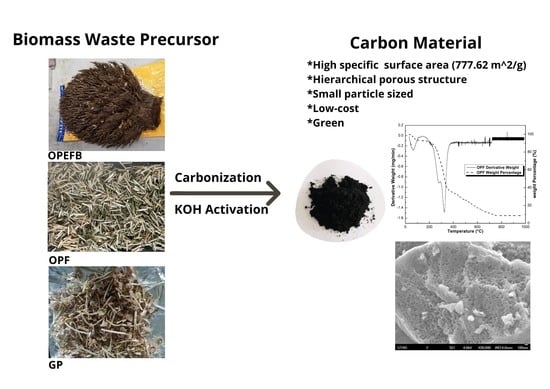
Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
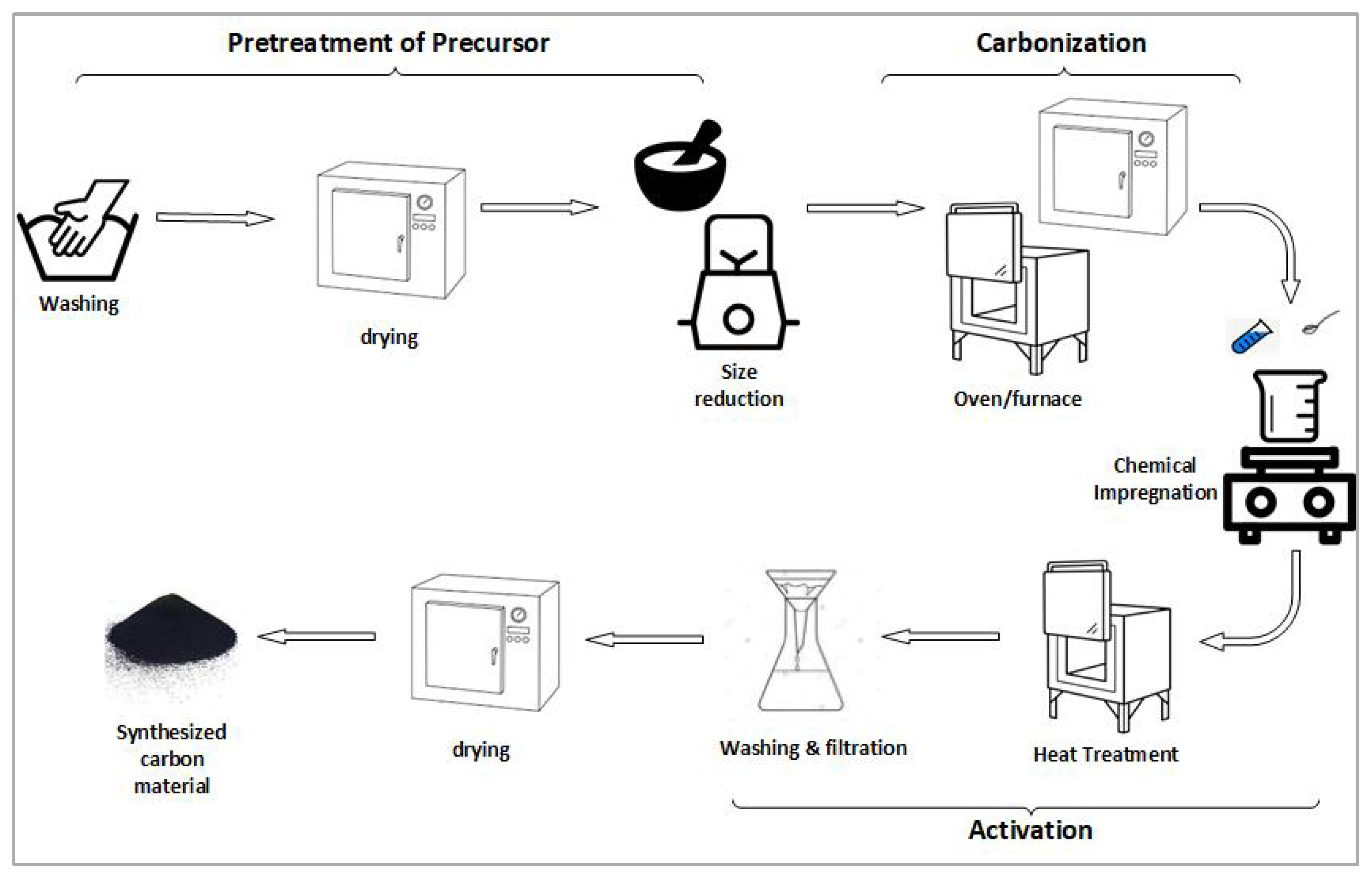
2.2. Synthesis of Carbon Material
2.3. Characterization of Carbon Material
2.3.1. Lignocellulosic Component Test
2.3.2. Chemical Characterization
2.3.3. Physical Characterization
3. Results and Discussion
3.1. Chemical Properties Characterization
3.1.1. Lignocellulosic Composition Test
3.1.2. SEM-EDX Analysis
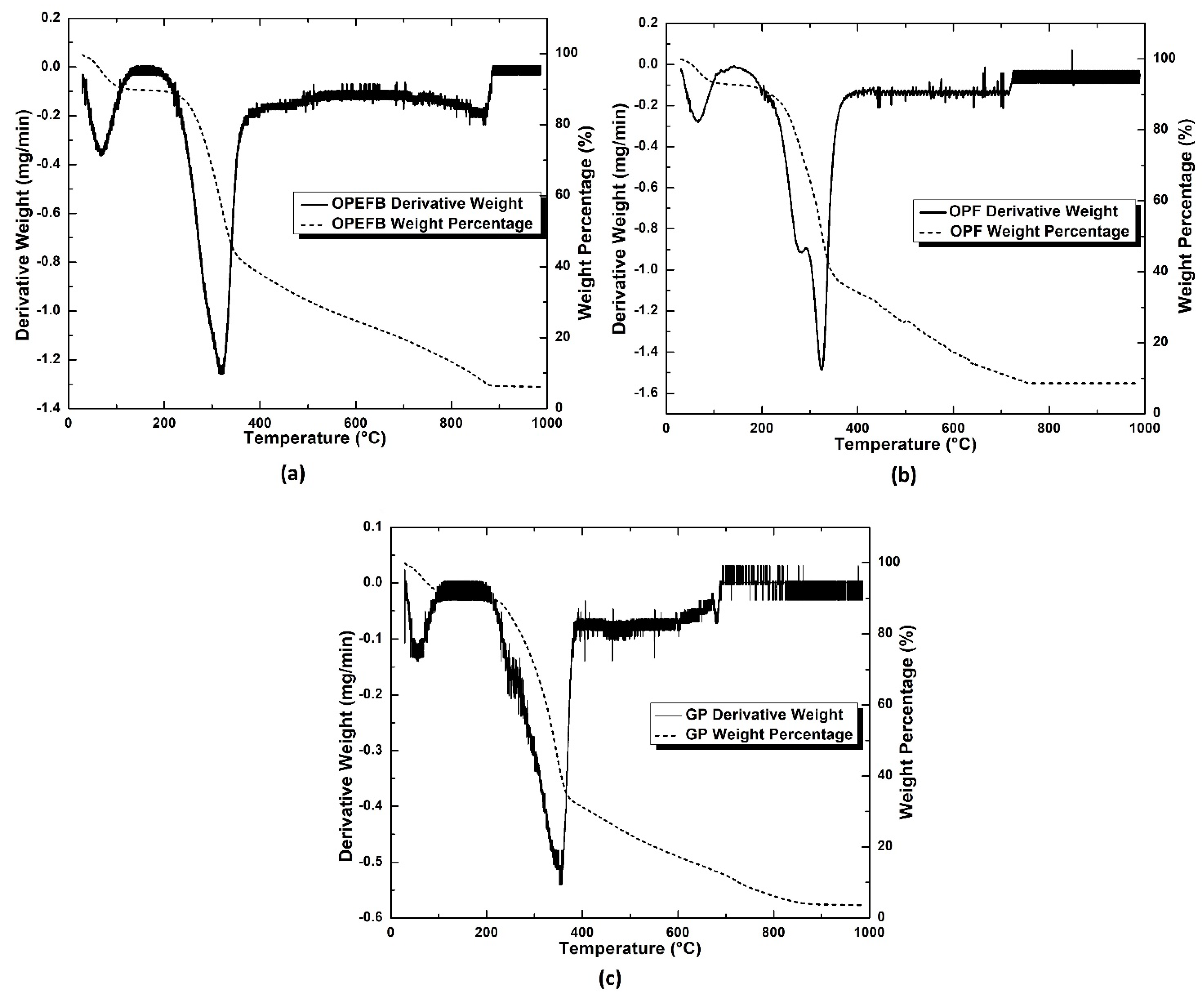
3.1.3. TGA Analysis
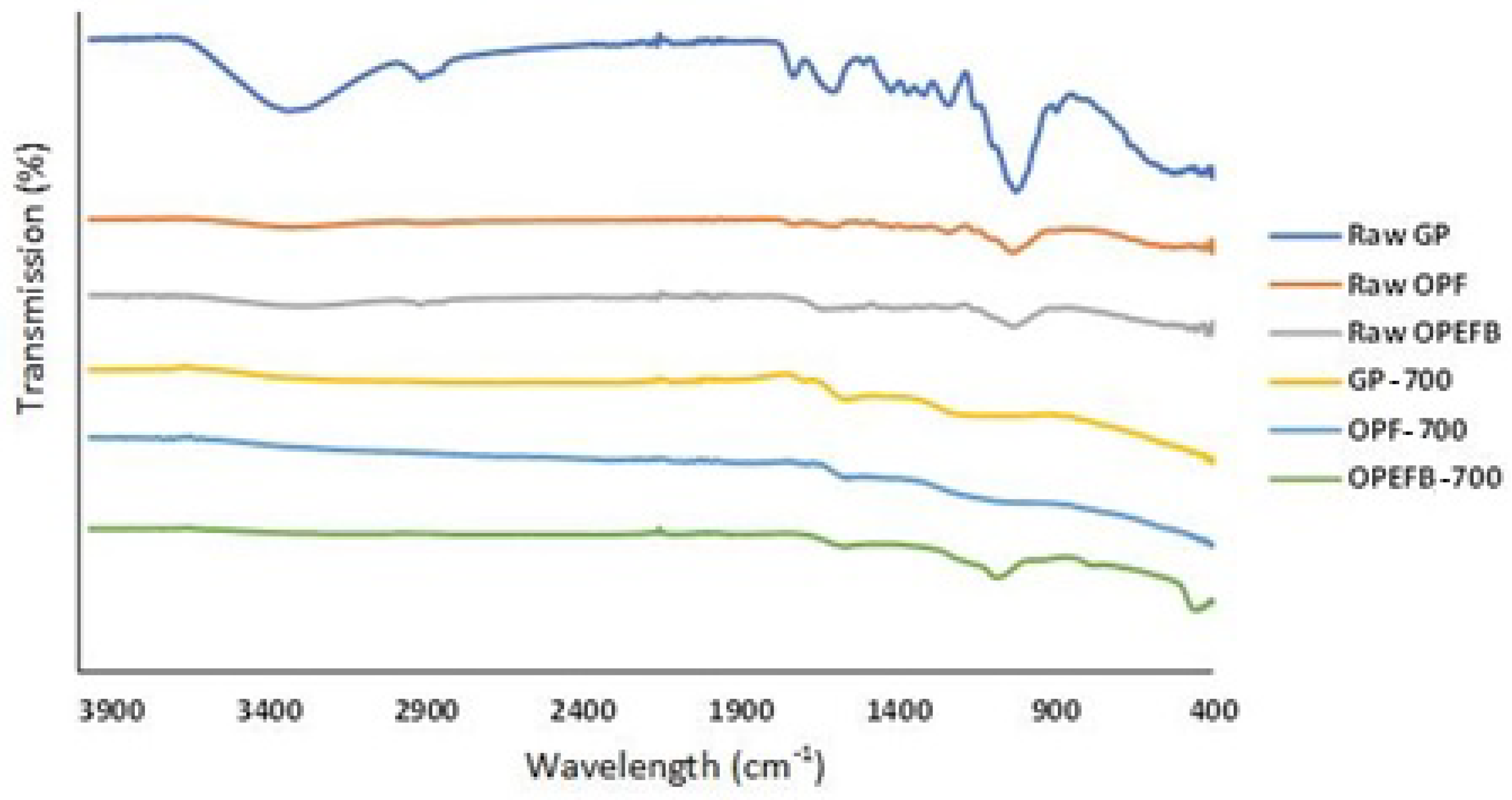
3.1.4. FTIR Analysis
3.2. Physical Properties Characterization
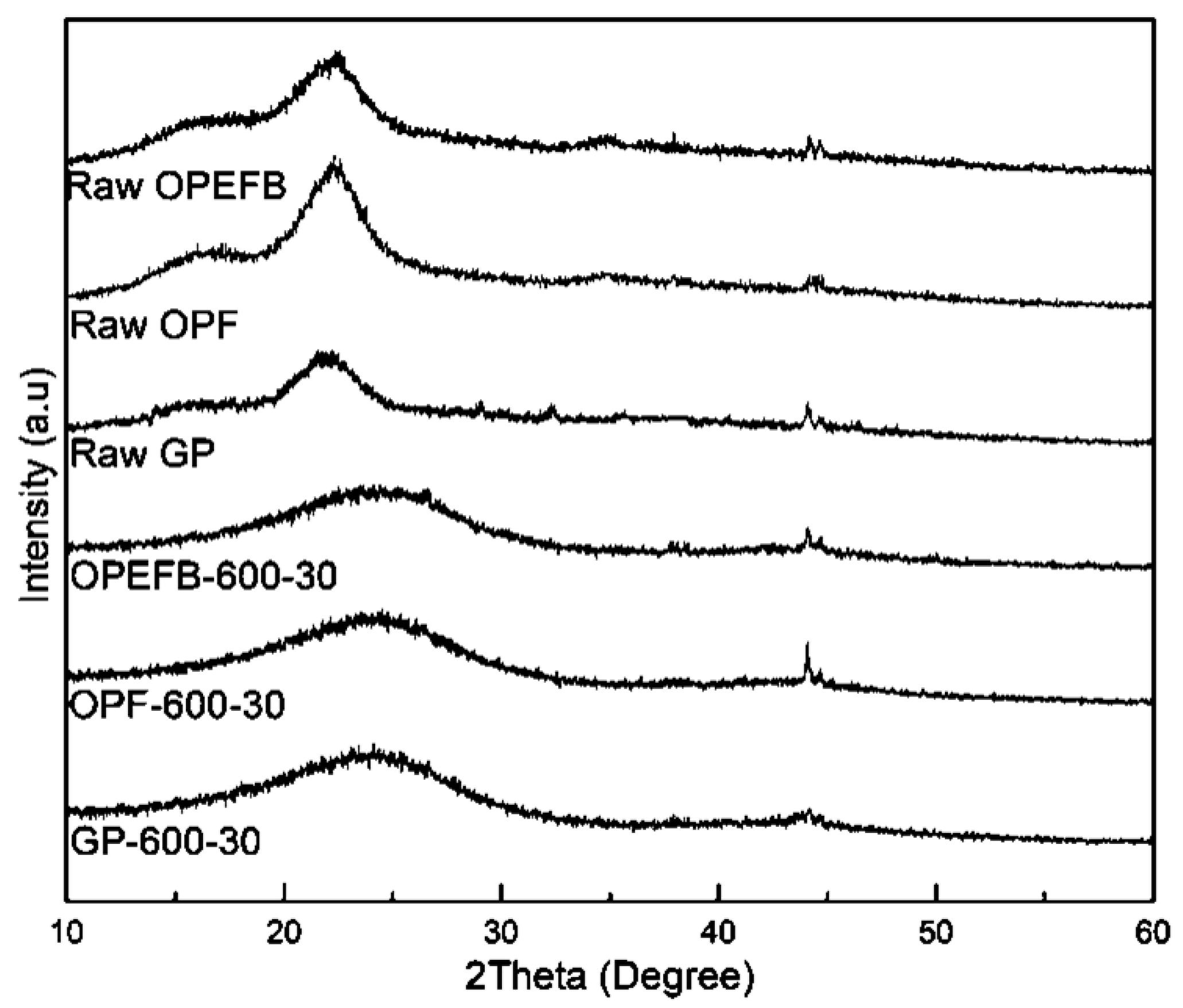
3.2.1. XRD Analysis
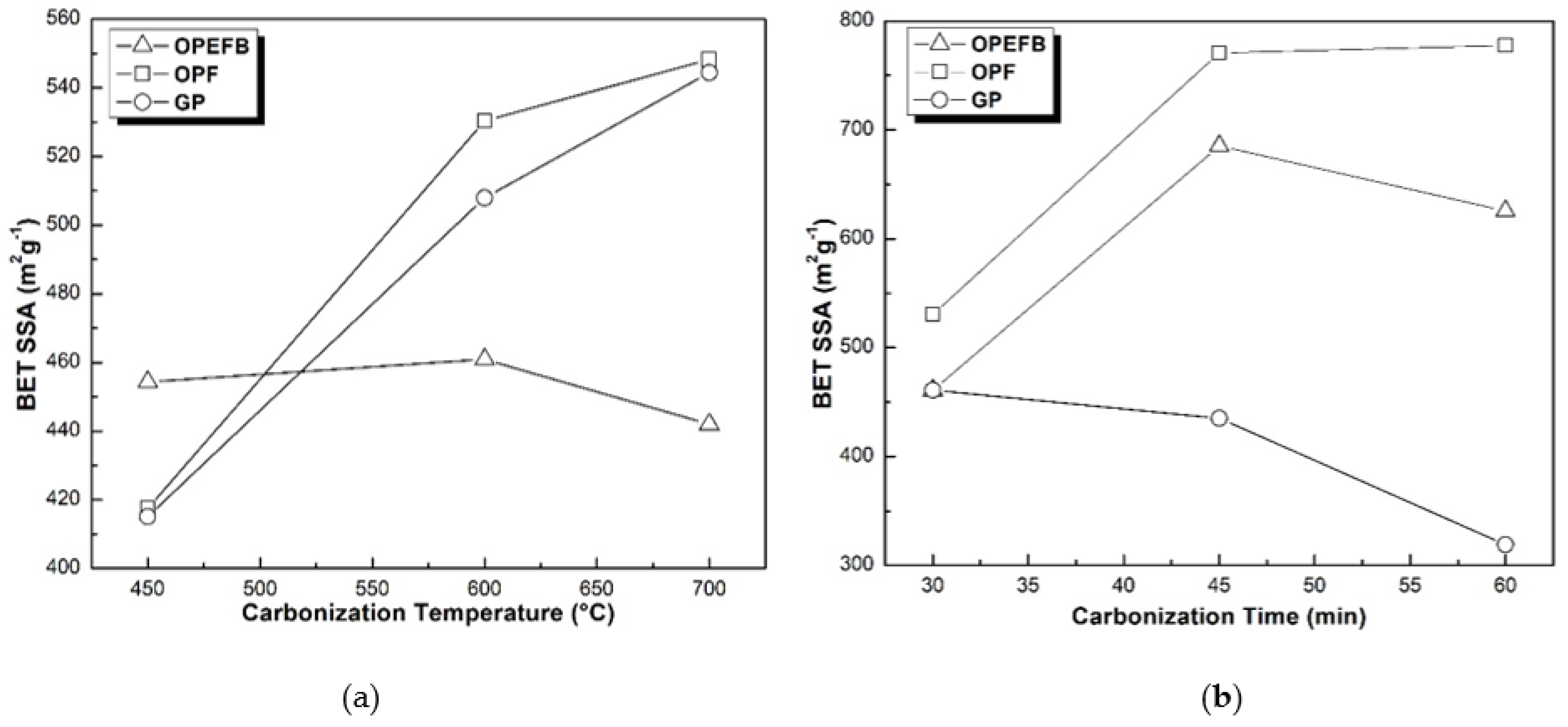
3.2.2. BET Analysis
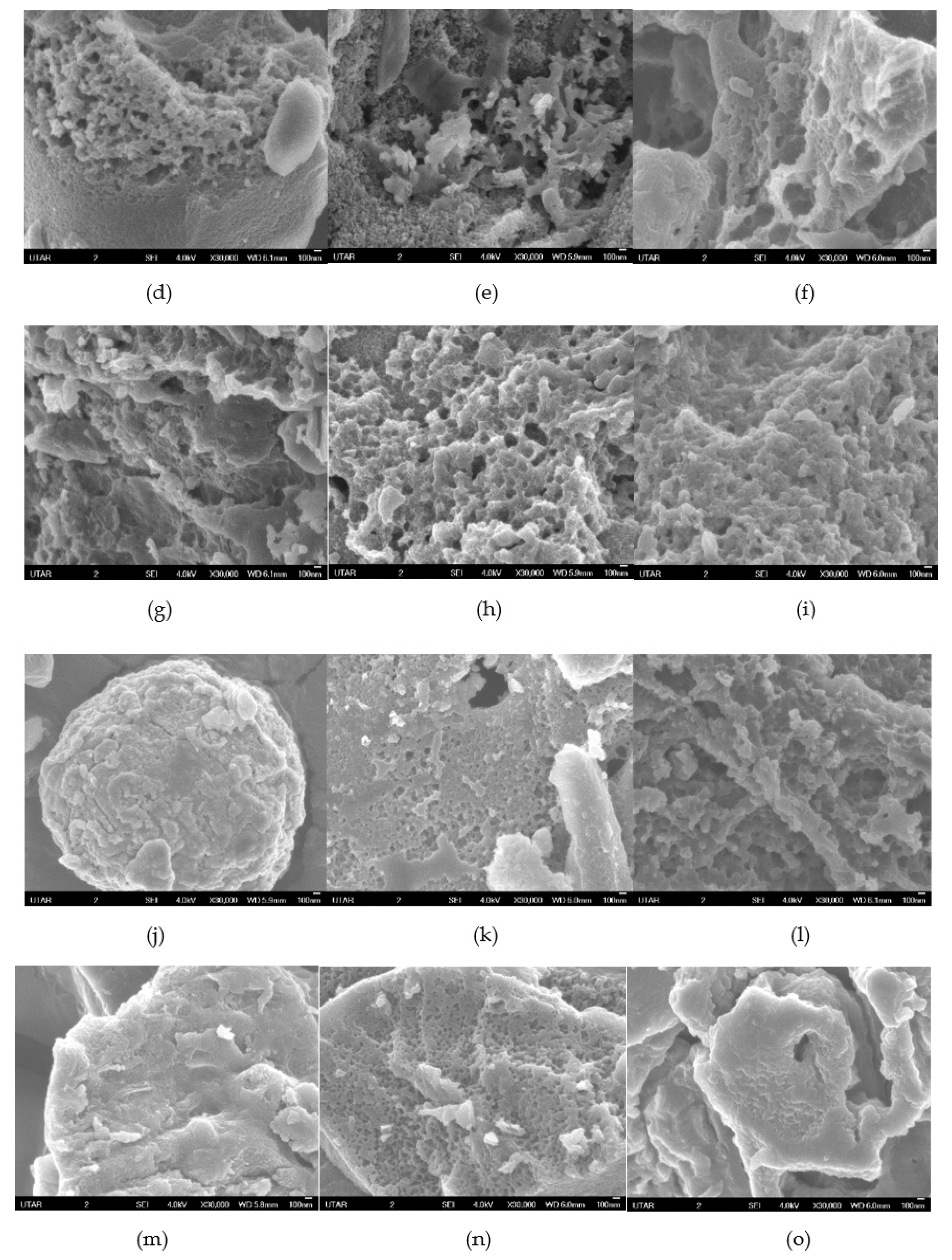
3.2.3. FESEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Praene, J.P.; Fakra, D.A.H.; Benard, F.; Ayagapin, L.; Rachadi, M.N.M. Comoros’s Energy Review for Promoting Renewable Energy Sources. Renew. Energy 2021, 169, 885–893. [Google Scholar] [CrossRef]
- Liedel, C. Sustainable Battery Materials from Biomass. ChemSusChem 2020, 13, 2110–2141. [Google Scholar] [CrossRef]
