paula, B.P.; Luchese, R.H.; Guerra, A.F.; et al. Use of probiotic strains to produce beers by axenic or semi-separated co-culture system. Food Bioprod. Process. 2020, 124, 408–418. [Google Scholar] [CrossRef]
- Silva, L.C.; Lago, H.S.; Rocha, M.O.T.; Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; Paula, B.P.; Martins, J.F.P.; Luchese, R.H.; Guerra, A.F.; et al. Craft beers fermented by potential probiotic yeast or lacticaseibacilli strains promote antidepressant-like behavior in swiss webster mice. Probiotics Antimicrob. Proteins 2021, 13, 698–708. [Google Scholar] [CrossRef]
- Oliveira, W.A.; Rodrigues, A.R.P.; Oliveira, F.A.; Oliveira, V.S.; Laureano-Melo, R.; Stutz, E.T.G.; Lemos Junior, W.J.F.; Paula, B.P.; Esmerino, E.A.; Corich, V.; et al. Potentially probiotic or postbiotic pre-converted nitrite from celery produced by an axenic culture system with probiotic lacticaseibacilli strain. Meat Sci. 2021, 174, 108408. [Google Scholar] [CrossRef]
- Alcine Chan, M.Z.; Chua, J.Y.; Toh, M.; Liu, S.-Q. Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef]
- Shao, L.; Tian, X.; Yu, Q.; Xu, L.; Li, X.; Dai, R. Inactivation and recovery kinetics of Escherichia coli O157:H7 treated with ohmic heating in broth. LWT 2019, 110, 1–7. [Google Scholar] [CrossRef]
- Paula, B.P.; Chávez, D.W.H.; Lemos Junior, W.J.F.; Guerra, A.F.; Corrêa, M.F.D.; Pereira, K.S.; Coelho, M.A.Z. Growth parameters and survivability of Saccharomyces boulardii for probiotic alcoholic beverages development. Front. Microbiol. 2019, 10, 2092. [Google Scholar] [CrossRef]
- Paula, B.P.; Lago, H.S.; Firmino, L.; Lemos Júnior, W.J.F.; Corrêa, M.F.D.; Guerra, A.F.; Pereira, K.S.; Coelho, M.A.Z. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Associação Brasileira de Proteína Animal. Protocolo de Bem-Estar para Frangos de Corte. Available online: http://www.abpa-br.org (accessed on 3 February 2022).
- ISO 4833-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ICS: Washington, DC, USA, 2013.
- Moradi, R.; Nosrati, R.; Zare, H.; Tahmasebi, T.; Saderi, H.; Owlia, P. Screening and characterization of in-vitro probiotic criteria of Saccharomyces and Kluyveromyces strains. Iran. J. Microbiol. 2018, 10, 123–131. [Google Scholar] [PubMed]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef] [Green Version]
- Angulo, M.T.; Moog, C.H.; Liu, Y.-Y. A theoretical framework for controlling complex microbial communities. Nat. Commun. 2019, 10, 1045. [Google Scholar] [CrossRef] [Green Version]
- Conte-Junior, C.A.; Monteiro, M.L.G.; Patrícia, R.; Mársico, E.T.; Lopes, M.M.; Alvares, T.S.; Mano, S.B. The effect of different packaging systems on the shelf life of refrigerated ground beef. Foods 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Food Safety Authority of Ireland. Guidande Note No. 18 Validation of Product Shelf-Life, 4th ed.; Food Safety Authority of Ireland: Dublin, Ireland, 2019.

 ) and S. boulardii 17 (
) and S. boulardii 17 (  ) and pH measurement (unconnected points) during potentially postbiotic-containing preservative (PPCP) production. (
) and pH measurement (unconnected points) during potentially postbiotic-containing preservative (PPCP) production. (  ) Standard Error.
) Standard Error.
 ) and S. boulardii 17 (
) and S. boulardii 17 (  ) and pH measurement (unconnected points) during potentially postbiotic-containing preservative (PPCP) production. (
) and pH measurement (unconnected points) during potentially postbiotic-containing preservative (PPCP) production. (  ) Standard Error.
