Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes
Abstract
:1. Introduction
2. Materials and Methods
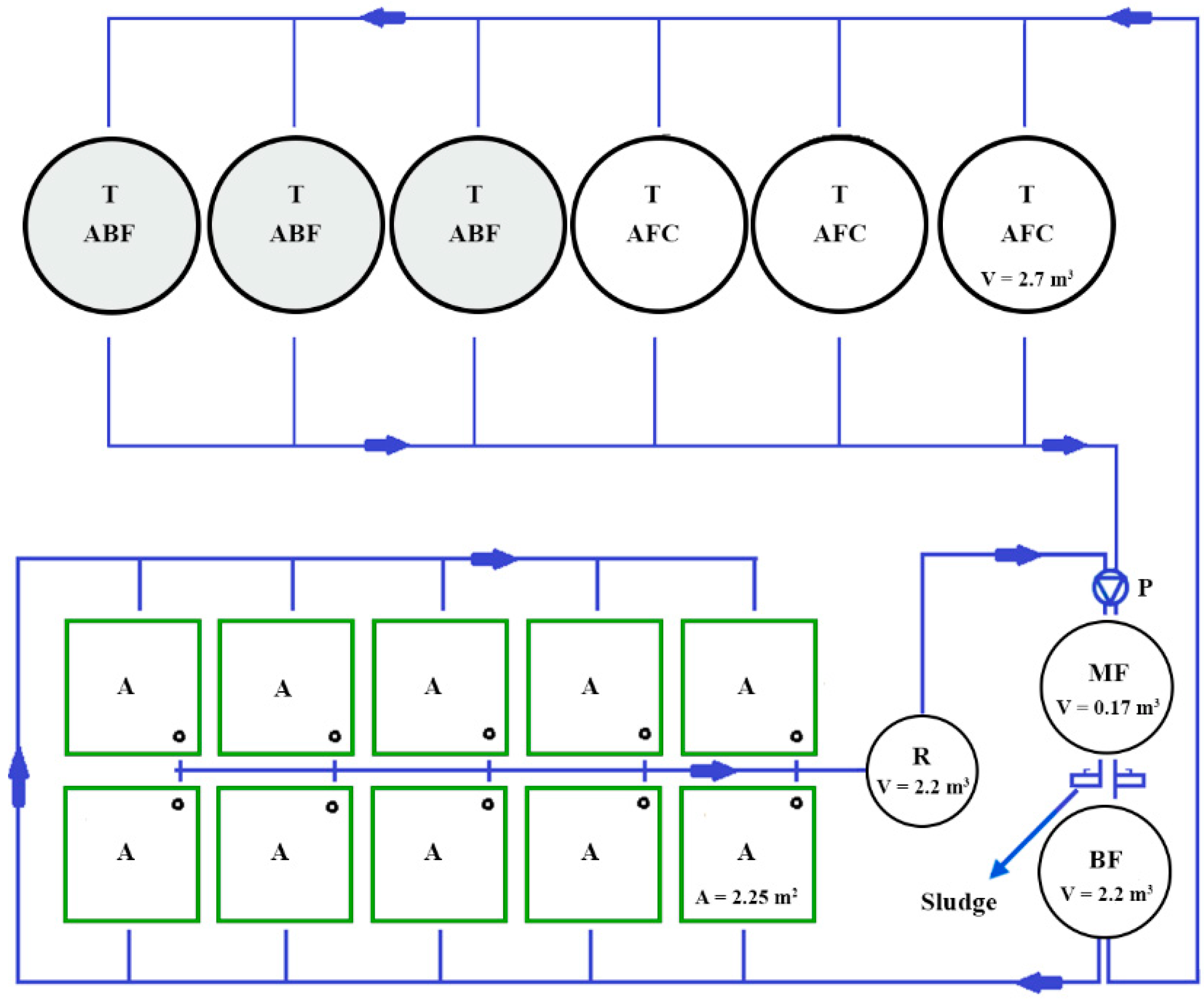
2.1. The Experimental Trial and Fish
2.2. Sample Collection
- FC—feed consumed (g);
- WG—weight gain (g);
- FN—final number of individuals;
- IN—initial number of individuals;
- W—fish weight (g);
- L—fish length (cm).
