Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach
Abstract
:1. Introduction
2. Experimental Description
2.1. Manometric Method of Vapor Pressure Measurement
2.2. Experimental Setup of Hybrid LDAC System
2.3. Uncertainty Assessment
3. Methodology
3.1. Experimental Design
3.2. DOE Structure
3.3. Performance Index
- (a)
- Specific humidity change (in grams per kg of dry air):
- (b)
- Heat load of dehumidifier (in ):
- (c)
- Coefficient of performance of the hybrid system ():
4. Results and Discussion
4.1. Regression Correlations
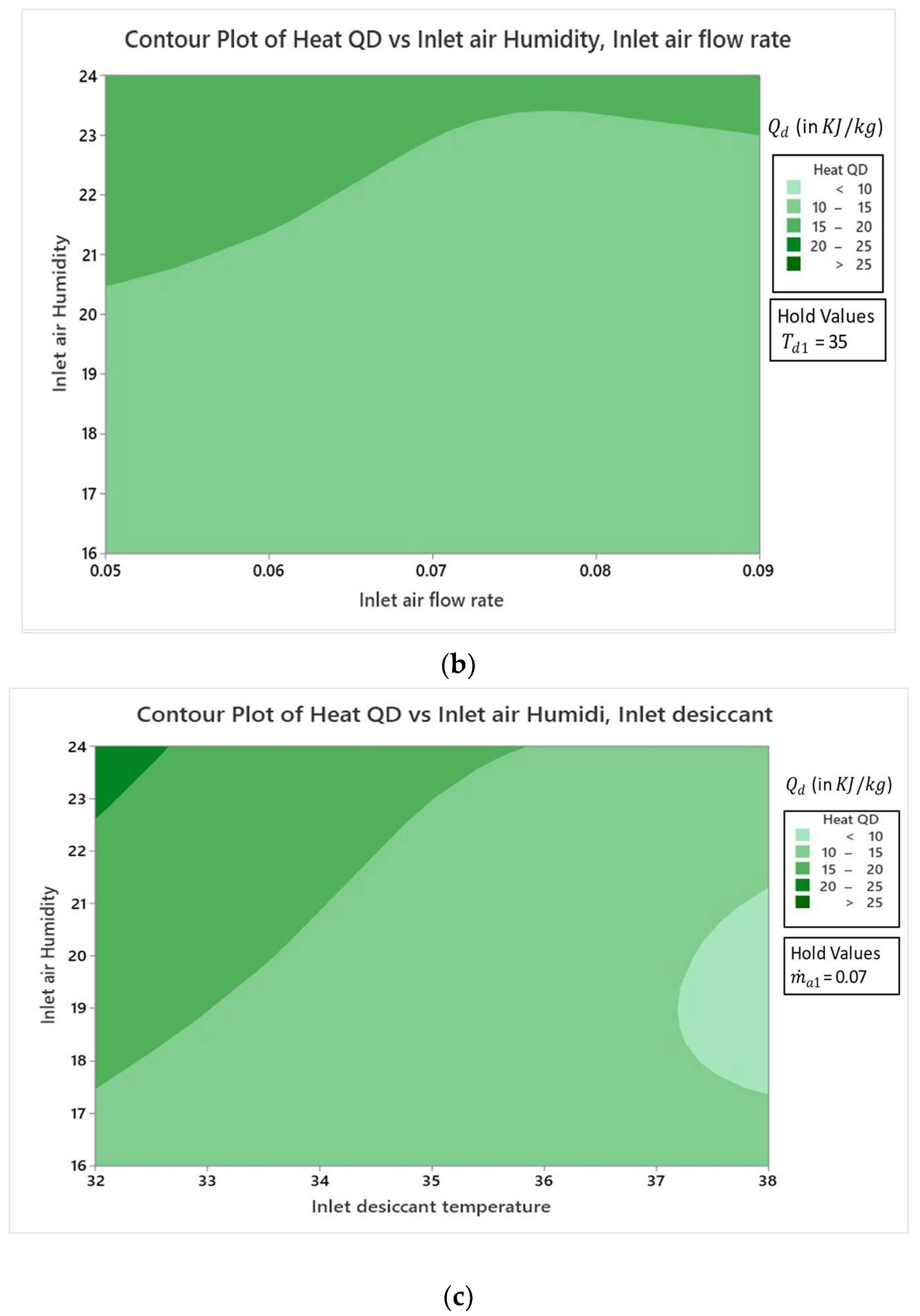
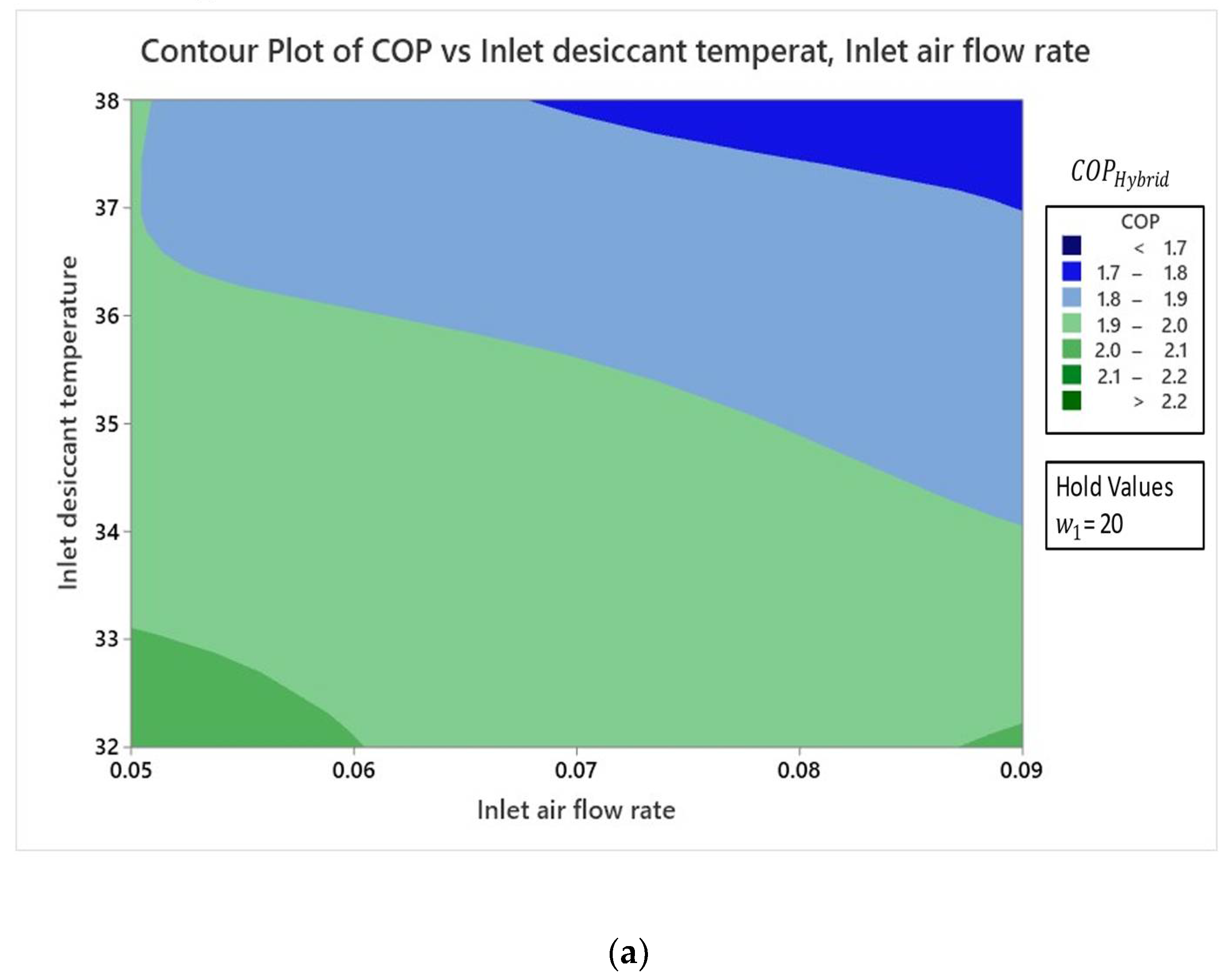
4.2. Effect of Variables
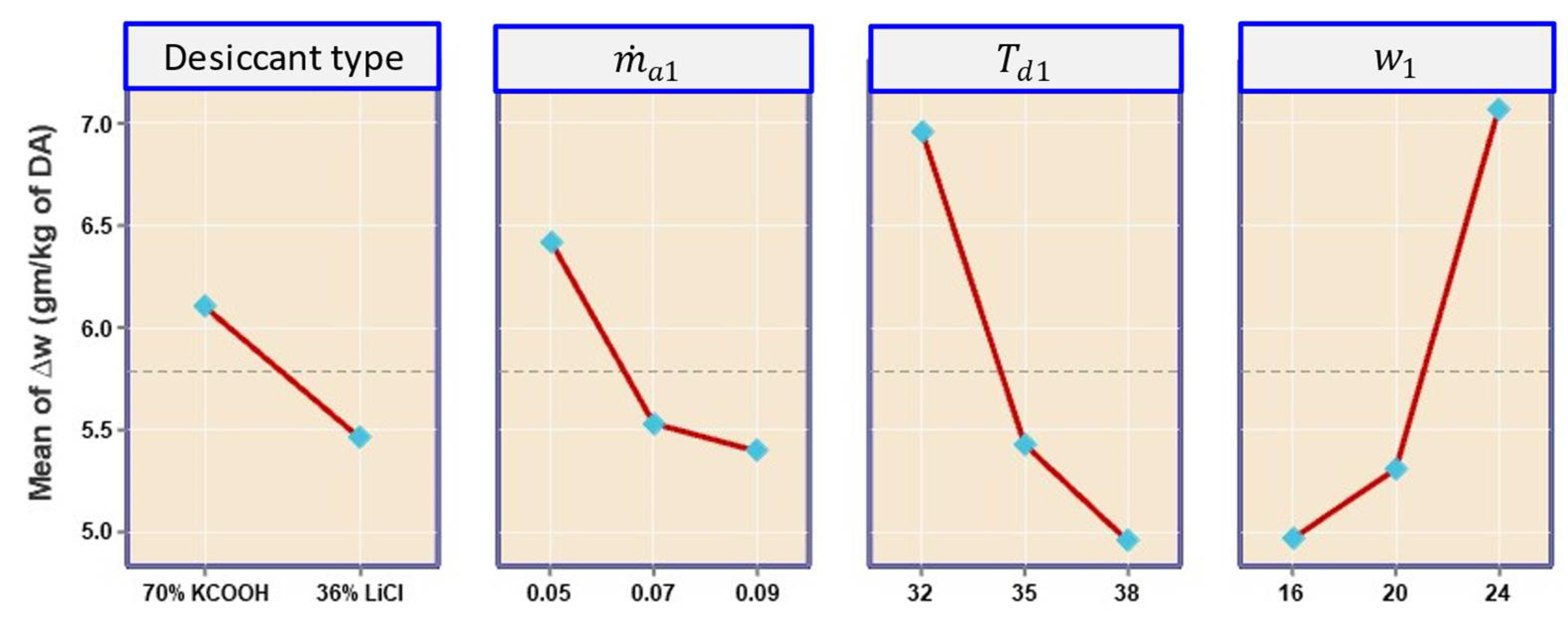
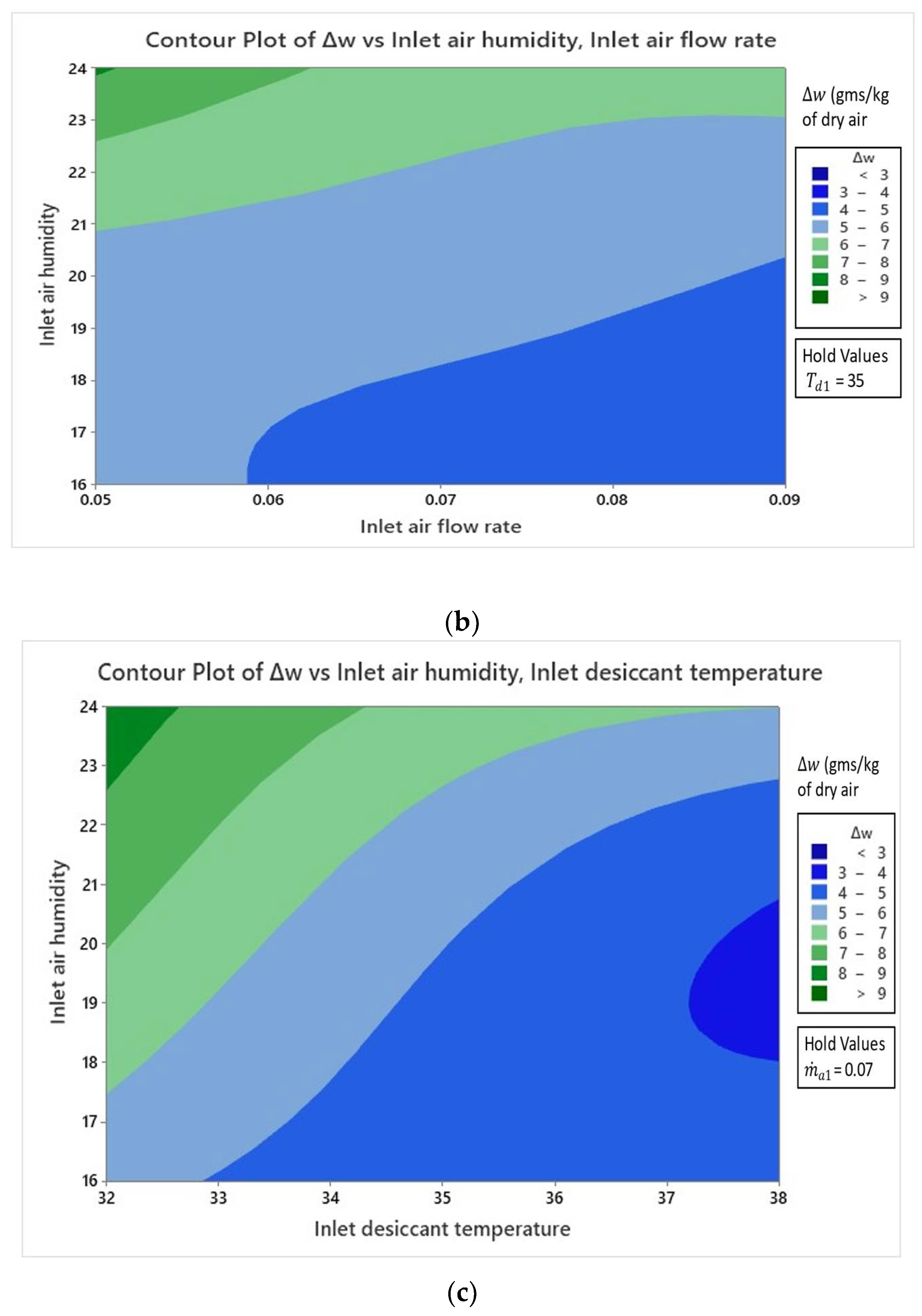
4.2.1. Effect on Specific Humidity Change
- The contributions of linear terms , , and were 30.97%, 35.81%, and 8.72%, respectively.
- The contribution of cross iteration () was only 0.29%, and the highest contribution was indicated by ( ) with 10.23%, indicating the least significance among all cross-interactions.
