Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation
Abstract
1. Introduction
2. Methodology
3. The Genus Pleurotus and Its Potential Uses
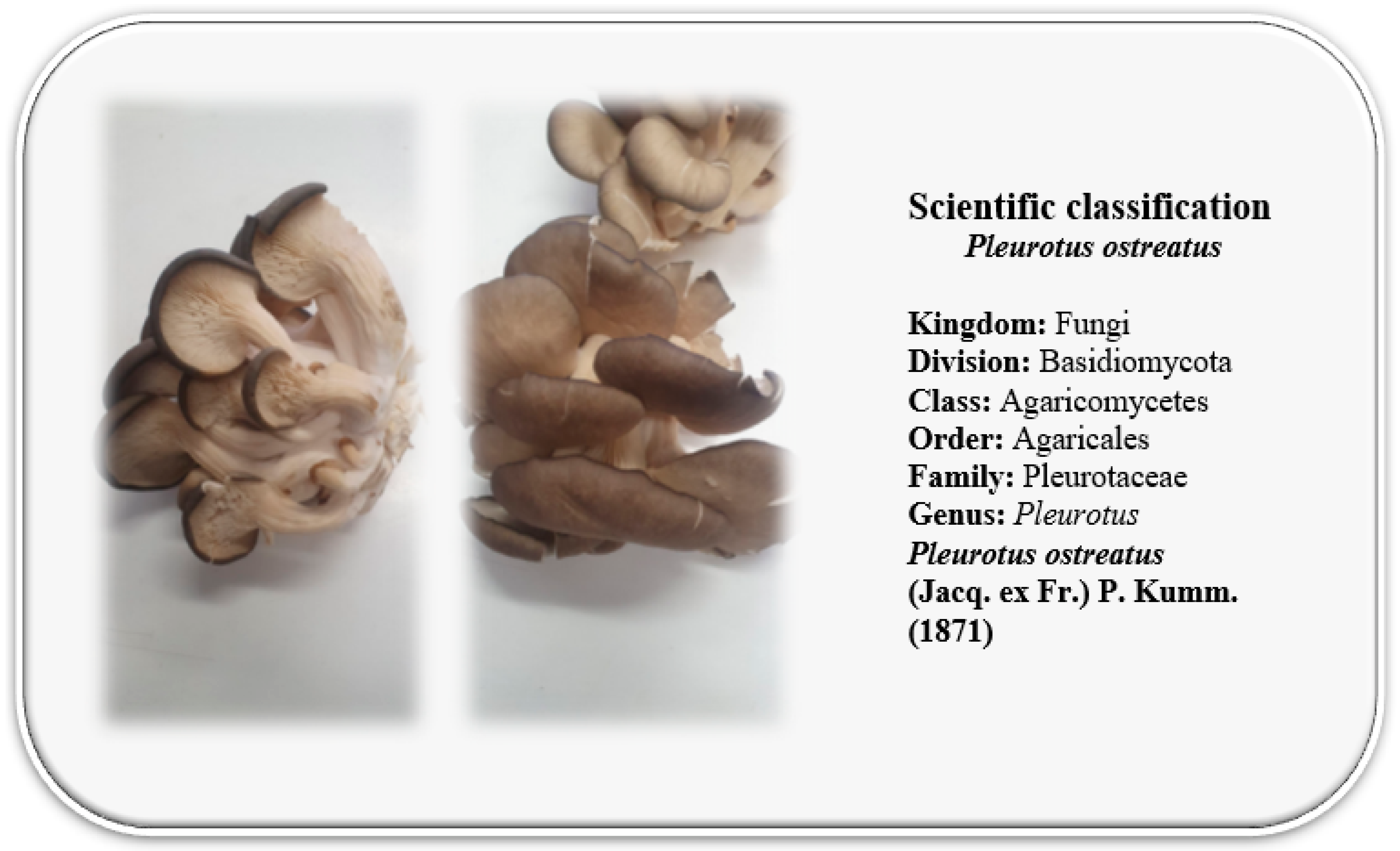
3.1. Taxonomy and Botanical Description
3.2. Food Importance of the Pleurotus Genus
3.3. Medicinal Importance of the Pleurotus Genus
4. General Features of Pleurotus ostreatus
5. Myco-Remediation by Pleurotus ostreatus
6. Recycling of Organic Solid Wastes by Pleurotus ostreatus
7. Enzyme Production by Pleurotus ostreatus
- Studies on the role of spent mushroom substrates after this cultivation produces many important materials, such as enzymes [12,19,126,127], biomass [118,128,129], bioethanol [19,127,130], feed ingredients, and functional foods [61,131,132]. Spent mushroom substrates can be recycled as a substrate for the “new cultivation cycle” of mushrooms, a feedstock for producing the second generation of biofuels, a bio-control agent, a biofertilizer, and for soil amendment [133,134,135];
- Studies on the biodegradation of agro-wastes or agro-industrial by-products through the solid-state fermentation or submerged fermentation by P. ostreatus, such as (a) using the deinking sludge as a substrate to produce lignocellulolytic enzymes (Vodovnik et al. [136], (b) producing exo-polygalacturonases using pomelo peel powder under submerged fermentation by P. ostreatus [122], and (c) producing laccases by white-rot fungi under solid-state fermentation conditions [137];
- Studies on the production of ligninolytic enzymes and their potential [129,138,139,140]. The most important ligninolytic enzymes, which can be produced by Pleurotus include manganese peroxidase, laccase, and lignin peroxidase through biodegradation, which varied from species to species. The main factors controlling the Pleurotus species and their ability to produce enzymes or to degrade wastes or pollutants include the pH, pollutant/waste concentration, and C:N ratio of the substrate [141];
- Studies on the role of nano-mycology, including the applications of Pleurotus spp. to the green or myco-synthesis of nano-silver [142,143,144], nano-TiO2 [145,146], nano-ZnO [147], and the production of fluorescent carbon quantum dots as a C-based nanomaterial [148]. The myco-synthesis of nano-nutrients (nano-Ag, nano-TiO2, and nano-ZnO) has been investigated for medical attributes, such as controlling mosquito larvae, and anti-cancer activities [145,146,147]. Nanomaterials conjugated lignocellulosic wastes for producing biofuels using immobilized enzymes [149];
- Sustainable management of agro-industrial wastes could be achieved by the reduction and conservation of wastes as well as different utilizations of wastes, including reuse and recycling [132,150]. The main agro-based industries that produce large amounts of waste may include plant-based foods (e.g., cereals, fermentation, sugar, food and fruit processing), animal-based foods (e.g., milk, dairy, fish and poultry products), and non-food industries, such as paper and textiles [132].
8. Bioethanol Production by Pleurotus ostreatus
- The chemical composition of agro-wastes (cellulose, hemicellulose, and lignin) or spent mushroom substrates (SMSs) is considered an important factor controlling the biodegradation of these wastes, as reported in Table 5. Every 1 kg of grown mushroom generates nearly 5 kg of SMSs, establishing a promising industry for SMSs [158]. The biodegradation mechanism of these SMSs by mushrooms (e.g., Pleurotus spp.) may include the enzymatic degradation of various substrates (mainly cellulose, hemicellulose, and lignin) into soluble compounds of a low molecular weight. A partial degradation of the lignocellulosic biomass by saccharification during the pre-treatment is needed;
- The condition of fertilization and its kind (solid-state fermentation or submerged), using media are the substrates as a source of carbon, and are the main factors that control the applications of Pleurotus ostreatus for the production of some cellulolytic enzymes and other by-products, such as reducing sugars, biofertilizers, and animal feeds, as reported in Table 6;
- The production of biofuel from saccharification and/or fermentation in the presence or absence of Pleurotus ostreatus or other mushrooms depends on the kind of applied substrate, mushroom species, and the oxidation or fermentation conditions, as reported in Table 7;
- The general structure of the lignocellulosic agro-wastes includes cellulose (30–50%), lignin (10–20%), and hemicelluloses (15–35%), in addition to some components, including minerals, extractives, and ash, in tiny amounts [167]. These agro-wastes are still underutilized, especially in developing countries. The unwise utilization of these wastes (mainly the burning of them) causes many environmental crises, such as global warming due to an increase in the emissions of gases, particularly carbon dioxide and sulfur dioxide, as well as underground water pollution [169];
- It is evident from Table 7 that the oyster mushroom (Pleurotus ostreatus) is a mushroom distinguished for its ability to produce of bioethanol compared to other mushrooms, even in the case of the genus Pleurotus. This rate of production is higher in Pleurotus ostreatus (up to 46 g L−1 with efficiency up to 70%), which is higher than Pleurotus florida or other species, such as Ganoderma lucidum.