- Mori, R. Recent Developments for Aluminum–Air Batteries. Electrochem. Energy Rev. 2020, 3, 344–369. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of Lithium-Ion Batteries in Grid-Scale Energy Storage Systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.; Latz, A.; Horstmann, B. A Review of Model-Based Design Tools for Metal-Air Batteries. Batteries 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent Progress of Metal–Air Batteries-A Mini Review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef] [Green Version]
- Zhong, M.; Liu, M.; Li, N.; Bu, X.H. Recent Advances and Perspectives of Metal/Covalent-Organic Frameworks in Metal-Air Batteries. J. Energy Chem. 2021, 63, 113–129. [Google Scholar] [CrossRef]
- Lu, G.; Li, Z.; Fan, W.; Wang, M.; Yang, S.; Li, J.; Chang, Z.; Sun, H.; Liang, S.; Liu, Z. Sponge-like N-Doped Carbon Materials with Co-Based Nanoparticles Derived from Biomass as Highly Efficient Electrocatalysts for the Oxygen Reduction Reaction in Alkaline Media. RSC Adv. 2019, 9, 4843–4848. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.A.; Pozo-Gonzalo, C.; Nairn, K.; MacFarlane, D.R.; Forsyth, M.; Howlett, P.C. An Investigation of Commercial Carbon Air Cathode Structure in Ionic Liquid Based Sodium Oxygen Batteries. Sci. Rep. 2020, 10, 7123. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Ox-ygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhon, S.S.; Lee, J.; Park, J.S. Biomass-Derived Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reaction: A Review. J. Energy Chem. 2021, 65, 149–172. [Google Scholar] [CrossRef]
