Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview
Abstract
:1. Introduction
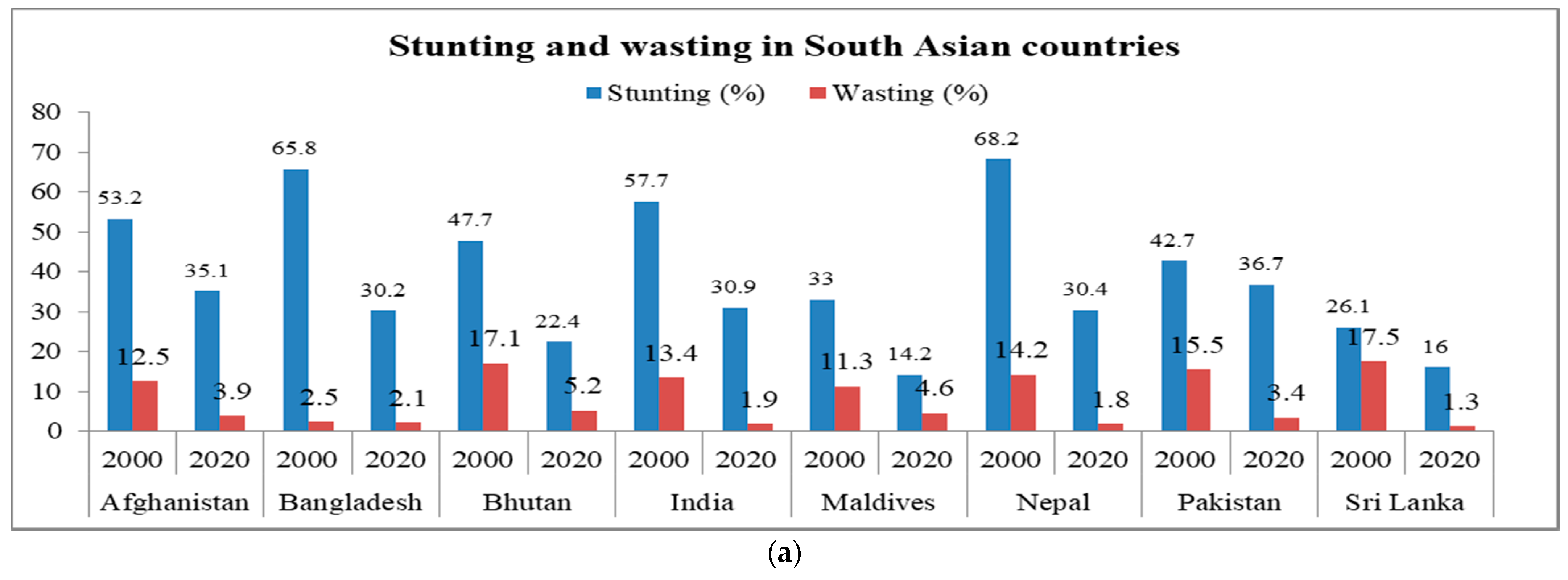
2. Health Issues and Nutritional Challenges Due to Malnutrition
Impact of the COVID-19 Pandemic on Food and Nutrition
3. Biofortification Approaches
3.1. Conventional Plant Breeding
3.2. Molecular Breeding
3.3. Genetic Engineering
3.4. Agronomic Biofortification
4. Current Efforts, Achievements, and Future Possibilities in the Biofortification of Food Crops
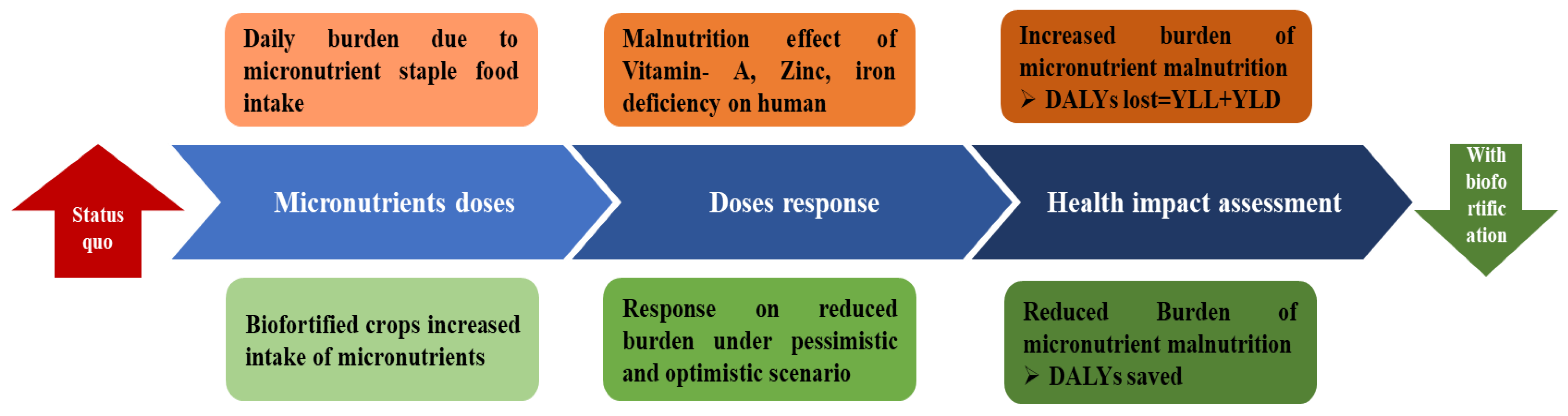
5. Impact of Biofortified Crop Cultivars in the Alleviation of Human Malnutrition
6. Cost-Effectiveness and Monetizing Benefits of Biofortification
7. Policy Support to Promote Biofortified Cultivars
8. Constraints and Challenges of Crop Biofortification
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. World Population Prospects, 2017. Available online: https://www.un.org/development/desa/publications/world-population-prospects-the-2017-revision.html (accessed on 27 August 2021).
- Kumar, S.; Lakhran, S.; Meena, R.S.; Jangir, C.K. Current need of sustainable food and forage production to eliminate food and forage insecurity under current climatic era. Forage Res. 2017, 44, 165–173. [Google Scholar]
- Von Grebmer, K.; Saltzman, A.; Birol, E.; Wiesman, D.; Prasai, N.; Yin, S.; Yohannes, Y.; Menon, P.; Thompson, J.; Sonntag, A. Synopsis: 2014 Global Hunger Index: The Challenge of Hidden Hunger; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2014. [Google Scholar]
- Pinstrup-Andersen, P. Agricultural research and policy for better health and nutrition in developing countries: A food systems approach. Agric. Econ. 2007, 37, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H. Reducing mineral and vitamin deficiencies through biofortification: Progress under HarvestPlus. In Hidden Hunger: Strategies to Improve Nutrition Quality. World Review of Nutrition and Dietetics; Biesalski, H.K., Birner, R., Eds.; Karger: Basel, Switzerland, 2018; pp. 112–122. [Google Scholar]
- International Food Policy Research Institute, Global Nutrition Report: From Promise to Impact: Ending Malnutrition by 2030. Available online: https://ebrary.ifpri.org/digital/collection/p15738coll2/id/130354 (accessed on 12 June 2021).
- Hodge, J. Hidden hunger: Approaches to tackling micronutrient deficiencies. In Nourishing Millions: Stories of Change in Nutrition; Gillespie, S., Hodge, J., Yosef, S., Pandya-Lorch, R., Eds.; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016. [Google Scholar]
- McGuire, M. FAO, IFAD, and WFP. The State of Food Insecurity in the World 2015: Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress. Rome: FAO, 2015. Adv. Nutr. 2015, 6, 623–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNICEF; WHO; WB Group. Joint Child Malnutrition Estimates—Levels and Trends in Child Malnutrition; UNICEF: New York, NY, USA; WHO: Geneva, Switzerland; The World Bank: Washington, DC, USA, 2021. [Google Scholar]
- Yang, Q.-Q.; Zhang, C.-Q.; Chan, M.-L.; Zhao, D.-S.; Chen, J.-Z.; Wang, Q.; Li, Q.-F.; Yu, H.-X.; Gu, M.-H.; Sun, S.S.-M.; et al. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J. Exp. Bot. 2016, 67, 4285–4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The Epidemiology of Global Micronutrient Deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Jangir, C.K.; Kumar, S.; Lakhran, H.; Meena, R.S. Towards mitigating malnutrition in pulses through biofortification. Trends Biosci. 2017, 10, 2999–3002. [Google Scholar]
- Kumar, S.; Karaliya, S.K.; Chaudhary, S. Precision Farming Technologies towards Enhancing Productivity and Sustainability of Rice-Wheat Cropping System. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 142–151. [Google Scholar] [CrossRef]
- Global Nutrition Report, Nourishing the SDGs (2017); Global Nutrition Report; Development Initiatives: Bristol, UK, 2017.
