Experimental Research on the Remediation Ability of Four Wetland Plants on Acid Mine Drainage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Test Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physiological Indicators of Four Plants
3.1.1. CAT Activity
3.1.2. GSH Content
3.1.3. The Plant Height
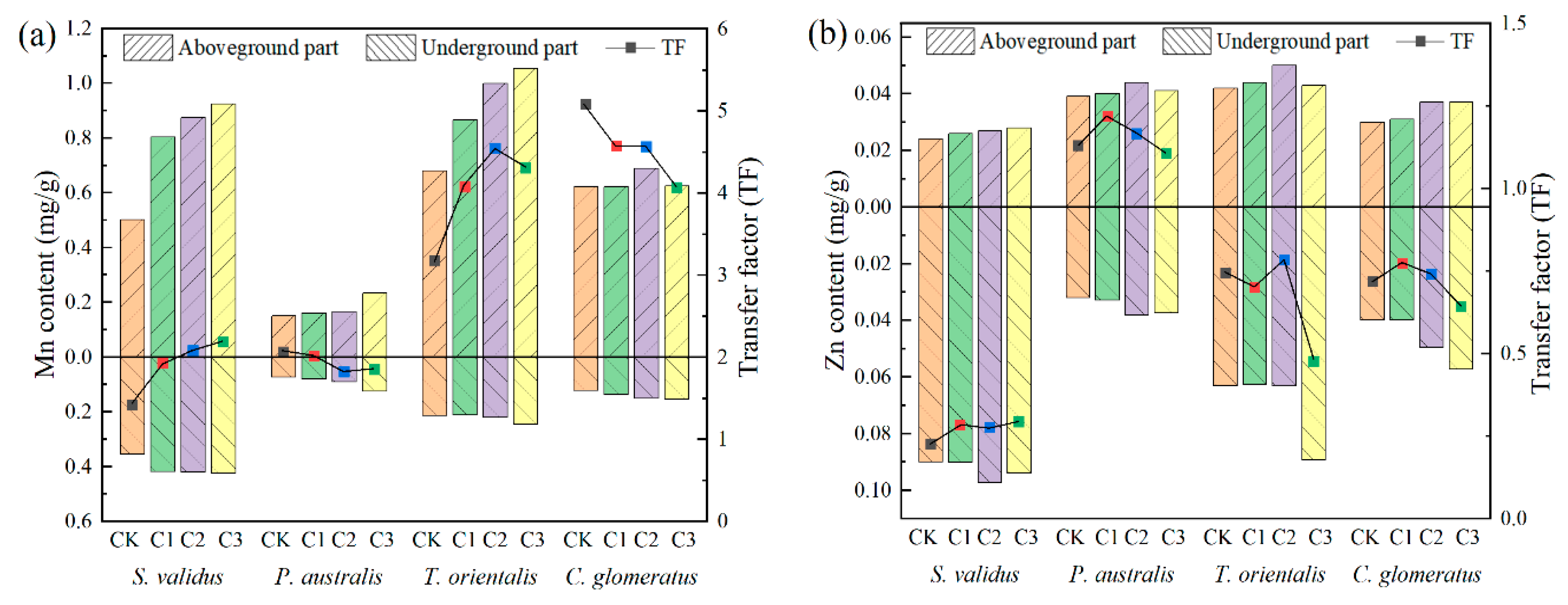
3.2. Plant Uptake of Sulfur, Mn, Zn
3.3. Transfer Factor of Plants to Metals
3.4. Microbial Biomass in Soil
4. Conclusions and Suggestion
- (1)
- The growth of S. validus and P. australis was inhibited under various concentrations of AMD treatment; the growth of C. glomeratus was inhibited under high concentrations of AMD treatment; and the growth of T. orientalis thrived as the concentration of AMD increased, indicating that T. orientalis was more resistant to AMD. therefore, T. orientalis was suitable for treating high concentrations of AMD (SO42− ≈ 9400 mg/L); C. glomeratus was suitable for treating medium concentrations of AMD (SO42− ≈ 4600 mg/L); and S. validus and P. australis could be used to treat low concentrations of AMD (SO42− ≈ 2300 mg/L).