2.3. Total RNA Extraction and Synthesis of the cDNA
2.4. Assessment of Gene Expression in Gills, Intestine and Kidney
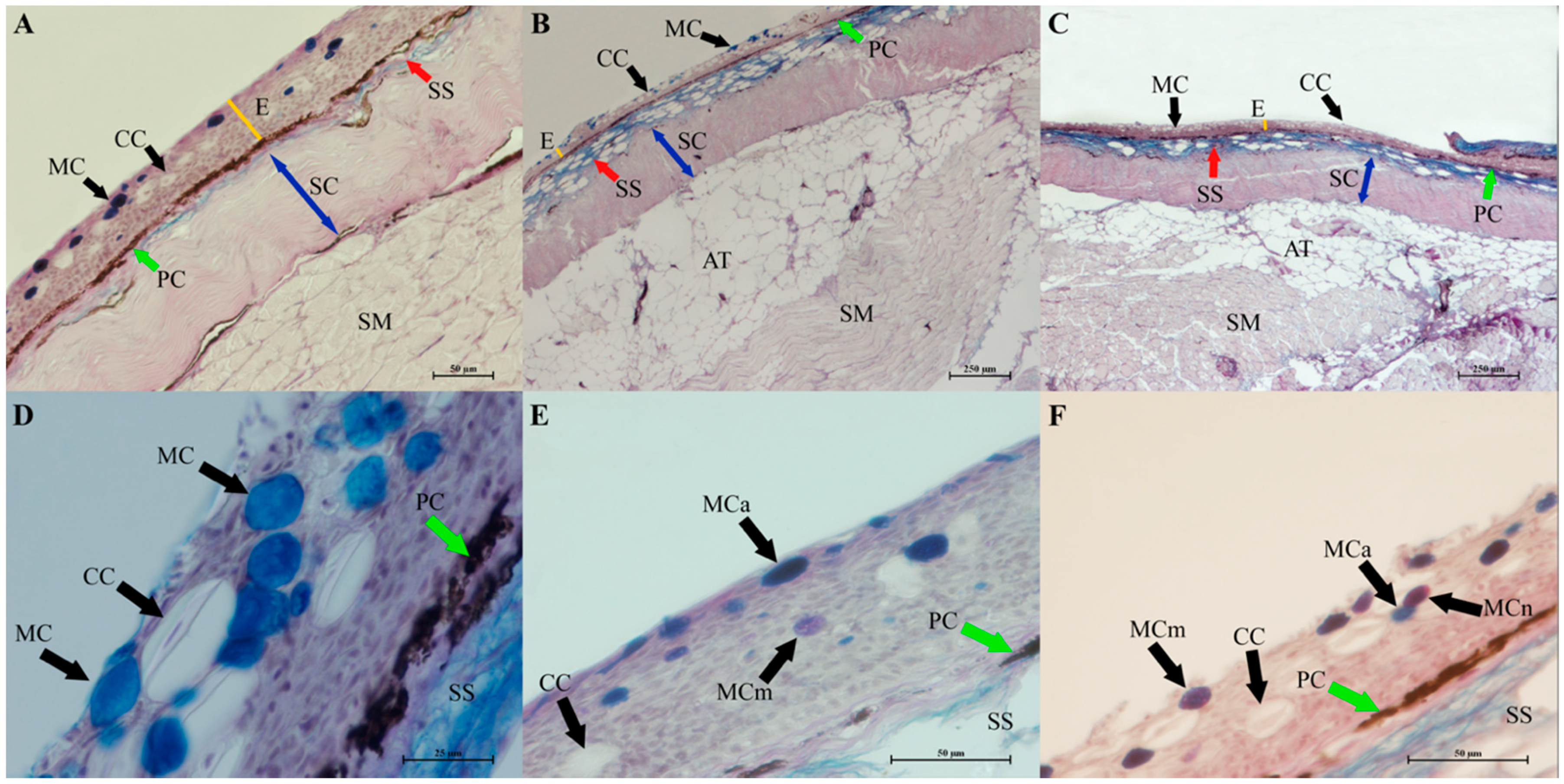
2.5. Histomorphology of Gills, Skin and Intestine
2.6. Statistical Analysis and Data Visualisation
3. Results
3.1. Basic Performance and Welfare Indices of Carp Wintering in Pond and RAS
3.2. Histomorphology of the Gills, Skin and Intestine of Carp Wintering in Pond and RAS
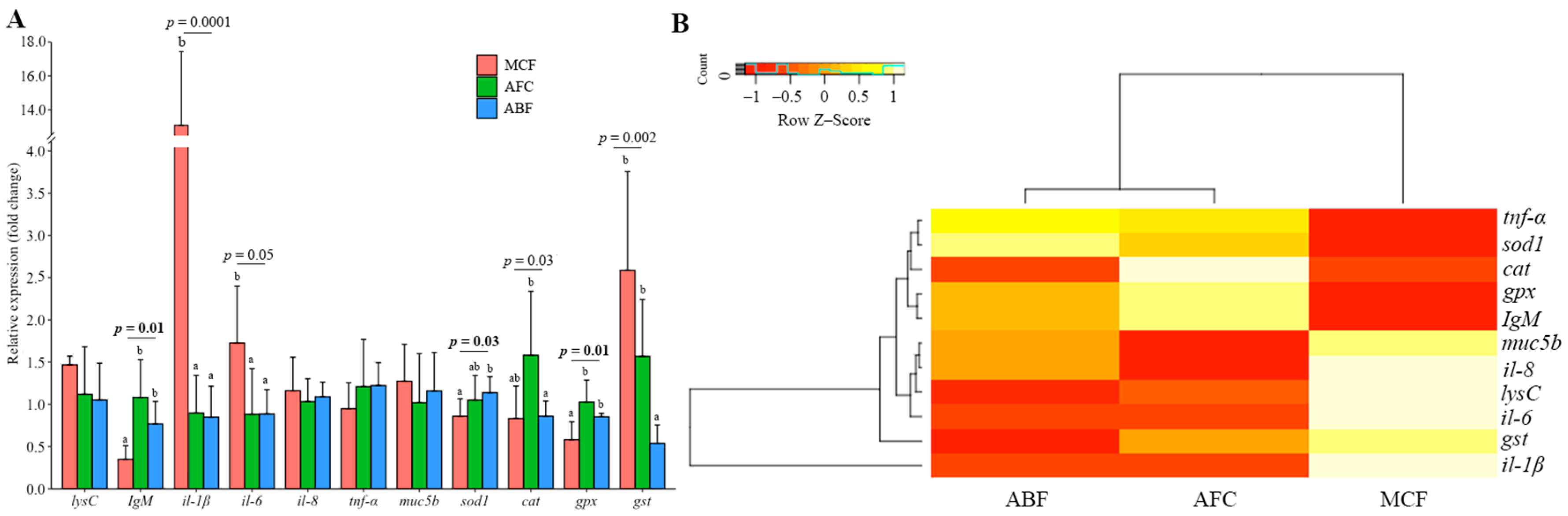
