Effect of Nitrogen Fertilizer and Biochar on Organic Matter Mineralization and Carbon Accretion in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Site and Soils
2.2. Experimental Design and Treatments
2.3. Collection of Soil Sample for Analysis
2.4. Statistical Analysis
3. Results
3.1. Carbon Reduction Trend and Degradation Rate Constant
3.2. Available Soil N
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K. Role of Soil Organic Matter in Maintaining Sustainability of Cropping Systems. Commun. Soil Sci. Plant Anal. 2012, 43, 2063–2113. [Google Scholar] [CrossRef]
- Rahman, M.M. Nutrient-Use and Carbon-Sequestration Efficiencies in Soils from Different Organic Wastes in Rice and Tomato Cultivation. Commun. Soil Sci. Plant Anal. 2013, 44, 1457–1471. [Google Scholar] [CrossRef]
- Rahman, F.; Rahman, M.; Rahman, G.M.; Saleque, M.; Hossain, A.S.; Miah, G. Effect of organic and inorganic fertilizers and rice straw on carbon sequestration and soil fertility under a rice–rice cropping pattern. Carbon Manag. 2016, 7, 41–53. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; Coleman, K. Soil organic matter: Its importance in sustainable agriculture and carbon dioxide fluxes. Adv. Agron. 2009, 101, 1–57. [Google Scholar]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Gnanavelrajah, N.; Shrestha, R.P.; Schmidt-Vogt, D.; Samarakoon, L. Carbon stock assessment and soil carbon management in agricultural land-uses in Thailand. Land Degrad. Dev. 2008, 19, 242–256. [Google Scholar] [CrossRef]
- Chapin, I.F.S.; Chapin, M.C.; Matson, P.A.; Vitousek, P. Principles of Terrestrial Ecosystem Ecology; Springer: Berlin, Germany, 2011. [Google Scholar]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The Global Stoichiometry of Litter Nitrogen Mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Majumder, B.; Bandyopadhyay, P.K.; Hazra, G.C.; Gangopadhyay, A.; Samantaray, R.N.; Mishra, A.K.; Chaudhury, J.; Saha, M.N.; Kundu, S. The potential of cropping systems and soil amendments for carbon sequestration in soils under long-term experiments in subtropical India. Glob. Change Biol. 2007, 13, 357–369. [Google Scholar] [CrossRef]
- Rahman, M.M. Carbon and nitrogen dynamics and carbon sequestration in soils under different residue management. Agriculture 2014, 12, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M. Carbon sequestration options in soils under different crops and their management practices. Agriculture 2010, 8, 90–101. [Google Scholar]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. Sustain. Agric. 2011, 2, 761–786. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, Sustainable Agriculture and Food Security: A Review in Sustainable Agriculture; Springer Science and Business Media LLC.: Berlin, Germany, 2009; pp. 635–651. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Fertilizer Recommendation Guide (FRG); Bangladesh Agricultural Research Council (BARC) Farmgate: Dhaka, Bangladesh, 2018; p. 274.
- Withers, P.J.A.; Neal, C.; Jarvie, H.P.; Doody, D.G. Agriculture and Eutrophication: Where Do We Go from Here? Sustainability 2014, 6, 5853–5875. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.; Rahman, M.; Biswas, J.C.; Akhter, S.; Maniruzzaman, M.; Choudhury, A.K.; Jahan, M.A.H.S.; Miah, M.U.; Sen, R.; Kamal, M.Z.U.; et al. Nitrogen transformation and carbon sequestration in wetland paddy field of Bangladesh. Paddy Water Environ. 2019, 17, 677–688. [Google Scholar] [CrossRef]
- Fisk, M.; Santangelo, S.; Minick, K. Carbon mineralization is promoted by phosphorus and reduced by nitrogen ad-dition in the organic horizon of northern hardwood forests. Soil Biol. Biochem. 2015, 81, 212–218. [Google Scholar] [CrossRef]
- Das, D.; Dwivedi, B.S.; Meena, M.C.; Singh, V.K.; Tiwari, K.N. Integrated nutrient management for improving soil health and crop productivity. Indian J. Fertil. 2015, 11, 64–83. [Google Scholar]
- Carbon and Nitrogen Cycling in Soil in Carbon and Nitrogen Cycling in Soil; Springer Science and Business Media LLC.: Berlin, Germany, 2020; pp. 45–85.
