Circular Economy: A Comprehensive Review of Eco-Friendly Wollastonite Applications
Abstract
:1. Introduction
2. Bibliography Review
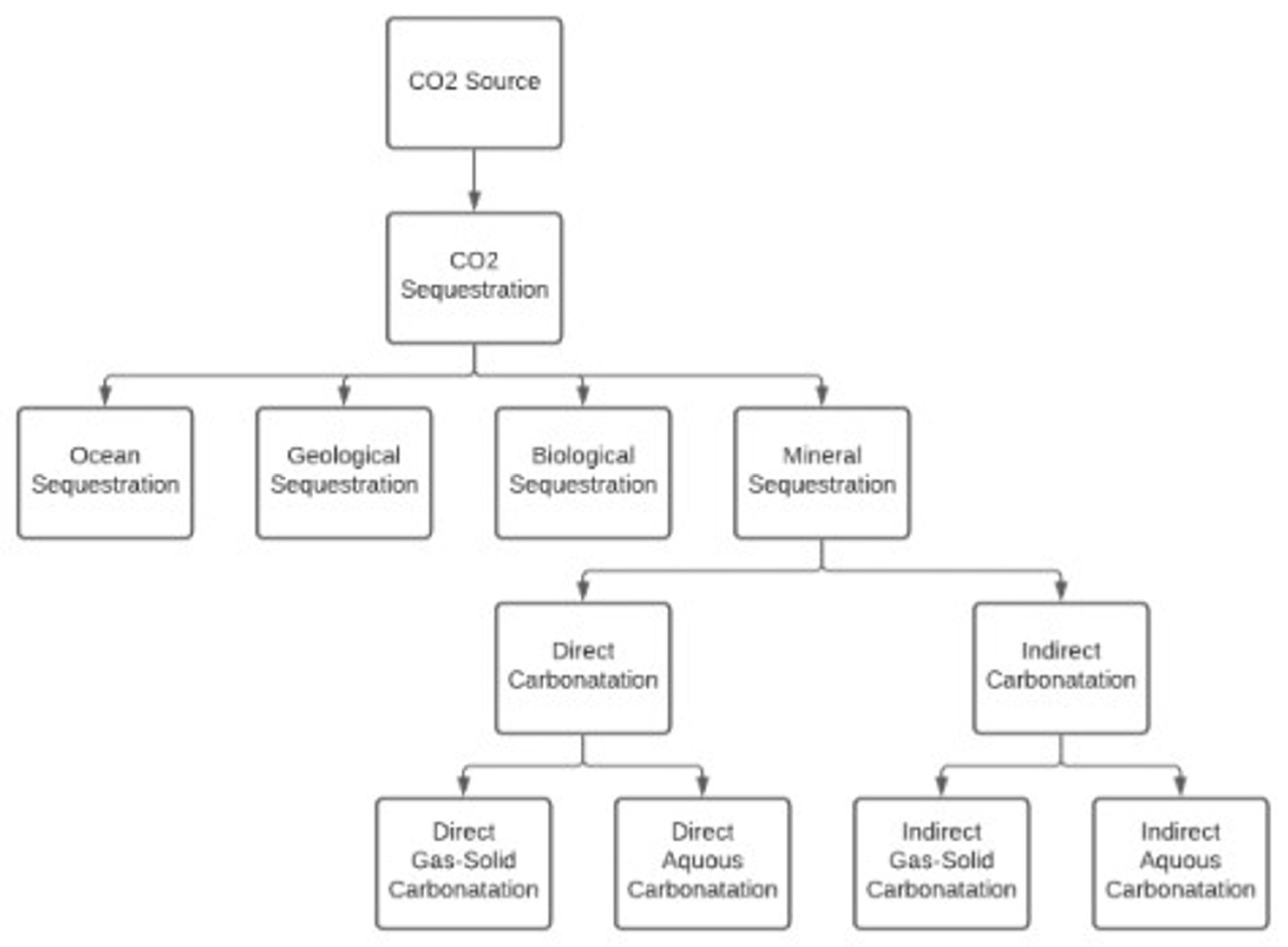
2.1. Carbon Capture and Storage (CCS)
- Post-combustion capture: Where the CO2 is removed after combustion process;
- Pre-combustion capture: Which involves the separation and storage of CO2 before combustion;
- Oxy-combustion: In which fuel is burned in the presence of oxygen to produce a concentrated current of CO2.
2.2. Carbon Mineralization
- Ex situ: In which the alkaline source is transported to the capture site of CO2, crushed into small particles, and combined with CO2 at high temperatures and pressure.
- Surficial: Where CO2-bearing and surface waters are reacted with reactive rock fragments, such as alkaline industrial waste (crushed mine tailings). For implementation cost, it is low cost, although a very large area footprint at the gigaton scale is proposed.