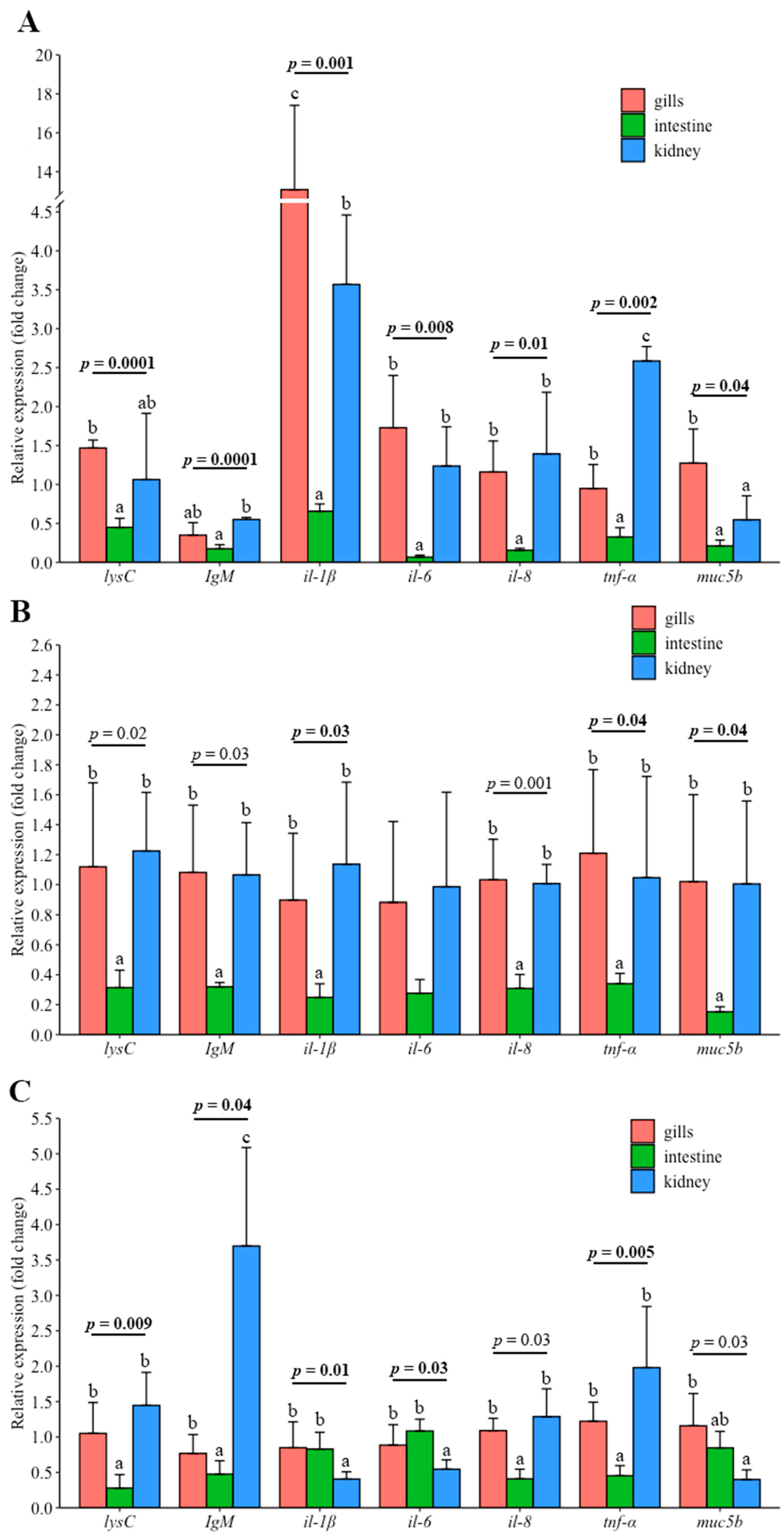
3.3. Gene Expression in the Gills and Intestine of Carp Wintering in Pond and RAS
4. Discussion
4.1. Differences in Weight Gain, Condition Factor and Mortality of Carp Overwintering in Earthen Ponds and RAS
4.2. Influence of Wintering Practice on the Histological and Molecular Indices of Carp Gills
4.3. Influence of Wintering Practice on the Histological and Molecular Indices of Carp Intestine
4.4. Influence of Wintering Practice on the Skin Histology of Carp from Pond and RAS
4.5. Gene Expression in Kidney under Carp Wintering in Ponds and RAS
4.6. Differences in Gene Expression between Gill, Intestine and Kidney in Fish under Different Wintering Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.A.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome. 2020. Available online: https://www.fao.org/documents/card/en/c/ca9229en (accessed on 6 September 2021).
