Effects of Imazethapyr on Soybean Root Growth and Soil Microbial Communities in Sloped Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characterization and Experiment Design
2.2. Sampling and Measurements
2.3. DNA Extraction and PCR Amplification
2.4. Function Group Analysis
2.5. Data Analysis and Statistics
3. Results
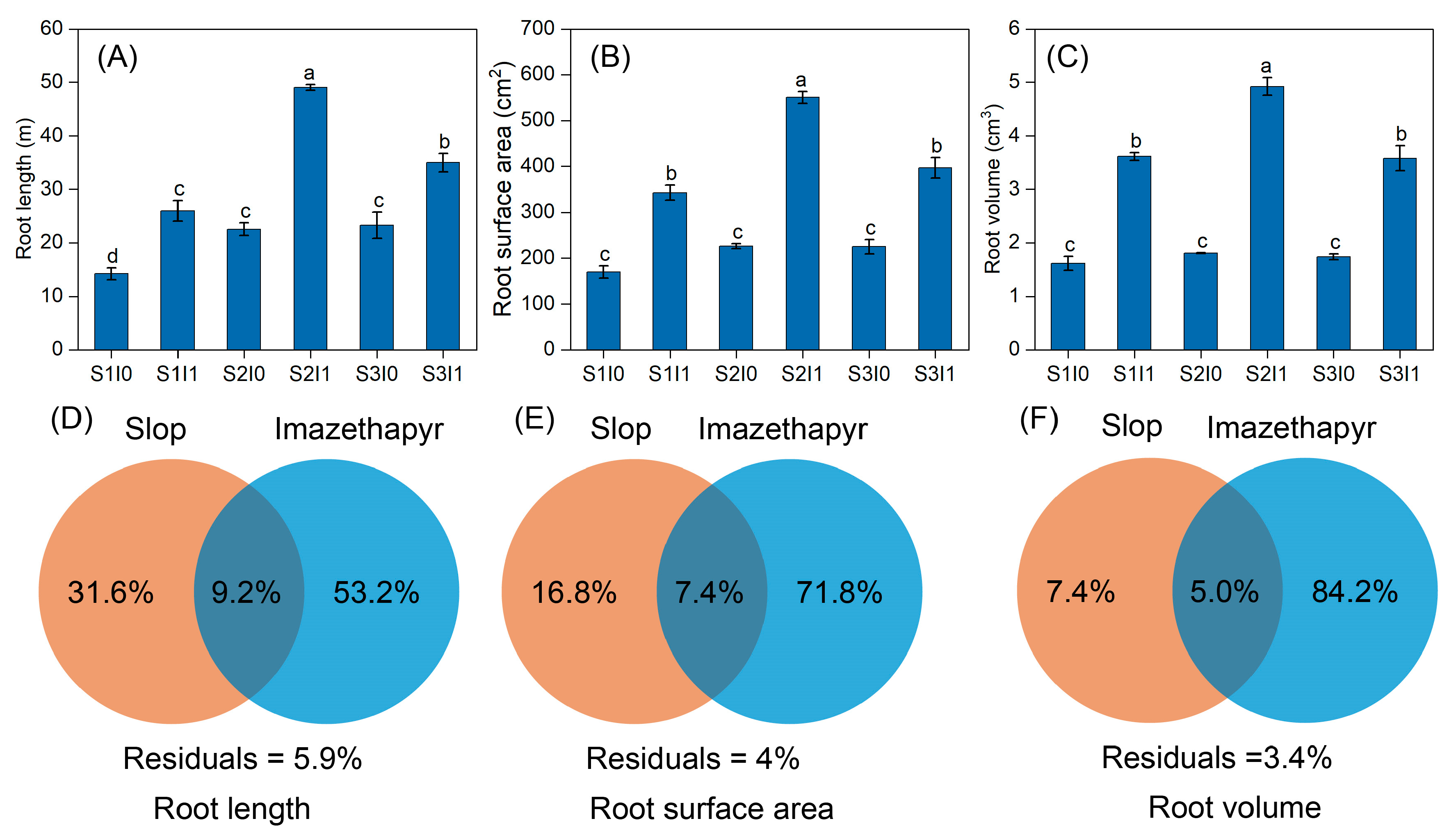
3.1. Root Growth
3.2. Soil Microbial Community Diversity and Composition
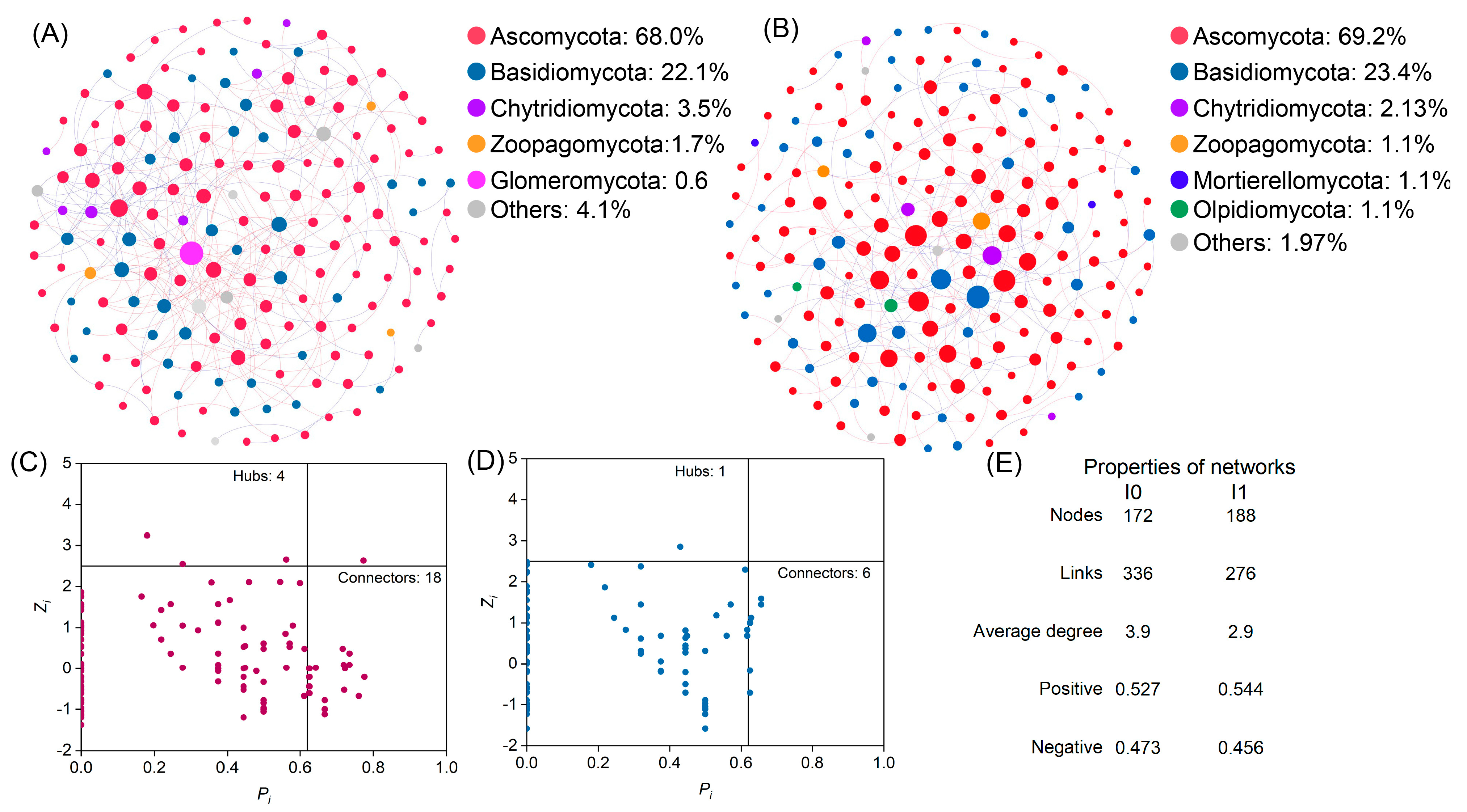
3.3. Co-Occurrence Networks
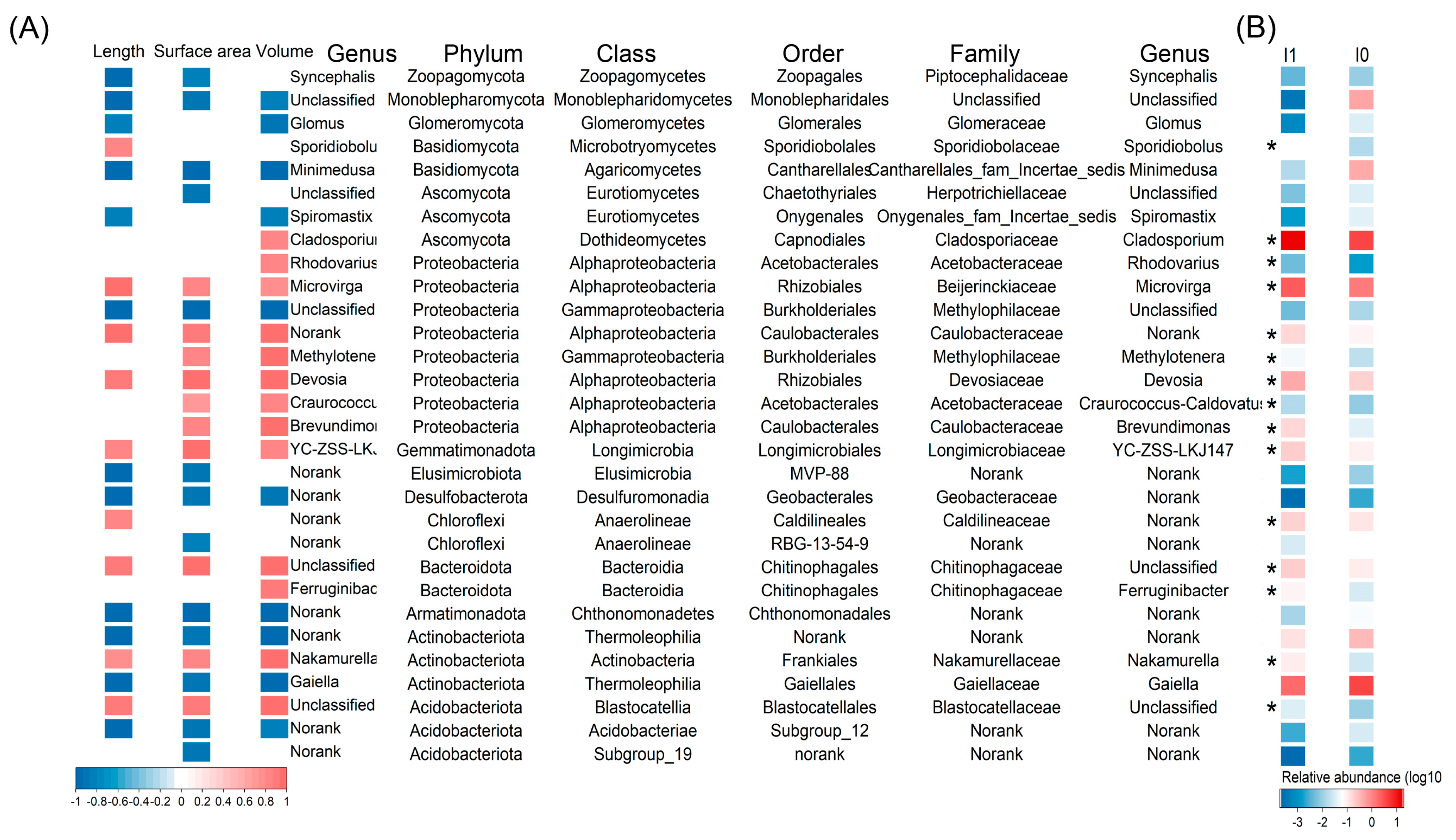
3.4. The Interaction between Biomarkers and Soybean Roots
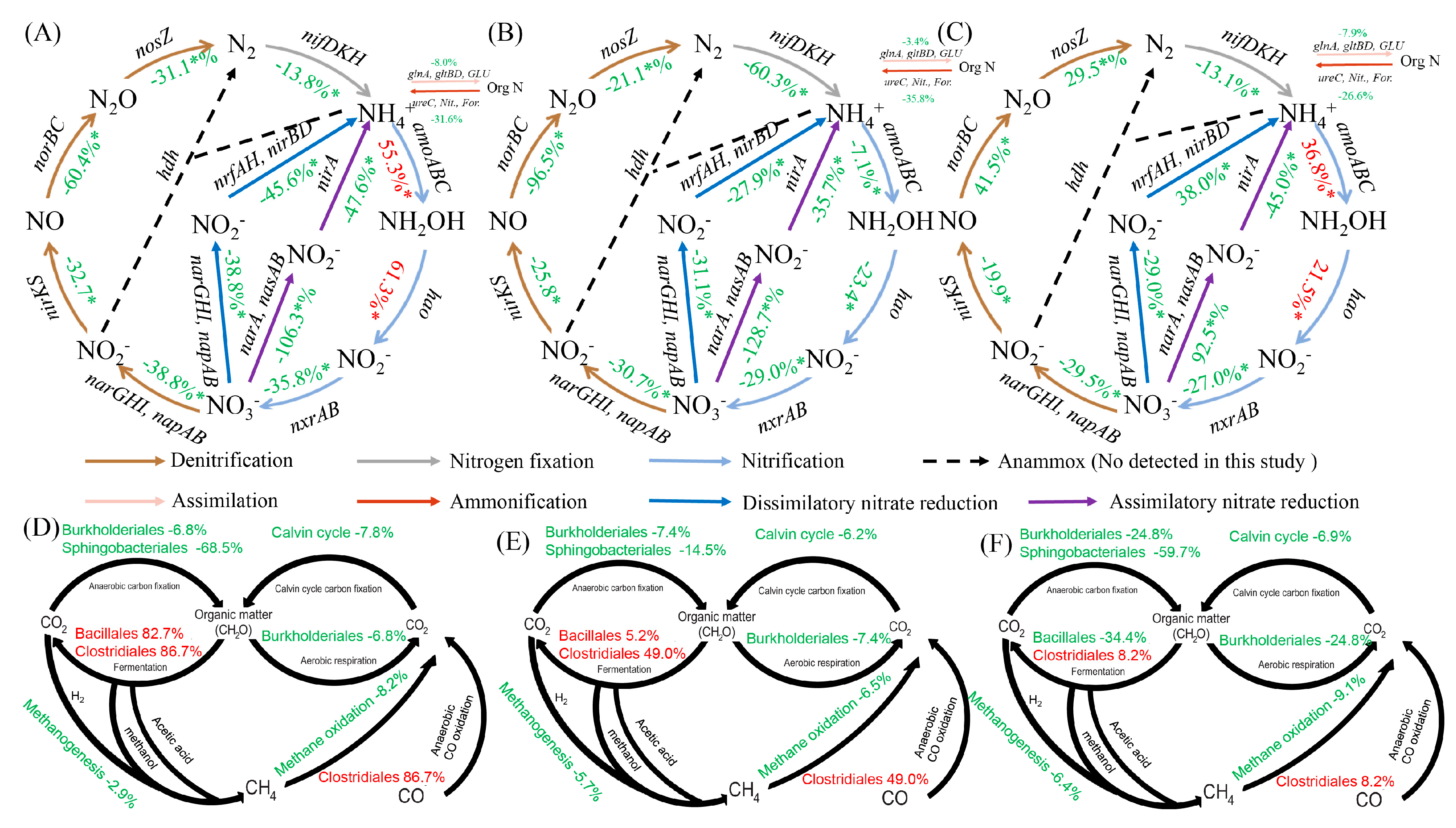
3.5. Nitrogen and Carbon Cycling Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qu, Q.; Li, Y.; Zhang, Z.; Cui, H.; Zhao, Q.; Liu, W.; Lu, T.; Qian, H. Effects of S-metolachlor on wheat (Triticum aestivum L.) seedling root exudates and the rhizosphere microbiome. J. Hazard. Mater. 2021, 411, 125137. [Google Scholar] [CrossRef]
- Wu, H.; Chen, H.; Jin, C.; Tang, C.; Zhang, Y. The chirality of imazethapyr herbicide selectively affects the bacterial community in soybean field soil. Environ. Sci. Pollut. Res. 2019, 26, 2531–2546. [Google Scholar] [CrossRef]