- In situ: In which fluids containing CO2 circulate through the porous subsurface in geological formations. These methods have a similar cost to the surficial method of carbon mineralization and a giant storage capacity, but they include uncertain feedback between permeability, reactive surface area, and reaction rate.
- Combined partial enrichment of CO2 using direct air capture with synthetic sorbents plus in situ carbon mineralization or surficial carbon mineralization. In terms of energy requirements and total costs for partial CO2 enrichment, this method involves lower energy than for enrichment to high purity.
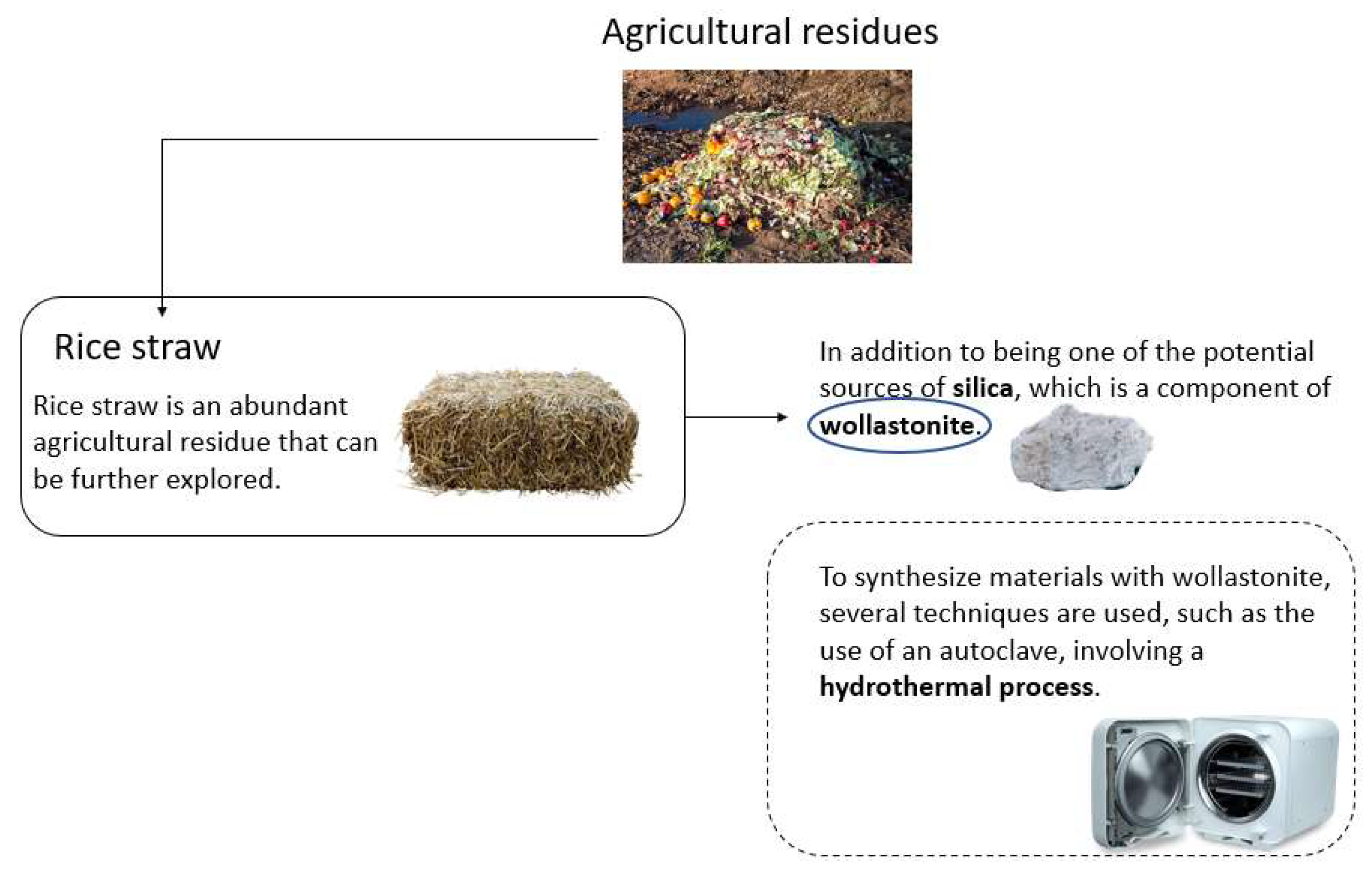
3. Wollastonite Synthesis and Some Eco-Friendly Applications
4. Global Impacts of Synthesis of Wollastonite by Rice Husk
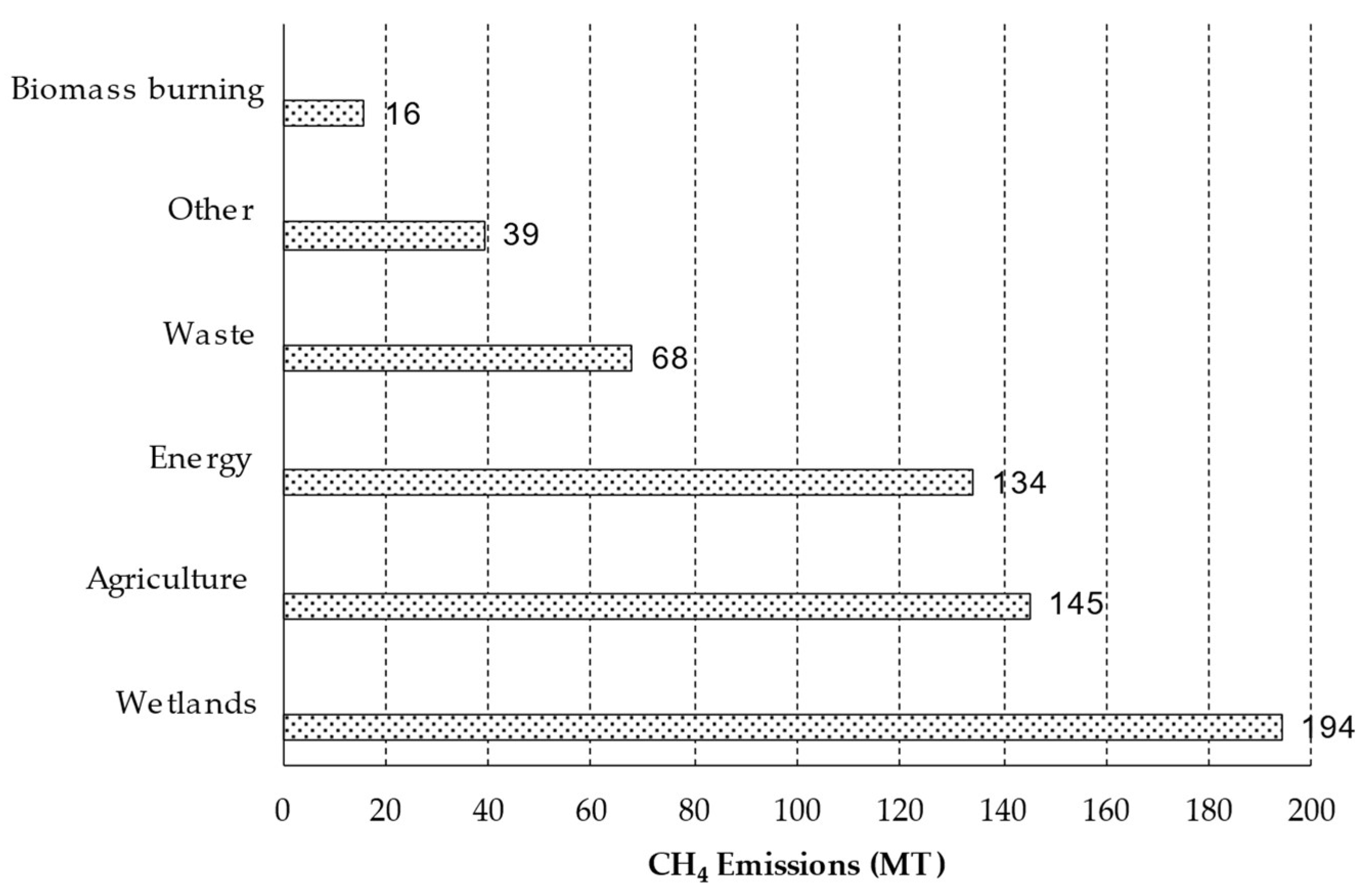
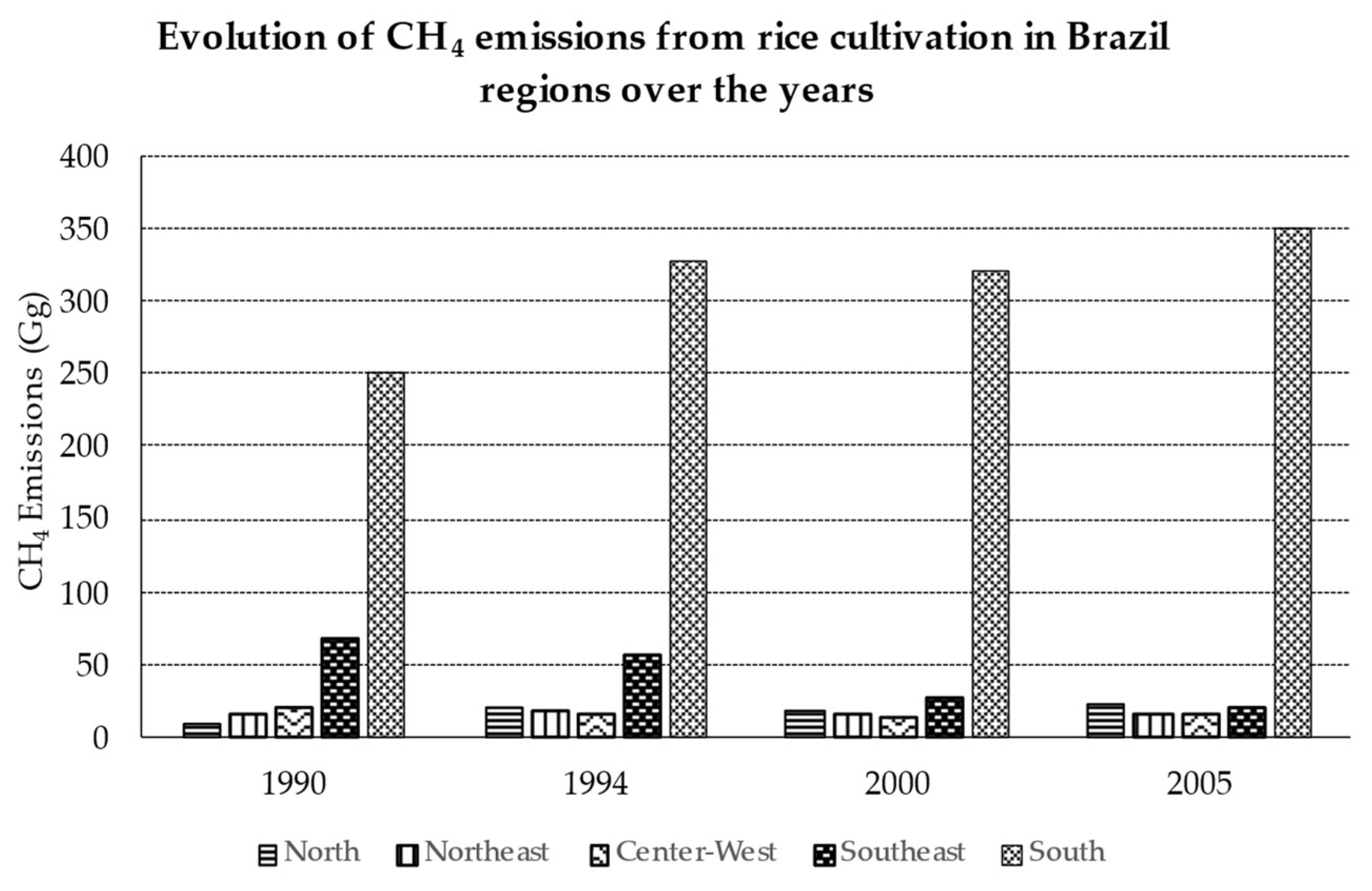
5. Energy and Biodiversity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, N.A.; Sairam, V. Research initiatives on the influence of wollastonite in cement-based construction material—A review. J. Clean. Prod. 2021, 283, 124665. [Google Scholar] [CrossRef]
- Gestão, R.D.E.; Sustentabilidade, A.E.; Luisa, A.; Netto, A.; Alves, V.H. Overview of public policies and strategies for the deployment of carbon capture and storage: Reflections for Brazil. J. Environ. Manag. Sustain. 2021, 10, 1–21. [Google Scholar]
- Kusin, F.M.; Hasan, S.N.M.S.; Hassim, M.A.; Molahid, V.L.M. Mineral carbonation of sedimentary mine waste for carbon sequestration and potential reutilization as cementitious material. Environ. Sci. Pollut. Res. 2020, 27, 12767–12780. [Google Scholar] [CrossRef] [PubMed]
- Alami, A.H.; Alasad, S.; Ali, M.; Alshamsi, M. Investigating algae for CO2 capture and accumulation and simultaneous production of biomass for biodiesel production. Sci. Total Environ. 2021, 759, 143529. [Google Scholar] [CrossRef]
- Bhola, V.; Swalaha, F.; Ranjith Kumar, R.; Singh, M.; Bux, F. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 2014, 11, 2103–2118. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Dhal, G.C. Materials progress in the control of CO and CO2 emission at ambient conditions: An overview. Mater. Sci. Energy Technol. 2019, 2, 607–623. [Google Scholar] [CrossRef]