- Wu, P.; Wang, Z.; Wang, H.; Bolan, N.S.; Wang, Y.; Chen, W. Visualizing the emerging trends of biochar research and applications in 2019: A scientometric analysis and review. Biochar 2020, 2, 135–150. [Google Scholar] [CrossRef]
- Zimmerman, A.R. Abiotic and Microbial Oxidation of Laboratory-Produced Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, W. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Michael, P.S. Cogon grass biochar amendment and Panicum coloratum planting improve selected properties of sandy soil under humid lowland tropical climatic conditions. Biochar 2020, 2, 489–502. [Google Scholar] [CrossRef]
- Brammer, H.; Antoine, J.; Kassam, A.H.; van Velthuizen, H.T. Land Resources Appraisal of Bangladesh for Agricultural Development in Report 3, Land Resources Data Base, Volume II; Soil, Landform and Hydrological Data Base; UNDP/FAO: Rome, Italy, 1988. [Google Scholar]
- Walkley, A.C.; Black, T.A. Estimation of soil organic carbon by chromic acid titration method. Soil Sci. 1935, 47, 29–38. [Google Scholar]
- Maynard, D.G.; Kalra, Y.P.; CruWAaugh, J.A. Nitrate and Exchangeable Ammonium Nitrogen in Soil Sampling and Methods of Analysis; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 1993. [Google Scholar]
