1. Introduction
In the palm oil industry, fresh fruit bunches (FFB) are required to create palm kernel oil (PKO), crude palm oil (CPO) and other biomass [
1]. Through palm oil milling activities, these FFB produce several wastes, including liquid and solid waste. These solid wastes are harmful if not properly regulated and monitored, which can result in soil contamination, air and water pollution, and the release of greenhouse gases [
2]. The use of these plant-derived materials can ease the load on landfill in the region; one such use is their utilization in the modification of asphalt binders [
3].
Various studies by different researchers and organizations have been conducted to utilize POBA in asphalt pavement as filler, fine aggregate or combination modifier, especially in hot mix asphalt. The utilization of 25% waste CPO ash as a filler in hot rolled sheet–wearing course mixture led to increased stability [
4]. Agusmaniza [
5] found that the use of combined palm oil shell ash (POSA) as filler and plastic substitution in asphalt concrete–wearing course mixtures can increase the value of stability and the Marshall quotient, with a 6.7% plastic and 5.8% POSA combination.
Ritonga et al. [
6] modified asphalt mixtures with cyclic natural rubber (CNR) and palm oil shell ash as filler material, and found that the asphalt stability values increased with 80 g CNR additions and with an optimum asphalt binder content of 6% in the asphalt mixtures. Rizal et al. [
7] investigated whether POBA can be used as reinforcement or filler in epoxy polymers, and they tested it as an alternative to silica-based inorganic fillers, used in order to improve the physical, mechanical and thermal characteristics.
In other industries, POBA can also substitute black carbon as a filler in the production of natural rubber compounds [
8]. Besides that, POBA can be utilized as sand and cement replacement in concrete: it was found that an increase in the POBA replacement level in a cement mixture will linearly reduce the compressive strength of the concrete mixer [
9]. POBA can also be used as an admixture in soil stabilization by mixing the variation of 6% POBA and 2% MATOS: this combination changes the California bearing ratio (CBR) value behavior; therefore, it is a viable soil stabilization solution [
10].
POBA is a solid waste generated by the palm oil industry and it is an environmental issue. After CPO extraction, a massive amount of solid biomass waste is produced, primarily consisting of empty fruit bunches (EFB), palm-pressed fiber (PPF), and palm kernel shells (PKS). Approximately 1.77 tons of solid biomass waste is created for every ton of CPO production [
11]. Based on the average palm oil mill’s capacity (10–60 tons FFB/h), an estimated 6–12 tons of solid biomass waste or 65–394 kg of ash is produced every hour [
12].
If it is not handled correctly, then this enormous volume of solid waste poses a severe threat to the environment. The studies of Rusbintardjo et al. [
13] and Babalghaith [
14] showed that POBA is a solid waste that has a high silica (SiO
2) content, which has the potential to pollute the ground. Natural radioactive radiation can have harmful health consequences, such as genetic abnormalities and cancer, that will cause damage to generations to come—POBA is categorized as hazardous waste due to its carcinogenic properties for humans, where the level of natural radioactivity potassium
40K is higher than the standard average range [
15].
POBA has been used as a geopolymer composite material [
16], as a heavy metal absorbent in treating wastewater [
17], as an absorbent of solvent-dispersed dyes [
18], as a source of nutrients for fertilizer production [
19], and as a coarse aggregate replacement in high-strength lightweight concrete [
20]. POBA was shown to be able to eliminate 98% of carotenoids, thus producing refined oil colors equivalent to those generated by traditional bleaching earths [
21]. Furthermore, the stiffness of POBA geopolymer brick was improved to a maximum strength of 16.1 MPa, making it a medium-weight, non-loading brick type [
16]. Additionally, POBA’s high organic carbon content was shown to boost nutrient-absorption efficiency, resulting in higher crop quality [
22]. These efforts, however, are not sufficient to make the palm oil industry waste-free. This is because wastes are still disposed of or left to degrade into the ground. This situation urges new inventions and in-depth research to turn POBA into a useful final product—especially in road construction industry applications—in order to reduce solid waste from the palm oil industry, lowering its negative environmental impact.