- Hajilary, N.; Rezakazemi, M.; Shahi, A. CO2 emission reduction by zero flaring startup in gas refinery. Mater. Sci. Energy Technol. 2020, 3, 218–224. [Google Scholar] [CrossRef]
- Neeraj, Y.S.; Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Mokhtara, C.; Negrou, B.; Bouferrouk, A.; Yao, Y.; Settou, N.; Ramadan, M. Integrated supply–demand energy management for optimal design of off-grid hybrid renewable energy systems for residential electrification in arid climates. Energy Convers. Manag. 2020, 221, 113192. [Google Scholar] [CrossRef]
- Ismail, H.; Shamsudin, R.; Abdul Hamid, M.A.; Jalar, A. Synthesis and characterization of nano-wollastonite from rice husk ash and limestone. Mater. Sci. Forum 2013, 756, 43–47. [Google Scholar] [CrossRef]
- Shamsudin, R.; Ismail, H.; Hamid, M.A.A. The suitability of rice straw ash as a precursor for synthesizing β-wollastonite. Mater. Sci. Forum 2016, 846, 216–222. [Google Scholar] [CrossRef]
- Ismail, H.; Shamsudin, R.; Hamid, M.A.A.; Awang, R. Characteristics of β-wollastonite derived from rice straw ash and limestone. J. Aust. Ceram. Soc. 2016, 52, 163–174. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [Green Version]
- Kelemen, P.; Benson, S.M.; Pilorgé, H.; Psarras, P.; Wilcox, J. An Overview of the Status and Challenges of CO2 Storage in Minerals and Geological Formations. Front. Clim. 2019, 1, 9. [Google Scholar] [CrossRef]
- Huang, H.; Guo, R.; Wang, T.; Hu, X.; Garcia, S.; Fang, M.; Luo, Z.; Maroto-Valer, M.M. Carbonation curing for wollastonite-Portland cementitious materials: CO2 sequestration potential and feasibility assessment. J. Clean. Prod. 2019, 211, 830–841. [Google Scholar] [CrossRef]
- Pastero, L.; Curetti, N.; Ortenzi, M.A.; Schiavoni, M.; Destefanis, E.; Pavese, A. CO2 capture and sequestration in stable Ca-oxalate, via Ca-ascorbate promoted green reaction. Sci. Total Environ. 2019, 666, 1232–1244. [Google Scholar] [CrossRef]