- FAO. Fishery and Aquaculture Statistics. Global Aquaculture Production 1950–2019 (FishStatJ). 2021. Available online: http://www.fao.org/3/cb5186en/cb5186en.pdf (accessed on 6 September 2021).
- Belton, B.; Little, D.C.; Zhang, W.; Edwards, P.; Skladany, M.; Thilsted, S.H. Farming fish in the sea will not nourish the world. Nat. Commun. 2020, 11, 5804. [Google Scholar] [CrossRef] [PubMed]
- Gál, D.; Kerepeczki, É.; Gyalog, G.; Pekar, F. Changing face of central European aquaculture: Sustainability issues. Surv. Fish. Sci. 2015, 2, 42–56. [Google Scholar] [CrossRef]
- EUMOFA. Freshwater Aquaculture in the EU. 2021. Available online: https://www.eumofa.eu/documents/20178/442176/Freshwater+aquaculture+in+the+EU.pdf (accessed on 6 September 2021).
- Bauer, C.; Schlott, G. Overwintering of farmed common carp (Cyprinus carpio L.) in the ponds of a central European aquaculture facility—Measurement of activity by radio telemetry. Aquaculture 2004, 241, 301–317. [Google Scholar] [CrossRef]
- Woynarovich, A.; Moth-Poulsen, T.; Péteri, A. Carp Polyculture in Central and Eastern Europe, the Caucasus and Central Asia: A Manual; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2010; Volume 554, p. 73. [Google Scholar]
- Biermann, G.; Geist, J. Life cycle assessment of common carp (Cyprinus carpio L.)—A comparison of the environmental impacts of conventional and organic carp aquaculture in Germany. Aquaculture 2020, 524, 735273. [Google Scholar] [CrossRef]
- Raftowicz, M.; Kalisiak-Mędelska, M.; Struś, M. Redefining the supply chain model on the Milicz carp market. Sustainability 2020, 12, 2934. [Google Scholar] [CrossRef] [Green Version]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Żochowska-Kujawska, J.; Barbosa, V.; Dias, J.; Marques, A. Nutritional value and sensory properties of common carp (Cyprinus carpio L.) fillets enriched with sustainable and natural feed ingredients. Food Chem. Toxicol. 2021, 152, 112197. [Google Scholar] [CrossRef]
- Eljasik, P.; Panicz, R.; Sobczak, M.; Sadowski, J.; Tórz, A.; Barbosa, V.; Marques, A.; Dias, J. Structural and molecular indices in common carp (Cyprinus carpio L.) fed n-3 PUFA enriched diet. Food Chem. Toxicol. 2021, 151, 112146. [Google Scholar] [CrossRef]
- Pietrzak, E.; Mazurkiewicz, J.; Slawinska, A. Innate immune responses of skin mucosa in common carp (Cyprinus carpio) fed a diet supplemented with galactooligosaccharides. Animals 2020, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Eljasik, P.; Panicz, R.; Sobczak, M.; Sadowski, J.; Barbosa, V.; Marques, A.; Dias, J. Plasma biochemistry, gene expression and liver histomorphology in common carp (Cyprinus carpio) fed with different dietary fat sources. Food Chem. Toxicol. 2020, 140, 111300. [Google Scholar] [CrossRef]
- Buchanan, K.; Burt de Perera, T.; Carere, C.; Carter, T.; Hailey, A.; Hubrecht, R.; Jennings, D.; Metcalfe, N.; Pitcher, T.; Peron, F.; et al. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [Google Scholar] [CrossRef]