9. General Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gullino, M.L. Green Biotechnologies. In Spores; Springer International Publishing: Cham, Switzerland, 2021; pp. 253–257. ISBN 978-3-030-69994-9. [Google Scholar]
- Chaurasia, P.K.; Bharati, S.L. Applicability of Fungi in Agriculture and Environmental Sustainability. In Microbes in Land Use Change Management; Elsevier: Amsterdam, The Netherlands, 2021; pp. 155–172. ISBN 978-0-12-824448-7. [Google Scholar]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Budzyńska, S.; Proch, J.; Gąsecka, M.; Niedzielski, P.; Rzymski, P. A Comparison of Toxic and Essential Elements in Edible Wild and Cultivated Mushroom Species. Eur. Food Res. Technol. 2021, 247, 1249–1262. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, M.; Yang, L.; Mi, F.; Cao, Y.; Liu, C.; Tang, X.; Wang, P.; Xu, J. Exploring the Species Diversity of Edible Mushrooms in Yunnan, Southwestern China, by DNA Barcoding. J. Fungi 2021, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable Improvement of Soil Health Utilizing Biochar and Arbuscular Mycorrhizal Fungi: A Review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The Antioxidant Potential of Different Edible and Medicinal Mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef] [PubMed]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y.B. Green Potential of Pleurotus spp. in Biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef]
- Xiao, P.; Wu, D.; Wang, J. Bibliometric Analysis of Global Research on White Rot Fungi Biotechnology for Environmental Application. Environ. Sci. Pollut. Res. 2022, 29, 1491–1507. [Google Scholar] [CrossRef]
- Khoo, S.C.; Ma, N.L.; Peng, W.X.; Ng, K.K.; Goh, M.S.; Chen, H.L.; Tan, S.H.; Lee, C.H.; Luang-In, V.; Sonne, C. Valorisation of Biomass and Diaper Waste into a Sustainable Production of the Medical Mushroom Lingzhi Ganoderma lucidum. Chemosphere 2022, 286, 131477. [Google Scholar] [CrossRef] [PubMed]
- Sithole, S.C.; Agboola, O.O.; Mugivhisa, L.L.; Amoo, S.O.; Olowoyo, J.O. Elemental Concentration of Heavy Metals in Oyster Mushrooms Grown on Mine Polluted Soils in Pretoria, South Africa. J. King Saud Univ. Sci. 2022, 34, 101763. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part II: Effect on Productivity and Quality of Carposomes. Carbon Resour. Convers. 2022, 5, 52–60. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Prokisch, J.; Törős, G.; El-Ramady, H. Edible Mushroom of Pleurotus spp.: A Case Study of Oyster Mushroom (Pleurotus ostreatus L.). EBSS 2021, 5, 1–2. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P.; Tripathi, A.; Sadh, P.K. A Comparative Study of Antimicrobial and Anti-Inflammatory Efficiency of Modified Solvent Evaporated and Vacuum Oven Dried Bioactive Components of Pleurotus floridanus. J. Food Sci. Technol. 2021, 58, 3328–3337. [Google Scholar] [CrossRef]
- Caldas, L.A.; Zied, D.C.; Sartorelli, P. Dereplication of Extracts from Nutraceutical Mushrooms Pleurotus Using Molecular Network Approach. Food Chem. 2022, 370, 131019. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C.F.R. Biotechnological, Nutritional and Therapeutic Uses of Pleurotus spp. (Oyster Mushroom) Related with Its Chemical Composition: A Review on the Past Decade Findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Mycoremediation Potential of Pleurotus Species for Heavy Metals: A Review. Bioresour. Bioprocess. 2017, 4, 32. [Google Scholar] [CrossRef]
- Durán-Aranguren, D.D.; Meléndez-Melo, J.P.; Covo-Ospina, M.C.; Díaz-Rendón, J.; Reyes-Gutiérrez, D.N.; Reina, L.C.; Durán-Sequeda, D.; Sierra, R. Biological Pretreatment of Fruit Residues Using the Genus Pleurotus: A Review. Bioresour. Technol. Rep. 2021, 16, 100849. [Google Scholar] [CrossRef]
- Ranjithkumar, M.; Uthandi, S.; Senthil Kumar, P.; Muniraj, I.; Thanabal, V.; Rajarathinam, R. Highly Crystalline Cotton Spinning Wastes Utilization: Pretreatment, Optimized Hydrolysis and Fermentation Using Pleurotus florida for Bioethanol Production. Fuel 2022, 308, 122052. [Google Scholar] [CrossRef]
- Kaewlaoyoong, A.; Cheng, C.-Y.; Lin, C.; Chen, J.-R.; Huang, W.-Y.; Sriprom, P. White Rot Fungus Pleurotus pulmonarius Enhanced Bioremediation of Highly PCDD/F-Contaminated Field Soil via Solid State Fermentation. Sci. Total Environ. 2020, 738, 139670. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Liu, L.; Qi, H.; You, H. Biodegradation of Decabromodiphenyl Ethane (DBDPE) by White-Rot Fungus Pleurotus ostreatus: Characteristics, Mechanisms, and Toxicological Response. J. Hazard. Mater. 2022, 424, 127716. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Ghosh, T.; Sengupta, A.; Das, A. Nutrition, Therapeutics and Environment Impact of Oyster Mushrooms: A Low Cost Proteinaceous Source. J. Gynecol. Women’s Health 2019, 14, 555876. [Google Scholar] [CrossRef]
- CABI Invasive Species Compendium: Datasheet. Pleurotus ostreatus (Oyster Mushroom). Available online: https://www.cabi.org/isc/datasheet/42037 (accessed on 8 February 2022).
- Espinosa-Páez, E.; Hernández-Luna, C.E.; Longoria-García, S.; Martínez-Silva, P.A.; Ortiz-Rodríguez, I.; Villarreal-Vera, M.T.; Cantú-Saldaña, C.M. Pleurotus ostreatus: A Potential Concurrent Biotransformation Agent/Ingredient on Development of Functional Foods (Cookies). LWT 2021, 148, 111727. [Google Scholar] [CrossRef]
- Menolli, N.; Breternitz, B.S.; Capelari, M. The Genus Pleurotus in Brazil: A Molecular and Taxonomic Overview. Mycoscience 2014, 55, 378–389. [Google Scholar] [CrossRef]
- Dicks, L.; Ellinger, S. Effect of the Intake of Oyster Mushrooms (Pleurotus ostreatus) on Cardiometabolic Parameters—A Systematic Review of Clinical Trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional Value and Biological Properties of Chilean Wild and Commercial Edible Mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic Composition and Antioxidant Properties of Pleurotus ostreatus and Pleurotus eryngii Enriched with Selenium and Zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Sardar, H.; Ali, M.A.; Anjum, M.A.; Nawaz, F.; Hussain, S.; Naz, S.; Karimi, S.M. Agro-Industrial Residues Influence Mineral Elements Accumulation and Nutritional Composition of King Oyster Mushroom (Pleurotus eryngii). Sci. Hortic. 2017, 225, 327–334. [Google Scholar] [CrossRef]
- Sardar, H.; Anjum, M.A.; Hussain, S.; Ali, S.; Shaheen, M.R.; Ahsan, M.; Ejaz, S.; Ahmad, K.S.; Naz, S.; Shafique, M. Deciphering the Role of Moringa Leaf Powder as a Supplement in the Cotton Waste Substrate for the Growth and Nutrition of King Oyster Mushroom. Sci. Hortic. 2022, 293, 110694. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V. Yield Performance and Nutritional Analysis of Pleurotus Citrinopileatus on Different Agrowastes and Vegetable Wastes. 2011. Available online: https://www.semanticscholar.org/paper/Yield-performance-and-nutritional-analysis-of-on-Singh-Singh/39e9fd8541f30a60aa6909903f881caa4266def3 (accessed on 10 February 2022).
