Transmission Factor (TF) Behavior of Bi2O3–TeO2–Na2O–TiO2–ZnO Glass System: A Monte Carlo Simulation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gamma Ray Shielding Parameters
2.2.1. Linear Attenuation Coefficient (μ, cm−1)
2.2.2. Tenth Value Layers (X1/10, cm)
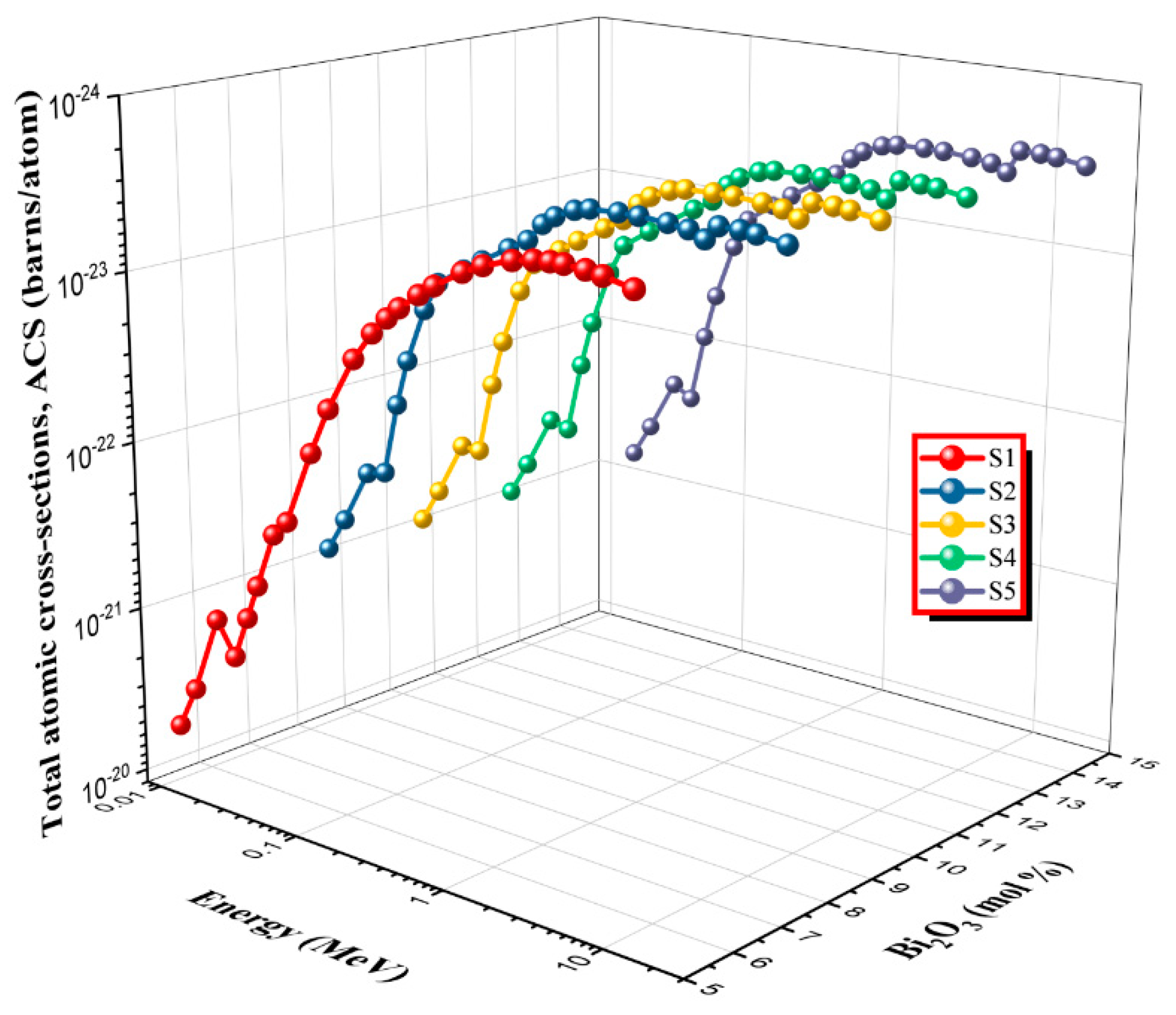
2.2.3. Total Atomic Cross Sections, ACS (σT)
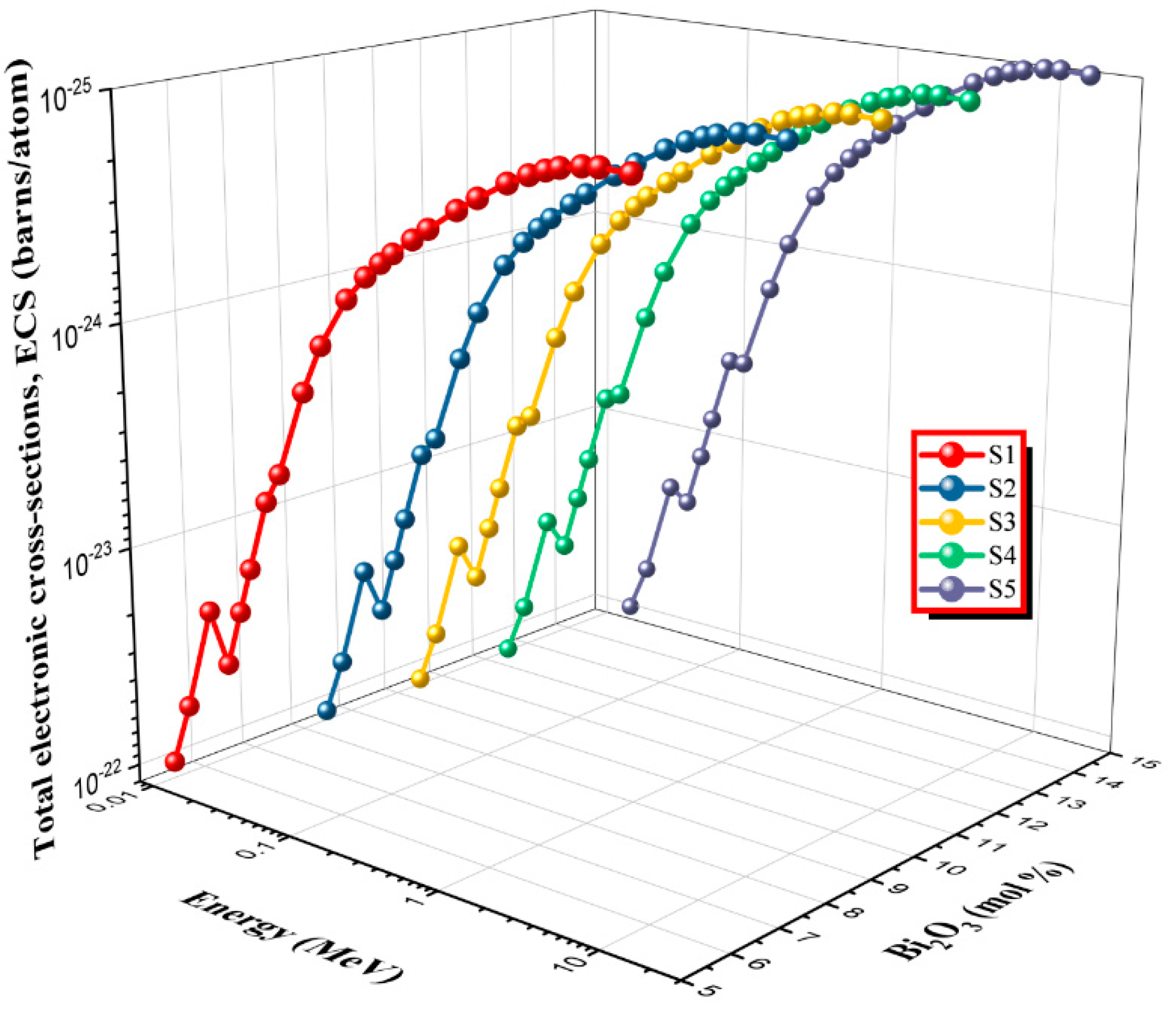
2.2.4. Total Electronic Cross Sections, ECS (σe)
2.2.5. Effective Atomic Number (Zeff)
2.3. MCNPX Monte Carlo Simulations for Transmission Factor (TF) Calculations
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fares, H.; Jlassi, I.; Elhouichet, H.; Férid, M. Investigations of thermal, structural and optical properties of tellurite glass with WO3 adding. J. Non-Cryst. Solids 2014, 396–397, 1–7. [Google Scholar] [CrossRef]
- Singh, V.P.; Badiger, N.; Kaewkhao, J. Radiation shielding competence of silicate and borate heavy metal oxide glasses: Comparative study. J. Non-Cryst. Solids 2014, 404, 167–173. [Google Scholar] [CrossRef]
- Waly, E.-S.A.; Al-Qous, G.S.; Bourham, M.A. Shielding properties of glasses with different heavy elements additives for radiation shielding in the energy range 15–300 keV. Radiat. Phys. Chem. 2018, 150, 120–124. [Google Scholar] [CrossRef]
- Rachniyom, W.; Chaiphaksa, W.; Limkitjaroeanporn, P.; Tuschaoen, S.; Sangwaranatee, N.; Kaewkhao, J. Effect of Bi2O3 on radiation shielding properties of glasses from coal fly ash. Mater. Today Proc. 2018, 5, 14046–14051. [Google Scholar] [CrossRef]
- Prabhu, N.; Hegde, V.; Sayyed, M.; Agar, O.; Kamath, S.D. Investigations on structural and radiation shielding properties of Er3+ doped zinc bismuth borate glasses. Mater. Chem. Phys. 2019, 230, 267–276. [Google Scholar] [CrossRef]
- Halimah, M.; Azuraida, A.; Ishak, M.; Hasnimulyati, L. Influence of bismuth oxide on gamma radiation shielding properties of boro-tellurite glass. J. Non-Cryst. Solids 2019, 512, 140–147. [Google Scholar] [CrossRef]
- Kavaz, E.; Tekin, H.O.; Yorgun, N.Y.; Özdemir, Ö.F.; Sayyed, M. Structural and nuclear radiation shielding properties of bauxite ore doped lithium borate glasses: Experimental and Monte Carlo study. Radiat. Phys. Chem. 2019, 162, 187–193. [Google Scholar] [CrossRef]
- Kirdsiri, K.; Kaewkhao, J.; Chanthima, N.; Limsuwan, P. Comparative study of silicate glasses containing Bi2O3, PbO and BaO: Radiation shielding and optical properties. Ann. Nucl. Energy 2011, 38, 1438–1441. [Google Scholar] [CrossRef]