WMA additive has been developed to enable the production of asphalt mixtures at temperatures lower than conventional hot mix asphalt, thus leading to reductions in fuel consumption and emissions of pollutants and greenhouse gases. Previous researchers have found that WMA additives can enhance asphalt mixture characteristics, such as ageing, viscosity and adhesion, in addition to lowering the mixing temperature. The objectives of this study were to investigate the physical and chemical properties of a 60/70 penetration-grade asphalt binder, modified with 2% of Rediset containing various percentages of POBA, and to determine the optimum percentage of POBA in the modified asphalt binder.
The unmodified asphalt binder (without POBA) was selected as the control mixture. The physical tests selected in this research included test of the penetration, softening temperature, ductility and viscosity of the mixtures, while the chemical tests included FTIR to identify the chemical compounds, SEM to identify the elemental composition of POBA, TGA to calculate the kinematic parameters for thermal degradation, and DSC to evaluate the changes of physicochemical transition in the modified asphalt binder.
2. Materials and Methods
2.1. Asphalt Binder
A 60/70 penetration-grade asphalt binder was utilized in the study, which was supplied by Sunway Quarry Industries Sdn. Bhd. (Semenyih) in Selangor, Malaysia. The properties of the asphalt binder used in this research are shown in
Table 1.
2.2. Rediset
The additive used in this study was a liquid chemical warm additive, namely Rediset LQ-1200 (hereafter referred to as Rediset), produced by AkzoNobel Company. Rediset is known to improve performance, especially the tensile strength [
23], the resistance to moisture damage [
24], the anti-rutting properties [
25], the stability [
26], and the fatigue lifespan [
27]. Rediset was added at the optimum content of 2% of the weight of the asphalt binder [
28,
29,
30,
31]. The physical properties of Rediset are presented in
Table 2.
2.3. POBA
POBA was used in this research as the modifier material of the asphalt binder. POBA was obtained from Sedenak Palm Oil Mill in Johor, Malaysia. POBA consists of large particles, which include unburned kernels, nutshells, and fibers. POBA was obtained as a powder and sieved using a 0.15 mm (#100) sieve size.
Figure 1 shows the surface morphology of POBA. The formation showed a good distribution in the pore structure of silica sand (Electron Image 1) with 1000 times magnification. With a 1500 times magnification, Electron Image 2 and 3 revealed an uneven surface with porous structure and flaky shape, demonstrating a highly absorbent surface. The sieving process altered the characteristics of POBA. The elemental composition of POBA, evaluated by scanning electron microscopy, is shown in
Table 3. It was also seen that the carbon content in the POBA was closer to the carbon content in the petroleum-based asphalt binder. That could indicate the compatibility of POBA with asphalt binder and its behavior could be similar to the asphalt. On the other hand, the oxygen content in POBA was much higher compared with the asphalt, which is expected, because the POBA material is derived from biomass resources as an organic material. However, compared with most of the biomass materials that contain oxygen content between 15% and 45%, the average oxygen content of 14% in POBA is considered below the normal range of oxygen in most biomass materials that have been used as modifiers for asphalt binder. Therefore, POBA-modified asphalt is expected to have an adequate aging resistance.
2.4. Sample Preparation
For preparation of the modifier for this project, POBA was heated at 70 °C for 20 h (according to ASTM C70—20) in the oven and sieved through a 0.15 mm (#100) sieve size. During the preparations of the blending, asphalt binder was transferred into a 500 mL tin can then heated 155 °C for about 10 min to avoid hardening during the blending procedure and testing. Based on the specification of the product, the blending would be applied beforehand, by adding Rediset into asphalt binder at 170 °C and mixing at 5000 rpm for 2 h to reach the homogeneity of the binder blending. The blending of the asphalt binder with POBA and Rediset was completed using a shear mixer and stirrer.