- Mshandete, A.M.; Cuff, J. Cultivation of Three Types of Indigenous Wild Edible Mushrooms: Coprinus cinereus, Pleurotus flabellatus and Volvariella volvocea on Composted Sisal Decortications Residue in Tanzania. Afr. J. Biotechnol. 2008, 7. [Google Scholar] [CrossRef]
- Jegadeesh, R.; Lakshmanan, H.; Kab-Yeul, J.; Sabar, V.; Raaman, N. Cultivation of Pink Oyster Mushroom Pleurotus Djamor Var. Roseus on Various Agro-Residues by Low Cost Technique. J. Mycopathol. Res. 2018, 56, 213–220. [Google Scholar]
- Silva, S.O.; Costa, S.M.G.D.; Clemente, E. Chemical Composition of Pleurotus pulmonarius (Fr.) Quél., Substrates and Residue after Cultivation. Braz. Arch. Biol. Technol. 2002, 45, 531–535. [Google Scholar] [CrossRef]
- Bumanlag, C.; Kalaw, S.; Dulay, R.; Reyes, R. Optimum conditions for mycelia growth and basidiocarp production of Pleurotus djamor on corn based media. Int. J.Biol. Pharm. Allied Sci. 2018, 7, 558–575. [Google Scholar] [CrossRef]
- Ijeh, I.I.; Okwujiako, I.A.; Nwosu, P.C.; Nnodim, H.I. Phytochemical Composition of Pleurotus Tuber Regium and Effect of Its Dietary Incorporation on Body /Organ Weights and Serum Triacylglycerols in Albino Mice. J. Med. Plants Res. 2009, 3, 939–943. [Google Scholar]
- Ahmed, S.; Kadam, J.; Mane, V.; Patil, S.; Mmv, B. Biological Efficiency and Nutritional Contents of Pleurotus florida (Mont.) Singer Cultivated On Different Agro-Wastes. Nat. Sci. Sleep 2008, 7, 44–48. [Google Scholar]
- Rajak, S.; Mahapatra, S.C.; Basu, M. Yield, Fruit Body Diameter and Cropping Duration of Oyster Mushroom (Pleurotus Sajor Caju) Grown on Different Grasses and Paddy Straw as Substrates. Eur. J. Med. Plants 2011, 1, 10–17. [Google Scholar] [CrossRef]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Change in Agrochemical and Biochemical Parameters during the Laboratory Vermicomposting of Spent Mushroom Substrate after Cultivation of Pleurotus ostreatus. Sci. Total Environ. 2020, 739, 140085. [Google Scholar] [CrossRef]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in Wild and Cultivated Mushrooms: Nutrition and Health. Phytochem. Rev. 2021. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A Critical Review on the Health Promoting Effects of Mushrooms Nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Vamanu, E. In Vitro Antimicrobial and Antioxidant Activities of Ethanolic Extract of Lyophilized Mycelium of Pleurotus ostreatus PQMZ91109. Molecules 2012, 17, 3653–3671. [Google Scholar] [CrossRef]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional Composition and Nutraceutical Properties of the Pleurotus Fruiting Bodies: Potential Use as Food Ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Talkad, M.S.; Das, R.K.; Bhattacharjee, P.; Ghosh, S.; Shivajirao, U.P. Establishment of Enzyme Inhibitory Activities of Lovastatin, Isolated From Pleurotus ostreatus. Int. J. Appl. Sci. Biotechnol. 2015, 3, 408–416. [Google Scholar] [CrossRef][Green Version]
- Anandhi, R.; Annadurai, T.; Anitha, T.S.; Muralidharan, A.R.; Najmunnisha, K.; Nachiappan, V.; Thomas, P.A.; Geraldine, P. Antihypercholesterolemic and Antioxidative Effects of an Extract of the Oyster Mushroom, Pleurotus ostreatus, and Its Major Constituent, Chrysin, in Triton WR-1339-Induced Hypercholesterolemic Rats. J. Physiol. Biochem. 2013, 69, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Golak-Siwulska, I.; Kałużewicz, A.; Spiżewski, T.; Siwulski, M.; Sobieralski, K. Bioactive Compounds and Medicinal Properties of Oyster Mushrooms (Pleurotus sp.). Folia Hortic. 2018, 30, 191–201. [Google Scholar] [CrossRef]
- Patel, Y.; Naraian, R.; Singh, V. Medicinal Properties of Pleurotus Species (Oyster Mushroom): A Review. World J. Fungal Plant Biol. 2012, 3, 1–12. [Google Scholar]
- Mitsou, E.K.; Saxami, G.; Stamoulou, E.; Kerezoudi, E.; Terzi, E.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mountzouris, K.C.; Pletsa, V.; et al. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules 2020, 25, 2806. [Google Scholar] [CrossRef]
- Piska, K. Edible Mushroom Pleurotus ostreatus (Oyster Mushroom)—Its Dietary Significance and Biological Activity. Acta Sci. Pol. Hortorum Cultus 2017, 16, 151–161. [Google Scholar]
- Kuo, M. Pleurotus ostreatus (MushroomExpert.Com). Available online: https://www.mushroomexpert.com/pleurotus_ostreatus.html (accessed on 9 February 2022).
- Włodarczyk, A.; Krakowska, A.; Sułkowska-Ziaja, K.; Suchanek, M.; Zięba, P.; Opoka, W.; Muszyńska, B. Pleurotus spp. Mycelia Enriched in Magnesium and Zinc Salts as a Potential Functional Food. Molecules 2020, 26, 162. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Aboshanab, K.M.; Yassien, M.A. Antiviral, Cytotoxic, and Antioxidant Activities of Three Edible Agaricomycetes Mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus Bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.S.; Yassein, M.A.; Aboshanab, K.M. Proteome Analysis and In Vitro Antiviral, Anticancer and Antioxidant Capacities of the Aqueous Extracts of Lentinula Edodes and Pleurotus ostreatus Edible Mushrooms. Molecules 2021, 26, 4623. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected Edible Medicinal Mushrooms from Pleurotus Genus as an Answer for Human Civilization Diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising Anticancer Activity of Polysaccharides and Other Macromolecules Derived from Oyster Mushroom (Pleurotus sp.): An Updated Review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors Affecting Mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Wan Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A Review on Valorization of Oyster Mushroom and Waste Generated in the Mushroom Cultivation Industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Abioye, S.A.; Akinyemi, D.D.; Fadairo, F.B.; Bolaniran, T.; Akinyele, B.J. Bioactivity Assessment of Ethanolic Extracts from Theobroma cacao and Cola spp. Wastes after Solid State Fermentation by Pleurotus ostreatus and Calocybe Indica. Adv. Tradit. Med. (ADTM) 2021. [Google Scholar] [CrossRef]
- Sardar, H.; Anjum, M.A.; Nawaz, A.; Ejaz, S.; Ali, M.A.; Khan, N.A.; Nawaz, F.; Raheel, M. Impact of various agro-industrial wastes on yield and quality of Pleurotus sajor-caju. Pak. J. Phytopathol. 2016, 28, 87–92. [Google Scholar]
- Zawadzka, A.; Janczewska, A.; Kobus-Cisowska, J.; Dziedziński, M.; Siwulski, M.; Czarniecka-Skubina, E.; Stuper-Szablewska, K. The Effect of Light Conditions on the Content of Selected Active Ingredients in Anatomical Parts of the Oyster Mushroom (Pleurotus ostreatus L.). PLoS ONE 2022, 17, e0262279. [Google Scholar] [CrossRef]
- Tagkouli, D.; Bekiaris, G.; Pantazi, S.; Anastasopoulou, M.E.; Koutrotsios, G.; Mallouchos, A.; Zervakis, G.I.; Kalogeropoulos, N. Volatile Profiling of Pleurotus eryngii and Pleurotus ostreatus Mushrooms Cultivated on Agricultural and Agro-Industrial By-Products. Foods 2021, 10, 1287. [Google Scholar] [CrossRef]
- Ahmad Zakil, F.; Mohd Isa, R.; Mohd Sueb, M.S.; Isha, R. Growth Performance and Mineral Analysis of Pleurotus ostreatus (Oyster Mushroom) Cultivated on Spent Mushroom Medium Mixed with Rubber Tree Sawdust. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and Chemical Diversity Go Hand in Hand: Basidiomycota as Source of New Pharmaceuticals and Agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef] [PubMed]
- Hazaimeh, M.D.; Ahmed, E.S. Bioremediation Perspectives and Progress in Petroleum Pollution in the Marine Environment: A Review. Environ. Sci. Pollut. Res. 2021, 28, 54238–54259. [Google Scholar] [CrossRef] [PubMed]
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead Pollution and Bacterial Bioremediation: A Review. Environ. Chem. Lett. 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Kumar, A.; Yadav, A.N.; Mondal, R.; Kour, D.; Subrahmanyam, G.; Shabnam, A.A.; Khan, S.A.; Yadav, K.K.; Sharma, G.K.; Cabral-Pinto, M.; et al. Myco-Remediation: A Mechanistic Understanding of Contaminants Alleviation from Natural Environment and Future Prospect. Chemosphere 2021, 284, 131325. [Google Scholar] [CrossRef] [PubMed]