- Malik, S.; Marchesan, S. Growth, Properties, and Applications of Branched Carbon Nanostructures. Nanomaterials 2021, 11, 2728. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.; Badhulika, S. Effect of Self-Doped Heteroatoms on the Performance of Biomass-Derived Carbon for Supercapacitor Applications. J. Power Sources 2020, 480, 228830. [Google Scholar] [CrossRef]
- He, G.; Yan, G.; Song, Y.; Wang, L. Biomass Juncus Derived Nitrogen-Doped Porous Carbon Materials for Supercapacitor and Oxygen Reduction Reaction. Front. Chem. 2020, 8, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Xiang, R.; Lin, J.; Cheng, Y.; Lu, C. Lignocellulosic Biomass-Derived Carbon Electrodes for Flexible Supercapacitors: An Overview. Materials 2021, 14, 4571. [Google Scholar] [CrossRef]
- Wang, N.; Li, T.; Song, Y.; Liu, J.; Wang, F. Metal-Free Nitrogen-Doped Porous Carbons Derived from Pomelo Peel Treated by Hypersaline Environments for Oxygen Reduction Reaction. Carbon 2018, 130, 692–700. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Kolanowski, L.; Graś, M.; Bartkowiak, M.; Doczekalska, B.; Lota, G. Electrochemical Capacitors Based on Electrodes Made of Lignocellulosic Waste Materials. Waste Biomass Valorization 2020, 11, 3863–3871. [Google Scholar] [CrossRef] [Green Version]