- Bao, J.; Lu, W.-H.; Zhao, J.; Bi, X.T. Greenhouses for CO2 sequestration from atmosphere. Carbon Resour. Convers. 2018, 1, 183–190. [Google Scholar] [CrossRef]
- SundarRajan, P.S.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Rosa, L.; Sanchez, D.L.; Realmonte, G.; Baldocchi, D.; D’Odorico, P. The water footprint of carbon capture and storage technologies. Renew. Sustain. Energy Rev. 2021, 138, 110511. [Google Scholar] [CrossRef]
- Wang, J.W.; Kang, J.N.; Liu, L.C.; Nistor, I.; Wei, Y.M. Research trends in carbon capture and storage: A comparison of China with Canada. Int. J. Greenh. Gas Control 2020, 97, 103018. [Google Scholar] [CrossRef]
- Vieira, L.C.; Amaral, F.G. Barriers and strategies applying Cleaner Production: A systematic review. J. Clean. Prod. 2016, 113, 5–16. [Google Scholar] [CrossRef]
- Soong, Y.; Howard, B.H.; Hedges, S.W.; Haljasmaa, I.; Warzinski, R.P.; Irdi, G.; McLendon, T.R. CO2 sequestration in saline formation. Aerosol Air Qual. Res. 2014, 14, 522–532. [Google Scholar] [CrossRef]
- Min, Y.; Jun, Y.S. Wollastonite carbonation in water-bearing supercritical CO2: Effects of water saturation conditions, temperature, and pressure. Chem. Geol. 2018, 483, 239–246. [Google Scholar] [CrossRef]
- Haque, F.; Santos, R.M.; Chiang, Y.W. CO2 sequestration by wollastonite-amended agricultural soils—An Ontario field study. Int. J. Greenh. Gas Control 2020, 97, 103017. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Hossain, S.S.; Roy, P.K. Study of physical and dielectric properties of bio-waste-derived synthetic wollastonite. J. Asian Ceram. Soc. 2018, 6, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y.; Bao, W.J.; Guo, Z.C.; Li, H.Q. Carbon Dioxide Sequestration via Steelmaking Slag Carbonation in Alkali Solutions: Experimental Investigation and Process Evaluation. Acta Metall. Sin. 2018, 31, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Kojima, T.; Nagamine, A.; Ueno, N.; Uemiya, S. Absorption and fixation of carbon dioxide by rock weathering. Energy Convers. Manag. 1997, 38, 461–466. [Google Scholar] [CrossRef]