- (2)
- All four plants had certain extraction effects on SO42−, Mn, and Zn, among which C. glomeratus and S. validus showed stronger extractions. Based on the TFs of each plant for heavy metals, it was known that all four plants could be used for phytoextraction for Mn-contaminated water; P. australis could be used for phytoextraction for Zn-contaminated water; and S. validus, T. orientalis, and C. glomeratus could be used for phytostabilisation for Zn-contaminated water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y. Mineral phases and mobility of trace metals in white aluminum precipitates found in acid mine drainage. Chemosphere 2015, 119, 803–811. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhang, Z.; Zhang, Y. Review: Acid Mine Drainage (AMD) in Abandoned Coal Mines of Shanxi, China. Water 2021, 13, 8. [Google Scholar] [CrossRef]
- Nyquist, J.; Greger, M. A field study of constructed wetlands for preventing and treating acid mine drainage. Ecol. Eng. 2008, 35, 630–642. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Kahlon, S.K.; Sharma, G.; Julka, J.M.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, P.; Guerreiro, N.; Simoes, I.; Imaginario, M.J.; Palma, P. Assessment of the Environmental Impact of Acid Mine Drainage on Surface Water, Stream Sediments, and Macrophytes Using a Battery of Chemical and Ecotoxicological Indicators. Water 2021, 13, 1436. [Google Scholar] [CrossRef]
- Oh, K.; Cao, T.; Tao, L.; Cheng, H. Study on Application of Phytoremediation Technology in Management and Remediation of Contaminated Soils. J. Clean Energy Technol. 2014, 2, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhu, H.; Banuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Wu, S.; Vymazal, J.; Brix, H. Critical Review: Biogeochemical Networking of Iron in Constructed Wetlands for Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 7930–7944. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; Ma, N.; Wang, W.; Ma, C.; Zhang, R. Cadmium removal capability and growth characteristics of Iris sibirica in subsurface vertical flow constructed wetlands. Ecol. Eng. 2015, 84, 443–450. [Google Scholar] [CrossRef]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Marchand, L.; Mench, M.; Jacob, D.L.; Otte, M.L. Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ. Pollut. 2010, 158, 3447–3461. [Google Scholar] [CrossRef]
- Mang, K.C.; Ntushelo, K. Phytoextraction and phytostabilisation approaches of heavy metal remediation in acid mine drainage with case studies: A review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments—An emerging remediation technology. Environ. Health Perspect. 2008, 116, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef]
- Leung, H.M.; Duzgoren-Aydin, N.S.; Au, C.K.; Krupanidhi, S.; Fung, K.Y.; Cheung, K.C.; Wong, Y.K.; Peng, X.L.; Ye, Z.H.; Yung, K.K.L.; et al. Monitoring and assessment of heavy metal contamination in a constructed wetland in Shaoguan (Guangdong Province, China): Bioaccumulation of Pb, Zn, Cu and Cd in aquatic and terrestrial components. Environ. Sci. Pollut. Res. 2017, 24, 9079–9088. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Lizama-Allende, K.; Roulet, E.; Rousset, C.; Bordas, F.; Mench, M. Selection of macrophytes with Cu-enriched root biomass intended for ecocatalyst production. Ecol. Eng. 2019, 138, 88–96. [Google Scholar] [CrossRef]
- Miloskovic, A.; Brankovic, S.; Simic, V.; Kovacevic, S.; Cirkovic, M.; Manojlovic, D. The Accumulation and Distribution of Metals in Water, Sediment, Aquatic Macrophytes and Fishes of the Gruža Reservoir, Serbia. Bull. Environ. Contam. Toxicol. 2013, 90, 563–569. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2008, 407, 3923–3930. [Google Scholar] [CrossRef]
- Ding, Z.; Fang, Q.; Daraz, U.; Sun, Q. Physiological responses and metal distributions of different organs of Phragmites australis shoots under acid mine drainage stress. Environ. Sci. Pollut. Res. 2021, 28, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- GB/T 39228-2020, Determination of Soil Microbial Biomass-Fumigation-Extraction Method [S]. (In Chinese). Available online: http://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=5026CEBF6F49CF5D1AB8FD6020CEB1C6 (accessed on 12 March 2022).
- Sasmaz, A.; Obek, E.; Hasar, H. The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecol. Eng. 2008, 33, 278–284. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Yang, Y.; Yuan, H.; Zhao, J.; Gu, J.; Huang, S. Pb uptake and toxicity to Iris halophila tested on Pb mine tailing materials. Environ. Pollut. 2016, 214, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Andra, S.S.; Datta, R.; Sarkar, D.; Makris, K.C.; Mullens, C.P.; Sahi, S.V.; Bach, S.B.H. Induction of Lead-Binding Phytochelatins in Vetiver Grass Vetiveria zizanioides (L.). J. Environ. Qual. 2009, 38, 868–877. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Loredo Portales, R.; Amabilis-Sosa, L.E.; Gomez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef] [Green Version]