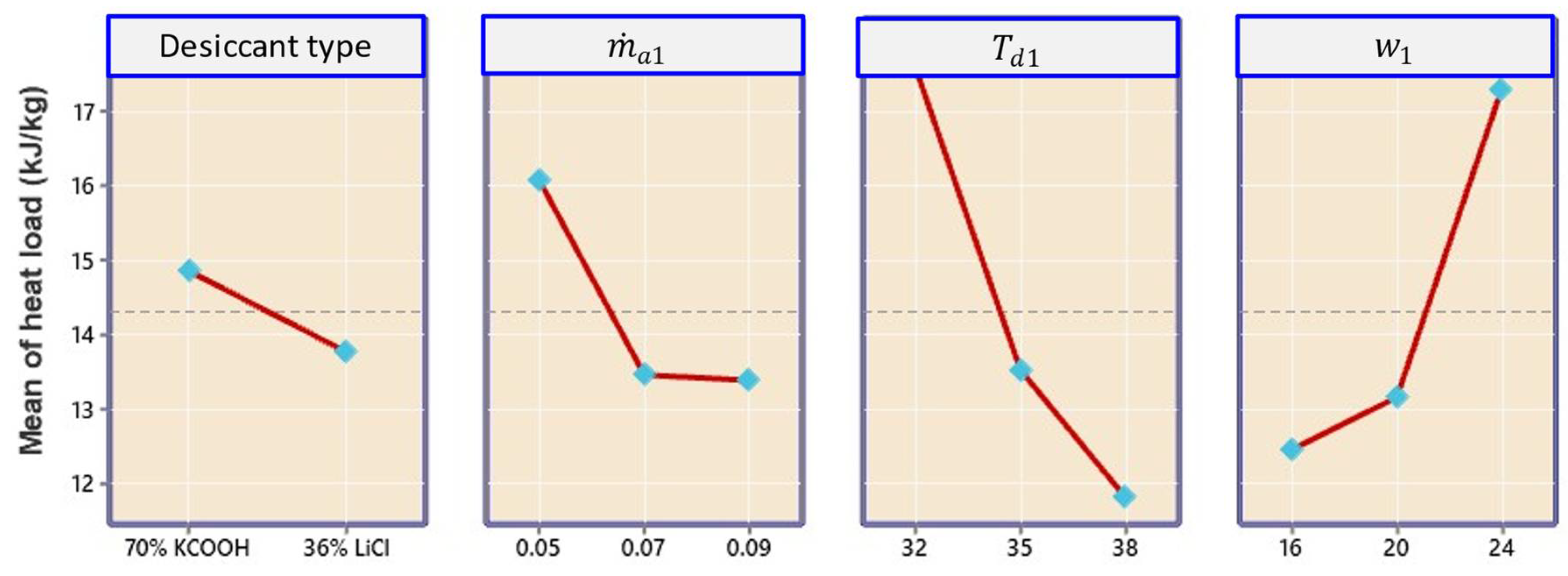
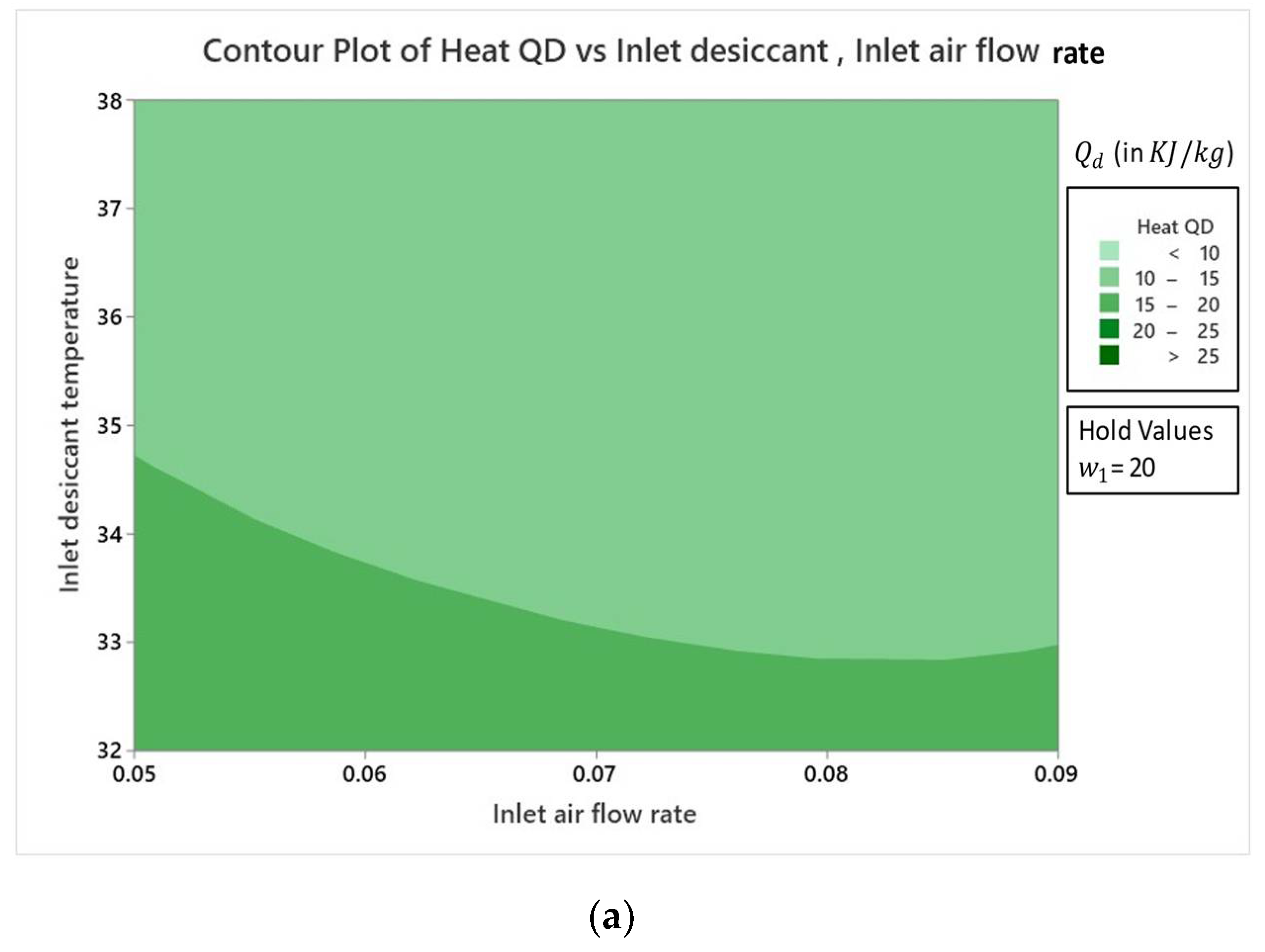
4.2.2. Effect on Dehumidifier Heat Load
- The contributions of linear terms , , and were 36.06%, 27.79%, and 9.41%, respectively.
- The contribution of cross-iteration () was only 1.04%, and the highest contribution was indicated by with 8.16%, signifying that cross-interaction had the least effect.
4.2.3. Effect on Coefficient of Performance
- The contributions of linear terms , , and were 9.13%, 33.28%, 34.42%, and 7.36%, respectively.
- The contribution of cross-iteration () was only 2.91%, which was the highest contribution among all cross-interactions, having the least effect on .
5. Conclusions and Future Research Scope
- The outcome of vapor pressure readings specifies that KCOOH solution with 70% concentration had similar vapor pressure as that of the LiCl solution with 36% concentration, and temperature range was between 20 and 47˚C. The cost of the KCOOH solution was approximately 22% of that of the LiCl solution, resulting in a more economical hybrid system while employing KCOOH as a liquid desiccant.
- In maximizing the response parameters, it is desirable to have lower , lower , and higher .
- From the experimental results, the superior dehumidification performance of the hybrid system could be observed while employing the KCOOH solution as compared to the LiCl solution, and KCOOH is highly environmentally friendly; therefore, KCOOH can be on the list of future-generation desiccants.
- Result analysis showed that the influence of inlet air humidity had a dominating impact on all three response parameters of the hybrid LDAC system as compared to the two other factors of and .
- MARD value of 7.65% was observed within the experimental and anticipated values, demonstrating the reliability of the correlation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Air conditioner |
| COP | Coefficient of performance |
| Coefficient of performance of a hybrid system | |
| DOE | Design of experiments |
| D1D4 | Desiccant solution state points |
| LDAC | Liquid desiccant air-conditioner |
| MARD | Mean absolute relative deviation |
| TR | Ton of refrigeration |
| VCR | Vapor compression refrigeration |
| Subscripts | |
| A1A7 | Air state points |
| Isobaric specific heat (kJ/kg.K) | |
| C1 | Concentration of LiCl solution |
| C2 | Concentration of KCOOH solution |
| hp | Heat pump |
| Latent heat of evaporation (kJ/kg) | |
| Absorption enthalpy (kJ/kg) | |
| Enthalpy of dilution (kJ/kg) | |
| Inlet airflow rate at level 1 | |
| Inlet airflow rate at level 2 | |
| Inlet airflow rate at level 3 | |
| sys | system |
| Temperature (K) | |
| Inlet desiccant temperature at level 1 | |
| Inlet desiccant temperature at level 2 | |
| Inlet desiccant temperature at level 3 | |
| Inlet air humidity at level 1 | |
| Inlet air humidity at level 2 | |
| Inlet air humidity at level 3 | |
| Specific humidity change | |
| Specific humidity | |
| Relative humidity |
References
- Bluyssen, P.M.; Cox, C. Indoor environment quality and upgrading of European office buildings. Energy Build. 2002, 34, 155–162. [Google Scholar] [CrossRef]
- Air Conditioners to Burn up Your Electricity Bill by 2030—The Financial Express. Available online: https://www.financialexpress.com/industry/air-conditioners-to-burn-up-your-electricity-bill-by-2030/1385105/ (accessed on 20 January 2021).