- Shamsudin, R.; Abdul Azam, F.A.; Abdul Hamid, M.A.; Ismail, H. Bioactivity and cell compatibility of β-wollastonite derived from rice husk ash and limestone. Materials 2017, 10, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savi, G.D.; Piacentini, K.C.; Rocha, L.O.; Carnielli-Queiroz, L.; Furtado, B.G.; Scussel, R.; Zanoni, E.T.; Machado-de-Ávila, R.A.; Corrêa, B.; Angioletto, E. Incidence of toxigenic fungi and zearalenone in rice grains from Brazil. Int. J. Food Microbiol. 2018, 270, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.F.; Moreira, G.M.; Nicolli, C.P.; Gomes, L.B.; Abreu, L.M.; Pfenning, L.H.; Haidukowski, M.; Moretti, A.; Logrieco, A.; del Ponte, E.M. Fusarium incarnatum-equiseti species complex associated with Brazilian rice: Phylogeny, morphology and toxigenic potential. Int. J. Food Microbiol. 2019, 306, 108267. [Google Scholar] [CrossRef]
- Heinemann, A.B.; Ramirez-Villegas, J.; Rebolledo, M.C.; Costa Neto, G.M.F.; Castro, A.P. Upland rice breeding led to increased drought sensitivity in Brazil. F. Crop. Res. 2019, 231, 57–67. [Google Scholar] [CrossRef]
- Fageria, N.K.; Wander, A.E.; Silva, S.C. Rice (Oryza sativa) cultivation in Brazil. Indian J. Agron. 2014, 59, 350–358. [Google Scholar]
- Hossain, S.S.; Yadav, S.; Majumdar, S.; Krishnamurthy, S.; Pyare, R.; Roy, P.K. A comparative study of physico-mechanical, bioactivity and hemolysis properties of pseudo-wollastonite and wollastonite glass-ceramic synthesized from solid wastes. Ceram. Int. 2020, 46, 833–843. [Google Scholar] [CrossRef]
- da Silva, M.T.; Martinazzo, R.; Silva, S.D.A.; Bamberg, A.L.; Stumpf, L.; Fermino, M.H.; Kohler, T.W.; Matoso, E.S.; Valgas, R.A. Innovative substrates for sugarcane seedling production: Sewage sludges and rice husk ash in a waste-to-product strategy. Ind. Crops Prod. 2020, 157, 112812. [Google Scholar] [CrossRef]
- Azam, F.A.A.; Shamsudin, R.; Ng, M.H.; Ahmad, A.; Akbar, M.A.M.; Rashidbenam, Z. Silver-doped pseudowollastonite synthesized from rice husk ash: Antimicrobial evaluation, bioactivity and cytotoxic effects on human mesenchymal stem cells. Ceram. Int. 2018, 44, 11381–11389. [Google Scholar] [CrossRef]