- Kalu, C.M.; Ogola, H.J.O.; Selvarajan, R.; Tekere, M.; Ntushelo, K. Fungal and metabolome diversity of the rhizosphere and endosphere of Phragmites australis in an AMD-polluted environment. Heliyon 2021, 7, e06399. [Google Scholar] [CrossRef]
- Clemens, S. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 2001, 212, 475–486. [Google Scholar] [CrossRef]
- Kiiskila, J.D.; Li, K.; Sarkar, D.; Datta, R. Metabolic response of vetiver grass (Chrysopogon zizanioides) to acid mine drainage. Chemosphere 2020, 240, 124961. [Google Scholar] [CrossRef]
- Moniuszko, G.; Sirko, A. Sulfur metabolism and its regulation in plants. Postepy Biochem. 2008, 54, 402–411. [Google Scholar]
- Rana, V.; Maiti, S.K. Metal Accumulation Strategies of Emergent Plants in Natural Wetland Ecosystems Contaminated with Coke-Oven Effluent. Bull. Environ. Contam. Toxicol. 2018, 101, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J. Hazard. Mater. 2020, 397, 122719. [Google Scholar] [CrossRef] [PubMed]
- Sochacki, A.; Guy, B.; Faure, O.; Surmacz-Gorska, J. Accumulation of Metals and Boron in Phragmites australis Planted in Constructed Wetlands Polishing Real Electroplating Wastewater. Int. J. Phytoremediat. 2015, 17, 1068–1072. [Google Scholar] [CrossRef]
- Dan, A.; Oka, M.; Fujii, Y.; Soda, S.; Ishigaki, T.; Machimura, T.; Ike, M. Removal of heavy metals from synthetic landfill leachate in lab-scale vertical flow constructed wetlands. Sci. Total Environ. 2017, 584, 742–750. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Daraz, U.; Li, Y.; Sun, Q.; Zhang, M.; Ahmad, I. Inoculation of Bacillus spp. Modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere 2021, 263, 128345. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Cutright, T.J. Remediation of acid mine drainage (AMD)-contaminated soil by Phragmites australis and rhizosphere bacteria. Environ. Sci. Pollut. Res. 2014, 21, 7350–7360. [Google Scholar] [CrossRef]
- Neculita, C.-M.; Zagury, G.J.; Bussiere, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Wu, Z.; Kong, Z.; Lu, S.; Huang, C.; Huang, S.; He, Y.; Wu, L. Isolation, characterization and the effect of indigenous heavy metal-resistant plant growth-promoting bacteria on sorghum grown in acid mine drainage polluted soils. J. Gen. Appl. Microbiol. 2019, 65, 254–264. [Google Scholar] [CrossRef] [Green Version]





| pH Value | Conductivity /(mS/cm) | SO42− | Fe | Mn | Zn | Ca2+ | K+ | Na+ | Pb | Cl− | F− | NO3− | NO2− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| /(mg/L) | |||||||||||||
| 3.62 | 7.44 | 9398.53 | 117.98 | 24.42 | 8.45 | 303 | 0.52 | 30.4 | 0.003 | 92.9 | 13 | 0.81 | 0.15 |
| Organic Matter | Total N | Total K | Total P | Total S | Mn | Zn | pH | CEC |
|---|---|---|---|---|---|---|---|---|
| g/kg | mg/kg | mmol/kg | ||||||
| 11.11 | 0.78 | 18.97 | 0.84 | 153.27 | 227.07 | 150.35 | 8.14 | 102.63 |
| Code | Treatment | Distilled Water: AMD | SO42− | Mn | Zn |
|---|---|---|---|---|---|
| mg/L | |||||
| CK | Control group | 1:0 | 0 | 0 | 0 |
| C1 | Low concentration AMD | 3:1 | 2315.51 | 6.24 | 2.34 |
| C2 | Medium concentration AMD | 1:1 | 4605.32 | 11.25 | 4.33 |
| C3 | High concentration AMD | 0:1 | 9398.53 | 24.42 | 8.45 |
| Pollution Element | Treatment | S. validus | P. australis | T. orientalis | C. glomeratus |
|---|---|---|---|---|---|
| Sulfur | CK | 1.00 | 1.00 | 1.00 | 1.00 |
| C1 | 1.85 | 1.65 | 1.35 | 1.22 | |
| C2 | 2.09 | 1.28 | 1.78 | 1.22 | |
| C3 | 1.42 | 1.21 | 1.29 | 1.54 | |
| Mn | CK | 1.00 | 1.00 | 1.00 | 1.00 |
| C1 | 1.43 | 1.06 | 1.20 | 1.02 | |
| C2 | 1.45 | 1.26 | 1.36 | 1.13 | |
| C3 | 1.57 | 1.48 | 1.45 | 1.05 | |
| Zn | CK | 1.00 | 1.00 | 1.00 | 1.00 |
| C1 | 1.03 | 1.03 | 1.01 | 1.06 | |
| C2 | 1.10 | 1.16 | 1.06 | 1.29 | |
| C3 | 1.08 | 1.11 | 1.25 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, A.; Zhang, Y.; Zhao, X.; Shi, H.; Xu, S.; Li, J.; Zhang, G.; Guo, L. Experimental Research on the Remediation Ability of Four Wetland Plants on Acid Mine Drainage. Sustainability 2022, 14, 3655. https://doi.org/10.3390/su14063655
Wu A, Zhang Y, Zhao X, Shi H, Xu S, Li J, Zhang G, Guo L. Experimental Research on the Remediation Ability of Four Wetland Plants on Acid Mine Drainage. Sustainability. 2022; 14(6):3655. https://doi.org/10.3390/su14063655
Chicago/Turabian StyleWu, Aijing, Yongbo Zhang, Xuehua Zhao, Hong Shi, Shuyuan Xu, Jiamin Li, Guowei Zhang, and Lina Guo. 2022. "Experimental Research on the Remediation Ability of Four Wetland Plants on Acid Mine Drainage" Sustainability 14, no. 6: 3655. https://doi.org/10.3390/su14063655