- Meena, R.S.; Yadav, A.; Kumar, S.; Jhariya, M.K.; Jatav, S.S. Agriculture ecosystem models for CO2 sequestration, improving soil physicochemical properties, and restoring degraded land. Ecol. Eng. 2022, 176, 106546. [Google Scholar] [CrossRef]
- Khush, G.S.; Lee, S.; Cho, J.-I.; Jeon, J.-S. Biofortification of crops for reducing malnutrition. Plant Biotechnol. Rep. 2012, 6, 195–202. [Google Scholar] [CrossRef]
- Pérez-Massot, E.; Banakar, R.; Gómez-Galera, S.; Zorrilla-López, U.; Sanahuja, G.; Arjó, G.; Miralpeix, B.; Vamvaka, E.; Farré, G.; Rivera, S.M.; et al. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes Nutr. 2012, 8, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Jakhar, S.R.; Kumar, S.; Jangir, C.K.; Meena, R.S. The role of mycorrhizal relationship in sustainable manner towards plant growth and soil fertility. Indian J. Agric. Allied Sci. 2018, 3, 19–24. [Google Scholar]
- Kakraliya, S.K.; Kumar, S.; Kakraliya, S.S.; Choudhary, K.K.; Singh, L.K. Remedial options for the sustainability of rice-wheat cropping system. J. Pharmacogn. Phytochem. 2018, 7, 163–171. [Google Scholar]
- Pingali, P.L. Green revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding Crops for Better Nutrition. Crop Sci. 2007, 47, S-88–S-105. [Google Scholar] [CrossRef]
- Bouis, H.E.; Graham, R.D.; Welch, R.M. The Consultative Group on International Agricultural Research (CGIAR) Micronutrients Project: Justification and Objectives. Food Nutr. Bull. 2000, 21, 374–381. [Google Scholar] [CrossRef] [Green Version]
- HarvestPlus, Breeding Crops for Better Nutrition: Harnessing Agricultural Technology to Improve Micronutrient Deficiencies; International Food Policy Research Institute: Washington, DC, USA, 2004; Available online: https://www.harvestplus.org/sites/default/files/brochure.pdf (accessed on 27 January 2022).
- Meenakshi, J.V. Biofortification. Best Practice Paper: New Advice from cc08; Copenhagen Consensus Center: Lowell, MA, USA, 2009. [Google Scholar]
- Buttriss, J.; Riley, H. Sustainable diets: Harnessing the nutrition agenda. Food Chem. 2013, 140, 402–407. [Google Scholar] [CrossRef]
- Akhtar, S. Vitamin D Status in South Asian Populations—Risks and Opportunities. Crit. Rev. Food Sci. Nutr. 2014, 56, 1925–1940. [Google Scholar] [CrossRef]
- Akhtar, S. Zinc Status in South Asian Populations—An Update. J. Heal. Popul. Nutr. 2013, 31, 139–149. [Google Scholar] [CrossRef] [Green Version]
- World Bank. South Asia Economic Focus, Spring 2016: Fading Tailwinds. World Bank. 2016. Available online: https://openknowledge.worldbank.org/handle/10986/24016 (accessed on 5 December 2021).
- Akhtar, S. Malnutrition in South Asia—A Critical Reappraisal. Crit. Rev. Food Sci. Nutr. 2015, 56, 2320–2330. [Google Scholar] [CrossRef]
- UNICEF. Child Nutrition and COVID-19. Available online: https://data.unicef.org/topic/nutrition/child-nutrition-and-covid-19/ (accessed on 4 September 2021).
- Jangir, C.K.; Singh, D.; Kumar, S. Yield and economic response of biofertilizer and fertility levels on black gram (Vigna mungo L.). Progress. Res. Int. J. 2016, 11, 5252–5254. [Google Scholar]
- Akhtar, S.; Ahmed, A.; Ahmad, A.; Ali, Z.; Riaz, M.; Ismail, T. Iron status of the Pakistani population-current issues and strategies. Asia Pac. J. Clin. Nutr. 2013, 22, 340–347. [Google Scholar]
- Yakoob, M.Y.; Bhutta, Z.A. Effect of routine iron supplementation with or without folic acid on anemia during pregnancy. BMC Public Health 2011, 11, S21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balarajan, Y.; Ramakrishnan, U.; Ozaltin, E.; Shankar, A.H.; Subramanian, S.V. Anemia in low-income and middle-income countries. Lancet 2012, 378, 2123–2135. [Google Scholar] [CrossRef]
- Bajiya, R.; Lakhran, H.; Kumar, S.; Ma, S. Biochar for Enhancing Agricultural Sustainability under Climate Change. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1876–1883. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Jakhar, S.R.; Jangir, C.K.; Gupta, A.; Meena, B.L. Adaptation strategies for enhancing agricultural and environmental sustainability under current climate. In Sustainable Agriculture; Meena, R.S., Ed.; Scientific Publisher: Jodhpur, India, 2019; pp. 226–274. [Google Scholar]
- Mrunalini, K.; Rolaniya, L.K.; Datta, D.; Kumar, S.; Behera, B.; Makarana, G.; Singh, A.; Prasad, J.V.N.S.; Pratibha, G.; Naik, M.R.; et al. Resource conservation technologies for climate change adaptation and mitigation. In Climate Change and Indian Agriculture: Challenges and Adaptation Strategies; Srinivasaraoet, C., Ed.; ICAR-National Academy of Agricultural Research Management: Hyderabad, India, 2020; pp. 1–22. [Google Scholar]
- Laborde, D.; Martin, W.; Vos, R. Poverty and Food Insecurity Could Grow Dramatically as COVID-19 Spreads; IFPRI Blog; International Food Policy Research Institute: Washington, DC, USA, 2020; Available online: https://www.ifpri.org/blog/poverty-and-food-insecurity-could-grow-dramatically-covid-19-spreads (accessed on 21 June 2021).
- Roberton, T.; Carter, E.; Chou, V.B.; Stegmuller, A.R.; Jackson, B.D.; Tam, Y.; Sawadogo-Lewis, T.; Walker, N. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: A modelling study. Lancet Glob. Health 2020, 8, e901–e908. [Google Scholar] [CrossRef]
- Akseer, N.; Kandru, G.; Keats, E.C.; Bhutta, Z.A. COVID-19 pandemic and mitigation strategies: Implications for maternal and child health and nutrition. Am. J. Clin. Nutr. 2020, 112, 251–256. [Google Scholar] [CrossRef]
- Headey, D.; Heidkamp, R.; Osendarp, S.; Ruel, M.; Scott, N.; Black, R.; Shekar, M.; Bouis, H.; Flory, A.; Haddad, L.; et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet 2020, 396, 519–521. [Google Scholar] [CrossRef]
- Fore, H.H.; Dongyu, Q.; Beasley, D.M.; Ghebreyesus, T.A. Child malnutrition and COVID-19: The time to act is now. Lancet 2020, 396, 517–518. [Google Scholar] [CrossRef]
- Martorell, R. Improved nutrition in the first 1000 days and adult human capital and health. Am. J. Hum. Biol. 2017, 29, e22952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poletti, S.; Sautter, C. Biofortification of the crops with micronutrients using plant breeding and/or transgenic strategies. Minerva Biotecnol. 2005, 17, 1–11. [Google Scholar]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.B.; Warkentin, T.D. Biofortification of Pulse Crops: Status and Future Perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, A.; Birol, E.; Oparinde, A.; Andersson, M.S.; Asaresson, W.D.; Diressie, M.T.; Zeller, M. Availability, production, and consumption of crops biofortified by plant breeding: Current evidence and future potential. Ann. N. Y. Acad. Sci. 2017, 1390, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, S.; Kumar, S.; Kumar, P.; Meena, R.S.; Rakshit, S. Nitrogen fixation in maize: Breeding opportunities. Theor. Appl. Genet. 2021, 134, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Reddy, B.V.; Ramaiah, B.; Sahrawat, K.L.; Pfeiffer, W.H. Genetic variability and character association for grain iron and zinc contents in sorghum germplasm accessions and commercial cultivars. Eur. J. Plant Sci. Biotechnol. 2012, 6, 1–5. [Google Scholar]
- Boy, E.; Haas, J.D.; Petry, N.; Cercamondi, C.I.; Gahutu, J.B.; Mehta, S.; Hurrell, R.F. Efficacy of iron-biofortified crops. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11879–11892. [Google Scholar] [CrossRef]
- Dutta, S.; Muthusamy, V.; Hossain, F.; Baveja, A.; Chhabra, R.; Jha, S.K.; Yadava, D.K.; Zunjare, R.U. Analysis of genetic variability for retention of kernel carotenoids in sub-tropically adapted biofortified maize under different storage conditions. J. Cereal Sci. 2020, 93, 102987. [Google Scholar] [CrossRef]
- Govindaraj, M.; Rai, K.N.; Kanatti, A.; Upadhyaya, H.D.; Shivade, H.; Rao, A.S. Exploring the genetic variability and diversity of pearl millet core collection germplasm for grain nutritional traits improvement. Sci. Rep. 2020, 10, 21177. [Google Scholar] [CrossRef]
- Maganti, S.; Swaminathan, R.; Parida, A. Variation in Iron and Zinc Content in Traditional Rice Genotypes. Agric. Res. 2019, 9, 316–328. [Google Scholar] [CrossRef]
- Unnevehr, L.; Pray, C.; Paarlberg, R. Addressing micronutrient deficiencies: Alternative interventions and technologies. AgBioforum 2007, 10, 124–134. [Google Scholar]
- Al Noor, M.; Quadir, Q.F.; Naher, J.; Jewel, Z.A.; Rashid, H.-O.; Chakrobarty, T.; Razia, S.; Nath, U.K. Inducing Variability in Rice for Enriched Iron and Zinc Content through In vitro Culture. Plant Tissue Cult. Biotechnol. 2019, 29, 161–174. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, G.B.; Senadhira, D.; Htut, H.; Graham, R.D. Breeding for Trace Mineral Density in Rice. Food Nutr. Bull. 2000, 21, 382–386. [Google Scholar] [CrossRef]