- Li, X.; Ke, M.; Zhang, M.; Peijnenburg, W.; Fan, X.; Xu, J.; Zhang, Z.; Lu, T.; Fu, Z.; Qian, H. The interactive effects of diclofop-methyl and silver nanoparticles on Arabidopsis thaliana: Growth, photosynthesis and antioxidant system. Environ. Pollut. 2017, 232, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.J.; Wang, Y. Study on temporal and spatial evolution of China’s pesticide application. Jiangsu Agric. Sci. 2019, 47, 327–331, (In Chinese with English). [Google Scholar]
- Su, S.Q. Herbicide Targets and New Variety Creation; Chemical Industry Press: Beijing, China, 2001. [Google Scholar]
- Zhang, C.P. Effect of Herbicide Imazethapyr on Soil Microbial Diversity and Environmental Behavior; Chinese Academy of Agricultural Sciences: Beijing, China, 2010; (In Chinese with English). [Google Scholar]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommenda-tions. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Cerdeira, A.L.; Duke, S.O. The current status and environmental impacts of glyphosate-resistant crops: A review. J. Environ. Qual. 2016, 35, 1633–1658. [Google Scholar] [CrossRef]
- Pertile, M.; Sousa, R.M.S.; Mendes, L.W.; Antunes, J.E.L.; Oliveira, L.M.D.S.; de Araujo, F.F.; Melo, V.M.M.; Araujo, A.S.F. Response of soil bacterial communities to the application of the herbicides imazethapyr and flumyzin. Eur. J. Soil Biol. 2021, 102, 103252. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Carvalhais, L.C.; Percy, C.D.; Verma, J.P.; Schenk, P.M.; Singh, B.K. Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytol. 2021, 229, 2873–2885. [Google Scholar] [CrossRef]