- Wagh, A.; Sayyed, M.; Askin, A.; Özpolat, Ö.F.; Şakar, E.; Lakshminarayana, G.; Kamath, S.D. Influence of RE oxides (Eu3+, Sm3+, Nd3+) on gamma radiation shielding properties of lead fluoroborate glasses. Solid State Sci. 2019, 96, 105959. [Google Scholar] [CrossRef]
- Wilson, M. Optimization of the radiation shielding capabilities of bismuth-borate glasses using the genetic algorithm. Mater. Chem. Phys. 2019, 224, 238–245. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.-U.-Z.; Rashid, A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Results Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Sayyed, M. Bismuth modified shielding properties of zinc boro-tellurite glasses. J. Alloys Compd. 2016, 688, 111–117. [Google Scholar] [CrossRef]
- Çelikbilek, M.; Ersundu, A.; Solak, N.; Aydin, S. Investigation on thermal and microstructural characterization of the TeO2–WO3 system. J. Alloys Compd. 2011, 509, 5646–5654. [Google Scholar] [CrossRef]
- Moiseev, A.; Dorofeev, V.; Chilyasov, A.; Kraev, I.; Churbanov, M.; Kotereva, T.; Pimenov, V.; Snopatin, G.; Pushkin, A.; Gerasimenko, V.; et al. Production and properties of high purity TeO2–ZnO–Na2O–Bi2O3 and TeO2–WO3–La2O3–MoO3 glasses. Opt. Mater. 2011, 33, 1858–1861. [Google Scholar] [CrossRef]
- Dorofeev, V.; Moiseev, A.; Churbanov, M.; Snopatin, G.; Chilyasov, A.; Kraev, I.; Lobanov, A.; Kotereva, T.; Ketkova, L.; Pushkin, A.; et al. High-purity TeO2–WO3–(La2O3,Bi2O3) glasses for fiber-optics. Opt. Mater. 2011, 33, 1911–1915. [Google Scholar] [CrossRef]
- Upender, G.; Ramesh, S.; Prasad, M.; Sathe, V.; Mouli, V. Optical band gap, glass transition temperature and structural studies of (100−2x)TeO2–xAg2O–xWO3 glass system. J. Alloys Compd. 2010, 504, 468–474. [Google Scholar] [CrossRef]
- Gunha, J.; Somer, A.; Gonçalves, A.; Sabino, S.D.R.; El-Mallawany, R.; Jacinto, C.; Novatski, A. Non-isothermal crystallization of TeO2-Na2O-TiO2 glasses. J. Non-Cryst. Solids 2019, 524, 119655. [Google Scholar] [CrossRef]
- Manning, S.; Ebendorff-Heidepriem, H.; Monro, T. Ternary tellurite glasses for the fabrication of nonlinear optical fibres. Opt. Mater. Express 2012, 2, 140–152. [Google Scholar] [CrossRef]
- Sayyed, M.; Elhouichet, H. Variation of energy absorption and exposure buildup factors with incident photon energy and penetration depth for boro-tellurite (B2O3-TeO2) glasses. Radiat. Phys. Chem. 2017, 130, 335–342. [Google Scholar] [CrossRef]
- Elkhoshkhany, N.; Mohamed, H.M.; Yousef, E.S. UV–Vis-NIR spectroscopy, structural and thermal properties of novel oxyhalide tellurite glasses with composition TeO2-B2O3-SrCl2-LiF-Bi2O3 for optical application. Results Phys. 2019, 13, 102222. [Google Scholar] [CrossRef]