The blending procedure started with a small tin can, then, the preheated asphalt binder weight was measured before 10 g of Rediset and varied weights of POBA were added. The percentages of POBA in each binder investigated in this research was 0%, 3%, 5%, 7% and 9%. The mixing temperature of warm mix additive specification according to the literature is between 16 and 33 °C less than the temperature of hot mix asphalt. Even though WMA binder is produced in a temperature range of 100–140 °C, these temperatures are only applicable to virgin binder. As for modified binder, the mixing temperature can be higher than 140 °C [
32,
33]. In addition, trial-and-error procedures have been conducted to control the storage stability of the blends and to ensure the homogeneity of the blends containing POBA and Rediset.
2.5. Storage Stability
The modified asphalt binder’s stability when stored at high temperatures was determined using a storage stability test. The heated modified asphalt binder was put into a 25 mm diameter aluminum tube with a length of 140 mm, and then heated for 48 h at 163 °C. After cooling, the aluminum tube was sliced into three pieces. A softening point test was used to determine the asphalt binder portions from the tube’s top and bottom. Based on ASTM D36, the difference between softening points must be less than or equal to 2.5 °C to pass the test.
2.6. Physical Test
Consistency tests were carried out to determine the physical parameters of the mixtures, in order to discover whether POBA can be utilized to improve or maintain the asphalt binder’s characteristics. Physical tests, such as penetration, softening point and ductility, were carried out in accordance with ASTM D5-13, ASTM D36-14 and ASTM D113-07, respectively.
2.7. Viscosity
In this study, the viscosity test was conducted according to ASTM D4402-15. Temperatures ranging from 100 to 200 °C were used. For each round of the test, around 10 ± 1 g of binder were required. A sample chamber was filled with a hot asphalt binder, which was then kept in a thermos container at the correct test temperature (38–260 °C). The test was performed by spinning the spindle at 20 rpm.
2.8. Dimensional Analysis
Dimensional analysis is a technique for reducing the number of relevant variables in a physical issue by utilizing dimensional homogeneity [
34]. In other words, it is an analysis of the connections between various physical quantities based on identification of their base quantities and their units of measurement. In this analysis, POBA—as the modifier binder—was the variable.
The viscosities of four asphalt binders, containing varying quantities of POBA, were evaluated in order to assess the efficiency of modifier in the asphalt binder system. The impact of POBA was assessed using rotational viscosity and viscosity-reduction efficiency (∇ŋ). ∇ŋ was defined as the contribution of the POBA percentage (3%, 5%, 7% and 9%) to the viscosity-reduction rate; that is, the higher the viscosity-reduction efficiency, the greater the cost effectiveness of the mixture.
A non-dimensional viscosity index was also utilized to evaluate and describe the changing rate of the asphalt binder’s rheological properties with POBA and Rediset against the asphalt 60/70 with Rediset. The viscosity was formulated by Equations (1) and (2), as follows:
and
where ŋ
R—the mixings relative viscosity;
v—the binder mix viscosity;
vc—asphalt 60/70 plus Rediset viscosity; ∇ŋ—the non-dimensional viscosity index.
The activation energy (Ea) of the flow was determined by the value of the asphalt binder viscosity over a predetermined temperature range.
In this research, the temperature range was set from 130 °C to 180 °C, with 10 °C increments. In addition, the activation energy of the flow was another index used to measure the temperature susceptibility of the asphalt binders. The calculation was based on
Arrhenius equation, as per Equation (3):
By introducing the ln factor to both sides of the equation, Equation (3) becomes:
Since ln
e = 1, Equation (4) can be arranged into:
In linear plot, the slope (
m) represents
. Since
R is a constant,
Ea can be calculated straight away. A higher value of
Ea means more energy is needed to make the material flow. For all types of asphalt binders, two dependent ([ln (
V)]) and independent variables ([
in (
K)]) were plotted.
Ea values were obtained from the slope parameter multiplied by the constant (
A). The calculation of the change in the
Ea of the asphalt 60/70 and Rediset–POBA blends is presented in Equation (6).