- Ambati, D.; Prasad, S.S.; Singh, J.B.; Verma, D.K.; Mishra, A.N.; Prakasha, T.L.; Phuke, R.M.; Sharma, K.C.; Singh, A.K.; Singh, G.P.; et al. High yielding durum wheat variety HI 8759 PusaTejas—A new rust and Karnal bunt resistant. Indian Farming 2019, 69, 20–22. [Google Scholar]
- Das, S.; Chaki, A.K.; Hossain, A. Breeding and agronomic approaches for the biofortification of zinc in wheat (Triticum aestivum L.) to combat zinc deficiency in millions of a population: A Bangladesh perspective. Acta Agrobot. 2019, 72, 1770. [Google Scholar] [CrossRef] [Green Version]
- Havrlentova, M.; Pšenáková, I.; Žofajová, A.; Rückschloss, L.; Kraic, J. Anthocyanins in wheat seed—A mini review. Nova Biotechnol. Chim. 2014, 13, 1–12. [Google Scholar] [CrossRef]
- Kumari, A.; Sharma, S.; Sharma, N.; Chunduri, V.; Kapoor, P.; Kaur, S.; Goyal, A.; Garg, M. Influence of Biofortified Colored Wheats (Purple, Blue, Black) on Physicochemical, Antioxidant and Sensory Characteristics of Chapatti (Indian Flatbread). Molecules 2020, 25, 5071. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Saltzman, A.; Virk, P.S.; Pfeiffer, W.H. Progress update: Crop development of biofortified staple food crops under HarvestPlus. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11905–11935. [Google Scholar] [CrossRef]
- Laurie, S.; Faber, M.; Adebola, P.; Belete, A. Biofortification of sweet potato for food and nutrition security in South Africa. Food Res. Int. 2015, 76, 962–970. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Gomez-Galera, S.; Pelacho, A.M.; Capell, T.; Christou, P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007, 12, 548–555. [Google Scholar] [CrossRef]
- Pray, C.E. The Asian Maize Biotechnology Network (AMBIONET): A Model for Strengthening National Agricultural Research Systems; CIMMYT: El Batán, Mexico, 2006; pp. 1–43. [Google Scholar]
- Rawat, N.; Neelam, K.; Tiwari, V.K.; Randhawa, G.S.; Friebe, B.; Gill, B.S.; Dhaliwal, H.S. Development and molecular characterization of wheat–Aegilops kotschyi addition and substitution lines with high grain protein, iron, and zinc. Genome 2011, 54, 943–953. [Google Scholar] [CrossRef]
- Muthusamy, V.; Hossain, F.; Thirunavukkarasu, N.; Pandey, N.; Vishwakarma, A.K.; Saha, S.; Gupta, H.S. Molecular Characterization of Exotic and Indigenous Maize Inbreds for Biofortification with Kernel Carotenoids. Food Biotechnol. 2015, 29, 276–295. [Google Scholar] [CrossRef]
- Boncompagni, E.; Arroyo, G.O.; Cominelli, E.; Gangashetty, P.I.; Grando, S.; Zu, T.T.K.; Daminati, M.G.; Nielsen, E.; Sparvoli, F. Antinutritional factors in pearl millet grains: Phytate and goitrogens content variability and molecular characterization of genes involved in their pathways. PLoS ONE 2018, 13, e0198394. [Google Scholar] [CrossRef] [PubMed]
- Oliva, N.; Cueto-Reaño, M.F.; Trijatmiko, K.R.; Samia, M.; Welsch, R.; Schaub, P.; Beyer, P.; Mackenzie, D.; Boncodin, R.; Reinke, R.; et al. Molecular characterization and safety assessment of biofortified provitamin A rice. Sci. Rep. 2020, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Swamy, B.P.M.; Rahman, M.A.; Inabangan-Asilo, M.A.; Amparado, A.; Manito, C.; Chadha-Mohanty, P.; Reinke, R.; Slamet-Loedin, I.H. Advances in breeding for high grain Zinc in Rice. Rice 2016, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Saini, D.K.; Kumar, A.; Kesh, H.; Kaushik, P. Breeding for Biofortification Traits in Rice: Means to Eradicate Hidden Hunger. In Agronomy-Climate Change & Food Security; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Jaiwal, P.K.; Chhillar, A.K.; Chaudhary, D.; Jaiwal, R. (Eds.) Nutritional Quality Improvement in Plants; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Bhatnagar-Mathur, P.; Reddy, D.S.; Anjaiah, V.; Sharma, K.K. Crop biofortification through genetic engineering: Present status and future directions. In Genomics and Crop Improvement: Relevance and Reservations; Institute of Biotechnology, Acharya NG Ranga Agricultural University: Hyderabad, India, 2011. [Google Scholar]
- Ricroch, A.; Clairand, P.; Harwood, W. Use of CRISPR systems in plant genome editing: Toward new opportunities in agriculture. Emerg. Top. Life Sci. 2017, 1, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, B.; Spalding, M.H.; Weeks, D.P.; Yang, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, J.; Li, Y.; Hu, W.; Chen, L.; Miao, Y.; Deng, P.; Yuan, C.; Ma, C.; Chen, X.; et al. Enrichment of provitamin A content in wheat (Triticum aestivum L.) by introduction of the bacterial carotenoid biosynthetic genes CrtB and CrtI. J. Exp. Bot. 2014, 65, 2545–2556. [Google Scholar] [CrossRef] [Green Version]
- Brooks, C.; Nekrasov, V.; Lippman, Z.B.; Van Eck, J. Efficient Gene Editing in Tomato in the First Generation Using the Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated9 System. Plant Physiol. 2014, 166, 1292–1297. [Google Scholar] [CrossRef] [Green Version]
- Curtin, S.J.; Xiong, Y.; Michno, J.M.; Campbell, B.W.; Stec, A.O.; Čermák, T.; Stupar, R.M. Crispr/cas9 and talen s generate heritable mutations for genes involved in small RNA processing of Glycine max and Medicago truncatula. Plant Biotechnol. J. 2018, 16, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Ansari, W.A.; Chandanshive, S.U.; Bhatt, V.; Nadaf, A.B.; Vats, S.; Katara, J.L.; Deshmukh, R. Genome editing in cereals: Approaches, applications and challenges. Int. J. Mol. Sci. 2020, 21, 4040. [Google Scholar] [CrossRef]
- Matres, J.M.; Arcillas, E.; Cueto-Reaño, M.F.; Sallan-Gonzales, R.; Trijatmiko, K.R.; Slamet-Loedin, I. Biofortification of Rice Grains for Increased Iron Content. In Rice Improvement; Ali, J., Wani, S.H., Eds.; Springer: Cham, Switzerland, 2021; pp. 471–486. [Google Scholar] [CrossRef]
- Ludwig, Y.; Slamet-Loedin, I.H. Genetic Biofortification to Enrich Rice and Wheat Grain Iron: From Genes to Product. Front. Plant Sci. 2019, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Elkonin, L.; Italyanskaya, J.; Panin, V. Genetic modification of sorghum for improved nutritional value: State of the problem and current approaches. J. Investig. Genom. 2018, 5, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Roy, O.; Meena, R.S.; Kumar, S.; Jhariya, M.K.; Pradhan, G. Assessment of land use systems for CO2 sequestration, carbon credit potential, and income security in Vindhyan region, India. Land Degrad. Dev. 2022, 33, 670–682. [Google Scholar] [CrossRef]
- Abid, N.; Khatoon, A.; Maqbool, A.; Irfan, M.; Bashir, A.; Asif, I.; Shahid, M.; Saeed, A.; Brinch-Pedersen, H.; Malik, K.A. Transgenic expression of phytase in wheat endosperm increases bioavailability of iron and zinc in grains. Transgenic Res. 2017, 26, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Gruissem, W.; Bhullar, N.K. Single genetic locus improvement of iron, zinc and β-carotene content in rice grains. Sci. Rep. 2017, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Y.; Che, P.; Glassman, K.; Albertsen, M. Nutritionally enhanced sorghum for the arid and semiarid tropical areas of Africa. In Sorghum; Humana Press: New York, NY, USA, 2019; pp. 197–207. [Google Scholar]
- Boonyaves, K.; Gruissem, W.; Bhullar, N.K. NOD promoter-controlled AtIRT1 expression functions synergistically with NAS and FERRITIN genes to increase iron in rice grains. Plant Mol. Biol. 2016, 90, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Boonyaves, K.; Wu, T.-Y.; Gruissem, W.; Bhullar, N.K. Enhanced Grain Iron Levels in Rice Expressing an iron-regulated metal transporter, nicotianamine synthase, and ferritin Gene Cassette. Front. Plant Sci. 2017, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Goto, F.; Yoshihara, T.; Saiki, H. Iron accumulation in tobacco plants expressing soybean ferritin gene. Transgenic Res. 1998, 7, 173–180. [Google Scholar] [CrossRef]
- Lucca, P.; Hurrell, R.; Potrykus, I. Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor. Appl. Genet. 2001, 102, 392–397. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the Provitamin A (β-Carotene) Biosynthetic Pathway into (Carotenoid-Free) Rice Endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Usuda, K.; Kobayashi, T.; Ishimaru, Y.; Kakei, Y.; Takahashi, M.; Higuchi, K.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Overexpression of the Barley Nicotianamine Synthase Gene HvNAS1 Increases Iron and Zinc Concentrations in Rice Grains. Rice 2009, 2, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Wirth, J.; Poletti, S.; Aeschlimann, B.; Yakandawala, N.; Drosse, B.; Osorio, S.; Tohge, T.; Fernie, A.R.; Günther, D.; Gruissem, W.; et al. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol. J. 2009, 7, 631–644. [Google Scholar] [CrossRef]