- Hossain, M.B.; Rahman, M.M.; Biswas, J.C.; Miah, M.M.U.; Akhter, S.; Maniruzzaman, M.; Choudhury, A.K.; Ahmed, F.; Shiragi, M.H.K.; Kalra, N. Carbon mineralization and carbon dioxide emission from organic matter added soil under different temperature regimes. Int. J. Recycl. Org. Waste Agric. 2017, 6, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen mineralization from organic residues: Research opportunities. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Nurliyana, M.; H’Ng, P.; Rasmina, H.; Kalsom, M.U.; Chin, K.L.; Lee, S.H.; Lum, W.; Khoo, G. Effect of C/N ratio in methane productivity and biodegradability during facultative co-digestion of palm oil mill effluent and empty fruit bunch. Ind. Crop. Prod. 2015, 76, 409–415. [Google Scholar] [CrossRef]
- Rahman, M. Carbon Dioxide Emission from Soil. Agric. Res. 2013, 2, 132–139. [Google Scholar] [CrossRef] [Green Version]
- NI, J.-Q.; Heber, A.; Hanni, S.; Lim, T.; Diehl, C. Characteristics of ammonia and carbon dioxide releases from layer hen manure. Br. Poult. Sci. 2010, 51, 326–334. [Google Scholar] [CrossRef]
- Rastogi, M.; Singh, S.; Pathak, H. Emission of carbon dioxide from soil. Curr. Sci. 2002, 82, 510–517. [Google Scholar]
- Russell, A.E.; Larid, D.A.; Parkin, T.B.; Mallarino, A.P. Impact of nitrogen fertilization and cropping system on carbon sequestration in Midwestern Mollisols. Soil Sci. Soc. Am. J. 2005, 69, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Kuzyakov, Y.; Friedel, J.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Nielsen, S.; Minchin, T.; Kimber, S.; Van Zwieten, L.; Gilbert, J.; Munroe, P.; Joseph, S.; Thomas, T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric. Ecosyst. Environ. 