The flow resistance of the asphalt binder decreased at lower
Ea. When the asphalt binder is less sensitive to temperature changes, it has lower
Ea, whereas a higher sensitivity to temperature changes indicates higher
Ea. A linear relationship between temperature and viscosity, referred to as the “
A-VTS” relationship, is a transformation from the nonlinear relationship between the two parameters. The relationship is shown mathematically in Equation (7), as follows:
where
η—viscosity (mPas);
A—intercept of temperature susceptibility relationship;
VTS—slope of temperature susceptibility;
TR—temperature in Rankine;
Tcritical—temperature in Rankine at which the viscosity was equal to 0.27 mPas.
With reference to Equation (7), the slope of the curve represents the VTS and the intercept is A. Flatter slope means a lower VTS, implying a reduced viscosity–temperature susceptibility of the asphalt binder to rutting distresses and thermal cracking.
2.9. Fourier Transform Infrared Test
The FTIR test is used to determine unknown materials, assess sample quality and investigate the individual elements in a mixture [
35]. Some of the infrared (IR) light passing through an asphalt binder sample was absorbed by the asphalt binder components. Then, a series of absorption bands at a specific wave number were recorded as transmittance or absorbance against the wave number. The spectra depicts molecule absorption and transmission, resulting in a molecular fingerprint of the asphalt binder’s constituents [
36]. The resulting wave number or wavelength indicates the existence of different functional groups. Wave numbers ranging from 4000 to 400 cm
−1 were used to record the whole spectrum of the asphalt binder’s functional groups.
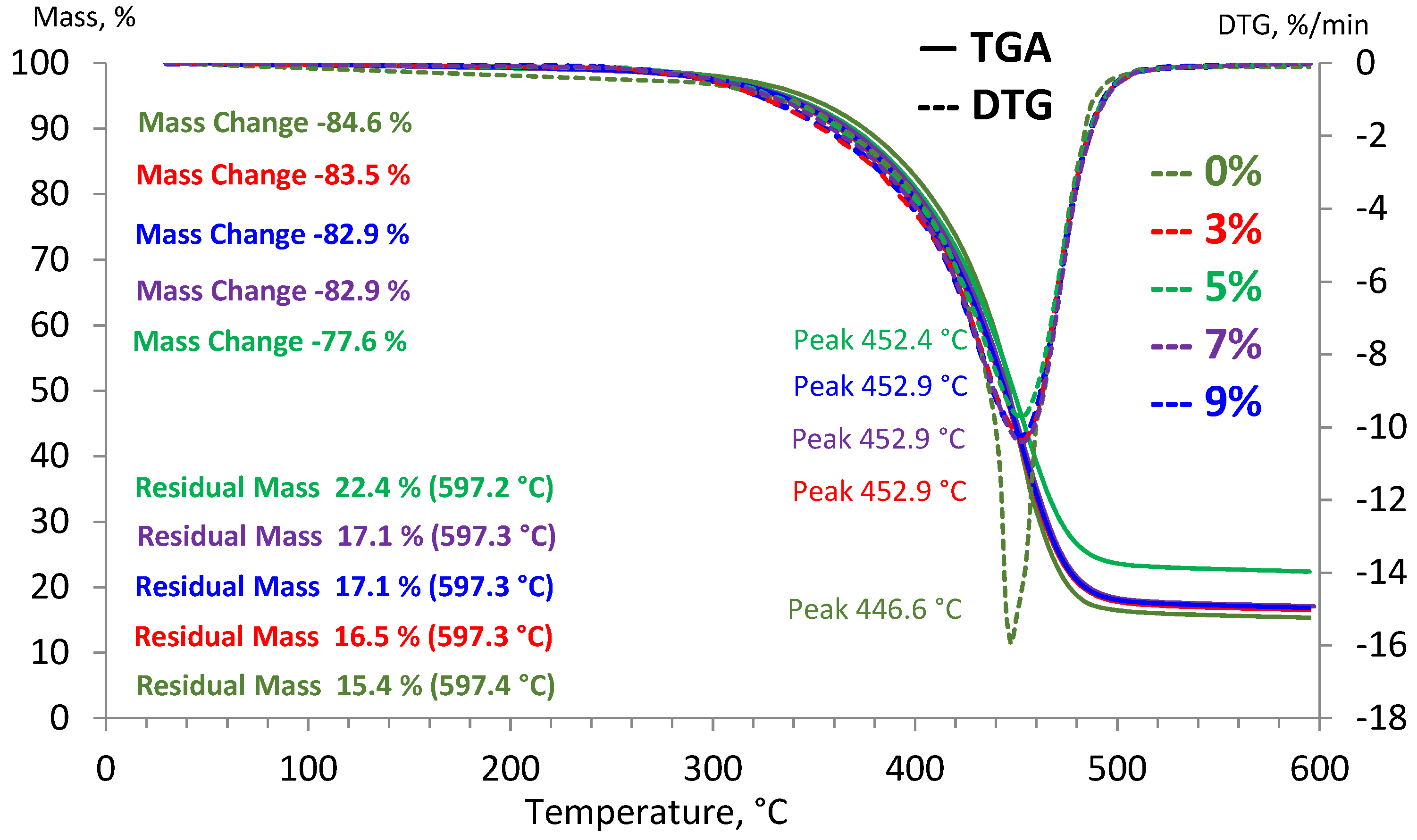
2.10. Thermogravimetric Analysis
TGA is an analytical technique for determining the physicochemical properties, thermal stability and volatile components fractions of materials by measuring the weight changes while a sample is heated at a constant rate; another definition of TGA is as a thermal analysis technique for measuring the mass of a sample over time, as the temperature changes [
37].
TGA is applied on a device known as a thermogravimetric analyzer. This instrument can function at maximum 600 °C, for which, mass, temperature and time are considered as the three basic measurements. The analysis was conducted by utilizing thermogravimetric analyzer in a nitrogen protective environment, with heating and cooling speeds ranging from 0.001 K/min to 50 K/min. To begin the analysis, the asphalt binders’ sample was maintained at room temperature and kept in an aluminum cell. The sample was then heated from ambient temperature to 600 °C. A microbalance was used to track their weight reduction.