- Lee, T.T.T.; Wang, M.M.C.; Hou, R.C.W.; Chen, L.-J.; Su, R.-C.; Wang, C.-S.; Tzen, J.T.C. Enhanced Methionine and Cysteine Levels in Transgenic Rice Seeds by the Accumulation of Sesame 2S Albumin. Biosci. Biotechnol. Biochem. 2003, 67, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, L.; Wang, C.; Chen, L.; Liu, H.; Yang, G.; He, G. Expression of phytoene synthase1 and carotene desaturase crtI genes result in an increase in the total carotenoids content in transgenic elite wheat (Triticum aestivum L.). J. Agric. Food Chem. 2009, 57, 8652–8660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyan, S.; Yan, Z.; Shubin, W. Improvement Fe content of wheat (Triticum aestivum) grain by soybean ferritin expression cassette without vector backbone sequence. J. Agric. Biotechnol. 2012, 20, 766–773. [Google Scholar]
- Borg, S.; Brinch-Pedersen, H.; Tauris, B.; Madsen, L.H.; Darbani, B.; Noeparvar, S.; Holm, P.B. Wheat ferritins: Improving the iron content of the wheat grain. J. Cereal Sci. 2012, 56, 204–213. [Google Scholar] [CrossRef]
- Brinch-Pedersen, H.; Olesen, A.; Rasmussen, S.K.; Holm, P.B. Generation of transgenic wheat (Triticum aestivum L.) for constitutive accumulation of an Aspergillus phytase. Mol. Breed. 2000, 6, 195–206. [Google Scholar] [CrossRef]
- Sestili, F.; Janni, M.; Doherty, A.; Botticella, E.; D’Ovidio, R.; Masci, S.; Lafiandra, D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010, 10, 144. [Google Scholar] [CrossRef] [Green Version]
- Doshi, K.M.; Eudes, F.; Laroche, A.; Gaudet, D. Transient embryo-specific expression of anthocyanin in wheat. Vitr. Cell. Dev. Biol. Plant 2006, 42, 432–438. [Google Scholar] [CrossRef]
- Decourcelle, M.; Perez-Fons, L.; Baulande, S.; Steiger, S.; Couvelard, L.; Hem, S.; Zhu, C.; Capell, T.; Christou, P.; Fraser, P.; et al. Combined transcript, proteome, and metabolite analysis of transgenic maize seeds engineered for enhanced carotenoid synthesis reveals pleotropic effects in core metabolism. J. Exp. Bot. 2015, 66, 3141–3150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahoon, E.B.; Hall, S.E.; Ripp, K.G.; Ganzke, T.S.; Hitz, W.D.; Coughlan, S.J. Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat. Biotechnol. 2003, 21, 1082–1087. [Google Scholar] [CrossRef]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin A content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluru, M.R.; Rodermel, S.R.; Reddy, M.B. Genetic Modification of Low Phytic Acid 1-1 Maize to Enhance Iron Content and Bioavailability. J. Agric. Food Chem. 2011, 59, 12954–12962. [Google Scholar] [CrossRef]
- Chen, R.; Xue, G.; Chen, P.; Yao, B.; Yang, W.; Ma, Q.; Fan, Y.; Zhao, Z.; Tarczynski, M.C.; Shi, J. Transgenic maize plants expressing a fungal phytase gene. Transgenic Res. 2008, 17, 633–643. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Schellin, K.; Li, B.; Faller, M.; Stoop, J.M.; Meeley, R.B.; Ertl, D.; Ranch, J.P.; Glassman, K. Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat. Biotechnol. 2007, 25, 930–937. [Google Scholar] [CrossRef]
- Yu, J.; Peng, P.; Zhang, X.; Zhao, Q.; Zhy, D.; Sun, X.; Liu, J.; Ao, G. Seed-specific expression of a lysine rich protein sb401 gene significantly increases both lysine and total protein content in maize seeds. Mol. Breed. 2004, 14, 1–7. [Google Scholar] [CrossRef]
- Lipkie, T.E.; De Moura, F.F.; Zhao, Z.-Y.; Albertsen, M.C.; Che, P.; Glassman, K.; Ferruzzi, M. Bioaccessibility of Carotenoids from Transgenic Provitamin A Biofortified Sorghum. J. Agric. Food Chem. 2013, 61, 5764–5771. [Google Scholar] [CrossRef]
- Kapusi, E.; Corcuera-Gómez, M.; Melnik, S.; Stoger, E. Heritable Genomic Fragment Deletions and Small Indels in the Putative ENGase Gene Induced by CRISPR/Cas9 in Barley. Front. Plant Sci. 2017, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schernthaner, J.; Labbé, N.; Hefford, M.A.; Zhao, J.; Simmonds, D.H. Improved protein quality in transgenic soybean expressing a de novo synthetic protein, MB-16. Transgenic Res. 2014, 23, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Kurtzer, R.; Eisenreich, W.; Schwab, W. The Carotenase AtCCD1 from Arabidopsis thaliana Is a Dioxygenase. J. Biol. Chem. 2006, 281, 9845–9851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.-J.; Kim, J.K.; Kim, H.J.; Pak, J.H.; Lee, J.-H.; Kim, D.-H.; Choi, H.K.; Jung, H.W.; Lee, J.-D.; Chung, Y.-S.; et al. Genetic Modification of the Soybean to Enhance the β-Carotene Content through Seed-Specific Expression. PLoS ONE 2012, 7, e48287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eenennaam, A.; Lincoln, K.; Durrett, T.P.; Valentin, H.E.; Shewmaker, C.K.; Thorne, G.M.; Jiang, J.; Baszis, S.R.; Levering, C.K.; Aasen, E.D.; et al. Engineering Vitamin E Content: From Arabidopsis Mutant to Soy Oil. Plant Cell 2003, 15, 3007–3019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkins, R.D.; Reddy, M.S.S.; Meurer, C.A.; Yan, B.; Trick, H.; Thibaud-Nissen, F.; Finer, J.J.; Parrott, W.A.; Collins, G.B. Increased sulfur amino acids in soybean plants overexpressing the maize 15 kDa zein protein. Vitr. Cell Dev. Biol. Plant 2001, 37, 742–747. [Google Scholar] [CrossRef] [Green Version]
- Aragao, F.J.L.; Barros, L.M.G.; De Sousa, M.V.; Grossi de Sá, M.F.; Almeida, E.R.P.; Gander, E.S.; Rech, E.L. Expression of a methionine-rich storage albumin from the Brazil nut (Bertholletia excelsa HBK, Lecythidaceae) in transgenic bean plants (Phaseolus vulgaris L., Fabaceae). Genet. Mol. Biol. 1999, 22, 445–449. [Google Scholar] [CrossRef]
- Lopez, A.B.; Van Eck, J.; Conlin, B.J.; Paolillo, D.J.; O’Neill, J.; Li, L. Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef]
- Upadhyay, C.P.H.; Ko, E.Y.; Nookaraju, A.; Kim, H.S.; Heung, J.J.; Oh, M.O.; Reddy, A.C.; Chun, S.C.; Kim, D.H.; Park, S.W. Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci. 2009, 177, 659–667. [Google Scholar]
- Dancs, G.; Kondrák, M.; Bánfalvi, Z. The effects of enhanced methionine synthesis on amino acid and anthocyanin content of potato tubers. BMC Plant Biol. 2008, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Joshi, V.; Jander, G. The catabolic enzyme methionine gamma-lyase limits methionine accumulation in potato tubers. Plant Biotechnol. J. 2014, 12, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Lukaszewicz, M.; Matysiak-Kata, I.; Skala, J.; Fecka, I.; Cisowski, W.; Szopa, J. Antioxidant Capacity Manipulation in Transgenic Potato Tuber by Changes in Phenolic Compounds Content. J. Agric. Food Chem. 2004, 52, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Beyene, G.; Chauhan, R.D.; Gaitán-Solís, E.; Gehan, J.; Butts, P.; Siritunga, D.; Okwuonu, I.; Woll, A.; Jiménez-Aguilar, D.M.; et al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat. Biotechnol. 2019, 37, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Arango, J.; Bär, C.; Salazar, B.; Al-Babili, S.; Beltran, J.; Chavarriaga, P.; Ceballos, H.; Tohme, J.; Beyer, P. Provitamin A Accumulation in Cassava (Manihot esculenta) Roots Driven by a Single Nucleotide Polymorphism in a Phytoene Synthase Gene. Plant Cell 2010, 22, 3348–3356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telengech, P.K.; Maling’a, J.N.; Nyende, A.B.; Gichuki, S.T.; Wanjala, B.W. Gene expression of beta carotene genes in transgenic biofortified cassava. 3 Biotech. 2015, 5, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Lorenc-Kukuła, K.; Wróbel-Kwiatkowska, M.; Starzycki, M.; Szopa, J. Engineering flax with increased flavonoid content and thus Fusarium resistance. Physiol. Mol. Plant Pathol. 2007, 70, 38–48. [Google Scholar] [CrossRef]
- Fujisawa, M.; Watanabe, M.; Choi, S.-K.; Teramoto, M.; Ohyama, K.; Misawa, N. Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J. Biosci. Bioeng. 2008, 105, 636–641. [Google Scholar] [CrossRef]
- Fujisawa, M.; Takita, E.; Harada, H.; Sakurai, N.; Suzuki, H.; Ohyama, K.; Shibata, D.; Misawa, N. Pathway engineering of Brassica napus seeds using multiple key enzyme genes involved in ketocarotenoid formation. J. Exp. Bot. 2009, 60, 1319–1332. [Google Scholar] [CrossRef]