- Udovic, M.; Thomas, P.; Mirgorodsky, A.; Durand, O.; Soulis, M.; Masson, O.; Merle-Méjean, T.; Champarnaud-Mesjard, J. Thermal characteristics, Raman spectra and structural properties of new tellurite glasses within the Bi2O3–TiO2–TeO2 system. J. Solid State Chem. 2006, 179, 3252–3259. [Google Scholar] [CrossRef]
- Halimah, M.K.; Daud, W.M.; Sidek, H.A.A.; Zaidan, A.W.; Zainal, A.S. Optical properties of ternary tellurite glasses. Mater. Sci.-Pol. 2010, 28, 173–180. [Google Scholar]
- Lakshminarayana, G.; Sayyed, M.I.; Baki, S.O.; Lira, A.; Dong, M.G.; Bashar, K.A.; Kityk, I.V.; Mahdi, M.A. Borotellurite Glasses for Gamma-Ray Shielding: An Exploration of Photon Attenuation Coefficients and Structural and Thermal Properties. J. Electron. Mater. 2018, 48, 930–941. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Bashar, K.; Baki, S.; Lira, A.; Caldiño, U.; Meza-Rocha, A.; Falcony, C.; Camarillo, E.; Kityk, I.; Mahdi, M. Er3+/Dy3+ codoped B2O3-TeO2-PbO-ZnO-Li2O-Na2O glasses: Optical absorption and fluorescence features study for visible and near-infrared fiber laser applications. J. Non-Cryst. Solids 2019, 503-504, 366–381. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Beegam, A.; Prasad, P.; Jose, J.; Oliveira, M.; Costa, F.G.; Soares, A.M.; Gonçalves, P.; Trindade, T.; Kalarikkal, N.; Thomas, S.; et al. Environmental Fate of Zinc Oxide Nanoparticles: Risks and Benefits. In Toxicology—New Aspects to This Scientific Conundrum; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef] [Green Version]
- Shimoji, N.; Hashimoto, T.; Nasu, H.; Kamiya, K. Non-linear optical properties of Li2O–TiO2–P2O5 glasses. J. Non-Cryst. Solids 2003, 324, 50–57. [Google Scholar] [CrossRef]
- Gowda, V.V.; Reddy, C.N.; Radha, K.; Anavekar, R.; Etourneau, J.; Rao, K. Structural investigations of sodium diborate glasses containing PbO, Bi2O3 and TeO2: Elastic property measurements and spectroscopic studies. J. Non-Cryst. Solids 2007, 353, 1150–1163. [Google Scholar] [CrossRef]
- Hirashima, H.; Arai, D.; Yoshida, T. Electrical Conductivity of PbO-P2O5-V2O5Glasses. J. Am. Ceram. Soc. 1985, 68, 486–489. [Google Scholar] [CrossRef]
- Elliott, S.R.; Rao, C.; Thomas, J.M. The chemistry of the non-crystalline state. Angew. Chem. Int. Ed. 1986, 25, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Fong, W.; Bashar, K.; Baki, S.; Zaid, M.; Goh, B.; Mahdi, M. Thermal, structural and optical properties of Bi2O3-Na2O-TiO2-ZnO-TeO2 glass system. J. Non-Cryst. Solids 2021, 555, 120621. [Google Scholar] [CrossRef]