- Jani, D.B.; Mishra, M.; Sahoo, P.K. Solid desiccant air conditioning—A state of the art review. Renew. Sustain. Energy Rev. 2016, 60, 1451–1469. [Google Scholar] [CrossRef]
- Pintaldi, S.; Perfumo, C.; Sethuvenkatraman, S.; White, S.; Rosengarten, G. A review of thermal energy storage technologies and control approaches for solar cooling. Renew. Sustain. Energy Rev. 2015, 41, 975–995. [Google Scholar] [CrossRef]
- Luo, Y.; Shao, S.; Xu, H.; Tian, C. Dehumidification performance of [EMIM] BF4. Appl. Therm. Eng. 2011, 31, 2772–2777. [Google Scholar] [CrossRef]
- Rafique, M.M.; Gandhidasan, P.; Bahaidarah, H.M.S. Liquid desiccant materials and dehumidifiers—A review. Renew. Sustain. Energy Rev. 2016, 56, 179–195. [Google Scholar] [CrossRef]
- Sharma, A.; Kaushal, R. Experimental investigation of a novel multi-channel flat plate liquid desiccant dehumidification system. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Sharma, A.; Kaushal, R. Experimental and statistical analysis of relative humidity and temperature of moist air in a flat Plate multi-layer desiccant cooling system. Energy Sources Part A Recover. Util. Environ. Eff. 2021, 1–19. [Google Scholar] [CrossRef]
- Mei, L.; Dai, Y. A technical review on use of liquid-desiccant dehumidification for air-conditioning application. Renew. Sustain. Energy Rev. 2008, 12, 662–689. [Google Scholar] [CrossRef]
- Gao, W.Z.; Liu, J.H.; Cheng, Y.P.; Zhang, X.L. Experimental investigation on the heat and mass transfer between air and liquid desiccant in a cross-flow dehumidifier. Renew. Energy 2012, 37, 117–123. [Google Scholar] [CrossRef]
- Salah Hassan, M.; Hassan, A.A.M. Performance of a proposed complete wetting surface counter flow channel type liquid desiccant air dehumidifier. Renew. Energy 2009, 34, 2107–2116. [Google Scholar] [CrossRef]
- Liu, X.; Qu, K.; Jiang, Y. Empirical correlations to predict the performance of the dehumidifier using liquid desiccant in heat and mass transfer. Renew. Energy 2006, 31, 1627–1639. [Google Scholar] [CrossRef]
- Qiu, G.; Liu, H.; Riffat, S.B. Experimental investigation of a liquid desiccant cooling system driven by flue gas waste heat of a biomass boiler. Int. J. Low-Carbon Technol. 2013, 8, 165–172. [Google Scholar] [CrossRef]
- Liang, J.-D.; Huang, B.-H.; Chiang, Y.-C.; Chen, S.-L. Experimental investigation of a liquid desiccant dehumidification system integrated with shallow geothermal energy. Energy 2020, 191, 116452. [Google Scholar] [CrossRef]
- Khalil, A. An experimental study on multi-purpose desiccant integrated vapor-compression air-conditioning system. Int. J. Energy Res. 2012, 36, 535–544. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Jeong, J.; Saito, K.; Miyauchi, H.; Harada, M. Hybrid liquid desiccant air-conditioning system: Experiments and simulations. Appl. Therm. Eng. 2011, 31, 3741–3747. [Google Scholar] [CrossRef]
- Lof GOG, Cooling with Solar Energy, Congress on Solar… Google Scholar. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Lof+GOG%2C+Cooling+with+Solar+Energy%2C+Congress+on+Solar+Energy%2C+1955%2C+pp.+117e189&btnG= (accessed on 25 January 2021).
- Zurigat, Y.H.; Abu-Arabi, M.K.; Abdul-Wahab, S.A. Air dehumidification by triethylene glycol desiccant in a packed column. Energy Convers. Manag. 2004, 45, 141–155. [Google Scholar] [CrossRef]
- Jain, S.; Bansal, P.K. Performance analysis of liquid desiccant dehumidification systems. Int. J. Refrig. 2007, 30, 861–872. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.; Zurigat, Y.; Abu-Arabi, M. Predictions of moisture removal rate and dehumidification effectiveness for structured liquid desiccant air dehumidifier. Energy 2004, 29, 19–34. [Google Scholar] [CrossRef]
- Elsarrag, E.; Ali, E.E.M.; Jain, S. Design Guidelines and performance study on a structured packed liquid desiccant air-conditioning system. HVAC&R Res. 2005, 11, 319–337. [Google Scholar] [CrossRef]
- Conde, M.R. Properties of aqueous solutions of lithium and calcium chlorides: Formulations for use in air conditioning equipment design. Int. J. Therm. Sci. 2004, 43, 367–382. [Google Scholar] [CrossRef]
- Shukla, D.L.; Modi, K.V. A technical review on regeneration of liquid desiccant using solar energy. Renew. Sustain. Energy Rev. 2017, 78, 517–529. [Google Scholar] [CrossRef]
- Yin, Y.; Qian, J.; Zhang, X. Recent advancements in liquid desiccant dehumidification technology. Renew. Sustain. Energy Rev. 2014, 31, 38–52. [Google Scholar] [CrossRef]
- Kaita, Y. Thermodynamic properties of lithium bromide–water solutions at high temperatures. Int. J. Refrig. 2001, 24, 374–390. [Google Scholar] [CrossRef]
- Pátek, J.; Klomfar, J. A computationally effective formulation of the thermodynamic properties of LiBr–H2O solutions from 273 to 500K over full composition range. Int. J. Refrig. 2006, 29, 566–578. [Google Scholar] [CrossRef]
- Mehta, J.R.; Shah, N.M.; Patel, K.N. A review on internally cooled liquid desiccant air dehumidifier. In Proceedings of the International Conference on Recent Trends in Engineering and Technology (ICRTET 2016), Nasik, India, 28–30 April 2016; pp. 5–10. [Google Scholar]
- Abdel-Salam, A.H.; Simonson, C.J. State-of-the-art in liquid desiccant air conditioning equipment and systems. Renew. Sustain. Energy Rev. 2016, 58, 1152–1183. [Google Scholar] [CrossRef]
- Abdel-Salam, M.R.; Besant, R.W.; Simonson, C.J. Sensitivity of the performance of a flat-plate liquid-to-air membrane energy exchanger (LAMEE) to the air and solution channel widths and flow maldistribution. Int. J. Heat Mass Transf. 2015, 84, 1082–1100. [Google Scholar] [CrossRef]
- Abdel-Salam, M.R.; Besant, R.W.; Simonson, C.J. Performance testing of a novel 3-fluid liquid-to-air membrane energy exchanger (3-fluid LAMEE) under desiccant solution regeneration operating conditions. Int. J. Heat Mass Transf. 2016, 95, 773–786. [Google Scholar] [CrossRef]
- Luo, C.; Su, Q.; Mi, W. Thermophysical properties and application of LiNO3–H2O working fluid. Int. J. Refrig. 2013, 36, 1689–1700. [Google Scholar] [CrossRef]
- Abdel-Salam, M.R.; Fauchoux, M.; Ge, G.; Besant, R.W.; Simonson, C.J. Expected energy and economic benefits, and environmental impacts for liquid-to-air membrane energy exchangers (LAMEEs) in HVAC systems: A review. Appl. Energy 2014, 127, 202–218. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Li, Z. Investigation on the coupled heat and mass transfer process between extremely high humidity air and liquid desiccant in the counter-flow adiabatic packed tower. Int. J. Heat Mass Transf. 2017, 110, 898–907. [Google Scholar] [CrossRef]
- Riffat, S.B.; James, S.E.; Wong, C.W. Experimental analysis of the absorption and desorption rates of hcook/H2O and LiBr/H2O. Int. J. Energy Res. 1998, 22, 1099–1103. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Wen, T.; Luo, Y.; Sheng, L. Experimental study on the corrosion behavior and regeneration performance of KCOOH aqueous solution. Sol. Energy 2020, 201, 638–648. [Google Scholar] [CrossRef]
- Ertas, A.; Anderson, E.; Kiris, I. Properties of a new liquid desiccant solution—Lithium chloride and calcium chloride mixture. Sol. Energy 1992, 49, 205–212. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Q.; Feng, W.; Xu, W. Experimental and theoretical research on the electrical conductivity of a liquid desiccant for the liquid desiccant air-conditioning system: LiCl aqueous solution. Int. J. Refrig. 2018, 91, 189–198. [Google Scholar] [CrossRef]
- Liu, X.; Yi, X.; Jiang, Y. Mass transfer performance comparison of two commonly used liquid desiccants: LiBr and LiCl aqueous solutions. Energy Convers. Manag. 2011, 52, 180–190. [Google Scholar] [CrossRef]
- Antony, J. Design of Experiments for Engineers and Scientists. Google Books. Available online: https://books.google.co.in/books?hl=en&lr=&id=p7pCAgAAQBAJ&oi=fnd&pg=PP1&dq=J.+Antony,+Design+of+Experiments+for+Engineers+and+Scientists,+Elsevier,&ots=BEoXVWuBMp&sig=uCaVIt5VXy4S04rHSuTKFNw2NYk#v=onepage&q=J.%20Antony%2C%20Design%20of%20Experiments%20for%20Engineers%20and%20Scientists%2C%20Elsevier%2C&f=false (accessed on 6 February 2021).