- Gotlib, E.; Tverdov, I.; Phuong, H.T.N.; Sokolova, A. The impact of production temperature of synthetic wollastonite filled with rice husk on its composition and modifying effect. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1030, 012004. [Google Scholar] [CrossRef]
- Hossain, S.S.; Mathur, L.; Roy, P.K. Rice husk ash as an alternative source of silica in ceramics: A review Rice husk/rice husk ash as an alternative source of silica in ceramics: A review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- de Miranda, M.S.; Fonseca, M.L.; Lima, A.; de Moraes, T.F.; Aparecido Rodrigues, F. Environmental Impacts of Rice Cultivation. Am. J. Plant Sci. 2015, 6, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Janssens-Maenhout, G.; Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Dentener, F.; Bergamaschi, P.; Pagliari, V.; Olivier, J.; Peters, J.; et al. EDGAR v4.3.2 Global Atlas of the three major Greenhouse Gas Emissions for the period 1970–2012. Earth Syst. Sci. Data Discuss. 2017, 3, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Bergier, I.; Silva, A.P.S.; de Abreu, U.G.P.; de Oliveira, L.O.F.; Tomazi, M.; Dias, F.R.T.; Urbanetz, C.; Nogueira, É.; Borges-Silva, J.C. Could bovine livestock intensification in Pantanal be neutral regarding enteric methane emissions? Sci. Total Environ. 2019, 655, 463–472. [Google Scholar] [CrossRef]
- Jing, L.; Chen, C.; Lu, Q.; Wang, Y.; Zhu, J.; Lai, S.; Wang, Y.; Yang, L. How do elevated atmosphere CO2 and temperature alter the physiochemical properties of starch granules and rice taste? Sci. Total Environ. 2021, 766, 142592. [Google Scholar] [CrossRef]
- Mofijur, M.; Mahlia, T.M.I.; Logeswaran, J.; Anwar, M.; Silitonga, A.S.; Rahman, S.M.A.; Shamsuddin, A.H. Potential of rice industry biomass as a renewable energy source. Energies 2019, 12, 4116. [Google Scholar] [CrossRef] [Green Version]
- Cheewaphongphan, P.; Junpen, A.; Kamnoet, O.; Garivait, S. Study on the potential of rice straws as a supplementary fuel in very small power plants in Thailand. Energies 2018, 11, 270. [Google Scholar] [CrossRef] [Green Version]