- Alım, B.; Şakar, E.; Baltakesmez, A.; Han, İ.; Sayyed, M.I.; Demir, L. Experimental investigation of radiation shielding perfor-mances of some important AISI-coded stainless steels: Part I. Radiat. Phys. Chem. 2020, 166, 108455. [Google Scholar] [CrossRef]
- Zakaly, H.M.; Saudi, H.; Tekin, H.; Rashad, M.; Issa, S.A.; Rammah, Y.; Elazaka, A.; Hessien, M.; Ene, A. Glass fabrication using ceramic and porcelain recycled waste and lithium niobate: Physical, structural, optical and nuclear radiation attenuation properties. J. Mater. Res. Technol. 2021, 15, 4074–4085. [Google Scholar] [CrossRef]
- Zakaly, H.M.; Ashry, A.; El-Taher, A.; Abbady, A.G.; Allam, E.A.; El-Sharkawy, R.M.; Mahmoud, M.E. Role of novel ternary nanocomposites polypropylene in nuclear radiation attenuation properties: In-depth simulation study. Radiat. Phys. Chem. 2021, 188, 109667. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Almatari, M.; Agar, O.; Altunsoy, E.; Kilicoglu, O.; Sayyed, M.; Tekin, H.O. Photon and neutron shielding characteristics of samarium doped lead alumino borate glasses containing barium, lithium and zinc oxides determined at medical diagnostic energies. Results Phys. 2019, 12, 2123–2128. [Google Scholar] [CrossRef]
- RSICC Computer Code Collection. In MCNPX ’User’s Manual Version 2.4.0. In Monte Carlo N-Particle Transport Code System for Multiple and High Energy Applications; Oak Ridge National Laboratory: Oak Ridge, TN, USA; Advanced Accelerator Applications Los Alamos National Laboratory: Los Alamos, NM, USA, 2002. [Google Scholar]
- Lakshminarayana, G.; Issa, S.A.; Saddeek, Y.; Tekin, H.; Al-Buriahi, M.; Dong, M.; Lee, D.-E.; Yoon, J.; Park, T. Analysis of physical and mechanical traits and nuclear radiation transmission aspects of Gallium(III) trioxide constituting Bi2O3-B2O3 glasses. Results Phys. 2021, 30, 104899. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kumar, A.; Tekin, H.; Issa, S.A.; Al-Buriahi, M.; Dong, M.; Lee, D.-E.; Yoon, J.; Park, T. Probing of nuclear radiation attenuation and mechanical features for lithium bismuth borate glasses with improving Bi2O3 content for B2O3 + Li2O amounts. Results Phys. 2021, 25, 104246. [Google Scholar] [CrossRef]
- Kurtulus, R.; Kavas, T.; Akkurt, I.; Gunoglu, K.; Tekin, H.O.; Kurtulus, C. A comprehensive study on novel alumino-borosilicate glass reinforced with Bi2O3 for radiation shielding applications: Synthesis, spectrometer, XCOM, and MCNP-X works. J. Mater. Sci. Mater. Electron. 2021, 32, 13882–13896. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kumar, A.; Tekin, H.; Issa, S.A.; Al-Buriahi, M.; Lee, D.-E.; Yoon, J.; Park, T. Binary B2O3–Bi2O3 glasses: Scrutinization of directly and indirectly ionizing radiations shielding abilities. J. Mater. Res. Technol. 2020, 9, 14549–14567. [Google Scholar] [CrossRef]