- Ratnasari, A.; Syafiuddin, A.; Kueh, A.B.H.; Suhartono, S.; Hadibarata, T. Opportunities and Challenges for Sustainable Bioremediation of Natural and Synthetic Estrogens as Emerging Water Contaminants Using Bacteria, Fungi, and Algae. Water Air Soil Pollut. 2021, 232, 242. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Kinetics of Nutrients Remediation from Sugar Industry Effluent-Treated Substrate Using Agaricus Bisporus: Mushroom Yield and Biochemical Potentials. 3 Biotech 2021, 11, 164. [Google Scholar] [CrossRef]
- Yadav, P.; Rai, S.N.; Mishra, V.; Singh, M.P. Mycoremediation of Environmental Pollutants: A Review with Special Emphasis on Mushrooms. Environ. Sustain. 2021, 4, 605–618. [Google Scholar] [CrossRef]
- Boujelben, R.; Ellouze, M.; Tóran, M.J.; Blánquez, P.; Sayadi, S. Mycoremediation of Tunisian Tannery Wastewater under Non-Sterile Conditions Using Trametes Versicolor: Live and Dead Biomasses. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Janyasuthwiong, S.; Rene, E. Bioprecipitation—A Promising Technique for Heavy Metal Removal and Recovery from Contaminated Wastewater Streams. MOJCE 2017, 2, 52. [Google Scholar] [CrossRef]
- Prigione, V.; Spina, F.; Tigini, V.; Giovando, S.; Varese, G.C. Biotransformation of Industrial Tannins by Filamentous Fungi. Appl. Microbiol. Biotechnol. 2018, 102, 10361–10375. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of Co-Contaminated Soil with Heavy Metals and Pesticides: Influence Factors, Mechanisms and Evaluation Methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Romero-Silva, R.; Sánchez-Reyes, A.; Díaz-Rodríguez, Y.; Batista-García, R.A.; Hernández-Hernández, D.; Tabullo de Robles, J. Bioremediation of Soils Contaminated with Petroleum Solid Wastes and Drill Cuttings by Pleurotus sp. under Different Treatment Scales. SN Appl. Sci. 2019, 1, 1209. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, D.; Sharma, A.; Bansal, S. Thermo Stable Tyrosinase Purified from Pleurotus Djamor Grown in Biomimetic Calcium Carbonate: A Biological Strategy to Industrial Waste Remediation. Environ. Technol. Innov. 2021, 21, 101294. [Google Scholar] [CrossRef]
- Golovko, O.; Kaczmarek, M.; Asp, H.; Bergstrand, K.-J.; Ahrens, L.; Hultberg, M. Uptake of Perfluoroalkyl Substances, Pharmaceuticals, and Parabens by Oyster Mushrooms (Pleurotus ostreatus) and Exposure Risk in Human Consumption. Chemosphere 2022, 291, 132898. [Google Scholar] [CrossRef]
- Bokade, P.; Purohit, H.J.; Bajaj, A. Myco-Remediation of Chlorinated Pesticides: Insights into Fungal Metabolic System. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Włodarczyk, A.; Lazur, J.; Opoka, W.; Muszyńska, B. The Remediation of Sulfonamides from the Environment by Pleurotus eryngii Mycelium. Efficiency, Products and Mechanisms of Mycodegradation. Chemosphere 2021, 262, 128026. [Google Scholar] [CrossRef]
- Golian, M.; Hegedűsová, A.; Mezeyová, I.; Chlebová, Z.; Hegedűs, O.; Urminská, D.; Vollmannová, A.; Chlebo, P. Accumulation of Selected Metal Elements in Fruiting Bodies of Oyster Mushroom. Foods 2021, 11, 76. [Google Scholar] [CrossRef]
- Akhtar, N.; Mannan, M.A. Mycoremediation: Expunging Environmental Pollutants. Biotechnol. Rep. 2020, 26, e00452. [Google Scholar] [CrossRef]
- Pini, A.K.; Geddes, P. Fungi Are Capable of Mycoremediation of River Water Contaminated by E. coli. Water Air Soil Pollut. 2020, 231, 83. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of Heavy Metals: Processes, Mechanisms, and Affecting Factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef] [PubMed]
- Jureczko, M.; Przystaś, W. Removal of Two Cytostatic Drugs: Bleomycin and Vincristine by White-Rot Fungi—A Sorption Study. J. Environ. Health Sci. Eng. 2021, 19, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Antón-Herrero, R.; Escolástico, C.; Segura, M.L.; Eymar, E. An Assessment of Pleurotus ostreatus to Remove Sulfonamides, and Its Role as a Biofilter Based on Its Own Spent Mushroom Substrate. Environ. Sci. Pollut. Res. 2021, 28, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Chen, P.; Li, H.; Qiao, S.; Wang, J.; Wang, Y.; Wang, X.; Wu, B.; Liu, H.; Wang, C.; et al. Comparative Transcriptome Analysis Reveals the Differential Response to Cadmium Stress of Two Pleurotus Fungi: Pleurotus cornucopiae and Pleurotus ostreatus. J. Hazard. Mater. 2021, 416, 125814. [Google Scholar] [CrossRef] [PubMed]
- Dickson, U.J.; Coffey, M.; George Mortimer, R.J.; Smith, B.; Ray, N.; Di Bonito, M. Investigating the Potential of Sunflower Species, Fermented Palm Wine and Pleurotus ostreatus for Treatment of Petroleum-Contaminated Soil. Chemosphere 2020, 240, 124881. [Google Scholar] [CrossRef]
- Hultberg, M.; Ahrens, L.; Golovko, O. Use of Lignocellulosic Substrate Colonized by Oyster Mushroom (Pleurotus ostreatus) for Removal of Organic Micropollutants from Water. J. Environ. Manag. 2020, 272, 111087. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Mączka, W.; Wińska, K.; Żarowska, B.; Maciejewska, G.; Gębarowska, E.; Jerzy Pietr, S. Antimicrobial Chloro-Hydroxylactones Derived from the Biotransformation of Bicyclic Halolactones by Cultures of Pleurotus ostreatus. Bioorg. Chem. 2020, 104, 104250. [Google Scholar] [CrossRef] [PubMed]
- Maadani Mallak, A.; Lakzian, A.; Khodaverdi, E.; Haghnia, G.H.; Mahmoudi, S. Effect of Pleurotus ostreatus and Trametes Versicolor on Triclosan Biodegradation and Activity of Laccase and Manganese Peroxidase Enzymes. Microb. Pathog. 2020, 149, 104473. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumar, A.; Kumar, S.; Barh, A.; Kamal, S. Substrate Sterilization with Thiophanate-Methyl and Its Biodegradation to Carbendazim in Oyster Mushroom (Pleurotus ostreatus Var. Florida). Environ. Sci. Pollut. Res. 2020, 27, 899–906. [Google Scholar] [CrossRef]
- Šrédlová, K.; Škrob, Z.; Filipová, A.; Mašín, P.; Holecová, J.; Cajthaml, T. Biodegradation of PCBs in Contaminated Water Using Spent Oyster Mushroom Substrate and a Trickle-Bed Bioreactor. Water Res. 2020, 170, 115274. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, B.; Marom, R.; Salton, O.; Oliferovsky, M.; Mordehay, V.; Ben-Ari, J.; Hadar, Y. Transformation of Lamotrigine by White-Rot Fungus Pleurotus ostreatus. Environ. Pollut. 2019, 250, 546–553. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; Amin, H.M.; Shebl, R.I. Modulation of Laccase Transcriptome during Biodegradation of Naphthalene by White Rot Fungus Pleurotus ostreatus. Int. Microbiol. 2019, 22, 217–225. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Q.; Su, Y.; Xue, Q.; Sun, L. Cobalt Speciation and Phytoavailability in Fluvo-Aquic Soil under Treatments of Spent Mushroom Substrate from Pleurotus ostreatus. Environ. Sci. Pollut. Res. 2019, 26, 7486–7496. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, B.; Sun, X.; Yu, L.; Wu, L.; Liu, W.; Wang, D.; Li, Y.; Jia, R.; Yu, H.; et al. Removal and Tolerance Mechanism of Pb by a Filamentous Fungus: A Case Study. Chemosphere 2019, 225, 200–208. [Google Scholar] [CrossRef]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in Mushroom Crops and Its Impact on Yield and Quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo, J.E.; Dias, E.S.; Rinker, D.L.; Caitano, C.E.C.; Zied, D.C. Optimization of Cultivation Techniques Improves the Agronomic Behavior of Agaricus Subrufescens. Sci. Rep. 2020, 10, 8154. [Google Scholar] [CrossRef]
- Prasad, S.; Rathore, H.; Sharma, S.; Tiwari, G. Yield and Proximate Composition of Pleurotus florida Cultivated on Wheat Straw Supplemented with Perennial Grasses. Indian J. Agric. Sci. 2018, 88, 91–94. [Google Scholar]
- Adenipekun, C.O.; Omolaso, P.O. Comparative Study on Cultivation, Yield Performance and Proximate Composition of Pleurotus pulmonarius Fries. (Quelet) on Rice Straw and Banana Leaves. World J. Agric. Sci. 2015, 11, 151–158. [Google Scholar]