2014, 191, 73–82. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K. Effects of biochars generated from crop residues on chemical properties of acid soils from tropical and subtropical China. Soil Res. 2012, 50, 570–578. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.; Kunhikrishnan, A.; Choppala, G.; Thangarajan, R.; Chung, J. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R.R.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
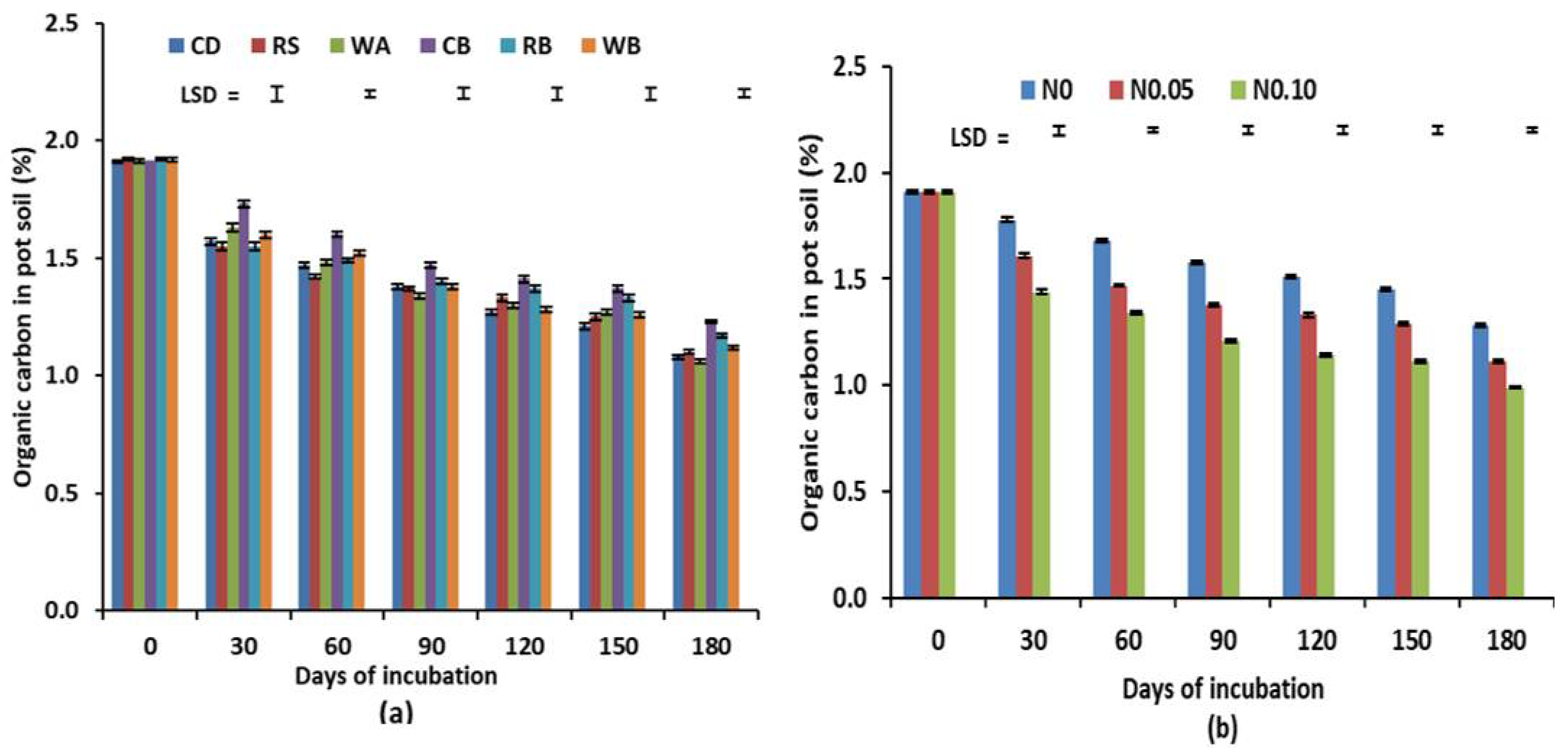
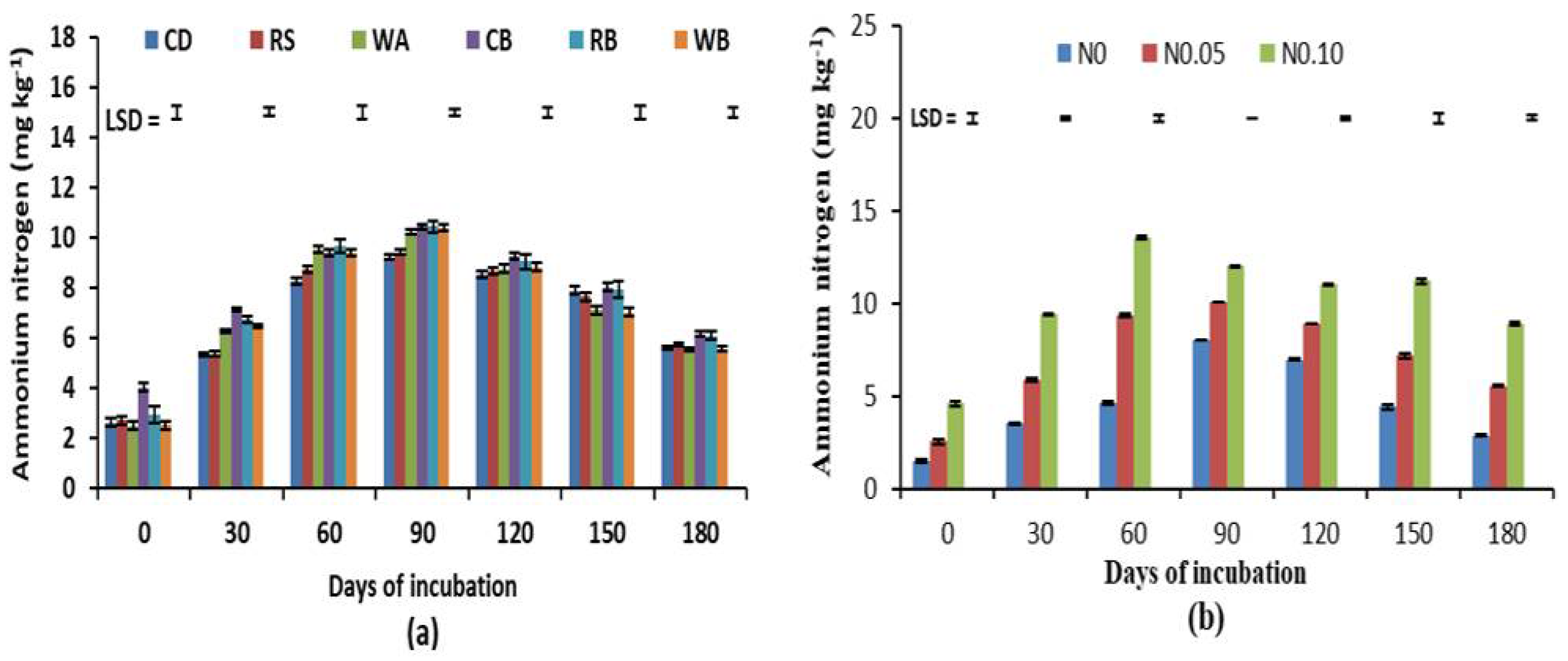
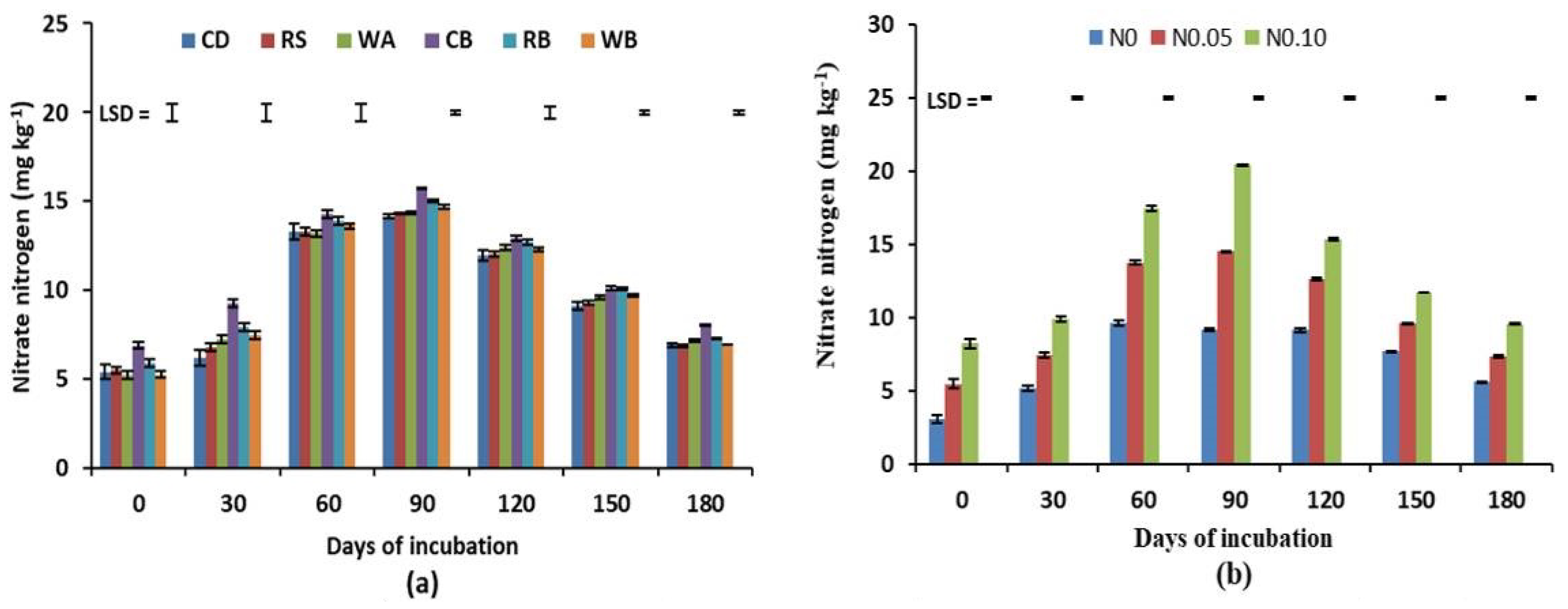
| Organic Materials | Moisture Content (%) | Total N (%) | Organic C (%) | C:N Ratio |
|---|---|---|---|---|
| Field soil | 27.59 ± 2.12 | 0.120 ± 0.01 | 1.68 ± 0.04 | 13.92 ± 0.37 |
| Cow dung | 29.58 ± 0.10 | 1.10 ± 0.10 | 25.82 ± 0.28 | 23.47 ± 0.63 |
| Rice straw | 16.38 ± 0.05 | 0.85 ± 0.08 | 40.00 ± 0.41 | 47.06 ± 0.03 |
| Wood ash | 16.91 ± 0.19 | 0.70 ± 0.11 | 20.40 ± 0.84 | 29.14 ± 0.06 |
| Cow dung biochar | 15.22 ± 0.40 | 0.95 ± 0.13 | 30.50 ± 0.67 | 32.11 ± 0.03 |
| Rice straw biochar | 14.35 ± 0.09 | 0.80 ± 0.08 | 45.50 ± 0.89 | 56.88 ± 0.81 |
| Wood biochar | 15.84 ± 0.04 | 0.75 ± 0.02 | 28.50 ± 0.85 | 38.00 ± 0.39 |
| Organic Materials | Nitrogen Fertilizer (g kg−1 Soil) | ||
|---|---|---|---|
| 0 | 0.05 | 0.10 | |
| 60 days | |||
| CD | 1.63 aa | 1.43 bc | 1.36 cb |
| RS | 1.66 aa | 1.45 bc | 1.15 cc |
| WA | 1.65 aa | 1.43 bc | 1.36 cb |