| Sample Code | Elemental Weight Fraction (wt%) | Density (g/cm3) | |||||
|---|---|---|---|---|---|---|---|
| Te | O | Zn | Ti | Na | Bi | ||
| S1 | 0.6048 | 0.1921 | 0.0413 | 0.0151 | 0.0145 | 0.1321 | 5.401 |
| S2 | 0.5488 | 0.1844 | 0.0391 | 0.0143 | 0.0137 | 0.1997 | 5.613 |
| S3 | 0.5147 | 0.1798 | 0.0377 | 0.0138 | 0.0132 | 0.2408 | 5.762 |
| S4 | 0.4829 | 0.1754 | 0.0364 | 0.0133 | 0.0128 | 0.2791 | 5.844 |
| S5 | 0.4392 | 0.1694 | 0.0346 | 0.0127 | 0.0122 | 0.3320 | 6.138 |
| Transmission Factors | ||||||||||||||
| S1 | Energy (MeV) | 0.5 m | 1 cm | 1.5 cm | 2 cm | 2.5 cm | 3 cm | S2 | 0.5 m | 1 cm | 1.5 cm | 2 cm | 2.5 cm | 3 cm |
| 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.023 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.053 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.071 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.080 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.006 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.081 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.122 | 0.214 | 0.010 | 0.001 | 0.000 | 0.000 | 0.000 | 0.121 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.135 | 0.411 | 0.041 | 0.004 | 0.000 | 0.000 | 0.000 | 0.264 | 0.017 | 0.001 | 0.000 | 0.000 | 0.000 | ||
| 0.136 | 0.437 | 0.046 | 0.005 | 0.000 | 0.000 | 0.000 | 0.285 | 0.020 | 0.001 | 0.000 | 0.000 | 0.000 | ||
| 0.141 | 0.509 | 0.064 | 0.008 | 0.001 | 0.000 | 0.000 | 0.341 | 0.029 | 0.002 | 0.000 | 0.000 | 0.000 | ||
| 0.167 | 1.027 | 0.261 | 0.068 | 0.017 | 0.004 | 0.001 | 0.794 | 0.154 | 0.030 | 0.005 | 0.001 | 0.000 | ||
| 0.171 | 1.102 | 0.303 | 0.082 | 0.022 | 0.006 | 0.002 | 0.868 | 0.183 | 0.040 | 0.008 | 0.002 | 0.000 | ||
| 0.184 | 1.349 | 0.451 | 0.149 | 0.050 | 0.017 | 0.005 | 1.102 | 0.301 | 0.083 | 0.022 | 0.005 | 0.001 | ||
| 0.245 | 2.198 | 1.216 | 0.669 | 0.363 | 0.194 | 0.107 | 1.989 | 0.992 | 0.493 | 0.239 | 0.119 | 0.058 | ||
| 0.276 | 2.476 | 1.548 | 0.961 | 0.594 | 0.365 | 0.219 | 2.297 | 1.331 | 0.767 | 0.439 | 0.246 | 0.139 | ||
| 0.284 | 2.535 | 1.622 | 1.029 | 0.653 | 0.409 | 0.253 | 2.369 | 1.408 | 0.831 | 0.489 | 0.284 | 0.165 | ||
| 0.303 | 2.652 | 1.781 | 1.187 | 0.790 | 0.522 | 0.342 | 2.497 | 1.578 | 0.988 | 0.616 | 0.382 | 0.233 | ||