- Froschel, C.; Komorek, J.; Attard, A.; Marsell, A.; Lopez-Arboleda, W.A.; Le Berre, J.; Wolf, E.; Gelner, N.; Waller, F.; Korte, A.; et al. Plant roots employ cell-layer-specific pro-grams to respond to pathogenic and beneficial microbes. Cell Host Microbe 2021, 29, 299–310.e7. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wei, Z.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Rhizosphere microbiome functional diversity and pathogen invasion resistance build up during plant development. Environ. Microbiol. 2020, 22, 5005–5018. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Lu, H.; Ding, H.; Lavoie, M.; Li, Y.; Liu, W.; Fu, Z. Analyzing Arabidopsis thaliana root proteome provides insights into themolecular bases of enantioselective imazethapyr toxicity. Sci. Rep. 2015, 5, 11975. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O.; Ayangbenro, A. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2019, 103, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ren, W.; Li, Y.; Xu, Y.; Teng, Y.; Christie, P.; Luo, Y. Nontargeted metabolomic analysis to unravel the impact of di (2-ethylhexyl) phthalate stress on root exudates of alfalfa (Medicago sativa). Sci. Total Environ. 2019, 646, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Xu, N.; Zhang, Q.; Zhang, Z.; Debognies, A.; Zhou, Z.; Sun, L.; Qian, H. Understanding the influence of glyphosate on the structure and function of freshwater microbial community in a microcosm. Environ. Pollut. 2020, 260, 114012. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Q.; Zhang, Z.; Li, Y.; Xu, N.; Qu, Q.; Lu, T.; Pan, X.; Qian, H. Enantioselective effects of imazethapyr on Arabidopsis thaliana root exudates and rhizosphere microbes. Sci. Total Environ. 2020, 716, 137121. [Google Scholar] [CrossRef]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, paraquat and para-quat-diquat on soil microbial activity and bacterial, archaeal and nematode diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Haskett, T.L.; Tkacz, A.; Poole, P.S. Engineering rhizobacteria for sustainable agriculture. ISME J. 2021, 15, 949–964. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, J.; Liu, X.; Dong, F.; Kong, Z.; Sheng, Y.; Zheng, Y. Impact of imazethapyr on the microbial community structure in agricultural soils. Chemosphere 2010, 81, 800–806. [Google Scholar] [CrossRef]
- Chu, H.; Feng, M.; Liu, X.; Shi, Y.; Yang, T.; Gao, G. Soil microbial biogeography: Recent advances in China and research frontiers in the World. Acta Pedol. Sin. 2020, 57, 515–529, (In Chinese with English). [Google