- Hendriansyah, R.; Prakoso, T.; Widiatmoko, P.; Nurdin, I.; Devianto, H. Manufacturing Carbon Material by Carbonization of Cellulosic Palm Oil Waste for Supercapacitor Material. MATEC Web Conf. 2018, 156, 03018. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Lee, J.H.; Roh, K.C. Herbaceous Biomass Waste-Derived Activated Carbons for Supercapacitors. J. Electrochem. Sci. Technol. 2018, 9, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated Carbons from KOH and H3PO4-Activation of Olive Residues and Its Application as Supercapacitor Electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.E.; Kim, S.; Lim, M.S.; Jin, A.; Kim, O.H.; Kim, M.J.; Lee, K.S.; Kim, J.; Kim, S.S.; et al. Biomass-Derived Air Cathode Materials: Pore-Controlled S,N-Co-Doped Carbon for Fuel Cells and Metal–Air Batteries. ACS Catal. 2019, 9, 3389–3398. [Google Scholar] [CrossRef]
- Hao, X.; Chen, W.; Jiang, Z.; Tian, X.; Hao, X.; Maiyalagan, T.; Jiang, Z.J. Conversion of Maize Straw into Nitrogen-Doped Porous Graphitized Carbon with Ultra-High Surface Area as Excellent Oxygen Reduction Electrocatalyst for Flexible Zinc–Air Batteries. Electrochim. Acta 2020, 362, 137143. [Google Scholar] [CrossRef]