| CB | 1.71 aa | 1.61 ba | 1.51 ca |
| RB | 1.68 aa | 1.41 bc | 1.38 cb |
| WB | 1.73 aa | 1.51 bb | 1.32 cb |
| 90 days | |||
| CD | 1.52 ab | 1.41 bb | 1.22 ca |
| RS | 1.57 ab | 1.41 bb | 1.16 ca |
| WA | 1.51 ab | 1.32 bb | 1.21 ca |
| CB | 1.66 aa | 1.43 ba | 1.25 ca |
| RB | 1.58 ab | 1.36 bb | 1.25 ca |
| WB | 1.58 ab | 1.38 bb | 1.18 ca |
| 120 days | |||
| CD | 1.41 ab | 1.25 ba | 1.16 cb |
| RS | 1.51 aa | 1.34 ba | 1.16 cb |
| WA | 1.53 aa | 1.36 ba | 1.01 cc |
| CB | 1.58 aa | 1.42 ba | 1.24 ca |
| RB | 1.51 aa | 1.36 ba | 1.25 ca |
| WB | 1.55 aa | 1.27 ba | 1.04 cc |
| 150 days | |||
| CD | 1.31 ab | 1.21 ba | 1.12 ca |
| RS | 1.41 aa | 1.29 ba | 1.06 ca |
| WA | 1.46 aa | 1.36 ba | 1.01 ca |
| CB | 1.53 aa | 1.36 ba | 1.22 ca |
| RB | 1.48 aa | 1.31 ba | 1.19 ca |
| WB | 1.52 aa | 1.23 ba | 1.04 ca |
| 180 days | |||
| CD | 1.25 ab | 1.08 bc | 0.91 cc |
| RS | 1.27 ab | 1.05 bc | 0.99 cb |
| WA | 1.25 ab | 1.06 bc | 0.89 cc |
| CB | 1.34 aa | 1.23 ba | 1.12 ca |
| RB | 1.34 aa | 1.16 bb | 1.00 cb |
| WB | 1.27 ab | 1.10 bc | 1.01 cb |
| Organic Materials and N Rates | Carbon Degradation Rate Constants (k) |
|---|---|
| Organic materials: | |
| Cow dung | 0.00463 c |
| Rice straw | 0.00473 c |
| Wood ash | 0.00448 d |
| Cow dung biochar | 0.00571 a |
| Rice straw biochar | 0.00522 b |
| Wood biochar | 0.00487 bc |
| S. E. (±) | 0.000238 |
| N rates (g/kg soil): | |
| 0 | 0.00616 a |
| 0.05 | 0.00484 b |
| 0.1 | 0.00405 c |