2.11. Differential Scanning Calorimetry
DSC analysis is a thermal analysis technique for determining the direct absorption of thermal energy in a sample during a controlled temperature increase or decrease, in which the heat flow into or out of a sample is monitored as a function of temperature or time [
38]. Calorimetric analysis is a general approach for examining such a process, since chemical processes and many physical transitions are connected to the creation or consumption of heat.
It is the primary approach for measuring the thermal characteristics of materials in order to create a link between temperature and particular physical properties of materials, and it is the sole method of determining the enthalpy associated with the desired process directly [
39]. When the sample was heated, the changes in the heat capacity of the asphalt binder were recorded as changes in the heat flow. The changes in structure (physicochemical processes), such as glass transition, melting, crystallization and oxidation phase changes, were identified.
2.12. Statistical Analysis
In this study, the results of the tests were computed using analysis of variance (ANOVA). ANOVA is a statistical test for finding differences in group means, when there is one parametric dependent variable and one or more independent variables. The one-way ANOVA technique was chosen for comparing the averages of two or more groups of samples, while also determining whether there is a correlation between them [
40].
The results—statistically analyzed using the data analysis tools in Microsoft Excel, ANOVA,
t-test, correlations and coefficient of variance—were included in testing the hypotheses in this study. ANOVA and
t-test were used to study whether a group of data was different from the other groups. To run these analyses, three assumptions had been made. First, the independence assumption, stating that the elements of one group were not related to the other group of samples. The second was a normality assumption, of which the samples were randomly picked from the sample population’s normal distribution with unknown population means. The equal variance assumption assumes that the variances of the two group’s populations are equal. If not, the means are no longer valid to measure the central tendency, and thus test will not be valid either.
Figure 2 shows the experimental flowchart of this study.