- Adedokun, O.M.; Akuma, A.H. Maximizing Agricultural Residues: Nutritional Properties of Straw Mushroom on Maize Husk, Waste Cotton and Plantain Leaves. Nat. Resour. 2013, 4, 534–537. [Google Scholar] [CrossRef]
- Triyono, S.; Haryanto, A.; Telaumbanua, M.; Dermiyati; Lumbanraja, J.; To, F. Cultivation of Straw Mushroom (Volvariella Volvacea) on Oil Palm Empty Fruit Bunch Growth Medium. Int. J. Recycl. Org. Waste Agric. 2019, 8, 381–392. [Google Scholar] [CrossRef]
- Iqbal, B.; Khan, H.; Saifullah; Khan, I.; Shah, B.; Naeem, A.; Ullah, W.; Khan, N.; Adnan, M.; Shah, S.R.A.; et al. Substrates Evaluation for the Quality, Production and Growth of Oyster Mushroom (Pleurotus florida Cetto). J. Entomol. Zool. Stud. 2016, 4, 98–107. [Google Scholar]
- De Souza, D.F.; da Silva, M.D.C.S.; de Paula Alves, M.; Fuentes, D.P.; Porto, L.E.O.; de Oliveira, P.V.; Kasuya, M.C.M.; Eller, M.R. By-Products as Substrates for Production of Selenium-Enriched Pleurotus ostreatus Mushrooms. Waste Biomass Valor. 2022, 13, 989–1001. [Google Scholar] [CrossRef]
- Sardar, A.; Satankar, V.; Jagajanantha, P.; Mageshwaran, V. Effect of Substrates (Cotton Stalks and Cotton Seed Hulls) on Growth, Yield and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus florida). J. Cotton Res. Dev. 2020, 34, 135–145. [Google Scholar]
- Grimm, A.; Eilertsen, L.; Chen, F.; Huang, R.; Atterhem, L.; Xiong, S. Cultivation of Pleurotus ostreatus Mushroom on Substrates Made of Cellulose Fibre Rejects: Product Quality and Spent Substrate Fuel Properties. Waste Biomass Valor. 2021, 12, 4331–4340. [Google Scholar] [CrossRef]
- Apetorgbor, M.M.; Apetorgbor, A.K. Comparative Studies On Yield Of Volvariella Volvacea Using Root And Tuber Peels For Improved Livelihood Of Communities. J. Ghana Sci. Assoc. 2015, 16, 34–43. [Google Scholar]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Ellah, N.H.A.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.-S.; Hetta, H.F.; Singh, M.P. Pleurotus Sajor-Caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Bohara, R.A.; Rai, S.N.; Aleya, L.; Singh, M.P. Two Birds with One Stone: Oyster Mushroom Mediated Bimetallic Au-Pt Nanoparticles for Agro-Waste Management and Anticancer Activity. Environ. Sci. Pollut. Res. 2021, 28, 13761–13775. [Google Scholar] [CrossRef]
- Zárate-Salazar, J.R.; Santos, M.N.; Caballero, E.N.M.; Martins, O.G.; Herrera, Á.A.P. Use of Lignocellulosic Corn and Rice Wastes as Substrates for Oyster Mushroom (Pleurotus ostreatus Jacq.) Cultivation. SN Appl. Sci. 2020, 2, 1904. [Google Scholar] [CrossRef]
- Tesfay, T.; Godifey, T.; Mesfin, R.; Kalayu, G. Evaluation of Waste Paper for Cultivation of Oyster Mushroom (Pleurotus ostreatus) with Some Added Supplementary Materials. AMB Expr. 2020, 10, 15. [Google Scholar] [CrossRef]
- Carrasco-Cabrera, C.P.; Bell, T.L.; Kertesz, M.A. Caffeine Metabolism during Cultivation of Oyster Mushroom (Pleurotus ostreatus) with Spent Coffee Grounds. Appl. Microbiol. Biotechnol. 2019, 103, 5831–5841. [Google Scholar] [CrossRef]
- Seekram, P.; Thammasittirong, A.; Thammasittirong, S.N.-R. Evaluation of Spent Mushroom Substrate after Cultivation of Pleurotus ostreatus as a New Raw Material for Xylooligosaccharides Production Using Crude Xylanases from Aspergillus Flavus KUB2. 3 Biotech 2021, 11, 176. [Google Scholar] [CrossRef]
- Anele, U.Y.; Anike, F.N.; Davis-Mitchell, A.; Isikhuemhen, O.S. Solid-State Fermentation with Pleurotus ostreatus Improves the Nutritive Value of Corn Stover-Kudzu Biomass. Folia Microbiol. 2021, 66, 41–48. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Arapoglou, D.; Chorianopoulos, N.; Markou, G.; Haroutounian, S.A. Conversion of Brewers’ Spent Grain into Proteinaceous Animal Feed Using Solid State Fermentation. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Teixeira, W.F.A.; Batista, R.D.; do Amaral Santos, C.C.A.; Júnior, A.C.F.; Terrasan, C.R.F.; de Santana, M.W.P.R.; de Siqueira, F.G.; de Paula-Elias, F.C.; de Almeida, A.F. Minimal Enzymes Cocktail Development by Filamentous Fungi Consortia in Solid-State Cultivation and Valorization of Pineapple Crown Waste by Enzymatic Saccharification. Waste Biomass Valor. 2021, 12, 2521–2539. [Google Scholar] [CrossRef]
- Zheng, M.; Zuo, S.; Niu, D.; Jiang, D.; Tao, Y.; Xu, C. Effect of Four Species of White Rot Fungi on the Chemical Composition and In Vitro Rumen Degradability of Naked Oat Straw. Waste Biomass Valor. 2021, 12, 435–443. [Google Scholar] [CrossRef]
- Majumder, K.; Paul, B.; Sundas, R. An Analysis of Exo-Polygalacturonase Bioprocess in Submerged and Solid-State Fermentation by Pleurotus ostreatus Using Pomelo Peel Powder as Carbon Source. J. Genet. Eng. Biotechnol. 2020, 18, 47. [Google Scholar] [CrossRef]
- Zamora Zamora, H.D.; Silva, T.A.L.; Varão, L.H.R.; Baffi, M.A.; Pasquini, D. Simultaneous Production of Cellulases, Hemicellulases, and Reducing Sugars by Pleurotus ostreatus Growth in One-Pot Solid State Fermentation Using Alstroemeria sp. Waste. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Atlı, B.; Yamaç, M.; Yıldız, Z.; Şőlener, M. Solid State Fermentation Optimization of Pleurotus ostreatus for Lovastatin Production. Pharm. Chem. J. 2019, 53, 858–864. [Google Scholar] [CrossRef]
- Giacobbe, S.; Piscitelli, A.; Raganati, F.; Lettera, V.; Sannia, G.; Marzocchella, A.; Pezzella, C. Butanol Production from Laccase-Pretreated Brewer’s Spent Grain. Biotechnol. Biofuels 2019, 12, 47. [Google Scholar] [CrossRef]
- Falade, A.O. Valorization of Agricultural Wastes for Production of Biocatalysts of Environmental Significance: Towards a Sustainable Environment. Environ. Sustain. 2021, 4, 317–328. [Google Scholar] [CrossRef]
- Fasiku, S.; Monilola Wakil, S. Pretreatment of Maize Straw with Pleurotus ostreatus and Lentinus Squarrosulus for Bioethanol Production Using Saccharomyces cerevisiae. Nov. Res. Microbiol. J. 2021, 5, 1480–1493. [Google Scholar] [CrossRef]
- Andrade, M.C.; Gorgulho Silva, C.D.O.; de Souza Moreira, L.R.; Ferreira Filho, E.X. Crop Residues: Applications of Lignocellulosic Biomass in the Context of a Biorefinery. Front. Energy 2021. [Google Scholar] [CrossRef]
- Tramontina, R.; Brenelli, L.B.; Sodré, V.; Franco Cairo, J.P.; Travália, B.M.; Egawa, V.Y.; Goldbeck, R.; Squina, F.M. Enzymatic Removal of Inhibitory Compounds from Lignocellulosic Hydrolysates for Biomass to Bioproducts Applications. World J. Microbiol. Biotechnol. 2020, 36, 166. [Google Scholar] [CrossRef]
- Moreira, B.R.D.A.; Viana, R.D.S.; Magalhães, A.C.; Caraschi, J.C.; Zied, D.C.; Dias, E.S.; Rinker, D.L. Production of Pleurotus ostreatus Var. Florida on Briquettes and Recycling Its Spent Substrate as Briquettes for Fuel Grade Biosolids. J. Clean. Prod. 2020, 274, 123919. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.M.; Orellana, L.; Calabi-Floody, M.; González, M.E.; Meier, S.; Borie, F.; Cornejo, P. Influence of Saprophytic Fungi and Inorganic Additives on Enzyme Activities and Chemical Properties of the Biodegradation Process of Wheat Straw for the Production of Organo-Mineral Amendments. J. Environ. Manag. 2020, 255, 109922. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Das, R.; Sangwan, S.; Rohatgi, B.; Khanam, R.; Peera, S.K.P.G.; Das, S.; Lyngdoh, Y.A.; Langyan, S.; Shukla, A.; et al. Utilisation of Agro-Industrial Waste for Sustainable Green Production: A Review. Environ. Sustain. 2021, 4, 619–636. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.-W.; Chang, J.-S.; Yang, F.-C. Recent Advances and Future Directions on the Valorization of Spent Mushroom Substrate (SMS): A Review. Bioresour. Technol. 2022, 344, 126157. [Google Scholar] [CrossRef]