) Standard Error.
| Ingredients | Semifinished Chicken Products | Raw Chicken Sausages | ||||
|---|---|---|---|---|---|---|
| Control | T1 | T2 | Control | T1 | T2 | |
| Chicken parts (slit back or thigh) | 92–93 | 91–92 | 90.5–91.5 | |||
| Minced chicken meat | 86.34 | 85.34 | 84.84 | |||
| Water | 2–5 | 2–5 | 2–5 | 8 | 8 | 8 |
| Seasoning 1 | 2.1 | 2.1 | 2.1 | 2.87 | 2.87 | 2.87 |
| Sodium phosphate | 0.5 | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 |
| Sodium trypoliphosphate | 2.5 | 2.5 | 2.5 | |||
| Sodium erythorbate | 0.6 | 0.6 | 0.6 | |||
| Annatto dye | 0.02 | 0.02 | 0.02 | |||
| Sodium lactate | 0–2 | 0–2 | 0–2 | |||
| Curing Salt 2 | 0.12 | 0.12 | 0.12 | |||
| Cochineal carmine dye | 0.02 | 0.02 | 0.02 | |||
| PPCP | 1.0 | 1.5 | 1.0 | 1.5 | ||
| Sample Incubation | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (Days) | Control | T1 | T2 | |||||||
| Laboratorial data (log cfu/g) | 0 | 3.80 | 3.75 | 3.72 | |||||||
| 3 | 2 | 3.83 | 3.77 | 3.72 | |||||||
| 4 | 3.84 | 3.76 | 3.74 | ||||||||
| 25 | 1 | 5.53 | 5.38 | 5.37 | |||||||
| 3 | 9.30 | 6.32 | 6.11 | ||||||||
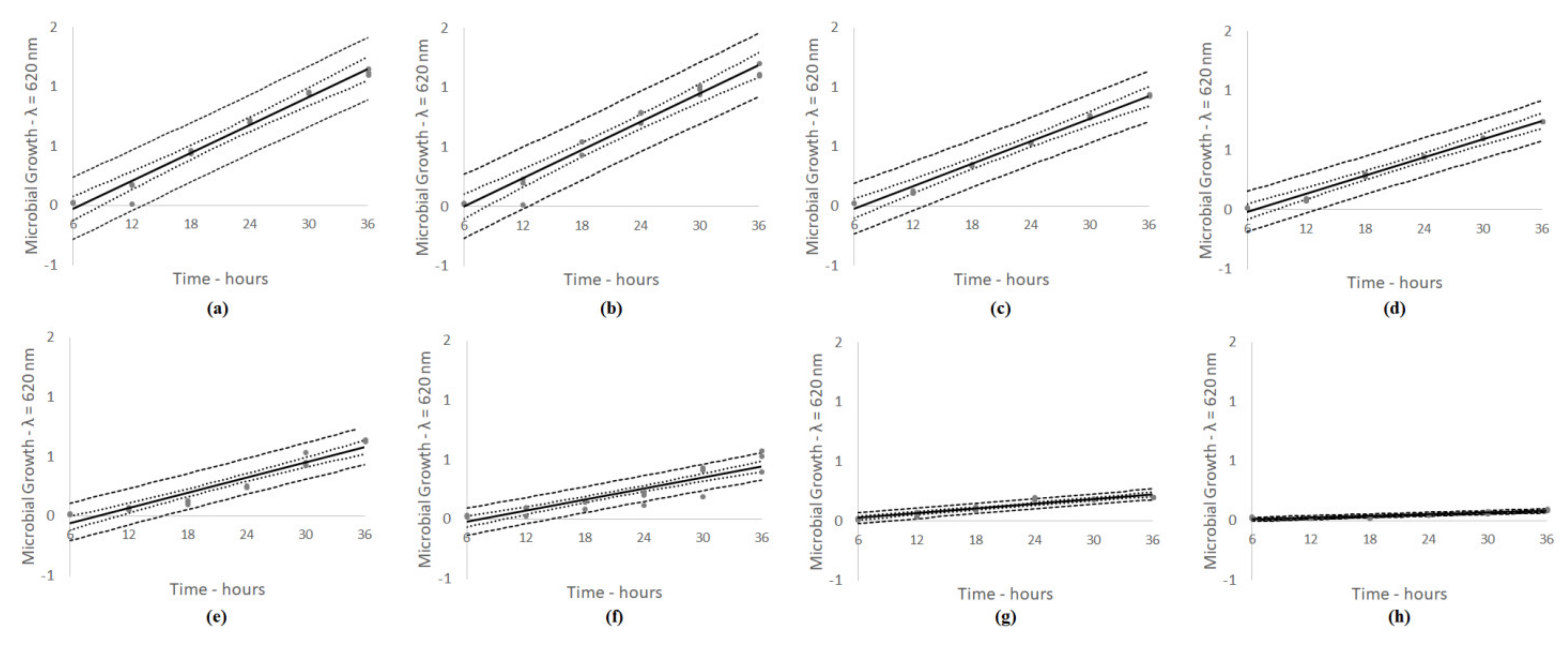
| Specific maximum growth rate (log cfu/g/day) | 3 | L phase | 0.0125 | 0.0062 | 0.0025 | ||||||
| D phase | 0.0110 | 0.0055 | 0.0022 | ||||||||
| 25 | L phase | 1.7817 | 1.2433 | 1.2233 | |||||||
| D phase | 1.5742 | 1.0986 | 1.0809 | ||||||||
| R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | |||
| 1st period | Ngrowth (log cfu/g/day) 1 | 0.0423 | 0.0423 | 0.0423 | 0.0292 | 0.0292 | 0.0292 | 0.0282 | 0.0282 | 0.0282 | |
| Ndeceleration (log cfu/g/day) 2 | 0.0374 | 0.0374 | 0.0374 | 0.0258 | 0.0258 | 0.0258 | 0.0249 | 0.0249 | 0.0249 | ||
| Ft(n) 3 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | ||
| 2nd period | Ngrowth (log cfu/g/day) 1 | 0.0026 | 0.0127 | 0.0013 | 0.0083 | 0.0005 | 0.0075 | ||||
| Ndeceleration (log cfu/g/day) 2 | 0.0025 | 0.0126 | 0.0012 | 0.0083 | 0.0005 | 0.0074 | |||||
| Ft(n) 3 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | |||||
| 3rd period | Ngrowth (log cfu/g/day) 1 | 0.0998 | 0.0127 | 0.0290 | 0.0679 | 0.0083 | 0.0185 | 0.0643 | 0.0075 | 0.0156 | |
| Ndeceleration (log cfu/g/day) 2 | 0.0806 | 0.0141 | 0.0252 | 0.0549 | 0.0093 | 0.0161 | 0.0519 | 0.0083 | 0.0136 | ||