- Zainol, M.M.; Amin, N.A.S.; Asmadi, M. Preparation and Characterization of Impregnated Magnetic Particles on Oil Palm Frond Activated Carbon for Metal Ions Removal. Sains Malays. 2017, 46, 773–782. [Google Scholar] [CrossRef]
- Muhamad, N.A.S.; Hanoin, M.A.H.M.; Mokhtar, N.M.; Lau, W.J.; Jaafar, J. Industrial Application of Membrane Distillation Technology Using Palm Oil Mill Effluent in Malaysia. Mater. Today Proc. 2021, 575. [Google Scholar] [CrossRef]
- Nurika, I.; Shabrina, E.N.; Azizah, N.; Suhartini, S.; Bugg, T.D.H.; Barker, G.C. Application of Ligninolytic Bacteria to the Enhancement of Lignocellulose Breakdown and Methane Production from Oil Palm Empty Fruit Bunches (OPEFB). Bioresour. Technol. Rep. 2022, 17, 100951. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Chong, K.P. Formulation of Biofertilizers from Oil Palm Empty Fruit Bunches and Plant Growth-Promoting Microbes: A Comprehensive and Novel Approach towards Plant Health. J. King Saud Univ.-Sci. 2021, 33, 101647. [Google Scholar] [CrossRef]
- Fatihah Salleh, S.; Abd Rahman, A.; Ab Rashid Tuan Abdullah, T. Potential of Deploying Empty Fruit Bunch (EFB) for Biomass Cofiring in Malaysia’s Largest Coal Power Plant. In Proceedings of the 2018 IEEE International Conference on Power and Energy (PECon2018), Kuala Lumpur, Malaysia, 3–4 December 2018. [Google Scholar]