| S.E. (±) | 0.000168 |
| Organic × nitrogen | ns |
| CV% | 7.26 |
| Organic Materials | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | ||||
|---|---|---|---|---|---|---|
| N0 | N0.05 | N0.1 | N0 | N0.05 | N0.1 | |
| 90 days | ||||||
| CD | 7.12 cb | 9.32 bb | 11.21 aa | 8.44 ac | 13.91 bc | 20.11 cb |
| RS | 7.43 cb | 10.27 ba | 11.47 aa | 8.39 ac | 14.21 bb | 20.31 cb |
| WA | 8.38 ca | 10.27 ba | 12.03 aa | 8.41 ac | 14.22 bb | 20.46 cb |
| CB | 8.47 ca | 10.44 ba | 12.44 aa | 10.31 aa | 15.38 ba | 21.50 ca |
| RB | 8.46 ca | 10.42 ba | 12.37 aa | 10.19 aa | 14.92 ba | 19.91 cb |
| WB | 8.34 ca | 10.46 ba | 12.37 aa | 9.23 ab | 14.43 bb | 20.34 cb |
| 120 days | ||||||
| CD | 3.05 ca | 5.39 ba | 8.42 aa | 5.32 cb | 6.61 bc | 8.83 ac |
| RS | 3.33 ca | 5.45 ba | 8.42 aa | 5.23 cb | 6.56 bc | 8.88 ac |
| WA | 2.93 ca | 4.99 ba | 8.69 aa | 5.12 cb | 7.14 bb | 9.23 ab |
| CB | 3.73 ca | 5.83 ba | 8.92 aa | 7.81 ca | 9.88 ba | 11.43 aa |
| RB | 2.57 ca | 6.31 ba | 9.37 aa | 5.27 cb | 7.21 bb | 9.45 ab |
| WB | 2.73 ca | 4.78 ba | 9.16 aa | 5.04 cb | 6.58 bc | 9.43 ab |
| 150 days | ||||||
| CD | 4.94 ca | 8.06 ba | 10.66 aa | 7.04 cc | 9.69 ba | 11.21 ac |
| RS | 3.15 ca | 7.21 ba | 12.61 aa | 7.43 cb | 9.21 bb | 11.24 ac |
| WA | 3.48 ca | 6.43 ba | 11.43 aa | 7.61 cb | 9.69 ba | 11.57 ab |
| CB | 5.27 ca | 7.99 ba | 10.84 aa | 8.29 ca | 9.67 ba | 12.33 aa |
| RB | 5.22 ca | 7.07 ba | 11.55 aa | 7.92 cb | 9.21 bb | 12.23 aa |