Scholar]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricul-tural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bian, Q.; Jiang, Y.; Zhu, L.; Chen, Y.; Liang, Y.; Sun, B. Organic amendments drive shifts in microbial community structure and keystone taxa which increase C mineralization across aggregate size classes. Soil Biol. Biochem. 2021, 153, 108062. [Google Scholar] [CrossRef]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Zhang, S.; Zhong, Q.; Gong, G.; Wang, G.; Guo, X.; Xu, X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2: An improved and extensible approach for metagenome inference. BioRxiv. 2019, 672295. Available online: https://www.biorxiv.org/content/10.1101/672295v1 (accessed on 1 February 2022).
- Kelly, C.N.; Schwaner, G.W.; Cumming, J.R.; Driscoll, T.P. Metagenomic reconstruction of nitrogen and carbon cycling pathways in forest soil: Influence of different hardwood tree species. Soil Biol. Biochem. 2021, 156, 108226. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, G.; Li, Y.; Zhang, X.; Xiong, Y.; Huang, Q.; Jin, S. Effects of the lignite bioorganic fertilizer on green-house gas emissions and pathways of nitrogen and carbon cycling in saline-sodic farmlands at Northwest China. J. Clean. Prod. 2022, 334, 130080. [Google Scholar] [CrossRef]