- Department of Statistics Malaysia Malaysian CPI Inflation Calculator. Available online: https://www.dosm.gov.my/cpi_calc/ (accessed on 22 December 2021).
- Lawal, A.A.; Hassan, M.A.; Farid, M.A.A.; Yasim-Anuar, T.A.T.; Yusoff, M.Z.M.; Zakaria, M.R.; Roslan, A.M.; Mokhtar, M.N.; Shirai, Y. Production of Biochar from Oil Palm Frond by Steam Pyrolysis for Removal of Residual Contaminants in Palm Oil Mill Effluent Final Discharge. J. Clean. Prod. 2020, 265, 121643. [Google Scholar] [CrossRef]
- Osman, N.B.; Shamsuddin, N.; Uemura, Y. Activated Carbon of Oil Palm Empty Fruit Bunch (EFB); Core and Shaggy. Procedia Eng. 2016, 148, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Zeng Wei, H. Effect of Pretreatment for Synthesis of Oil Palm Frond Based Catalyst for Biodiesel Production. Ph.D. Thesis, Universiti Tunku Abdul Rahman, Kampar, Perak, Malaysia, May 2019. [Google Scholar]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Characterization of Fennel Extracts and Quantification of Estragole: Optimization and Comparison of Accelerated Solvent Extraction and Soxhlet Techniques. Ind. Crops Prod. 2014, 52, 528–536. [Google Scholar] [CrossRef]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass: Laboratory Analytical Procedure (LAP). 2008. Available online: https://www.nrel.gov/docs/gen/fy08/42619.pdf (accessed on 19 December 2021).