- Kumar, R.; Dhar, P.; Jain, S. Development of new wire mesh packings for improving the performance of zero carryover spray tower. Energy 2011, 36, 1362–1374. [Google Scholar] [CrossRef]
- Afzal, A.; Samee, A.D.M.; Javad, A.; Shafvan, S.A.; Ajinas, P.V.A.; Kabeer, K.M.A. Heat transfer analysis of plain and dimpled tubes with different spacings. Heat Transf.-Asian Res. 2017, 47, 556–568. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Safaei, M.R.; Manokar, A.M.; El-Seesy, A.I.; Mujtaba, M.A.; Samuel, O.D.; Badruddin, I.A.; Ahmed, W.; Shahapurkar, K.; et al. Investigation on the effect of cottonseed oil blended with different percentages of octanol and suspended MWCNT nanoparticles on diesel engine characteristics. J. Therm. Anal. 2020, 147, 525–542. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Kareemullah, M. Waste coconut oil methyl ester with and without additives as an alternative fuel in diesel engine at two different injection pressures. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–19. [Google Scholar] [CrossRef]
- Verma, T.N.; Nashine, P.; Chaurasiya, P.K.; Rajak, U.; Afzal, A.; Kumar, S.; Singh, D.V.; Azad, A.K. The effect of ethanol-diesel-biodiesel blends on combustion, performance and emissions of a direct injection diesel engine. Sustain. Energy Tech-Nologies Assess. 2020, 42, 100851. [Google Scholar] [CrossRef]
- Afzal, A.; Aabid, A.; Khan, A.; Khan, S.A.; Rajak, U.; Verma, T.N.; Kumar, R. Response surface analysis, clustering, and random forest regression of pressure in suddenly expanded high-speed aerodynamic flows. Aerosp. Sci. Technol. 2020, 107, 106318. [Google Scholar] [CrossRef]
- Afzal, A.; Saleel, C.A.; Badruddin, I.A.; Khan, T.Y.; Kamangar, S.; Mallick, Z.; Samuel, O.D.; Soudagar, M.E. Human thermal comfort in passenger vehicles using an organic phase change material—An experimental investigation, neural network modelling, and optimization. Build. Environ. 2020, 180, 107012. [Google Scholar] [CrossRef]
- Aneeque, M.; Alshahrani, S.; Kareemullah, M.; Afzal, A.; Saleel, C.; Soudagar, M.; Hossain, N.; Subbiah, R.; Ahmed, M. The combined effect of alcohols and Calophyllum inophyllum biodiesel using response surface methodology optimization. Sustainability 2021, 13, 7345. [Google Scholar] [CrossRef]
- Afzal, A.; Mokashi, I.; Khan, S.A.; Abdullah, N.A.; Bin Azami, M.H. Optimization and analysis of maximum temperature in a battery pack affected by low to high Prandtl number coolants using response surface methodology and particle swarm optimization algorithm. Numer. Heat Transf. Part A Appl. 2020, 79, 406–435. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Chaluvaraju, B.V.; Afzal, A.; Vinnik, D.A.; Kaladgi, A.; Alamri, S.; Ahamed, S.C.; Tirth, V. Mechanical and corrosion studies of friction stir welded nano Al2O3 reinforced Al-Mg matrix composites: RSM-ANN modelling approach. Symmetry 2021, 13, 537. [Google Scholar] [CrossRef]