- Mohd Hanafi, F.H.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally Sustainable Applications of Agro-Based Spent Mushroom Substrate (SMS): An Overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Umor, N.A.; Ismail, S.; Abdullah, S.; Huzaifah, M.H.R.; Huzir, N.M.; Mahmood, N.A.N.; Zahrim, A.Y. Zero Waste Management of Spent Mushroom Compost. J. Mater. Cycles Waste Manag. 2021, 23, 1726–1736. [Google Scholar] [CrossRef]
- Vodovnik, M.; Vrabec, K.; Hellwig, P.; Benndorf, D.; Sežun, M.; Gregori, A.; Gottumukkala, L.D.; Anderson, R.C.; Reichl, U. Valorisation of Deinking Sludge as a Substrate for Lignocellulolytic Enzymes Production by Pleurotus ostreatus. J. Clean. Prod. 2018, 197, 253–263. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The Production of Laccases by White-Rot Fungi under Solid-State Fermentation Conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Ligninolytic Enzymes Mediated Ligninolysis: An Untapped Biocatalytic Potential to Deconstruct Lignocellulosic Molecules in a Sustainable Manner. Catal. Lett. 2020, 150, 524–543. [Google Scholar] [CrossRef]
- Brugnari, T.; Braga, D.M.; dos Santos, C.S.A.; Torres, B.H.C.; Modkovski, T.A.; Haminiuk, C.W.I.; Maciel, G.M. Laccases as Green and Versatile Biocatalysts: From Lab to Enzyme Market—An Overview. Bioresour. Bioprocess. 2021, 8, 131. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Bilal, M.; Chandra, R. Extremophilic Ligninolytic Enzymes: Versatile Biocatalytic Tools with Impressive Biotechnological Potential. Catal. Lett. 2021. [Google Scholar] [CrossRef]
- Kunjadia, P.D.; Sanghvi, G.V.; Kunjadia, A.P.; Mukhopadhyay, P.N.; Dave, G.S. Role of Ligninolytic Enzymes of White Rot Fungi (Pleurotus spp.) Grown with Azo Dyes. SpringerPlus 2016, 5, 1487. [Google Scholar] [CrossRef]
- Irshad, A.; Sarwar, N.; Sadia, H.; Riaz, M.; Sharif, S.; Shahid, M.; Khan, J.A. Silver Nano-Particles: Synthesis and Characterization by Using Glucans Extracted from Pleurotus ostreatus. Appl. Nanosci. 2020, 10, 3205–3214. [Google Scholar] [CrossRef]
- Owaid, M.N. Green Synthesis of Silver Nanoparticles by Pleurotus (Oyster Mushroom) and Their Bioactivity: Review. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100256. [Google Scholar] [CrossRef]
- Martínez-Flores, H.E.; Contreras-Chávez, R.; Garnica-Romo, M.G. Effect of Extraction Processes on Bioactive Compounds from Pleurotus ostreatus and Pleurotus Djamor: Their Applications in the Synthesis of Silver Nanoparticles. J. Inorg. Organomet. Polym. 2021, 31, 1406–1418. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of TiO2 Nanoparticles Using Edible Mushroom (Pleurotus Djamor) Extract: Mosquito Larvicidal, Histopathological, Antibacterial and Anticancer Effect. J. Clust. Sci. 2021, 32, 1229–1240. [Google Scholar] [CrossRef]
- Manimaran, K.; Natarajan, D.; Balasubramani, G.; Murugesan, S. Pleurotus Sajor Caju Mediated TiO2 Nanoparticles: A Novel Source for Control of Mosquito Larvae, Human Pathogenic Bacteria and Bone Cancer Cells. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological Applications of Synthesized ZnO Nanoparticles Using Pleurotus Djamor Against Mosquito Larvicidal, Histopathology, Antibacterial, Antioxidant and Anticancer Effect. J. Clust. Sci. 2021, 32, 1635–1647. [Google Scholar] [CrossRef]
- Sargin, I.; Karakurt, S.; Alkan, S.; Arslan, G. Live Cell Imaging With Biocompatible Fluorescent Carbon Quantum Dots Derived From Edible Mushrooms Agaricus Bisporus, Pleurotus ostreatus, and Suillus Luteus. J. Fluoresc. 2021, 31, 1461–1473. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thakur, M.; Tondan, D.; Bamrah, G.K.; Misra, S.; Kumar, P.; Pandohee, J.; Kulshrestha, S. Nanomaterial Conjugated Lignocellulosic Waste: Cost-Effective Production of Sustainable Bioenergy Using Enzymes. 3 Biotech 2021, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, P.M.; de Figueroa, L.I.C.; Pajot, H.F. Dual Purpose of Ligninolytic- Basidiomycetes: Mycoremediation of Bioethanol Distillation Vinasse Coupled to Sustainable Bio-Based Compounds Production. Fungal Biol. Rev. 2020, 34, 25–40. [Google Scholar] [CrossRef]
- Karimi, F.; Mazaheri, D.; Saei Moghaddam, M.; Mataei Moghaddam, A.; Sanati, A.L.; Orooji, Y. Solid-State Fermentation as an Alternative Technology for Cost-Effective Production of Bioethanol as Useful Renewable Energy: A Review. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Naresh Kumar, M.; Ravikumar, R.; Thenmozhi, S.; Ranjith Kumar, M.; Kirupa Shankar, M. Choice of Pretreatment Technology for Sustainable Production of Bioethanol from Lignocellulosic Biomass: Bottle Necks and Recommendations. Waste Biomass Valor. 2019, 10, 1693–1709. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; CFRFerreira, I.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef]
- Ryden, P.; Efthymiou, M.-N.; Tindyebwa, T.A.M.; Elliston, A.; Wilson, D.R.; Waldron, K.W.; Malakar, P.K. Bioethanol Production from Spent Mushroom Compost Derived from Chaff of Millet and Sorghum. Biotechnol. Biofuels 2017, 10, 195. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, H.; Li, X. Multi-Perspective Analyses of Rice Straw Modification by Pleurotus ostreatus and Effects on Biomethane Production. Bioresour. Technol. 2020, 296, 122365. [Google Scholar] [CrossRef]
- Richard, E.N.; Hilonga, A.; Machunda, R.L.; Njau, K.N. Two-Stage Banana Leaves Wastes Utilization towards Mushroom Growth and Biogas Production. 3 Biotech 2020, 10, 542. [Google Scholar] [CrossRef]
- Devi, R.; Kapoor, S.; Thakur, R.; Sharma, E.; Tiwari, R.K.; Joshi, S.J. Lignocellulolytic Enzymes and Bioethanol Production from Spent Biomass of Edible Mushrooms Using Saccharomyces Cerevisiae and Pachysolen Tannophilus. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Sotthisawad, K.; Mahakhan, P.; Vichitphan, K.; Vichitphan, S.; Sawaengkaew, J. Bioconversion of Mushroom Cultivation Waste Materials into Cellulolytic Enzymes and Bioethanol. Arab. J. Sci. Eng. 2017, 42, 2261–2271. [Google Scholar] [CrossRef]
- Sonwani, R.; Gupta, S.B.; Soni, R. Production of Bioethanol from Biodegraded Alkali Pretreated Rice Straw. Vegetos 2020, 33, 128–134. [Google Scholar] [CrossRef]
- Suri, P.; Dwivedi, D.; Rathour, R.K.; Rana, N.; Sharma, V.; Bhatia, R.K.; Bhatt, A.K. Enhanced C-5 Sugar Production from Pine Needle Waste Biomass Using Bacillus sp. XPB-11 Mutant and Its Biotransformation to Bioethanol. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Ramzan, M. Enhanced Bio-Ethanol Production from Old Newspapers Waste Through Alkali and Enzymatic Delignification. Waste Biomass Valor. 2017, 8, 2271–2281. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Simultaneous Saccharification and Fermentation of Ethanol from Potato Waste by Co-Cultures of Aspergillus Niger and Saccharomyces Cerevisiae in Biofilm Reactors. Fuel 2017, 202, 260–270. [Google Scholar] [CrossRef]
- Shankar, K.; Kulkarni, N.S.; Jayalakshmi, S.K.; Sreeramulu, K. Saccharification of the Pretreated Husks of Corn, Peanut and Coffee Cherry by the Lignocellulolytic Enzymes Secreted by Sphingobacterium sp. Ksn for the Production of Bioethanol. Biomass Bioenergy 2019, 127, 105298. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Ravel, M.; Perumal, K. Pretreatment and Process Optimization of Bioethanol Production from Spent Biomass of Ganoderma Lucidum Using Saccharomyces Cerevisiae. Fuel 2021, 306, 121680. [Google Scholar] [CrossRef]
- Holmatov, B.; Schyns, J.F.; Krol, M.S.; Gerbens-Leenes, P.W.; Hoekstra, A.Y. Can Crop Residues Provide Fuel for Future Transport? Limited Global Residue Bioethanol Potentials and Large Associated Land, Water and Carbon Footprints. Renew. Sustain. Energy Rev. 2021, 149, 111417. [Google Scholar] [CrossRef]
- Periyasamy, S.; Karthik, V.; Senthil Kumar, P.; Isabel, J.B.; Temesgen, T.; Hunegnaw, B.M.; Melese, B.B.; Mohamed, B.A.; Vo, D.-V.N. Chemical, Physical and Biological Methods to Convert Lignocellulosic Waste into Value-Added Products. A Review. Environ. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Tyagi, V.K.; Gunasekaran, M.; Karthikeyan, O.P.; Kumar, G. Lignocellulosic Biomass Based Biorefinery: A Successful Platform towards Circular Bioeconomy. Fuel 2021, 302, 121086. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]