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lim, C.H.; Shuit, S.H.; Lee, K.M.; Chong, C.T. Effects of Organic Solvents on the Organosolv Pretreatment of Degraded Empty Fruit Bunch for Fractionation and Lignin Removal. Sustainability 2021, 13, 6757. [Google Scholar] [CrossRef]
- Wang, Y.J.; Fang, B.; Zhang, D.; Li, A.; Wilkinson, D.P.; Ignaszak, A.; Zhang, L.; Zhang, J. A Review of Carbon-Composited Materials as Air-Electrode Bifunctional Electrocatalysts for Metal-Air Batteries; Springer: Singapore, 2018; Volume 1, ISBN 0123456789. [Google Scholar]
- Del Mar Saavedra Rios, C.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from Various Biomass Types as Precursors for Hard Carbon Anodes in Sodium-Ion Batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of Lignocellulose into Cellulose, Hemicellulose, and Lignin for Preparation of Porous Carbon Materials with Enhanced Performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Hendrawan, Y.; Sajidah, N.; Umam, C.; Fauzy, M.R.; Wibisono, Y.; Hawa, L.C. Effect of Carbonization Temperature Variations and Activator Agent Types on Activated Carbon Characteristics of Sengon Wood Waste (Paraserianthes Falcataria (L.) Nielsen). In Proceedings of the 12th International Interdisciplinary Studies Seminar—Environmental Conservation and Education for Sustainable Development, Malang, Indonesia, 14–15 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 239. [Google Scholar]
- Zhao, R.; Ni, B.; Wu, L.; Sun, P.; Chen, T. Carbon-Based Iron-Cobalt Phosphate FeCoP/C as an Effective ORR/OER/HER Triunctional Electrocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128118. [Google Scholar] [CrossRef]
- Oh, W.-D.; Lisak, G.; Webster, R.D.; Liang, Y.N.; Veksha, A.; Giannis, A.; Moo, J.G.S.; Lim, J.W.; Lim, T.T. Insights into the Thermolytic Transformation of Lignocellulosic Biomass Waste to Redox-Active Carbocatalyst: Durability of Surface Active Sites. Appl. Catal. B Environ. 2018, 233, 120–129. [Google Scholar] [CrossRef]
- Zhao, L.; Ning, G.; Zhang, S. Green Synthesis of S-Doped Carbon Nanotubes via Gaseous Post-Treatment and Their Application as Conductive Additive in Li Ion Batteries. Carbon 2021, 179, 425–434. [Google Scholar] [CrossRef]
- Babinszki, B.; Jakab, E.; Terjék, V.; Sebestyén, Z.; Várhegyi, G.; May, Z.; Mahakhant, A.; Attanatho, L.; Suemanotham, A.; Thanmongkhon, Y.; et al. Thermal Decomposition of Biomass Wastes Derived from Palm Oil Production. J. Anal. Appl. Pyrolysis 2021, 155, 105069. [Google Scholar] [CrossRef]
- Sunphorka, S.; Chalermsinsuwan, B.; Piumsomboon, P. Artificial Neural Network Model for the Prediction of Kinetic Parameters of Biomass Pyrolysis from Its Constituents. Fuel 2017, 193, 142–158. [Google Scholar] [CrossRef]
- Xu, C.A.; Qu, Z.; Meng, H.; Chen, B.; Wu, X.; Cui, X.; Wang, K.; Wu, K.; Shi, J.; Lu, M. Effect of Polydopamine-Modified Multi-Walled Carbon Nanotubes on the Thermal Stability and Conductivity of UV-Curable Polyurethane/Polysiloxane Pressure-Sensitive Adhesives. Polymer 2021, 223, 123615. [Google Scholar] [CrossRef]
- Bhat, V.S.; Kanagavalli, P.; Sriram, G.; B, R.P.; John, N.S.; Veerapandian, M.; Kurkuri, M.; Hegde, G. Low Cost, Catalyst Free, High Performance Supercapacitors Based on Porous Nano Carbon Derived from Agriculture Waste. J. Energy Storage 2020, 32, 101829. [Google Scholar] [CrossRef]
- Li, J.; Dou, B.; Zhang, H.; Zhang, H.; Chen, H.; Xu, Y. Thermochemical Characteristics and Non-Isothermal Kinetics of Camphor Biomass Waste. J. Environ. Chem. Eng. 2021, 9. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of Garlic Skin-Derived 3D Hierarchical Porous Carbon for High-Performance Supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, G.; Sun, N.; Zhang, X.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Biomass-Derived N-Doped Porous Carbon as Electrode Materials for Zn-Air Battery Powered Capacitive Deionization. Chem. Eng. J. 2018, 334, 1270–1280. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, I.; Sathe, S.M.; Dubey, B.K.; Ghangrekar, M.M. Waste-Derived Biochar: Applications and Future Perspective in Microbial Fuel Cells. Bioresour. Technol. 2020, 312, 123587. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, C.; Guo, Y.; Hassan, A.; Li, S.; Zhang, Y.; Wang, J. Dimethylimidazole and Dicyandiamide Assisted Synthesized Rich-Defect and Highly Dispersed CuCo-Nx Anchored Hollow Graphite Carbon Nanocages as Efficient Trifunctional Electrocatalyst in the Same Electrolyte. J. Power Sources 2022, 517, 230721. [Google Scholar] [CrossRef]
- Chen, W.; Gong, Y.F.; Liu, J.H. Recent Advances in Electrocatalysts for Non-Aqueous Li–O2 Batteries. Chin. Chem. Lett. 2017, 28, 709–718. [Google Scholar] [CrossRef]