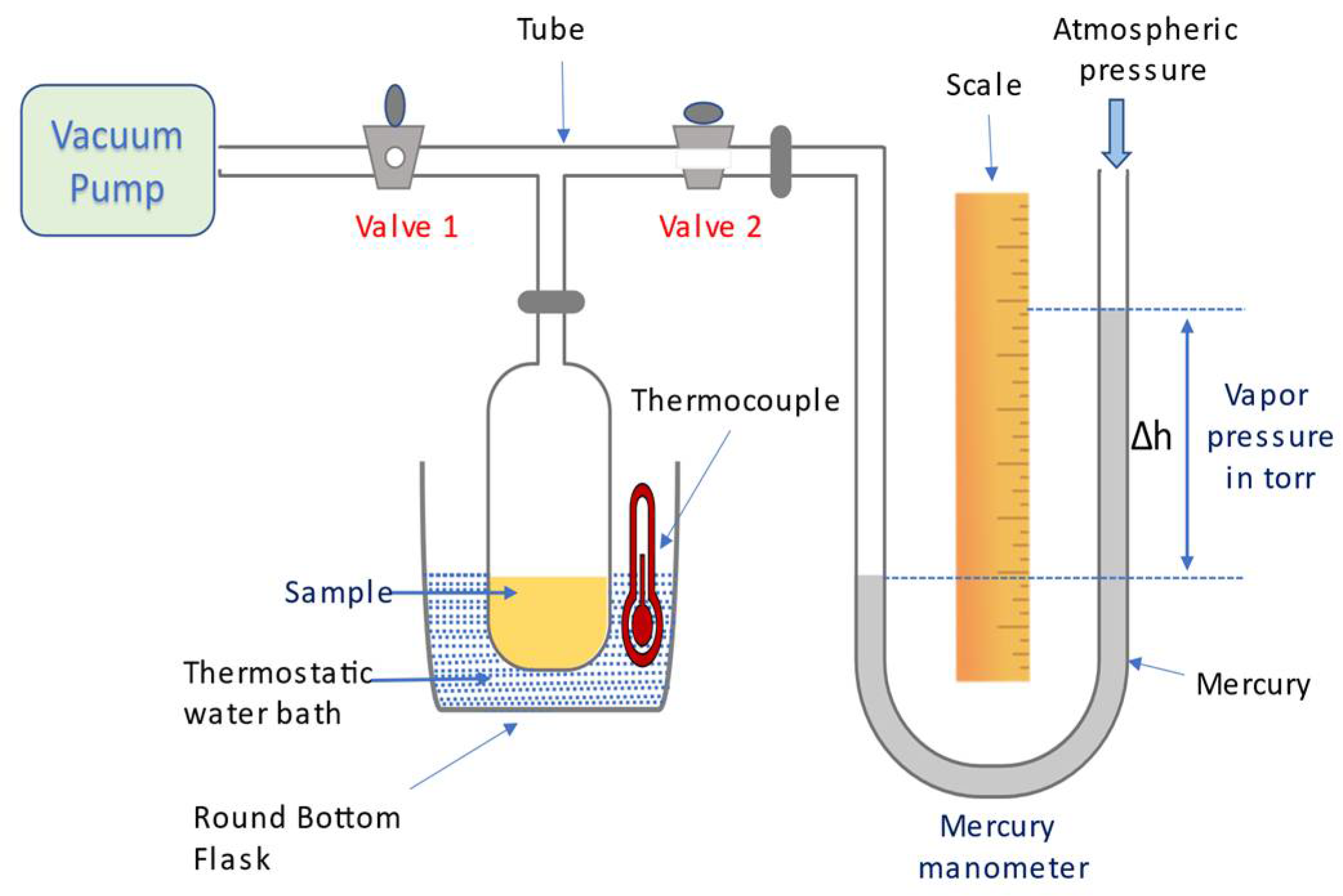

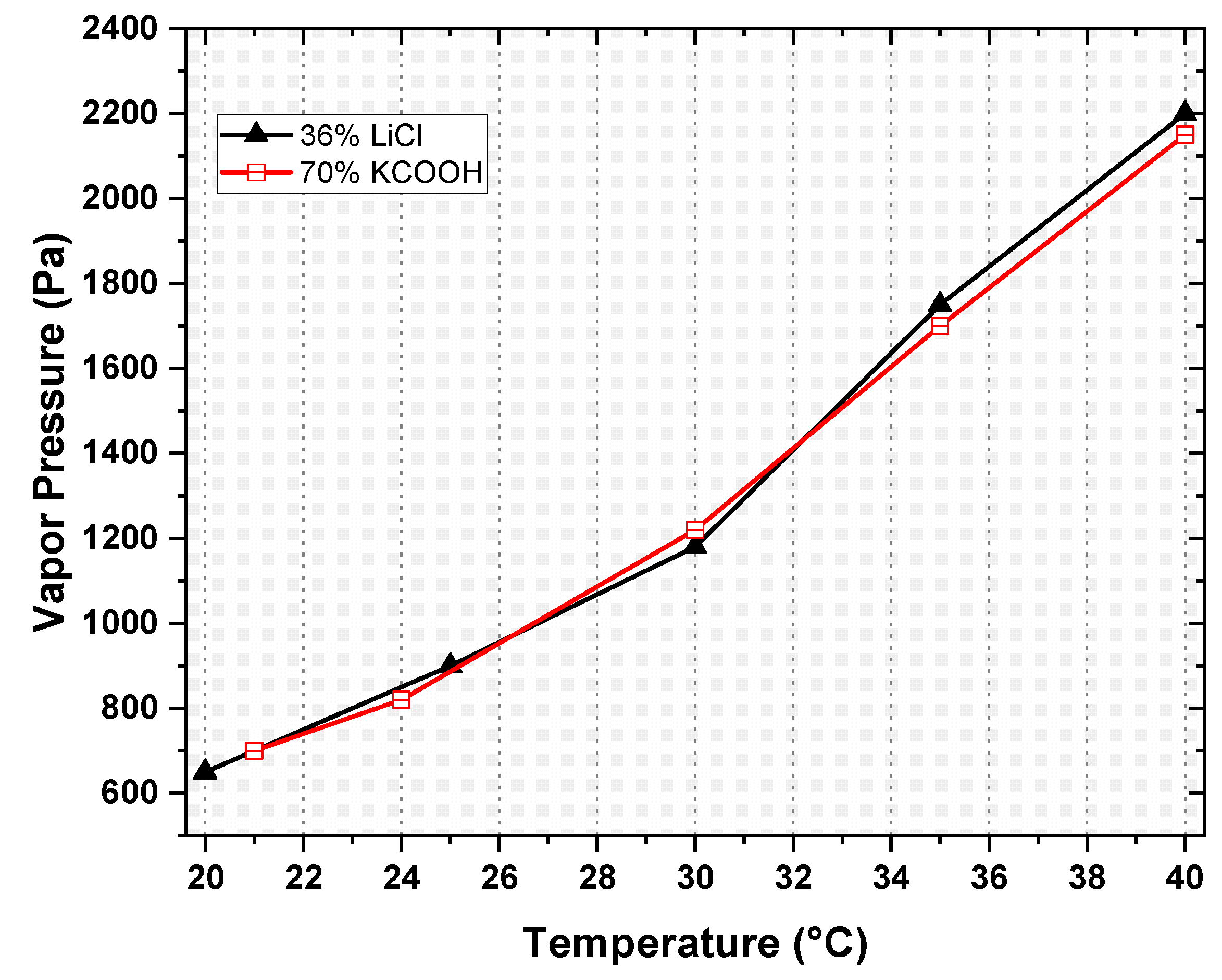
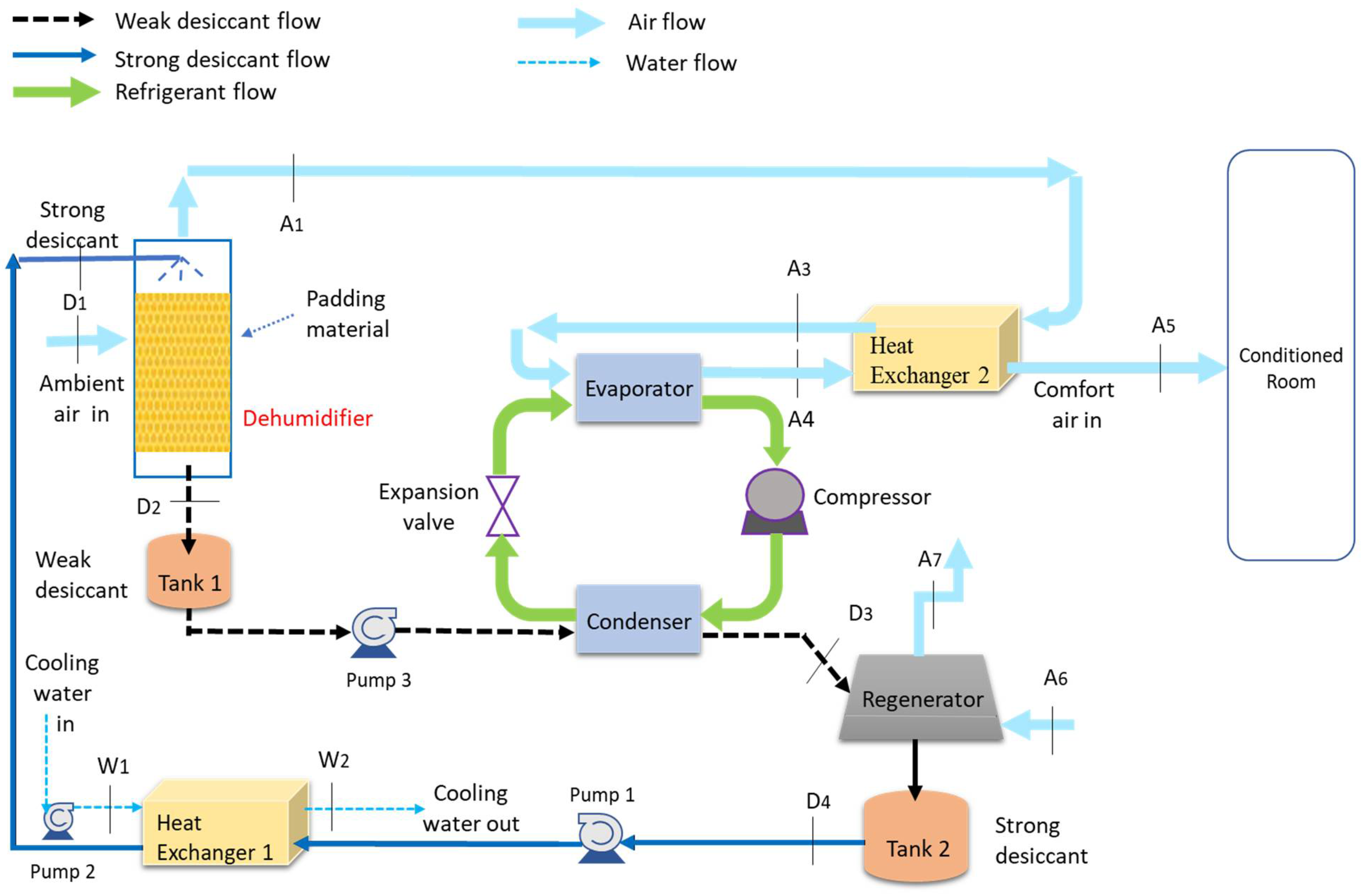
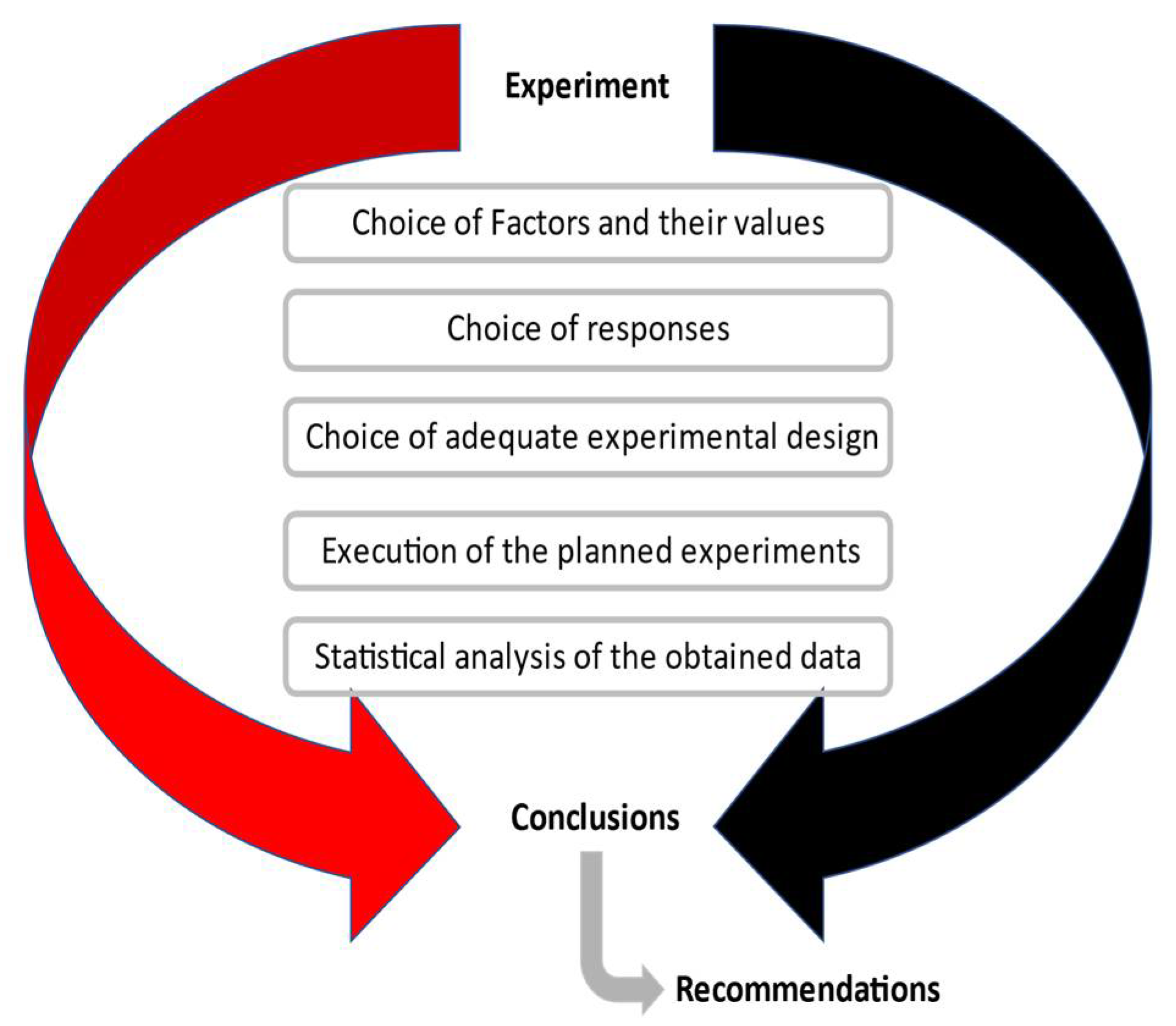



| T (°C) | 20 | 22 | 24 | 27 | 30 | 33 | 37 | 41 | 44 | 47 |
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. (Pa) | 2289 | 2679 | 2984 | 3540 | 4202 | 4985 | 6159 | 7627 | 8955 | 10,489 |
| Ref. (Pa) | 2333 | 2639 | 2986 | 3559 | −4239 | 5026 | 6279 | 7772 | 9105 | 10,612 |
| MD (%) | 1.92 | 1.49 | 0.06 | 0.53 | 0.88 | 0.82 | 1.94 | 1.90 | 1.67 | 1.17 |
| Parameters | Instruments | Range of Measurement | Accuracy |
|---|---|---|---|
| Temperature | PT-100 RTD | −50 to 200 °C | 0.1 °C |
| Air relative humidity | Multispan MTC-1101 | 0–100% | 1% |
| Solution density | Specific gravity hydrometer | 1.0–2.0 (specific gravity) | 1 Kg/m3 |
| Air velocity | Anemometer HTC AVM-06 | 0.8–30 m/s | 2% |
| Airflow rate | Anemometer HTC AVM-06 | 09999 m3/min | - |
| Parameters | Range | Uncertainties |
|---|---|---|
| Relative air humidity (%) | 40–70 | 1 |
| Air temperature (°C) | 20–45 | 0.2 |
| Desiccant inlet temperature, (°C) | 15–45 | 0.3 |
| Solution concertation | 0.2% | |
| Humidity, (gm/kg of dry air) | 2.455% | |
| COP of hybrid system, COPhybrid | ||
| Dehumidifier heat load, (kJ/kg) | 5.323% |
| SI. No | Parameters | Units | Low Level | Medium Level | High Level |
|---|---|---|---|---|---|
| 1 | Type of desiccant | 36% LiCl | 70% KCOOH | ||
| 2 | Inlet air flow rate | kg/s | 0.05 | 0.07 | 0.09 |
| 3 | Inlet desiccant temperature (°C) | °C | 32 | 35 | 38 |
| 4 | Inlet air humidity | g/Kg | 16 | 20 | 24 |
| Run Order | Desiccant Type | |||
|---|---|---|---|---|
| 1 | 70% KCOOH | 0.07 | 38 | 16 |
| 2 | 70% KCOOH | 0.05 | 38 | 24 |
| 3 | 70% KCOOH | 0.07 | 38 | 24 |
| 4 | 36% LiCl | 0.09 | 32 | 16 |
| 5 | 70% KCOOH | 0.09 | 38 | 24 |
| 6 | 70% KCOOH | 0.05 | 32 | 20 |
| 7 | 70% KCOOH | 0.07 | 32 | 20 |
| 8 | 36% LiCl | 0.05 | 35 | 24 |
| 9 | 36% LiCl | 0.05 | 32 | 24 |
| 10 | 70% KCOOH | 0.05 | 32 | 16 |
| 11 | 36% LiCl | 0.09 | 32 | 24 |
| 12 | 36% LiCl | 0.07 | 35 | 16 |
| 13 | 70% KCOOH | 0.05 | 38 | 16 |
| 14 | 70% KCOOH | 0.05 | 38 | 20 |
| 15 | 36% LiCl | 0.09 | 35 | 16 |
| 16 | 70% KCOOH | 0.09 | 32 | 24 |
| 17 | 70% KCOOH | 0.09 | 35 | 16 |
| 18 | 36% LiCl | 0.07 | 32 | 24 |
| 19 | 70% KCOOH | 0.09 | 32 | 20 |
| 20 | 70% KCOOH | 0.05 | 32 | 24 |
| 21 | 36% LiCl | 0.07 | 38 | 20 |
| 22 | 70% KCOOH | 0.07 | 35 | 24 |
| 23 | 36% LiCl | 0.07 | 32 | 16 |
| 24 | 36% LiCl | 0.07 | 35 | 24 |
| 25 | 36% LiCl | 0.05 | 32 | 20 |
| 26 | 70% KCOOH | 0.09 | 32 | 16 |
| 27 | 36% LiCl | 0.05 | 38 | 20 |
| 28 | 36% LiCl | 0.07 | 38 | 16 |
| 29 | 36% LiCl | 0.05 | 35 | 20 |
| 30 | 36% LiCl | 0.09 | 38 | 20 |
| 31 | 70% KCOOH | 0.07 | 32 | 24 |
| 32 | 36% LiCl | 0.09 | 38 | 24 |
| 33 | 70% KCOOH | 0.09 | 38 | 16 |
| 34 | 70% KCOOH | 0.05 | 35 | 24 |
| 35 | 70% KCOOH | 0.05 | 35 | 16 |