| Ft(n) 3 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | ||
| Use-by date—days | 16 | 91 | 43 | 22 | 146 | 69 | 24 | 167 | 83 | ||
| Sample Incubation | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (Days) | Control | T1 | T2 | |||||||
| Laboratorial data (log cfu/g) | 0 | 3.90 | 3.92 | 4.01 | |||||||
| 3 | 2 | 4.08 | 4.07 | 4.01 | |||||||
| 4 | 4.03 | 4.01 | 4.05 | ||||||||
| 25 | 1 | 7.11 | 5.32 | 5.25 | |||||||
| 3 | 9.40 | 6.91 | 7.34 | ||||||||
| Specific maximum growth rate (log cfu/g/day) | 3 | L phase | 0.0613 | 0.0488 | 0.0050 | ||||||
| D phase | 0.0541 | 0.0431 | 0.0044 | ||||||||
| 25 | L phase | 2.5217 | 1.1983 | 1.1750 | |||||||
| D phase | 2.2281 | 1.0588 | 1.0382 | ||||||||
| R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | |||
| 1st period | Ngrowth (log cfu/g/day) 1 | 0.0661 | 0.0661 | 0.0661 | 0.0343 | 0.0343 | 0.0343 | 0.0274 | 0.0274 | 0.0274 | |
| Ndeceleration (log cfu/g/day) 2 | 0.0584 | 0.0584 | 0.0584 | 0.0303 | 0.0303 | 0.0303 | 0.0242 | 0.0242 | 0.0242 | ||
| Ft(n)3 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | ||
| 2nd period | Ngrowth (log cfu/g/day) 1 | 0.0128 | 0.0267 | 0.0102 | 0.0167 | 0.0010 | 0.0077 | ||||
| Ndeceleration (log cfu/g/day) 2 | 0.0121 | 0.0266 | 0.0096 | 0.0166 | 0.0010 | 0.0077 | |||||
| Ft(n) 3 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | |||||
| 3rd period | Ngrowth (log cfu/g/day) 1 | 0.1718 | 0.0267 | 0.0713 | 0.0953 | 0.0167 | 0.0476 | 0.0636 | 0.0077 | 0.0168 | |
| Ndeceleration (log cfu/g/day) 2 | 0.1387 | 0.0298 | 0.0620 | 0.0770 | 0.0186 | 0.0414 | 0.0514 | 0.0086 | 0.0146 | ||
| Ft(n) 3 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | ||
| Use-by date—days | 10 | 39 | 18 | 15 | 63 | 26 | 19 | 124 | 60 | ||
| Sample Incubation | Treatments | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (Days) | Control | T1 | T2 | |||||||
| Laboratorial data (log cfu/g) | 0 | 3.91 | 3.82 | 3.79 | |||||||
| 3 | 2 | 4.00 | 3.83 | 3.84 | |||||||
| 4 | 4.03 | 3.92 | 3.93 | ||||||||
| 25 | 1 | 6.78 | 6.29 | 4.41 | |||||||
| 3 | 9.40 | 8.01 | 5.83 | ||||||||
| Specific maximum growth rate (log cfu/g/day) | 3 | L phase | 0.0375 | 0.0150 | 0.0300 | ||||||
| D phase | 0.0331 | 0.0133 | 0.0265 | ||||||||
| 25 | L phase | 2.3500 | 1.9333 | 0.6500 | |||||||
| D phase | 2.0764 | 1.7082 | 0.5743 | ||||||||