- Llorens-Mares, T.; Yooseph, S.; Goll, J.; Hoffman, J.; Vila-Costa, M.; Borrego, C.M.; Dupont, C.L.; Casamayor, E.O. Connecting biodiversity and potential functional role in modern euxinic environments by microbial metagenomics. ISME J. 2015, 9, 1648–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louca, S.; Parfrey, L.W.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.A.U.; Lavoie, M.; Song, H.; Jin, Y.; Fu, Z.; Qian, H. Interaction of chiral herbicides with soil microorganisms, algae and vascular plants. Sci. Total Environ. 2017, 580, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Klier, B.; Häfner, E.; Albert, H.; Binder, G.; Knödler, M.; Kühn, M.; Schenk, A.; Steinhoff, B. Pesticide residues in herbal drugs: Evaluation of a database. J. Appl. Res. Med. Aromat. Plants 2019, 15, 100223. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Li, Y.; Ke, M.; Qu, Q.; Yuan, W.; Pan, X.; Qian, H. Enantioselective effects of imazethapyr residues on Arabidopsis thaliana metabolic profile and phyllosphere microbial communities. J. Environ. Sci. 2020, 93, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhao, Q.; Feng, L.; Zhang, Z.; Zhang, Q.; Zhang, F.; Deng, Y.; Lu, T.; Qian, H. Regulative effect of imazethapyr on Arabidopsis thaliana growth and rhizosphere microbial community through multiple generations of culture. Plant Soil. 2022. Available online: https://link.springer.com/article/10.1007/s11104-022-05318-3 (accessed on 1 February 2022).