| 36 | 36% LiCl | 0.07 | 38 | 24 |
| 37 | 70% KCOOH | 0.07 | 32 | 16 |
| 38 | 70% KCOOH | 0.09 | 35 | 24 |
| 39 | 70% KCOOH | 0.07 | 38 | 20 |
| 40 | 36% LiCl | 0.07 | 32 | 20 |
| 41 | 36% LiCl | 0.05 | 35 | 16 |
| 42 | 70% KCOOH | 0.07 | 35 | 20 |
| 43 | 36% LiCl | 0.07 | 35 | 20 |
| 44 | 36% LiCl | 0.05 | 38 | 16 |
| 45 | 36% LiCl | 0.09 | 32 | 20 |
| 46 | 36% LiCl | 0.05 | 32 | 16 |
| 47 | 36% LiCl | 0.09 | 35 | 20 |
| 48 | 70% KCOOH | 0.09 | 35 | 20 |
| 49 | 70% KCOOH | 0.09 | 38 | 20 |
| 50 | 70% KCOOH | 0.05 | 35 | 20 |
| 51 | 70% KCOOH | 0.07 | 35 | 16 |
| 52 | 36% LiCl | 0.09 | 38 | 16 |
| 53 | 36% LiCl | 0.05 | 38 | 24 |
| 54 | 36% LiCl | 0.09 | 35 | 24 |
| Run Order | Parameters | Result | |||||
|---|---|---|---|---|---|---|---|
| Type of Desiccant | Inlet Desiccant Temperature | Inlet Air Humidity | |||||
| 1 | 70% KCOOH | 0.07 | 38 | 16 | 4.78 | 11.231 | 1.799 |
| 2 | 70% KCOOH | 0.05 | 38 | 24 | 7.9 | 17.34 | 2.12 |
| 3 | 70% KCOOH | 0.07 | 38 | 24 | 5.13 | 12.325 | 1.893 |
| 4 | 36% LiCl | 0.09 | 32 | 16 | 4.51 | 11.998 | 1.851 |
| 5 | 70% KCOOH | 0.09 | 38 | 24 | 6.1 | 15.156 | 1.912 |
| 6 | 70% KCOOH | 0.05 | 32 | 20 | 8.12 | 21.853 | 2.117 |
| 7 | 70% KCOOH | 0.07 | 32 | 20 | 6.987 | 17.153 | 2.123 |
| 8 | 36% LiCl | 0.05 | 35 | 24 | 7.21 | 15.156 | 1.912 |
| 9 | 36% LiCl | 0.05 | 32 | 24 | 8.98 | 24.456 | 2.182 |
| 10 | 70% KCOOH | 0.05 | 32 | 16 | 5.9 | 14.169 | 1.89 |
| 11 | 36% LiCl | 0.09 | 32 | 24 | 7.9 | 21.0467 | 2.105 |
| 12 | 36% LiCl | 0.07 | 35 | 16 | 3.35 | 7.867 | 1.789 |
| 13 | 70% KCOOH | 0.05 | 38 | 16 | 6.23 | 15.132 | 1.902 |
| 14 | 70% KCOOH | 0.05 | 38 | 20 | 4.1 | 9.342 | 1.834 |
| 15 | 36% LiCl | 0.09 | 35 | 16 | 4.56 | 12.127 | 1.729 |
| 16 | 70% KCOOH | 0.09 | 32 | 24 | 8.1 | 22.5467 | 2.211 |
| 17 | 70% KCOOH | 0.09 | 35 | 16 | 5.23 | 13.563 | 1.799 |
| 18 | 36% LiCl | 0.07 | 32 | 24 | 7.59 | 17.569 | 1.959 |
| 19 | 70% KCOOH | 0.09 | 32 | 20 | 7.1 | 14.23 | 1.989 |
| 20 | 70% KCOOH | 0.05 | 32 | 24 | 9.56 | 25.156 | 2.256 |
| 21 | 36% LiCl | 0.07 | 38 | 20 | 4.13 | 9.869 | 1.756 |
| 22 | 70% KCOOH | 0.07 | 35 | 24 | 7.12 | 17.256 | 2.154 |
| 23 | 36% LiCl | 0.07 | 32 | 16 | 5.25 | 14.987 | 1.894 |
| 24 | 36% LiCl | 0.07 | 35 | 24 | 6.6 | 16.786 | 2.0156 |
| 25 | 36% LiCl | 0.05 | 32 | 20 | 7.29 | 20.239 | 2.089 |
| 26 | 70% KCOOH | 0.09 | 32 | 16 | 5.13 | 12.562 | 1.978 |
| 27 | 36% LiCl | 0.05 | 38 | 20 | 3.72 | 8.856 | 1.794 |
| 28 | 36% LiCl | 0.07 | 38 | 16 | 4.27 | 9.779 | 1.671 |
| 29 | 36% LiCl | 0.05 | 35 | 20 | 5.2 | 13.112 | 1.898 |
| 30 | 36% LiCl | 0.09 | 38 | 20 | 2.91 | 7.2392 | 1.765 |
| 31 | 70% KCOOH | 0.07 | 32 | 24 | 8.85 | 19.125 | 2.019 |
| 32 | 36% LiCl | 0.09 | 38 | 24 | 5.82 | 14.8956 | 1.865 |
| 33 | 70% KCOOH | 0.09 | 38 | 16 | 5.12 | 11.112 | 1.725 |
| 34 | 70% KCOOH | 0.05 | 35 | 24 | 8.13 | 17.56 | 1.989 |
| 35 | 70% KCOOH | 0.05 | 35 | 16 | 5.23 | 14.569 | 1.956 |