- Ai, N.; Chen, L.; Fu, Y. A novel analysis on pyrolysis and gasification process of rice straw feedstock. Sustain. Energy Technol. Assess. 2022, 51, 101866. [Google Scholar] [CrossRef]
- Lopes Grotto, C.G.; Gomes Colares, C.J.; Lima, D.R.; Pereira, D.H.; Teixeira do Vale, A. Energy potential of biomass from two types of genetically improved rice husks in Brazil: A theoretical-experimental study. Biomass Bioenergy 2020, 142, 105816. [Google Scholar] [CrossRef]



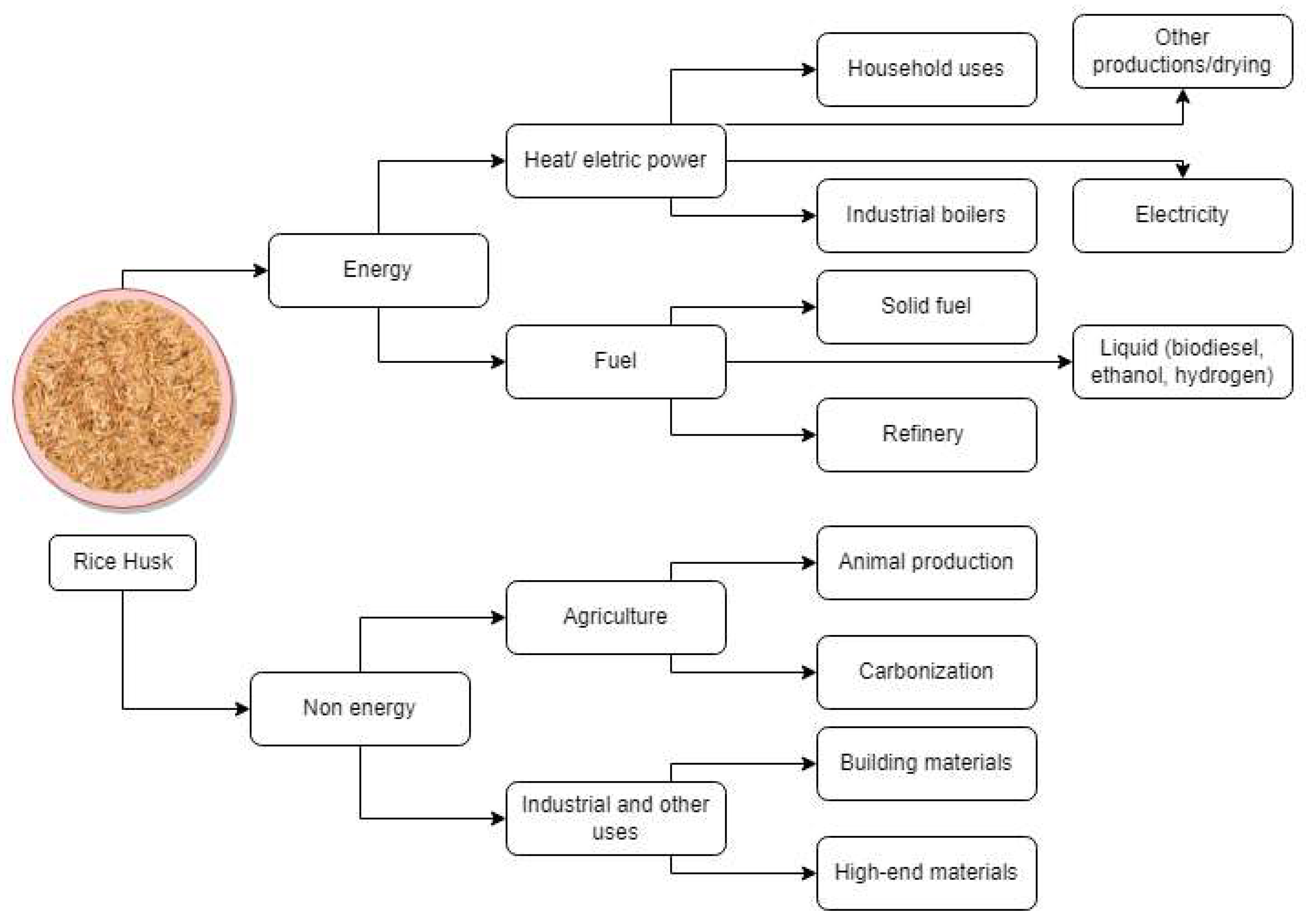
| Sector | CO2 Emission (%) |
|---|---|
| Land-use change and forests | 64.90 |
| Energy | 27.68 |
| Industrial processes | 5.60 |
| Agriculture | 1.76 |
| Waste | 0.06 |
| Country | Project | In Operation Since | Source of CO2 | CO2 Capture Capacity (Mt/Year) |
|---|---|---|---|---|
| US | Shute Creek gas processing facility | 1986 | Natural gas processing | 7.0 |
| US/Canada | Great Plains Synfuels (Weyburn/Midale) | 2000 | Synthetic natural gas | 3.0 |
| US | Century plant | 2010 | Natural gas processing | 8.4 |
| Brazil | Petrobras Santos Basin pre-salt oilfield CCS | 2013 | Natural gas processing | 3.0 |
| Canada | Boundary Dam CCS | 2014 | Power generation (coal) | 1.0 |
| Saudi Arabia | Uthmaniyah CO2-EOR demonstration | 2015 | Natural gas processing | 0.8 |
| Canada | Quest | 2015 | Hydrogen production | 1.0 |
| United Arab Emirates | Abu Dhabi CCS | 2016 | Iron and steel production | 0.8 |
| US | Petra Nova | 2017 | Power generation (coal) | 1.4 |
| US | Illinois Industrial | 2017 | Ethanol production | 1.0 |
| China | Jilin oilfield CO2-EOR | 2018 | Natural gas processing | 0.6 |
| Australia | Gorgon Carbon Dioxide Injection | 2019 | Natural gas processing | 3.4–4.0 |
| Canada | Alberta Carbon Trunk Line (ACTL) with Agrium CO2 stream | 2020 | Fertilizer production | 0.3–0.6 |
| Canada | ACTL with North West Sturgeon Refinery CO2 stream | 2020 | Hydrogen production | 1.2–1.4 |
| Storage Types | Main Benefits | Cost (BRL/ton CO2) |
|---|---|---|
| Geological | Economical Public acceptance | 0.5–8 |
| Ocean | High potential Universal availability | 6–31 |