| Pleurotus spp. | Moisture (%) | Proteins (%) | Carbohydrates (%) | Fats (%) | Ash (%) | Fiber (%) | Refs. |
|---|---|---|---|---|---|---|---|
| Pleurotus ostreatus | 90.7 | 18.3 | 71.25 | 2.58 | 7.82 | 14.31 | [28] |
| Pleurotus eryngii | 91.0 | 11.9 | 39.85 | 7.50 | 4.89 | 28.29 | [29] |
| Pleurotus eryngii | 88 | 20 | 53 | 2.8 | 7.5 | 7.5 | [30] |
| Pleurotus eryngii | 88 | 18.8 | 57 | 2.3 | 5.5 | 10 | [31] |
| P. citrinopileatus | 88.9 | 30.0 | 42.50 | 3.90 | 7.65 | 20.78 | [32] |
| Pleurotus flabellatus | 91.0 | 21.6 | 57.40 | 1.80 | 10.7 | 11.90 | [33] |
| P. djamor var. roseus | 79.5 | 35.5 | 44.75 | 1.72 | 5.90 | 14.60 | [34] |
| Pleurotus pulmonarius | 78.8 | 20.3 | 34.00 | 2.62 | 7.33 | 9.00 | [35] |
| Pleurotus djamor | 86.8 | 24.1 | 45.59 | 4.73 | 9.84 | 15.91 | [36] |
| Pleurotus tuber-regium | 87.1 | 22.1 | 63.03 | 1.06 | 2.97 | 10.86 | [37] |
| Pleurotus florida | 87.5 | 20.5 | 42.83 | 2.31 | 9.02 | 11.50 | [38] |
| Pleurotus sajor-caju | 87.0 | 24.6 | 39.82 | 2.29 | 8.28 | 10.90 | [39] |
| Pleurotus cystidiosus | 91.1 | 15.6 | 55.92 | 2.05 | 6.30 | 20.05 | [22] |
| Activity | Bioactive Compound | Mode of Action | Refs. |
|---|---|---|---|
| Anti-oxidative | Lectins | The dendritic cells were activated using the pathway of “Toll-like receptor 6 signal” | [42] |
| Polysaccharides | Increasing the activities of SOD, CAT, GST, GR, APx and reducing superoxide radicals, and the activity of GPx | [43] | |
| Phenols | Inhibits the growth of HL-60 cells by inducing apoptosis | [44] | |
| Flavonoids, ascorbic acid and β-carotene | Induces apoptosis by inhibiting HL-60 cell growth | [44] | |
| Vitamin E | Lipid peroxidation is prevented in cell membranes | [43] | |
| Immuno-modulatory | Polysaccharides | The toxicity of cyclophosphamide in mice was decreased due to the immune-modulatory activity | [43] |
| Anti-inflammatory | Polysaccharides (β-glucans) | Methotrexate may have a synergistic effect on the arthritis of rats | [45] |
| Anti-hypercholesterolemic | Statins (lovastatin) | In the cholesterol synthesis pathway, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase is inhibited due to the conversion of enzymes to mevalonic acid | [46] |
| Flavons (chrysin) | Non-enzymatic antioxidant parameters in hypercholesterolemic rats, the blood/serum levels of lipid profile parameters and hepatic marker enzymes decreased | [47] | |
| Anti-cancer and anti-tumor | Polysaccharides | In HeLa cell lines, cytotoxic activity inhibited the development of Ehrlich Tumor and Sarcoma 180 (S-180) | [45,48] |
| Pleuran (β-glucan) | Anti-neoplastic properties of different cells (breast, colorectal and prostate cancers) | [48] | |
| Proteins | In cell line SW 480, therapeutic effects on colorectal cancer and monocytic leukemia by inducing apoptosis | [48] | |
| Lectins | Tumor burden in Sarcoma S180 reduced by 88.4% and hepatoma H-22 by 75.4% in mice; increase in survival time | [45] | |
| Anti-viral and anti-microbial | Laccase | Anti-viral effects against hepatitis C | [48] |
| Ubiquitin-like protein | Anti-viral effects in human immunodeficiency viruses, such as HIV-1 | [45] | |
| Nanoparticles mixed with aqueous extract | Inhibiting the growth of Gram-negative bacteria | [45] | |
| Ribonucleases | Degradation of viral genetic materials to neutralize HIV | [49] | |
| Hepatoprotective | Poly-saccharopeptides | Thioacetamide is alleviated, inducing alterations in inflammation, steatosis, fibrosis and necrosis | [49] |
| Anti-aging | Mushroom powder | Significant bifidogenic and then strong lactogenic effects | [50] |
| Approach or Mechanism | Kinds of Pollutants | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Biosorption | Mainly metal pollutants | Simple process; highly cost-effective way to produce biomass; removes various HMs at the same time without using chemicals | Many adsorbent types are required; reversible sorption of metals on biomass; suffers from the saturation and clogging of reactors; expensive regeneration | [74] |
| Bioaccumulation or precipitation | All pollutants | To remediate wastewater, it is the simplest and cheapest method; very efficient for removing sulfides and metals; non-selective of metals; does not require chemicals | A difficult method to maintain; the key for success by precipitation is the genetic engineering; the oxidation step is required for complex metals | [75] |
| Biotransformation or bioconversion | Agro-industrial wastes by biological catalysts | It is a time-saving technology and has low operational control; produces biodegradable compounds by green chemistry | Depending on enzymes (high cost), biocatalysts require narrow operation parameters, which are susceptible to the inhibition of products or substrates | [76] |
| Biodegradation | Pollutants from human activities | High reduction pollutant rate; can be used in entirely polluted areas depending upon its characteristics; economically viable; can clean-up with time | Removing other beneficial elements during the natural attenuation of pollutants, the mobility and toxicity of pollutants may be too high; monitoring and groundwater controls are required | [77] |
| Pollutant Details | Growth Conditions | The Main Findings or the Mechanism | Refs. |
|---|---|---|---|
| Decabromo-diphenyl ethane (5, 20 mg L−1) | Biodegradation after 120 h | Biodegradation of pollutants by enzyme P450, manganese peroxidase, lignin peroxidase, and laccase | [21] |
| Cytostatic drugs include vincristine and bleomycin (5, 10 and 15 mg L−1) | Cultivated in liquid medium for 30 days before the test | Studied drugs as anticancer treatments can be removed by biosorption on fungal biomass during wastewater treatment | [87] |
| Sulfonamide antibiotics (0.1 mM) | Biodegradation after 14 d in polluted wastewater | Mushrooms as biofilters removed sulfonamides by up to 83–91% of the applied doses over 14 d from polluted wastewater | [88] |
| Cadmium at doses ranging from 0.5 to 20 mg L−1 Cd | Removal rate up to 54.6% for 7 days | Cd detoxification pathways included 26 enzymes, including catalase, superoxide dismutase, and peroxisomal enzymes | [89] |
| Petroleum hydrocarbons in soils (339 g kg−1 dry weight) for 90 days | Mushroom spawn (10 g) added to pot (1.5 kg soil) | Myco-remediation efficiency was 85% from polluted soil, depending on the type of substrates and application method | [90] |
| Organic micro-pollutants, such as diclofenac and bicalutamide | Substrate content was 200 g L−1 during 14 and 36 d | Removal efficiency of bicalutamide, lamotrigine, and metformin was 43%, 73%, and 59%, respectively, from water | [91] |
| Chloro-hydroxyl-actones | Culture medium for 72 h | Mushroom bio-transformed bicyclic halolactones to chlorolactones | [92] |
| Triclosan (5, 10, 20, 30, and 50 mg L−1) | Biodegradation at 4, 7, and 10 days in liquid medium | Complete biodegradation within the first day of sampling through manganese peroxidase and laccase activity | [93] |
| Pesticide of carbendazim residue (up to 25 days) using wheat straw | Biodegradable in spawned bags (at 22–26 °C) | Mushroom can bioremediate both thiophanate-methyl (up to 60 ppm) and fungicides with a similar chemistry | [94] |
| Polychlorinated biphenyls (PCBs at 0.1–1.0 µg L−1) | Contaminated groundwater for 30–71 days | Spent oyster substrate degraded PCBs and aerobic and/or anaerobic bacteria (87 %) | [95] |
| Lamotrigine, C9H7Cl2N5 (100 mg L−1) | Transformation on culture medium within 20 days | Oxidation of cytochrome P450, where, after 10 days, ~50% of the pollutant was removed | [96] |
| Polycyclic aromatic hydrocarbons (50 mg L−1) | Biodegradative effect up to 14 d in liquid medium | Naphthalene was completely degraded within 5 days (86.47%) by laccase or dioxygenase and ligninolytic enzymes | [97] |