| 36 | 36% LiCl | 0.07 | 38 | 24 | 4.25 | 10.235 | 1.823 |
| 37 | 70% KCOOH | 0.07 | 32 | 16 | 6.12 | 15.789 | 1.912 |
| 38 | 70% KCOOH | 0.09 | 35 | 24 | 5.95 | 14.671 | 1.998 |
| 39 | 70% KCOOH | 0.07 | 38 | 20 | 4.98 | 11.579 | 1.813 |
| 40 | 36% LiCl | 0.07 | 32 | 20 | 6.25 | 16.458 | 1.933 |
| 41 | 36% LiCl | 0.05 | 35 | 16 | 4.89 | 13.231 | 1.823 |
| 42 | 70% KCOOH | 0.07 | 35 | 20 | 5.1 | 13.567 | 1.913 |
| 43 | 36% LiCl | 0.07 | 35 | 20 | 4.75 | 12.136 | 1.834 |
| 44 | 36% LiCl | 0.05 | 38 | 16 | 5.46 | 13.982 | 1.812 |
| 45 | 36% LiCl | 0.09 | 32 | 20 | 6.56 | 13.56 | 1.952 |
| 46 | 36% LiCl | 0.05 | 32 | 16 | 5.23 | 13.892 | 1.85 |
| 47 | 36% LiCl | 0.09 | 35 | 20 | 4.56 | 12.099 | 1.808 |
| 48 | 70% KCOOH | 0.09 | 35 | 20 | 5.1 | 12.987 | 1.897 |
| 49 | 70% KCOOH | 0.09 | 38 | 20 | 3.24 | 7.956 | 1.812 |
| 50 | 70% KCOOH | 0.05 | 35 | 20 | 5.5 | 14.567 | 1.987 |
| 51 | 70% KCOOH | 0.07 | 35 | 16 | 4.1 | 8.567 | 1.814 |
| 52 | 36% LiCl | 0.09 | 38 | 16 | 4.11 | 9.576 | 1.615 |
| 53 | 36% LiCl | 0.05 | 38 | 24 | 7 | 16.8678 | 1.984 |
| 54 | 36% LiCl | 0.09 | 35 | 24 | 5.17 | 13.569 | 1.926 |
| Regression Correlation | |
|---|---|
| ] | |
| ] | |
| ] | |
| Sum of Square Values | p-Value | Sum of Square Values | p-Value | Sum of Squares Values | p-Value | |
|---|---|---|---|---|---|---|
| Model | 127.478 | <0.0001 | 874.041 | <0.0001 | 0.98190 | <0.0001 |
| Desiccant type | 5.598 | <0.0001 | 16.089 | <0.0001 | 0.08780 | <0.0001 |
| 11.201 | <0.0001 | 85.009 | <0.0001 | 0.07031 | <0.0001 | |
| 39.760 | <0.0001 | 318.988 | <0.0001 | 0.32877 | <0.0001 | |
| 45.964 | <0.0001 | 248.956 | <0.0001 | 0.34548 | <0.0001 | |
| 0.377 | 0.046 | 9.196 | <0.0001 | 0.02924 | <0.0001 | |
| 2.992 | <0.0001 | 21.801 | <0.0001 | 0.00608 | 0.175 | |
| 13.137 | <0.0001 | 71.737 | <0.0001 | 0.01503 | 0.009 | |
| 8.447 | <0.0001 | 102.265 | <0.0001 | 0.09919 | <0.0001 | |
| R2 value | 99.32% | 99.53% | 97.72% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, K.; Singh, A.; Shaik, S.; Saleel, C.A.; Aabid, A.; Baig, M. Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach. Sustainability 2022, 14, 3441. https://doi.org/10.3390/su14063441
Kumar K, Singh A, Shaik S, Saleel CA, Aabid A, Baig M. Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach. Sustainability. 2022; 14(6):3441. https://doi.org/10.3390/su14063441
Chicago/Turabian StyleKumar, Kashish, Alok Singh, Saboor Shaik, C Ahamed Saleel, Abdul Aabid, and Muneer Baig. 2022. "Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach" Sustainability 14, no. 6: 3441. https://doi.org/10.3390/su14063441
APA StyleKumar, K., Singh, A., Shaik, S., Saleel, C. A., Aabid, A., & Baig, M. (2022). Comparative Analysis on Dehumidification Performance of KCOOH–LiCl Hybrid Liquid Desiccant Air-Conditioning System: An Energy-Saving Approach. Sustainability, 14(6), 3441. https://doi.org/10.3390/su14063441