| 0.320 | 2.745 | 1.911 | 1.321 | 0.909 | 0.621 | 0.425 | 2.602 | 1.716 | 1.123 | 0.734 | 0.473 | 0.304 | ||
| 0.356 | 2.903 | 2.135 | 1.562 | 1.138 | 0.829 | 0.595 | 2.784 | 1.965 | 1.374 | 0.959 | 0.665 | 0.458 | ||
| 0.364 | 2.932 | 2.179 | 1.615 | 1.192 | 0.873 | 0.637 | 2.821 | 2.016 | 1.430 | 1.010 | 0.710 | 0.496 | ||
| 0.384 | 2.994 | 2.275 | 1.724 | 1.298 | 0.973 | 0.730 | 2.891 | 2.119 | 1.545 | 1.120 | 0.810 | 0.580 | ||
| 0.511 | 3.249 | 2.695 | 2.221 | 1.826 | 1.494 | 1.215 | 3.187 | 2.587 | 2.091 | 1.683 | 1.344 | 1.070 | ||
| 0.637 | 3.376 | 2.913 | 2.497 | 2.140 | 1.829 | 1.555 | 3.333 | 2.834 | 2.396 | 2.025 | 1.704 | 1.423 | ||
| 0.662 | 3.394 | 2.945 | 2.539 | 2.189 | 1.881 | 1.609 | 3.353 | 2.871 | 2.442 | 2.077 | 1.761 | 1.484 | ||
| 0.723 | 3.433 | 3.010 | 2.628 | 2.288 | 1.988 | 1.727 | 3.395 | 2.945 | 2.540 | 2.191 | 1.877 | 1.605 | ||
| 0.811 | 3.476 | 3.090 | 2.737 | 2.414 | 2.130 | 1.872 | 3.445 | 3.032 | 2.660 | 2.322 | 2.023 | 1.763 | ||
| 1.173 | 3.591 | 3.291 | 3.014 | 2.752 | 2.503 | 2.275 | 3.574 | 3.257 | 2.963 | 2.685 | 2.424 | 2.191 | ||
| 1.332 | 3.622 | 3.352 | 3.093 | 2.847 | 2.614 | 2.400 | 3.606 | 3.321 | 3.047 | 2.791 | 2.548 | 2.324 | ||
| Transmission Factors | ||||||||||||||
| S3 | Energy (MeV) | 0.5 m | 1 cm | 1.5 cm | 2 cm | 2.5 cm | 3 cm | S4 | 0.5 m | 1 cm | 1.5 cm | 2 cm | 2.5 cm | 3 cm |
| 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.023 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.053 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.071 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.080 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.081 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.122 | 0.079 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.058 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.135 | 0.186 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.145 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.136 | 0.203 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.159 | 0.006 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.141 | 0.250 | 0.015 | 0.001 | 0.000 | 0.000 | 0.000 | 0.197 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 | ||
| 0.167 | 0.651 | 0.103 | 0.017 | 0.002 | 0.000 | 0.000 | 0.562 | 0.078 | 0.010 | 0.001 | 0.000 | 0.000 | ||
| 0.171 | 0.718 | 0.124 | 0.022 | 0.003 | 0.001 | 0.000 | 0.626 | 0.094 | 0.015 | 0.002 | 0.000 | 0.000 | ||
| 0.184 | 0.942 | 0.217 | 0.051 | 0.011 | 0.003 | 0.001 | 0.838 | 0.171 | 0.035 | 0.007 | 0.001 | 0.000 | ||
| 0.245 | 1.845 | 0.849 | 0.387 | 0.173 | 0.081 | 0.036 | 1.743 | 0.756 | 0.324 | 0.137 | 0.059 | 0.025 | ||