| Applied cobalt (Co) of up to 20 mg kg−1 to the soil | Spent mushroom substrate for 30 d in fluvo-aquic soil | Mushroom reduced Co phyto-availability if added to cultivated soil at a range of 8.86 to 9.51 g kg−1 with pakchois plants | [98] |
| Lead (Pb) from liquid media | Removal rate of Pb was 53.7% | Mushroom removed Pb by biosorption, precipitation, and bioaccumulation | [99] |
| Agro-Industrial Wastes | Biological Efficiency (%) | Crude Proteins (%) | Carbohydrates (%) | Fats (%) | Fiber (%) | Ash (%) | Refs. |
|---|---|---|---|---|---|---|---|
| I. Applied individual agro-industrial waste | |||||||
| Wheat straw | 37.6 | 13.6 | 60.5 | 2.3 | 22.7 | 10.3 | [102] |
| Barley straw | 21.3 | 12.8 | 54.7 | 29.9 | 0.90 | 1.2 | [103] |
| Rice straw | 55.6 | 17.9 | 56.4 | 8.4 | 4.30 | 9.6 | [104] |
| Maize cob | 46.4 | 23.4 | 50.8 | 3.1 | 22.0 | 7.6 | [105] |
| Soya stalk | 85.2 | 24.7 | 53.2 | 2.8 | 7.2 | 6.7 | [104] |
| Cotton stalk | 44.3 | 30.1 | 40.2 | 2.1 | 17.2 | 8.4 | [106] |
| Cotton seed hull | 8.9 | 17.5 | 65.9 | 1.2 | 10.2 | 5.2 | [107] |
| Rice husk | 9.5 | 5.9 | 48.5 | 30.9 | 0.3 | 14.3 | [103] |
| Sugarcane bagasse | 65.7 | 27.1 | 34.9 | 2.0 | 29.3 | 6.7 | [105] |
| Sugarcane bagasse | 52.3 | 17.1 | - | 1.18 | 12.1 | 4.5 | [108] |
| Cassava peel | 25.1 | 10.6 | 73.7 | 2.2 | 8.7 | 7.6 | [109] |
| Acacia sawdust | 46.4 | 19.5 | 51.3 | 1.3 | 22.0 | 5.9 | [105] |
| Beech sawdust | 46.8 | 16.1 | 73.6 | 3.5 | 15.8 | 6.2 | [102] |
| Birch sawdust | 42.5 | 21.0 | 67.6 | 1.0 | 6.4 | 6.4 | [110] |
| II. Applied combined agro-industrial wastes | |||||||
| Soya stalk + rice straw | 81.7 | 23.0 | 50.5 | 2.7 | 7.7 | 6.4 | [111] |
| Soya stalk + wheat straw | 77.7 | 21.1 | 52.0 | 2.6 | 7.4 | 6.2 | [111] |
| Wheat and rice straw | 71.8 | 20.3 | 56.0 | 2.6 | 7.5 | 5.9 | [111] |
| Cotton stalk + cottonseed hull | 20.2 | 22.8 | 58.0 | 2.9 | 10.8 | 5.5 | [107] |
| Acacia sawdust + maize cob | 58.8 | 18.7 | 46.9 | 3.3 | 24.5 | 6.7 | [105] |
| Acacia sawdust + sugarcane bagasse | 58.9 | 24.2 | 37.8 | 2.5 | 28.8 | 6.7 | [105] |
| Wheat straw + olive pruning residues | 56.8 | 19.9 | 71.7 | 1.9 | 16.5 | 6.5 | [111] |
| Cassava peel + maize cobs | 32.4 | 10.7 | 73.8 | 2.2 | 8.7 | 7.6 | [109] |
| Media | Fermentation Conditions and Its Purpose | Substrate (Source of Carbon) | Refs. |
|---|---|---|---|
| Malt extract agar (up 36 days) 26 °C, RH 75% | Production of laccases and endoglucanases were recorded for oat straw, rice bark, and poplar wood sawdust (26–51 days) | Oat straw, rice bark, poplar wood sawdust, olive pulp, and wheat straw | [12] |
| Malt extract agar (up to 36 days) 25 °C, RH 80% | Rice bark presented the highest productivity, with the highest biological efficiency > 70% (during a cropping period of 51 days) | Rice bark, wheat straw, coffee residue, barley and oat straw | [11] |
| Potato dextrose agar for 5 days | Incubated at 25 °C and sampled after 77 days for improved ruminant animal feed | Maize stover and kudzu (Pueraria montana) | [118] |
| Potato dextrose agar for 7 days | Incubated at 27 °C for 15 days to produce phenolics and flavonoids | Cocoa pod husk and kolanut pod | [61] |
| Fungi stock culture for 12 days | Inoculation at 25°C and 60% humidity for 12 d for proteinaceous animal feed | Brewer’s spent grain | [119] |
| Bacteriological agar for 5 days | Solid culture media at 30 °C, carbon source (sugarcane bagasse), to produce cellulases, pectinases, and xylanase | Pineapple wastes (fruits, leaves, and stalks) | [120] |
| Potato dextrose agar for 28 days | Inoculated straw with fungus at 28 °C, humidity 80% for rumen degradability | Naked oat straw | [121] |
| Potato extract for 5 days | Produce exopoly-galacturonases after 7 or 10 days at 30 °C | Peel of pomelo (Citrus maxima) | [122] |
| Potato dextrose agar for 8 days | Production at 28–32 °C of cellulases, hemi-cellulases, and reducing sugars | Leaf-and-stem mixture of Alstroemeria sp. | [123] |
| Potato dextrose broth for 6 days | Incubated at 28 °C to produce lovastatin at a rate of 34.97 mg g−1 | Barley, wheat bran, rice husk, and oat grains | [124] |
| Culture media (50 °C for 72 h) | Butanol production through treatment by laccase (saccharification yield 99%) | Brewer’s spent grain | [125] |
| Fungi Species | Fermentation Conditions | Biofuel Production | Refs. |
|---|---|---|---|
| Pleurotus florida | Cotton-spinning waste mixture using solid-state cultivation for 14 d; hydrolysis at 32 °C for 72 h | Ethanol at 1.18 g L−1 (64 % at 60 h) | [19] |
| Pleurotus ostreatus | Mushroom compost derived from millet and sorghum produced ethanol by saccharification and fermentation (applied substrate at 5–30% w/v) | Ethanol at 45.8 g L−1 dry weight (70%) | [155] |
| Pleurotus ostreatus | Rice straw was biodegraded by ligninolytic enzymes (cellulase and xylanase) up to 45 days | Biomethane yield 269 mL·g−1 (at 25 d) | [156] |
| Pleurotus ostreatus | Using 181 g of mushrooms per wet 2 kg waste of banana leaves with a biological efficiency of 37% | Biogas yield (282 mL g−1 VS−1) | [157] |
| Pachysolen tannophilus | Spent mushroom substrate of Agaricus bisporus was enzymatic hydrolysis (30 °C after 48 h) incubation | Ethanol at 6.41 g L−1 (76.13%) | [156] |
| Saccharomyces cerevisiae | Spent mushroom substrate of Pleurotus forida was enzymatic hydrolysis (30 °C after 48 h) incubation | Ethanol yield was 5.8 g L−1 (58.12%) | [158] |
| Saccharomyces cerevisiae | Cultivation wastes of Aspergillus tubingensis, which produced cellulolytic enzymes (at 30 °C for 10 days) | Ethanol yield was 17.3 g L−1 (48 h) | [159] |
| Saccharomyces cerevisiae | Rice straw was biodegraded by Trichoderma reesei and mushroom for 7 days and incubated at 32 °C | Bioethanol produced by S. cerevisiae | [160] |
| Kluyveromyces marxianus | Pine needle wastes were catalyzed by xylanase from Bacillus sp., fermented by fungi (at 40 °C for 96 h) | Bioethanol was 5.34 g L−1 (3.89% yield) | [161] |
| Ganoderma lucidum | Substrate of old newspapers was alkali (4% NaOH) or enzymatic fermented using Trichoderma harzianum (30 °C for 5 d) | Ethanol production: 17.8 and 20.4 g L−1, respectively, for 2 methods | [162] |
| Aspergillus niger and Saccharomyces cerevisiae | Rice straw was pretreated (4% NaOH; incubated 40 °C for 24 h), saccharified, and fermented at 37 °C by S. cerevisiae | Ethanol yield was 31.9 g L−1 after the incubation | [163] |
| Aspergillus niger and Saccharomyces cerevisiae | Potato wastes were pretreated, saccharified, and fermented at pH 5.8, 35 °C, and no-aeration by co-cultures of A. niger and S. cerevisiae | Ethanol yield was 37.93 g L−1 | [163] |
| Saccharomyces cerevisiae | Maize husks, peanut husks, and husks of coffee cherry were pretreated using 3 methods (acid or H2SO4, alkaline or NaOH and steam) | Ethanol yield was 20.61, 18.21 and 6.86 g L−1, respectively, for each one | [164] |
| Ganoderma lucidum | Spent substrate of G. lucidum was acid-pretreated by H2SO4 using Saccharomyces cerevisiae (30 °C for 5 d) | Ethanol yield was 0.91 g L−1 | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. https://doi.org/10.3390/su14063667
El-Ramady H, Abdalla N, Fawzy Z, Badgar K, Llanaj X, Törős G, Hajdú P, Eid Y, Prokisch J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability. 2022; 14(6):3667. https://doi.org/10.3390/su14063667
Chicago/Turabian StyleEl-Ramady, Hassan, Neama Abdalla, Zakaria Fawzy, Khandsuren Badgar, Xhensila Llanaj, Gréta Törős, Peter Hajdú, Yahya Eid, and József Prokisch. 2022. "Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation" Sustainability 14, no. 6: 3667. https://doi.org/10.3390/su14063667
APA StyleEl-Ramady, H., Abdalla, N., Fawzy, Z., Badgar, K., Llanaj, X., Törős, G., Hajdú, P., Eid, Y., & Prokisch, J. (2022). Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability, 14(6), 3667. https://doi.org/10.3390/su14063667