- Falco, S.C.; Guida, T.; Locke, M.; Mauvais, J.; Sanders, C.; Ward, R.T.; Webber, P. Transgenic Canola and Soybean Seeds with Increased Lysine. Nat. Biotechnol. 1995, 13, 577–582. [Google Scholar] [CrossRef]
- Dehesh, K.; Jones, A.; Knutzon, D.S.; Voelker, T.A. Production of high levels of 8: 0 and 10: 0 fatty acids in transgenic canola by overexpression of Ch FatB2, a thioesterase cDNA from Cuphea hookeriana. Plant J. 1996, 9, 167–172. [Google Scholar] [CrossRef]
- Rosati, C.; Aquilani, R.; Dharmapuri, S.; Pallara, P.; Marusic, C.; Tavazza, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000, 24, 413–420. [Google Scholar] [CrossRef]
- Apel, W.; Bock, R. Enhancement of Carotenoid Biosynthesis in Transplastomic Tomatoes by Induced Lycopene-to-Provitamin A Conversion. Plant Physiol. 2009, 151, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-C.; Zhong, Y.-J.; Liu, J.; Sandmann, G.; Chen, F. Metabolic engineering of tomato for high-yield production of astaxanthin. Metab. Eng. 2013, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Dharmapuri, S.; Rosati, C.; Pallara, P.; Aquilani, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of xanthophyll content in tomato fruits. FEBS Lett. 2002, 519, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Halka, M.; Smoleń, S.; Czernicka, M.; Klimek-Chodacka, M.; Pitala, J.; Tutaj, K. Iodine biofortification through expression of HMT, SAMT and S3H genes in Solanum lycopersicum L. Plant Physiol. Biochem. 2019, 144, 35–48. [Google Scholar] [CrossRef]
- Morineau, C.; Bellec, Y.; Tellier, F.; Gissot, L.; Kelemen, Z.; Nogué, F.; Faure, J.-D. Selective gene dosage by CRISPR-Cas9 genome editing in hexaploid Camelina sativa. Plant Biotechnol. J. 2017, 15, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Hanania, U.; Ariel, T.; Tekoah, Y.; Fux, L.; Sheva, M.; Gubbay, Y.; Weiss, M.; Oz, D.; Azulay, Y.; Turbovski, A.; et al. Establishment of a tobacco BY2 cell line devoid of plant-specific xylose and fucose as a platform for the production of biotherapeutic proteins. Plant Biotechnol. J. 2017, 15, 1120–1129. [Google Scholar] [CrossRef]
- Mercx, S.; Smargiasso, N.; Chaumont, F.; De Pauw, E.; Boutry, M.; Navarre, C. Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 Cells by a Multiplex CRISPR/Cas9 Strategy Results in Glycoproteins without Plant-Specific Glycans. Front. Plant Sci. 2017, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Meena, N.; Fathima, P.S. Nutrient uptake of rice as influenced by agronomic biofortification of Zn and Fe under methods of rice cultivation. Int. J. Pure App. Biosci. 2017, 5, 456–459. [Google Scholar]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Shivay, Y.S.; Singh, U.; Prasad, R.; Kaur, R. Agronomic interventions for micronutrient biofortification of pulses. Indian J. Agron. 2016, 61, 161–172. [Google Scholar]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Pang, L.-L.; Yan, P.; Liu, D.-Y.; Zhang, W.; Yost, R.; Zhang, F.-S.; Zou, C.-Q. Zinc fertilizer placement affects zinc content in maize plant. Plant Soil 2013, 372, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Ohtani, Y.; Tatsukami, Y.; Aoki, W.; Amemiya, T.; Sukekiyo, Y.; Kubokawa, S.; Ueda, M. Folate biofortifi-cation in hydroponically cultivated spinach by the addition of phenylalanine. J. Agric. Food Chem. 2017, 65, 4605–4610. [Google Scholar] [CrossRef]
- Montesano, F.F.; D’Imperio, M.; Parente, A.; Cardinali, A.; Renna, M.; Serio, F. Green bean biofortification for Si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci. Rep. 2016, 6, 31662. [Google Scholar] [CrossRef] [Green Version]
- Barrameda-Medina, Y.; Blasco, B.; Lentini, M.; Esposito, S.; Baenas, N.; Moreno, D.A.; Ruiz, J.M. Zinc biofortification improves phytochemicals and amino-acidic profile in Brassica oleracea cv. Bronco. Plant Sci. 2017, 258, 45–51. [Google Scholar] [CrossRef]
- Zou, C.; Du, Y.; Rashid, A.; Ram, H.; Savasli, E.; Pieterse, P.J.; Ortiz-Monasterio, I.; Yazici, A.; Kaur, C.; Mahmood, K.; et al. Simultaneous Biofortification of Wheat with Zinc, Iodine, Selenium, and Iron through Foliar Treatment of a Micronutrient Cocktail in Six Countries. J. Agric. Food Chem. 2019, 67, 8096–8106. [Google Scholar] [CrossRef] [Green Version]
- Prom-U-Thai, C.; Rashid, A.; Ram, H.; Zou, C.; Guilherme, L.R.G.; Corguinha, A.P.B.; Guo, S.; Kaur, C.; Naeem, A.; Yamuangmorn, S.; et al. Simultaneous Biofortification of Rice with Zinc, Iodine, Iron and Selenium Through Foliar Treatment of a Micronutrient Cocktail in Five Countries. Front. Plant Sci. 2020, 11, 1516. [Google Scholar] [CrossRef]
- Sahin, O. Combined biofortification of soilless grown lettuce with iodine, selenium and zinc and its effect on essential and non-essential elemental composition. J. Plant Nutr. 2020, 44, 673–678. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir, A. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Ramzani, P.M.A.; Khalid, M.; Naveed, M.; Ahmad, R.; Shahid, M. Iron biofortification of wheat grains through integrated use of organic and chemical fertilizers in pH affected calcareous soil. Plant Physiol. Biochem. 2016, 104, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, M.; Nadeem, F.; Rehman, H.U.; Wahid, A.; Basra, S.M.A.; Siddique, K. Seed priming in field crops: Potential benefits, adoption and challenges. Crop Pasture Sci. 2019, 70, 731–771. [Google Scholar] [CrossRef]
- Raj, A.B.; Raj, S.K. Seed priming: An approach towards agricultural sustainability. J. Appl. Nat. Sci. 2019, 11, 227–234. [Google Scholar] [CrossRef]
- Praharaj, S.; Singh, R.; Singh, V.K.; Chandra, R. Yield and grain zinc concentration of wheat as affected by nutri priming and foliar application of zinc. J. Pharm. Phytochem. 2019, 8, 503–505. [Google Scholar]
- Naveed, M.; Khalid, H.; Ayub, M.A.; Rehman, M.Z.U.; Rizwan, M.; Rasul, A.; Haq, M.A.U. Biofortification of Cereals with Zinc and Iron: Recent Advances and Future Perspectives. In Resources Use Efficiency in Agriculture; Springer: Cham, Switzerland, 2020; pp. 615–646. [Google Scholar] [CrossRef]
- Omari, R.; Zotor, F.; Tagwireyi, J.; Lokosang, L. Advocacy for scaling up biofortified crops for improved micronutrient status in Africa: Approaches, achievements, challenges and lessons. Proc. Nutr. Soc. 2019, 78, 567–575. [Google Scholar] [CrossRef]
- Mohapatra, T.; Yadava, D.K.; Hossain, F. Nutritional security through crop biofortification in India: Status & future prospects. Indian J. Med. Res. 2018, 148, 621–631. [Google Scholar] [CrossRef]
- Owens, B.F.; Lipka, A.E.; Magallanes-Lundback, M.; Tiede, T.; Diepenbrock, C.H.; Kandianis, C.B.; Kim, E.; Cepela, J.; Mateos-Hernandez, M.; Buell, C.R.; et al. A Foundation for Provitamin A Biofortification of Maize: Genome-Wide Association and Genomic Prediction Models of Carotenoid Levels. Genetics 2014, 198, 1699–1716. [Google Scholar] [CrossRef] [Green Version]
- Diepenbrock, C.H.; Gore, M.A. Closing the Divide between Human Nutrition and Plant Breeding. Crop Sci. 2015, 55, 1437–1448. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.P.; Herrera, L.A.C.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Stangoulis, J.; Sohu, V.S.; Mavi, G.S.; Mishra, V.K.; et al. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2018, 8, 13526. [Google Scholar] [CrossRef] [PubMed]
- Raza, Q.; Riaz, A.; Sabar, M.; Atif, R.M.; Bashir, K. Meta-analysis of grain iron and zinc associated QTLs identified hotspot chromosomal regions and positional candidate genes for breeding biofortified rice. Plant Sci. 2019, 288, 110214. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Coyne, C.J.; Grusak, M.A.; Mazourek, M.; Cheng, P.; Main, R.; McGee, R.J. Genome-wide SNP identification, linkage map construction and QTL mapping for seed mineral concentrations and contents in pea (Pisum sativum L.). BMC Plant Biol. 2017, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Vandemark, G.J.; Grusak, M.A.; McGee, R.J. Mineral concentrations of chickpea and lentil cultivars and breeding lines grown in the U.S. Pacific Northwest. Crop J. 2018, 6, 253–262. [Google Scholar] [CrossRef]
- Neeraja, C.N.; Babu, V.R.; Ram, S.; Hossain, F.; Hariprasanna, K.; Rajpurohit, B.S.; Prabhakar; Longvah, T.; Prasad, K.S.; Sandhu, J.S.; et al. Biofortification in Cereals: Progress and Prospects. Curr. Sci. 2017, 113, 1050–1057. [Google Scholar] [CrossRef]
- Coghlan, A. GM Golden Rice Gets Approval from Food Regulators in the US. NewScientist, 30 May 2018. Available online: https://www.newscientist.com/article/mg23831802-500-gm-golden-rice-gets-approval-from-food-regulators-in-the-us/ (accessed on 8 October 2021).