- Peng, L.; Liang, Y.; Dong, H.; Hu, H.; Zhao, X.; Cai, Y.; Xiao, Y.; Liu, Y.; Zheng, M. Super-Hierarchical Porous Carbons Derived from Mixed Biomass Wastes by a Stepwise Removal Strategy for High-Performance Supercapacitors. J. Power Sources 2018, 377, 151–160. [Google Scholar] [CrossRef]
- Lei, W.; Deng, Y.P.; Li, G.; Cano, Z.P.; Wang, X.; Luo, D.; Liu, Y.; Wang, D.; Chen, Z. Two-Dimensional Phosphorus-Doped Carbon Nanosheets with Tunable Porosity for Oxygen Reactions in Zinc-Air Batteries. ACS Catal. 2018, 8, 2464–2472. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A Review on Biochar Production from Different Biomass Wastes by Recent Carbonization Technologies and Its Sustainable Applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Herawan, S.G.; Yusof, A.A. Effect of Activation Time on the Pinang Frond Based Activated Carbon for Remazol Brilliant Blue R Removal. J. Mech. Eng. Sci. 2014, 7, 1085–1093. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated Bio-Chars Derived from Rice Husk via One- and Two-Step KOH-Catalyzed Pyrolysis for Phenol Adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, H.L.; Ornaghi, F.G.; Neves, R.M.; Monticeli, F.; Bianchi, O. Mechanisms Involved in Thermal Degradation of Lignocellulosic Fibers: A Survey Based on Chemical Composition. Cellulose 2020, 27, 4949–4961. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, K.; Qiu, Y.; Liu, X.; Cao, C.; Feng, Y.; Hu, P.A. Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Bio-Waste as a Promising Electrocatalyst for Zinc-Air Battery. Energy 2018, 143, 43–55. [Google Scholar] [CrossRef]
- Amosa, M.K.; Jami, M.S.; AlKhatib, M.F.R.; Jimat, D.N.; Muyibi, S.A. Comparative and Optimization Studies of Adsorptive Strengths of Activated Carbons Produced from Steam- and CO2-Activation for BPOME Treatment. Adv. Environ. Biol. 2014, 8, 603–612. [Google Scholar]



| Components (%) | EFB | PF | GP |
|---|---|---|---|
| Extractives | 22.55 ± 3.30 | 31.10 ± 4.88 | 15.85 ± 0.07 |
| Ash | 6.827 ± 0.87 | 3.71 ± 0.18 | 3.84 ± 0.05 |
| Lignin | 24.67 ± 3.49 | 17.91 ± 2.11 | 19.30 ± 3.57 |
| Hemicellulose | 14.93 ± 0.20 | 39.51 ± 0.22 | 26.62 ± 0.23 |
| Cellulose | 40.69 ± 0.20 | 19.61 ± 0.22 | 37.68 ± 0.23 |
| Elements (At %) | Raw OPEFB | EFB-600-30 | Raw OPF | PF-600-30 | Raw GP | GP-600-30 |
|---|---|---|---|---|---|---|
| C | 54.16 | 80.44 | 53.52 | 79.11 | 51.61 | 86.42 |
| N | 1.29 | 3.07 | 1.46 | 1.35 | 1.63 | 0.82 |
| O | 44.28 | 16.41 | 44.85 | 19.49 | 46.62 | 12.60 |
| S | 0.26 | 0.08 | 0.18 | 0.05 | 0.14 | 0.16 |
| Precursor | Purpose | Synthesis Condition | SSABET (m2/g) | Remark | |
|---|---|---|---|---|---|
| Carbonization | Activation | ||||
| Garlic Peel | Supercapacitor | 1000 °C, 1 h, N2 flow 250 mL cm−3, 10 °C min−1 | - | 436.2 | [47] |
| Garlic Stem | ZAB | HTC 180 °C, 6 h, 900 °C, 75 min, Ar flow 100 sccm | - | 991.0 | [60] |
| Garlic Peel | Supercapacitor | 600 °C, 2 h, N2 flow 5 °C min−1 | 4:1(KOH:C) ratio, 800 °C, 2.5 h N2 flow 5 °C min−1 | 2818.0 | [49] |
| Garlic Peel | MAB | 700 °C, 30 min | 1:3(KOH:C) ratio, 600 °C, 30 min | 541.0 | This study |
| Empty Fruit Bunch | Wastewater treatment | 900 °C, 15 min, CO2 0.1 Lmin−1 | - | 345.0 | [61] |
| Empty Fruit Bunch | Wastewater treatment | 900 °C, 15 min, Steam 2.0 mL min−1 | - | 635.6 | [61] |
| Empty Fruit Bunch | MAB | 600 °C, 30 min | 1:3(KOH:C) ratio, 600 °C, 30 min | 460.9 | This study |
| Oil Palm Frond | Wastewater treatment | 500 °C, 2 h, Steam 100 cm3 min−1, 10 °C min−1 | - | 457.7 | [30] |
| Oil Palm Frond | MAB | 700 °C, 30 min | 1:3(KOH:C) ratio, 600 °C, 30 min | 548.26 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, B.A.-L.; Lim, S.; Pang, Y.L.; Shuit, S.H.; Wong, K.H.; Ooi, J.B. Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application. Sustainability 2022, 14, 2919. https://doi.org/10.3390/su14052919
Lim BA-L, Lim S, Pang YL, Shuit SH, Wong KH, Ooi JB. Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application. Sustainability. 2022; 14(5):2919. https://doi.org/10.3390/su14052919
Chicago/Turabian StyleLim, Brenda Ai-Lian, Steven Lim, Yean Ling Pang, Siew Hoong Shuit, Kam Huei Wong, and Jong Boon Ooi. 2022. "Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application" Sustainability 14, no. 5: 2919. https://doi.org/10.3390/su14052919
APA StyleLim, B. A.-L., Lim, S., Pang, Y. L., Shuit, S. H., Wong, K. H., & Ooi, J. B. (2022). Investigation on the Potential of Various Biomass Waste for the Synthesis of Carbon Material for Energy Storage Application. Sustainability, 14(5), 2919. https://doi.org/10.3390/su14052919