| WB | 4.42 ca | 6.46 ba | 10.21 aa | 7.72 cb | 9.68 ba | 11.72 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasnat, M.; Alam, M.A.; Khanam, M.; Binte, B.I.; Kabir, M.H.; Alam, M.S.; Kamal, M.Z.U.; Rahman, G.K.M.M.; Haque, M.M.; Rahman, M.M. Effect of Nitrogen Fertilizer and Biochar on Organic Matter Mineralization and Carbon Accretion in Soil. Sustainability 2022, 14, 3684. https://doi.org/10.3390/su14063684
Hasnat M, Alam MA, Khanam M, Binte BI, Kabir MH, Alam MS, Kamal MZU, Rahman GKMM, Haque MM, Rahman MM. Effect of Nitrogen Fertilizer and Biochar on Organic Matter Mineralization and Carbon Accretion in Soil. Sustainability. 2022; 14(6):3684. https://doi.org/10.3390/su14063684
Chicago/Turabian StyleHasnat, Momtahina, Mohammad Ashraful Alam, Mariam Khanam, Bushra Islam Binte, Mohammad Humayun Kabir, Mohammad Saiful Alam, Mohammed Zia Uddin Kamal, Golum Kibria Muhammad Mustafizur Rahman, Mohammad Manjurul Haque, and Mohammad Mizanur Rahman. 2022. "Effect of Nitrogen Fertilizer and Biochar on Organic Matter Mineralization and Carbon Accretion in Soil" Sustainability 14, no. 6: 3684. https://doi.org/10.3390/su14063684
APA StyleHasnat, M., Alam, M. A., Khanam, M., Binte, B. I., Kabir, M. H., Alam, M. S., Kamal, M. Z. U., Rahman, G. K. M. M., Haque, M. M., & Rahman, M. M. (2022). Effect of Nitrogen Fertilizer and Biochar on Organic Matter Mineralization and Carbon Accretion in Soil. Sustainability, 14(6), 3684. https://doi.org/10.3390/su14063684