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.; Praharaj, C.S.; Singh, S.S.; Singh, N.P. (Eds.) Biofortification of Food Crops; Springer: New Delhi, India, 2016. [Google Scholar]
- Kumar, S.; Palve, A.; Joshi, C.; Srivastava, R.K. Crop biofortification for iron (Fe), zinc (Zn) and vitamin A with transgenic approaches. Heliyon 2019, 5, e01914. [Google Scholar] [CrossRef] [Green Version]
- Lockyer, S.; White, A.; Buttriss, J.L. Biofortified crops for tackling micronutrient deficiencies—What impact are these having in developing countries and could they be of relevance within Europe? Nutr. Bull. 2018, 43, 319–357. [Google Scholar] [CrossRef]
- La Frano, M.R.; De Moura, F.F.; Boy, E.; Lönnerdal, B.; Burri, B.J. Bioavailability of iron, zinc, and provitamin A carotenoids in biofortified staple crops. Nutr. Rev. 2014, 72, 289–307. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Beard, J.L.; Murray-Kolb, L.E.; Del Mundo, A.M.; Felix, A.; Gregorio, G.B. Iron-Biofortified Rice Improves the Iron Stores of Nonanemic Filipino Women. J. Nutr. 2005, 135, 2823–2830. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Mehta, S.; Udipi, S.A.; Ghugre, P.S.; Luna, S.V.; Wenger, M.; Murray-Kolb, L.E.; Przybyszewski, E.M.; Haas, J.D. A Randomized Trial of Iron-Biofortified Pearl Millet in School Children in India. J. Nutr. 2015, 145, 1576–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, J.D.; Luna, S.V.; Lung’Aho, M.G.; Wenger, M.J.; Murray-Kolb, L.E.; Beebe, S.; Gahutu, J.B.; Egli, I.M. Consuming Iron Biofortified Beans Increases Iron Status in Rwandan Women after 128 Days in a Randomized Controlled Feeding Trial. J. Nutr. 2016, 146, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Low, J.W.; Arimond, M.; Osman, N.; Cunguara, B.; Zano, F.; Tschirley, D. A Food-Based Approach Introducing Orange-Fleshed Sweet Potatoes Increased Vitamin A Intake and Serum Retinol Concentrations in Young Children in Rural Mozambique. J. Nutr. 2007, 137, 1320–1327. [Google Scholar] [CrossRef] [Green Version]
- Hotz, C.; Loechl, C.; De Brauw, A.; Eozenou, P.; Gilligan, D.; Moursi, M.; Munhaua, B.; Van Jaarsveld, P.; Carriquiry, A.; Meenakshi, J.V. A large-scale intervention to introduce orange sweet potato in rural Mozambique increases vitamin A intakes among children and women. Br. J. Nutr. 2012, 108, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Van Jaarsveld, P.J.; Faber, M.; Tanumihardjo, S.A.; Nestel, P.; Lombard, C.J.; Benadé, A.J.S. β-Carotene–rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am. J. Clin. Nutr. 2005, 81, 1080–1087. [Google Scholar] [CrossRef]
- Jamil, K.M.; Brown, K.H.; Jamil, M.; Peerson, J.M.; Keenan, A.H.; Newman, J.W.; Haskell, M.J. Daily Consumption of Orange-Fleshed Sweet Potato for 60 Days Increased Plasma β-Carotene Concentration but Did Not Increase Total Body Vitamin A Pool Size in Bangladeshi Women. J. Nutr. 2012, 142, 1896–1902. [Google Scholar] [CrossRef] [Green Version]
- Talsma, E.F.; Brouwer, I.; Verhoef, H.; Mbera, G.N.K.; Mwangi, A.M.; Demir, A.Y.; Maziya-Dixon, B.; Boy, E.; Zimmermann, M.B.; Melse-Boonstra, A. Biofortified yellow cassava and vitamin A status of Kenyan children: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 103, 258–267. [Google Scholar] [CrossRef]
- Gannon, B.; Kaliwile, C.; Arscott, S.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.; Masi, C.; A Tanumihardjo, S. Biofortified orange maize is as efficacious as a vitamin A supplement in Zambian children even in the presence of high liver reserves of vitamin A: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef]
- Sheftel, J.; Gannon, B.M.; Davis, C.R.; Tanumihardjo, S.A. Provitamin A-biofortified maize consumption increases serum xanthophylls and 13C-natural abundance of retinol in Zambian children. Exp. Biol. Med. 2017, 242, 1508–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, A.C.; Craft, N.E.; Schulze, K.J.; Barffour, M.; Chileshe, J.; Siamusantu, W.; West, K.P. Impact of biofortified maize consumption on serum carotenoid concentrations in Zambian children. Eur. J. Clin. Nutr. 2018, 72, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Gunaratna, N.S.; De Groote, H.; Nestel, P.; Pixley, K.V.; McCabe, G.P. A meta-analysis of community-level studies on quality protein maize. Food Policy 2010, 35, 202–210. [Google Scholar] [CrossRef]
- Nuss, E.T.; Tanumihardjo, S.A.; Fretham, S.J.B.; Carlson, E.S.; Georgieff, M.K. Quality Protein Maize for Africa: Closing the Protein Inadequacy Gap in Vulnerable Populations. Adv. Nutr. Int. Rev. J. 2011, 2, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Paroda, R.S.; Joshi, P.K. Proceedings of the National Conference on Sustainable Development Goals: India’s Preparedness and the Role of Agriculture; Indian Council of Agricultural Research (ICAR), Trust for Advancement of Agricultural Sciences (TAAS), International Food Policy Research Institute (IFPRI): New Delhi, India, 2017; p. 48. [Google Scholar]
- De Steur, H.; Gellynck, X.; Blancquaert, D.; Lambert, W.; Van Der Straeten, D.; Qaim, M. Potential impact and cost-effectiveness of multi-biofortified rice in China. New Biotechnol. 2011, 29, 432–442. [Google Scholar] [CrossRef]
- World Bank World Development Report 1993; Oxford University Press: Washington, DC, USA, 1993.