- Singh, R.; Singh, G. Influence of herbicides on symbiotic parameters, growth, yield and nutrient uptake in mungbean [Vigna radiata (L.) Wilczek]. Arch. Agron. Soil Sci. 2021, 67, 410–425. [Google Scholar] [CrossRef]
- Pertile, M.; Antunes, J.E.L.; Araujo, F.F.; Mendes, L.W.; Brink, P.V.D.; Araujo, A.S.F. Responses of soil microbial biomass and enzyme activity to herbicides imazethapyr and flumioxazin. Sci. Rep. 2020, 10, 7694. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, G. Effect of pendimethalin and imazethapyr on the development of microorganisms in vitro and at field conditions. Toxicol. Environ. Chem. 2020, 102, 439–454. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Ulanova, D.; Wick, L.Y.; Bode, H.B.; Garbeva, P. Microbe-driven chemical ecology: Past, present and future. ISME J. 2019, 13, 2656–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H. The Effects of Imazethapyron Soil Microbial Community Structure and the Chiral Differences of Degradationin; Zhejiang University: Zhejiang, China, 2019; (In Chinese with English). [Google Scholar]
- Cai, Z.C.; Huang, X.Q. Soil-borne Pathogens Should not Be Ignored by Soil Science. Acta Pedol. Sin. 2016, 53, 305–310, (In Chinese with English). [Google Scholar]
- Mijangos, I.; Becerril, J.M.; Albizu, I.; Epelde, L.; Garbis, C. Effects of glyphosate on rhizosphere soil microbial communities under two different plant compositions by cultivation-dependent and -independent methodologies. Soil Biol. Biochem. 2009, 41, 505–513. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Chen, S.; Jin, Y.; Song, H.; Ruan, S.; Fu, Z.; Asad, M.A.U.; Qian, H. Effects of the Herbicide Imazethapyr on Photosynthesis in PGR5- and NDH-Deficient Arabidopsis thaliana at the Biochemical, Transcriptomic, and Proteomic Levels. J. Agric. Food Chem. 2016, 64, 4497–4504. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Zheng, X.; Zhang, Y.; Chen, D.; Ding, H. Divergent modulation of land use-driven changes in soil properties and herbicide acetochlor application on soil nitrogen cycling. Soil Till. Res. 2022, 215. [Google Scholar] [CrossRef]



| Treatment | Bacterial Community | Fungal Community | ||
|---|---|---|---|---|
| Shannon | Sobs | Shannon | Sobs | |
| S1I0 | 3.47 ± 0.4 ab | 611 ± 59 a | 3.43 ± 0.4 ab | 362.25 ± 59 b |
| S1I1 | 3.46 ± 0.3 ab | 592.75 ± 38.5 a | 3.44 ± 0.3 ab | 551 ± 38.5 a |
| S2I0 | 3.85 ± 0.1 ab | 563.25 ± 15.9 a | 3.69 ± 0.1 ab | 611 ± 15.9 a |
| S2I1 | 3.7 ± 0.1 ab | 551 ± 52.9 a | 3.84 ± 0.1 ab | 520.5 ± 52.9 a |
| S3I0 | 4.23 ± 0.1 a | 520.5 ± 31 a | 4.23 ± 0.1 a | 592.75 ± 31 a |
| S3I1 | 3.14 ± 0.2 b | 362.25 ± 37.5 b | 3.12 ± 0.2 b | 563.25 ± 37.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Wang, T. Effects of Imazethapyr on Soybean Root Growth and Soil Microbial Communities in Sloped Fields. Sustainability 2022, 14, 3518. https://doi.org/10.3390/su14063518
Wang Z, Wang X, Wang T. Effects of Imazethapyr on Soybean Root Growth and Soil Microbial Communities in Sloped Fields. Sustainability. 2022; 14(6):3518. https://doi.org/10.3390/su14063518
Chicago/Turabian StyleWang, Zhidan, Xuan Wang, and Tieliang Wang. 2022. "Effects of Imazethapyr on Soybean Root Growth and Soil Microbial Communities in Sloped Fields" Sustainability 14, no. 6: 3518. https://doi.org/10.3390/su14063518