| Biological | Low cost High potential | 3–10 |
| Carbon mineralization | Environmentally safe Abundance of feedstock Utilization of industrial waste | 50–100 |
| Region | Area (103 ha) | Yield (kg/ha) | Production (103 t) |
|---|---|---|---|
| North | 233.5 | 4.334 | 1012 |
| Northeast | 168.8 | 2.051 | 342.3 |
| Center-West | 156 | 3.935 | 613.8 |
| Southeast | 10.1 | 4.077 | 41.3 |
| South | 1139.3 | 7.863 | 8958.1 |
| Agriculture Sector | Methane Emissions (%) |
|---|---|
| Enteric fermentation | 66.89 |
| Rice cultivation | 24.26 |
| Manure management | 7.70 |
| Agricultural waste burning | 1 |
| Countries | Countries Rice Crop | Predicted Rice Husk | Predicted Rice Straw | Energy Potential (PJ) |
|---|---|---|---|---|
| China, mainland | 212.68 | 42.54 | 212.68 | 638.03 |
| India | 81.38 | 33.70 | 168.50 | 505.50 |
| Indonesia | 48.98 | 16.28 | 81.38 | 244.15 |
| Bangladesh | 42.76 | 9.80 | 48.98 | 146.94 |
| Vietnam | 33.38 | 8.55 | 42.76 | 128.29 |
| Thailand | 25.62 | 6.68 | 33.38 | 100.15 |
| Myanmar | 19.28 | 5.12 | 25.62 | 76.87 |
| Philippines | 12.47 | 3.86 | 19.28 | 57.83 |
| Brazil | 212.68 | 2.49 | 12.47 | 37.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peres, C.B.; Resende, P.R.; Nunes, L.J.R.; de Morais, L.C. Circular Economy: A Comprehensive Review of Eco-Friendly Wollastonite Applications. Sustainability 2022, 14, 3070. https://doi.org/10.3390/su14053070
Peres CB, Resende PR, Nunes LJR, de Morais LC. Circular Economy: A Comprehensive Review of Eco-Friendly Wollastonite Applications. Sustainability. 2022; 14(5):3070. https://doi.org/10.3390/su14053070
Chicago/Turabian StylePeres, Christiano Bruneli, Pedro R. Resende, Leonel J. R. Nunes, and Leandro Cardoso de Morais. 2022. "Circular Economy: A Comprehensive Review of Eco-Friendly Wollastonite Applications" Sustainability 14, no. 5: 3070. https://doi.org/10.3390/su14053070
APA StylePeres, C. B., Resende, P. R., Nunes, L. J. R., & de Morais, L. C. (2022). Circular Economy: A Comprehensive Review of Eco-Friendly Wollastonite Applications. Sustainability, 14(5), 3070. https://doi.org/10.3390/su14053070