- Meenakshi, J.; Johnson, N.L.; Manyong, V.M.; De Groote, H.; Javelosa, J.; Yanggen, D.R.; Naher, F.; Gonzalez, C.; García, J.; Meng, E. How Cost-Effective is Biofortification in Combating Micronutrient Malnutrition? An Ex ante Assessment. World Dev. 2010, 38, 64–75. [Google Scholar] [CrossRef] [Green Version]
- De Steur, H.; Gellynck, X.; Storozhenko, S.; Liqun, G.; Lambert, W.; Van Der Straeten, D.; Viaene, J. Health impact in China of folate-biofortified rice. Nat. Biotechnol. 2010, 28, 554–556. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Sachdev, H.; Qaim, M. Potential impact and cost-effectiveness of Golden Rice. Nat. Biotechnol. 2006, 24, 1200–1201. [Google Scholar] [CrossRef]
- Zimmermann, R.; Qaim, M. Potential health benefits of golden rice: A Philippine case study. Food Policy 2004, 29, 147–168. [Google Scholar] [CrossRef]
- Stein, A.J.; Meenakshi, J.; Qaim, M.; Nestel, P.; Sachdev, H.; Bhutta, Z.A. Potential impacts of iron biofortification in India. Soc. Sci. Med. 2008, 66, 1797–1808. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.J. Plant breeding to control zinc deficiency in India: How cost effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qaim, M.; Stein, A.J.; Meenakshi, J.V. Economics of biofortification. Agric. Econ. 2007, 37, 119–133. [Google Scholar] [CrossRef] [Green Version]
- Qaim, M. Benefits of genetically modified crops for the poor: Household income, nutrition, and health. New Biotechnol. 2010, 27, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Meenakshi, J.V.; Qaim, M.; Nestel, P.; Sachdev, H.P.S.; Bhutta, Z.A. Health Benefits of Biofortification-An ex-Ante Analysis of Iron-Rich Rice and Wheat in India. In Proceedings of the 2005 Annual Agricultural and Applied Economics Association (AAEA) Conferences, Providence, RI, USA, 24–27 July 2005. [Google Scholar] [CrossRef]
- Rani, A.; Rani, K.; Tokas, J.; Anamika; Singh, A.; Kumar, R.; Punia, H.; Kumar, S. Nanomaterials for agriculture input use efficiency. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Mulongo, G.; Munyua, H.; Mbabu, A.; Maru, J. What is required to scale-up and sustain biofortification? Achievements, challenges and lessons from scaling-up Orange-Fleshed Sweetpotato in Sub-Sahara Africa. J. Agric. Food Res. 2021, 4, 100102. [Google Scholar] [CrossRef] [PubMed]
- Bafana, B. Biofortification offers hope for Africa’s malnourished. Afr. Renew. 2014, 28, 22–23. [Google Scholar] [CrossRef]
- Ismail, A.M.; Heuer, S.; Thomson, M.J.; Wissuwa, M. Genetic and genomic approaches to develop rice germplasm for problem soils. Plant Mol. Biol. 2007, 65, 547–570. [Google Scholar] [CrossRef]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Al-Babili, S.; Beyer, P. Golden Rice—Five years on the road—Five years to go? Trends Plant Sci. 2004, 10, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Holme, I.B.; Dionisio, G.; Brinch-Pedersen, H.; Wendt, T.; Madsen, C.K.; Vincze, E.; Holm, P.B. Cisgenic barley with improved phytase activity. Plant Biotechnol. J. 2012, 10, 237–247. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.K.; Thakral, S.K.; Bhardwaj, K.K.; Jhariya, M.K.; Meena, R.S.; Jangir, C.K.; Bedwal, S.; Jat, R.D.; Gaber, A.; et al. Integrated Nutrient Management Improves the Productivity and Nutrient Use Efficiency of Lens culinaris Medik. Sustainability 2022, 14, 1284. [Google Scholar] [CrossRef]


| Crop | Targeted Nutrient | Variety | Level of Target Nutrient | Breeding Approach | Country | References |
|---|---|---|---|---|---|---|
| Rice | Fe and Zn | BRRI dhan 62, BRRI dhan 72, BRRI dhan 64 | 18–25 mg kg−1 Zn | Conventional breeding | Bangladesh | CIAT, HarvestPlus |
| Binadhan-20 | 20–31 mg/L Fe | MABB | Bangladesh | [55] | ||
| IR68144-3B-2-2-3, Jalmagna | 21 mg/kg Fe | Selection | India | [56] | ||
| Zn | DRR Dhan 49, DRR Dhan 48, DRR Dhan 45 | 22.6–25.2 ppm | Backcross and pedigree selection | India | IIRR, India | |
| (https://www.icar-iirr.org/index.php/institute-research/institue-technologies-developed/33-iirr-technologies/107-technology-5; assessed on 26 November 2021) | ||||||
| Zinco Rice MS | 27.4 ppm | Pure line selection | India | IGKV, India | ||
| Protein | CR Dhan 311 (Mukul), CR Dhan 315 | 10.2% | Backcross followed by pedigree selection | India | NRRI, India | |
| (https://icar-nrri.in/wp-content/uploads/2019/06/2.-leaflet_highprotein_final.pdf; assessed on 6 June 2021) | ||||||
| Wheat | Zn | BHU 1, BHU 3, BHU 5, BHU 6, BHU 17, BHU 18, Zinc Shakti (Chitra) | 40–45 ppm | Conventional methods | India | CIAT, CIMMYT, Harvest Plus |
| PBW1Zn | 40.6 ppm Zn | Conventional | India | PAU, India | ||
| Fe, Zn, and protein | Pusa Tejas (HI 8759) (durum), MACS 4028 (durum) | 42.1 ppm Fe, 42.8 ppm Zn, 12% protein | Pure line selection | India | [57] | |
| Protein and Fe | Pusa Ujala (HI 1605) | 43 ppm Fe, 35 ppm Zn, 13% protein | Pure line selection | India | IARI India | |
| Protein | PBW 752 | 12.5% protein | Conventional | India | PAU, India | |
| Zn | HD 3171, PBW 757 | 47.1 ppm Zn, 42.3 ppm Zn | Hybridization and selection | India | IARI; PAU, India | |
| BARI Gom 33 | - | Conventional breeding | Bangladesh | [58] | ||
| Zincol 2016, NR 419, 421 | 33.9 ppm Zn, | -do- | Pakistan | CIMMYT | ||
| Zinc Gahun 1, Zinc Gahun 2, Borlaug 2020, | - | -do- | Nepal | CIMMYT | ||
| Fe and Zn | WB2 | 40 ppm Fe, 42 ppm Zn | Pure line selection | India | IIWBR, India | |
| HPBW-01 | 40 ppm Fe, | -do- | India | PAU, India | ||
| 40.6 ppm Zn | ||||||
| HI 8777 (durum) | 48.7 ppm Fe, 43.6 ppm Zn | Conventional | IARI, India | |||
| breeding | ||||||
| Carotene | HI 8627 | 6–9 ppm | -do- | India | IARI, India | |
| Anthocyanins | Black-grained wheat | 17.71% protein | -do- | China | [59] | |
| NABIMG-9, NABIMG-10, NABIMG-11 | - | Backcross | India | [60] | ||
| Indigo | Conventional | Austria | [59] | |||
| breeding | ||||||
| Maize | Lysine and tryptophan | Pusa HM4 Improved, Pusa HM8 Improved, Pusa HM9 Improved, IQMH 201 (LQMH 1), IQMH 202 (LQMH 2), IQMH 203 (LQMH 3) | 3.62% lysine, 0.91% tryptophan (HM4) | MAS | India | CIMMYT; VPKAS, India; IARI, India |
| 4.18% lysine | - | |||||
| 1.06% tryptophan (HM8) | ||||||
| CML140, CML194, P70 | - | Selection | China | CIMMYT | ||
| BR-451, BR-473 | - | Conventional | Brazil | CIMMYT | ||
| QS-7705 | - | Hybrid | South Africa | CIMMYT | ||
| CML176, CML170 | - | Selection | Mexico | CIMMYT | ||
| Provitamin A, lysine and tryptophan | Pusa Vivek QPM9 Improved, Pusa HQPM 5 Improved, Pusa HQPM 7 Improved | 8.15 ppm provitamin, 2.67% lysine, 0.74% tryptophan | MABB | India | IARI, India | |
| Provitamin A | Pusa VH 27 Improved | 5.49 ppm | -do- | India | IARI, India | |
| CSIR-CRI Honampa (OPV) | 6.2 µg/g | Conventional | Africa | CIMMYT | ||
| Ife maizehyb-3, Ife maizehyb-4, Sammaz 38 (OPV), | 6.3–8.0 µg/g | -do- | Nigeria | CIIMYT | ||
| Sammaz 39 (OPV) | ||||||
| Pearl millet | Fe and Zn | HHB 299, AHB 1269Fe, ABV 04, Phule Mahashakti, RHB 233, RHB 234, Dhanashakti | 73.0 ppm Fe, 41.0 ppm Zn (HHB 299), 91.0 ppm Fe, and 43.0 ppm Zn (AHB1269), 70 ppm Fe, and 63 ppm Zn (ABV 04) | Conventional | India | HAU, VNMKV, India with ICRISAT; MPKV, India |
| Hybrid ICMH 1201 (Shakti-1201) | breeding | |||||
| Fe | AHB 1200Fe | 73.0 ppm, 83.0 ppm | -do- | India | VNMKV and HAU in collaboration with ICRISAT | |
| HHB 311 | ||||||
| GB 8735 and ICTP 8203 (OPV) | 53.60 mg, 55.07 mg | -do- | West Africa | [61] | ||
| Sorghum | Fe | ICSR 14001, ICSH 14002 | 45 ppm Fe and 32 ppm Zn | -do- | India | ICRISAT, HarvestPlus |
| 12KNICSV (Deko)-188 12KNICSV-22 (Zabuwa) | 128.99 ppm Fe | -do- | Nigeria | ICRISAT, HarvestPlus | ||
| Finger millet (Eleusine coracana) | Fe | VR 929 (Vegavathi) | 131.8 mg/kg Fe and 33.2 mg/kg Zn | Pedigree selection | India | ANGRAU, India |
| Ca, Fe, Zn | CFMV1 (Indravati), | 58.0 ppm Fe, 44.0 ppm Zn, 428 mg/100 g Ca, | - | India | ANGRAU, India; NAU, India | |