| R1 | R2 | R3 | R1 | R2 | R3 | R1 | R2 | R3 | |||
| 1st period | Ngrowth (log cfu/g/day) 1 | 0.0588 | 0.0588 | 0.0588 | 0.0461 | 0.0461 | 0.0461 | 0.0191 | 0.0191 | 0.0191 | |
| Ndeceleration (log cfu/g/day) 2 | 0.0520 | 0.0520 | 0.0520 | 0.0407 | 0.0407 | 0.0407 | 0.0169 | 0.0169 | 0.0169 | ||
| Ft(n)3 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | 1.1318 | ||
| 2nd period | Ngrowth (log cfu/g/day) 1 | 0.0062 | 0.0210 | 0.0140 | 0.0140 | 0.0078 | 0.0098 | ||||
| Ndeceleration (log cfu/g/day) 2 | 0.0059 | 0.0209 | 0.0140 | 0.0140 | 0.0074 | 0.0097 | |||||
| Ft(n) 3 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | 1.0548 | 1.0034 | |||||
| 3rd period | Ngrowth (log cfu/g/day) 1 | 0.1464 | 0.0210 | 0.0528 | 0.1093 | 0.0140 | 0.0324 | 0.0542 | 0.0098 | 0.0283 | |
| Ndeceleration (log cfu/g/day) 2 | 0.1182 | 0.0234 | 0.0459 | 0.0883 | 0.0156 | 0.0282 | 0.0438 | 0.0109 | 0.0246 | ||
| Ft(n) 3 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | 1.2381 | 0.8971 | 1.1501 | ||
| Use-by date—days | 11 | 49 | 23 | 14 | 80 | 38 | 26 | 122 | 46 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida Godoy, C.L.; Costa, L.M.; Guerra, C.A.; de Oliveira, V.S.; de Paula, B.P.; Lemos Junior, W.J.F.; da Silva Duarte, V.; Luchese, R.H.; Bautitz, I.R.; Guerra, A.F. Potentially Postbiotic-Containing Preservative to Extend the Use-By Date of Raw Chicken Sausages and Semifinished Chicken Products. Sustainability 2022, 14, 2646. https://doi.org/10.3390/su14052646
de Almeida Godoy CL, Costa LM, Guerra CA, de Oliveira VS, de Paula BP, Lemos Junior WJF, da Silva Duarte V, Luchese RH, Bautitz IR, Guerra AF. Potentially Postbiotic-Containing Preservative to Extend the Use-By Date of Raw Chicken Sausages and Semifinished Chicken Products. Sustainability. 2022; 14(5):2646. https://doi.org/10.3390/su14052646
Chicago/Turabian Stylede Almeida Godoy, Carolyne Luciane, Lucas Marques Costa, Carlos Alberto Guerra, Vanessa Sales de Oliveira, Breno Pereira de Paula, Wilson José Fernandes Lemos Junior, Vinícius da Silva Duarte, Rosa Helena Luchese, Ivonete Rossi Bautitz, and André Fioravante Guerra. 2022. "Potentially Postbiotic-Containing Preservative to Extend the Use-By Date of Raw Chicken Sausages and Semifinished Chicken Products" Sustainability 14, no. 5: 2646. https://doi.org/10.3390/su14052646