| 0.276 | 2.164 | 1.181 | 0.642 | 0.344 | 0.182 | 0.098 | 2.075 | 1.082 | 0.561 | 0.286 | 0.146 | 0.075 | ||
| 0.284 | 2.235 | 1.260 | 0.707 | 0.390 | 0.212 | 0.117 | 2.147 | 1.159 | 0.624 | 0.330 | 0.174 | 0.093 | ||
| 0.303 | 2.384 | 1.434 | 0.853 | 0.508 | 0.298 | 0.175 | 2.302 | 1.337 | 0.772 | 0.442 | 0.248 | 0.140 | ||
| 0.320 | 2.497 | 1.578 | 0.988 | 0.616 | 0.382 | 0.232 | 2.424 | 1.486 | 0.901 | 0.544 | 0.327 | 0.193 | ||
| 0.356 | 2.694 | 1.839 | 1.242 | 0.836 | 0.558 | 0.373 | 2.630 | 1.750 | 1.157 | 0.760 | 0.496 | 0.320 | ||
| 0.364 | 2.735 | 1.893 | 1.300 | 0.887 | 0.600 | 0.407 | 2.672 | 1.807 | 1.213 | 0.809 | 0.534 | 0.354 | ||
| 0.384 | 2.814 | 2.007 | 1.417 | 0.997 | 0.700 | 0.489 | 2.758 | 1.925 | 1.334 | 0.920 | 0.627 | 0.430 | ||
| 0.511 | 3.139 | 2.510 | 1.994 | 1.579 | 1.240 | 0.973 | 3.109 | 2.457 | 1.927 | 1.510 | 1.171 | 0.907 | ||
| 0.637 | 3.299 | 2.776 | 2.320 | 1.940 | 1.612 | 1.330 | 3.277 | 2.737 | 2.269 | 1.883 | 1.551 | 1.268 | ||
| 0.662 | 3.322 | 2.816 | 2.372 | 1.995 | 1.673 | 1.392 | 3.301 | 2.779 | 2.320 | 1.941 | 1.612 | 1.331 | ||
| 0.723 | 3.368 | 2.896 | 2.472 | 2.111 | 1.797 | 1.523 | 3.351 | 2.862 | 2.432 | 2.065 | 1.745 | 1.467 | ||
| 0.811 | 3.421 | 2.990 | 2.604 | 2.257 | 1.951 | 1.680 | 3.406 | 2.962 | 2.564 | 2.213 | 1.902 | 1.629 | ||
| 1.173 | 3.556 | 3.230 | 2.925 | 2.636 | 2.372 | 2.132 | 3.546 | 3.214 | 2.899 | 2.607 | 2.338 | 2.095 | ||
| 1.332 | 3.594 | 3.299 | 3.014 | 2.749 | 2.497 | 2.265 | 3.586 | 3.285 | 2.994 | 2.723 | 2.464 | 2.232 | ||
| Transmission Factors | ||||||||||||||
| S5 | Energy (MeV) | 0.5 m | 1 cm | 1.5 cm | 2 cm | 2.5 cm | 3 cm | |||||||
| 0.0086 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0093 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0144 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0230 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0532 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0710 | 0.0003 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0796 | 0.0037 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.0810 | 0.0050 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.1221 | 0.0324 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.1350 | 0.0921 | 0.0020 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.1365 | 0.1011 | 0.0025 | 0.00003 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.1405 | 0.1301 | 0.0038 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | ||||||||
| 0.1670 | 0.4227 | 0.0432 | 0.0040 | 0.0004 | 0.00003 | 0.00003 | ||||||||