| CFMV 2 | 39.0 ppm Fe, 25.0 ppm Zn, 454 mg/100 g Ca | - | ||||
| Little millet (Panicum sumatrense) | Fe and Zn | CLMV1 | 59.0 ppm Fe, 35.0 ppm Zn | - | India | IIMR, India |
| Lentil (Lens culinaris) | Fe | Pusa Ageti Masoor | 65.0 ppm Fe | Conventional | India | IARI, India |
| Fe and Zn | IPL 220, L4704, Pusa Vaibhav | 73.0 ppm Fe, 51.0 ppm Zn (IPL 220) | -do- | India | IARI India, ICARDA, HarvestPlus | |
| Idlib-2, Idlib-3 | - | Syria | ICARDA, HarvestPlus | |||
| Alemaya | - | Ethiopia | ICARDA, HarvestPlus | |||
| Barimasur-6, | 86 ppm Fe and 63 ppm Zn | -do- | Bangladesh | ICARDA, HarvestPlus | ||
| Barimasur-4, | 86 ppm Fe and 51 ppm Zn | -do- | ||||
| Barimasur-7 | 81 ppm Fe and 61 ppm Zn | -do- | ||||
| Cowpea (Vigna unguiculata) | Fe | Pant Lobia-1, | 82 ppm Fe and 40 ppm Zn (Pant Lobia-1), 100 ppm Fe, and 37 ppm Zn (Pant Lobia-2), 67 ppm Fe, and 38 ppm Zn (Pant Lobia-3), 51 ppm Fe, and 36 ppm Zn (Pant Lobia-4) | -do- | India | GBPAUT, HarvestPlus |
| Pant Lobia-2, | ||||||
| Pant Lobia-3, | ||||||
| Pant Lobia-4, | ||||||
| Pant Lobia-7 | ||||||
| Groundnut (Arachis hypogea) | Oleic acid | Girnar 4, Girnar 5 | 78.4–78.5% | Marker-assisted breeding | India | DGR, India |
| Linseed (Linum usitatissimum) | Linoleic acid | TL 99 | 58.9% Linoleic acid | Mutagenesis | India | BARC, India |
| Mustard (Brassica rapa) | Erucic acid | Pusa Mustard 30, | 1.20%, | Pedigree selection | India | IARI, India |
| Pusa Mustard 32 | 1.32% | |||||
| Erucic acid and Glucosinolates | Pusa Double Zero Mustard 31 | 0.76% Erucic acid and 29.41 ppm Glucosinolates | -do- | India | IARI, India | |
| Soybean (Glysine max) | Kunitz Trypsin Inhibitor Free | NRC 127 | - | Marker-assisted backcrossing | India | IISR, India |
| Lipoxigenase-2 free | NRC 132 | - | Modified marker-assisted backcrossing | India | IISR, India | |
| Oleic acid | NRC 147 | 42.00% | Pedigree selection | India | IISR, India | |
| Potato (Solanum tuberosum) | Anthocyanin | Kufri Manik, | 0.68 ppm, | - | India | CPRI, India |
| Kufri Neelkanth | 1.0 ppm | Hybridization and selection | ||||
| Sweet potato (Ipomoea batatas) | Provitamin A | Bhu Sona | 14.0 mg/100 g | Pure line selection | India | CTCRI, India |
| Kokota, Olympia, Zambezi | - | - | Zambia | CIP, HarvestPlus | ||
| Vita, Naspot 13 O, Ejumula | - | Clonal selection | Uganda | CIP, HarvestPlus | ||
| Beauregard, Resisto, W-119 | - | Conventional | USA | [62] | ||
| Cauliflower (Brassica oleracea var. botrytis) | Provitamin A | Pusa Beta Kesari 1 | 8.0–10.0 ppm | Pure line selection | India | IARI, India |
| Tomato | Anthocyanin | Sun Black | 7.1 mg/100 FW | Conventional breeding | Italy | [62] |
| Black Galaxy | - | -do- | Israel | [63] | ||
| Greater yam (Dioscorea alata) | Anthocyanin, protein, Zn | Sree Neelima | 50 mg/100 g anthocyanin, 15.4% protein, and 49.8 ppm Zn | Selection | India | CTCRI, India |
| Anthocyanin, Fe, Ca | Da 340 | 141.4 mg/100 g anthocyanin, 136.2 ppm Fe, and 1890 ppm Ca | - | India | CTCRI, India | |
| Cassava | Vitamin A | NR07/0220-UMUCASS44, TMS01/1368-UMUCASS36 | - | - | Nigeria | IITA, HarvestPlus |
| Kindisa (TMS 2001/1661); I011661 | - | DRC | IITA, HarvestPlus | |||
| Pomegranate (Punica granatum) | Fe, Zn, vitamin C | Solapur Lal | 5.6–6.1 mg/100 g Fe, 0.64–0.69 mg/100 g Zn, and 19.4–19.8 mg/100 g Vit C | Conventional breeding | India | NRCP, India |
| Targeted Crop | Targeted Nutrients | Gene | Donor Organism or Technique | References |
|---|---|---|---|---|
| Rice | Fe | AtIRT1, AtNAS1, PvFER | Arabidopsis, common bean | [86,87] |
| Fe | Soyfer H-1 | Soybean | [88] | |
| Phaseolus ferritin | Common bean | [89] | ||
| Fe, Zn, β-carotene | AtNAS1, PvFERRITIN, CRTI, ZmPSY | Arabidopsis, common bean, maize | [84] | |
| Vitamin A | Phytoene synthase (PSY), phytoene desaturase (CrtI) | Daffodil, Erwinia uredovora, maize | [90,91] | |
| Zn | HvNAS1 | Barley (Hordeum vulgare) | [92] | |
| Ferritin, phytase, OsNAS1 | Soybean, Aspergillus flavus, rice | [93] | ||
| Methionine and cysteine | Sulfur-rich protein, S2SA | Sesame (Sesamum indicum) | [94] | |
| Lysine | lysC, dapA | Bacteria | [10] | |
| Wheat | Vitamin A | psy1, crtI, CrtB+ Crtl | Maize, bacteria | [95,96] |
| Fe | Ferritin | Soybean | [97] | |
| TaFer1 and TaFer2 | Wheat | [98] | ||
| Low-phytate | phyA | Aspergillus niger | [99] | |
| Low-phytate | phyA | Aspergillus japonicus | [83] | |
| Amylose | SBEIIa | Wheat | [100] | |
| Anthocyanin | Dhn12, Itr1, and Ltp1 | Barley | [101] | |
| Maize | Carotenoid | crtI | Bacteria | [102] |
| Vitamin E | HGGT | Barley | [103] | |
| Vitamin A or multivitamin | crtB and crtI, psy1 | Bacteria | [104] | |
| Fe | lpa1-1, ferritin | Maize and soybean | [105] | |
| Low-phytate | phyA2 | Aspergillus niger | [106] | |
| MRP ATP-binding cassette | Maize | [107] | ||
| Lysin and total protein | sb401 | Solanum berthaultii | [108] | |
| Lipid, protein (lysin) and starch | AtGIF1, OstGIF1, ZmGIF1 | Arabidopsis, rice, maize | [108] | |
| Sorghum | Carotenoids | - | - | [109] |
| Lysin, vitamin A, Fe and Zn | PSY1, CRTI, At-DXS HGGT | Maize, Pantoea ananatis, Arabidopsis, barley | [85] | |
| Asparagine content | ENGase | CRISPR/Cas9 | [110] | |
| Soybean | Amino acid | MB-16 | Soybean | [111] |
| β-carotenoid | PSY | Pantoea ananatis | [112] | |
| PAC | Capsicum and Pantoea ananatis | [113] | ||
| Vitamin E | At-VTE3 | Arabidopsis | [114] | |
| Sulfur | Zein | Maize | [115] | |
| Common bean (Phaseolus vulgaris) | Methionine and cysteine | uidA and be2s2 | - | [116] |
| Potato | Beta carotene | Or | Cauliflower | [117] |
| Vitamin C | GalUR | Strawberry | [118] | |
| Methionine and anthocyanin | CgS, PAL | Arabidopsis | [119] | |
| Methionine | StMGL1 | Solanum tuberosum | [120] | |
| Phenolic acids and anthocyanins | CHS, CHI, DFR | Barley and Petunia hybrida | [121] | |
| Cassava | Fe | Vascular iron transporter VIT1, iron transporter IRT1, ferritin(FER1) | Arabidopsis | [122] |
| Beta carotene Provitamin A | PSY, CrtI, nptII, crtB and DXS | Pantoea ananatis | [123,124] | |
| Linseed | Flavonoid | CHS, CHI, DFR | Petunia hybrida | [125] |
| Carotenoid | crtB | Pantoea ananatis | [126] | |
| Canola (Brassica napus) | Carotenoid | crtB, crtE, crtZ, crtY, crtI, crtW, and idi | Pantoea ananatis and Brevundimonas sp. | [127] |
| Lysine | AK and DHDPS | Corynebacterium and Escherichia coli | [128] | |
| Fatty acids | Ch FatB2 | Cuphea hookeriana | [129] | |
| Tomato | β-carotene | β-Lcy | Arabidopsis | [130] |
| β-cyclase | Erwinia herbicola, Narcissus pseudonarcissus | [131] | ||
| Astaxanthin | - | Chlamydomonas reinhardtii and Haematococcus pluvialis | [132] | |
| Xanthophyll | b-Lcy, b-Chy | Arabidopsis and pepper | [133] | |
| Iodine | HMT, S3H, and SAMT | Solanum lycopersicum L. | [134] | |
| Camelina sativa | Low polyunsaturated fatty acids | FAD2 | Targeted mutagenesis by CRISPR/Cas9 | [135] |
| Tobacco | Protein | XylT, FucT | CRISPR/Cas9 | [136,137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheoran, S.; Kumar, S.; Ramtekey, V.; Kar, P.; Meena, R.S.; Jangir, C.K. Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview. Sustainability 2022, 14, 3301. https://doi.org/10.3390/su14063301
Sheoran S, Kumar S, Ramtekey V, Kar P, Meena RS, Jangir CK. Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview. Sustainability. 2022; 14(6):3301. https://doi.org/10.3390/su14063301
Chicago/Turabian StyleSheoran, Seema, Sandeep Kumar, Vinita Ramtekey, Priyajoy Kar, Ram Swaroop Meena, and Chetan Kumar Jangir. 2022. "Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview" Sustainability 14, no. 6: 3301. https://doi.org/10.3390/su14063301
APA StyleSheoran, S., Kumar, S., Ramtekey, V., Kar, P., Meena, R. S., & Jangir, C. K. (2022). Current Status and Potential of Biofortification to Enhance Crop Nutritional Quality: An Overview. Sustainability, 14(6), 3301. https://doi.org/10.3390/su14063301