| 0.1710 | 0.4784 | 0.0552 | 0.0062 | 0.0007 | 0.00003 | 0.00003 | ||||||||
| 0.1840 | 0.6758 | 0.1093 | 0.0173 | 0.0027 | 0.0004 | 0.00003 | ||||||||
| 0.2450 | 1.5604 | 0.6071 | 0.2286 | 0.0899 | 0.0339 | 0.0127 | ||||||||
| 0.2764 | 1.9171 | 0.9188 | 0.4355 | 0.2040 | 0.0970 | 0.0452 | ||||||||
| 0.2843 | 1.9902 | 0.9915 | 0.4911 | 0.2397 | 0.1185 | 0.0579 | ||||||||
| 0.3029 | 2.1534 | 1.1662 | 0.6314 | 0.3358 | 0.1776 | 0.0958 | ||||||||
| 0.3201 | 2.2848 | 1.3180 | 0.7538 | 0.4273 | 0.2370 | 0.1348 | ||||||||
| 0.3560 | 2.5129 | 1.5928 | 1.0020 | 0.6243 | 0.3877 | 0.2363 | ||||||||
| 0.3645 | 2.5586 | 1.6526 | 1.0579 | 0.6725 | 0.4237 | 0.2649 | ||||||||
| 0.3838 | 2.6503 | 1.7764 | 1.1800 | 0.7796 | 0.5107 | 0.3330 | ||||||||
| 0.5110 | 3.0380 | 2.3395 | 1.7971 | 1.3693 | 1.0352 | 0.7831 | ||||||||
| 0.6370 | 3.2264 | 2.6540 | 2.1653 | 1.7613 | 1.4272 | 1.1449 | ||||||||
| 0.6617 | 3.2547 | 2.6972 | 2.2176 | 1.8216 | 1.4873 | 1.2062 | ||||||||
| 0.7229 | 3.3084 | 2.7894 | 2.3331 | 1.9507 | 1.6241 | 1.3425 | ||||||||
| 0.8108 | 3.3693 | 2.8979 | 2.4795 | 2.1109 | 1.7939 | 1.5160 | ||||||||
| 1.1732 | 3.5225 | 3.1716 | 2.8400 | 2.5294 | 2.2529 | 1.9987 | ||||||||
| 1.3325 | 3.5671 | 3.2455 | 2.9392 | 2.6507 | 2.3865 | 2.1460 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, H.O.; ALMisned, G.; Susoy, G.; Ali, F.T.; Baykal, D.S.; Ene, A.; Issa, S.A.M.; Rammah, Y.S.; Zakaly, H.M.H. Transmission Factor (TF) Behavior of Bi2O3–TeO2–Na2O–TiO2–ZnO Glass System: A Monte Carlo Simulation Study. Sustainability 2022, 14, 2893. https://doi.org/10.3390/su14052893
Tekin HO, ALMisned G, Susoy G, Ali FT, Baykal DS, Ene A, Issa SAM, Rammah YS, Zakaly HMH. Transmission Factor (TF) Behavior of Bi2O3–TeO2–Na2O–TiO2–ZnO Glass System: A Monte Carlo Simulation Study. Sustainability. 2022; 14(5):2893. https://doi.org/10.3390/su14052893
Chicago/Turabian StyleTekin, Huseyin O., Ghada ALMisned, Gulfem Susoy, Fatema T. Ali, Duygu Sen Baykal, Antoaneta Ene, Shams A. M. Issa, Yasser S. Rammah, and Hesham M. H. Zakaly. 2022. "Transmission Factor (TF) Behavior of Bi2O3–TeO2–Na2O–TiO2–ZnO Glass System: A Monte Carlo Simulation Study" Sustainability 14, no. 5: 2893. https://doi.org/10.3390/su14052893
APA StyleTekin, H. O., ALMisned, G., Susoy, G., Ali, F. T., Baykal, D. S., Ene, A., Issa, S. A. M., Rammah, Y. S., & Zakaly, H. M. H. (2022). Transmission Factor (TF) Behavior of Bi2O3–TeO2–Na2O–TiO2–ZnO Glass System: A Monte Carlo Simulation Study. Sustainability, 14(5), 2893. https://doi.org/10.3390/su14052893