- Burck, H.C. Technika Histologiczna; PZWL: Warszawa, Poland, 1975; pp. 139–140. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Korytář, T.; Wiegertjes, G.F.; Zusková, E.; Tomanová, A.; Lisnerová, M.; Patra, S.; Sieranski, V.; Šíma, R.; Born-Torrijos, A.; Wentzel, A.S.; et al. Holzer The kinetics of cellular and humoral immune responses of common carp to presporogonic development of the myxozoan Sphaerospora molnari. Parasites Vectors 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolte, E.H.; Nabuurs, S.B.; Bury, N.R.; Sturm, A.; Flik, G.; Savelkoul, H.F.; Verburg-van Kemenade, B.L. Stress and innate immunity in carp: Corticosteroid receptors and pro-inflammatory cytokines. Mol. Immunol. 2008, 46, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Diaz, D.A.; Schmidt, J.G.; Vera-Jimenez, N.I.; Steinhagen, D.; Nielsen, M.E. β-Glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2013, 35, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Wang, Z.; Wu, H.; Zhao, X.; Liu, T.; Li, S.; Xu, S. Assessment of pesticide residues and gene expression in common carp exposed to atrazine and chlorpyrifos: Health risk assessments. Ecotoxicol. Environ. Saf. 2015, 113, 491–498. [Google Scholar] [CrossRef]
- Pu, Y.; Zhu, J.; Wang, H.; Zhang, X.; Hao, J.; Wu, Y.; Geng, Y.; Wang, K.; Li, Z.; Zhou, J.; et al. Molecular characterization and expression analysis of Hsp90 in Schizothorax prenanti. Cell Stress Chaperones 2016, 21, 983–991. [Google Scholar] [CrossRef] [Green Version]
- Shahi, N.; Ardó, L.; Fazekas, G.; Gócza, E.; Kumar, S.; Rèvèsz, N.; Kumar, S.; Jeney, Z.; Sándor, Z.J.; Molnár, Z.; et al. Immunogene expression in head kidney and spleen of common carp (Cyprinus carpio L.) following thermal stress and challenge with Gram-negative bacterium, Aeromonas hydrophila. Aquac. Int. 2018, 26, 727–741. [Google Scholar] [CrossRef]
- Agus, H.H.; Sümer, S.; Erkoç, F. Toxicity and molecular effects of di-n-butyl phthalate (DBP) on CYP1A, SOD, and GPx in Cyprinus carpio (common carp). Environ. Monit. Assess. 2015, 187, 423. [Google Scholar] [CrossRef]
- Jiang, W.D.; Hu, K.; Zhang, J.X.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Soyabean glycinin depresses intestinal growth and function in juvenile Jian carp (Cyprinus carpio var Jian): Protective effects of glutamine. Br. J. Nutr. 2015, 114, 1569–1583. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, J.; Winter, M.J.; Lange, A.; Cumming, R.; Owen, S.F.; Tyler, C.R. Effects of the lipid regulating drug clofibric acid on PPARα-regulated gene transcript levels in common carp (Cyprinus carpio) at pharmacological and environmental exposure levels. Aquat. Toxicol. 2015, 161, 127–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickley, L.K.; Lange, A.; Winter, M.J.; Tyler, C.R. Evaluation of a carp primary hepatocyte culture system for screening chemicals for oestrogenic activity. Aquat. Toxicol. 2009, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.F.; Huising, M.O.; Stakauskas, R.; Forlenza, M.; Verburg-van Kemenade, B.L.; Buchmann, K.; Nielsen, M.E.; Wiegertjes, G.F. Real-time gene expression analysis in carp (Cyprinus carpio L.) skin: Inflammatory responses to injury mimicking infection with ectoparasites. Dev. Comp. Immunol. 2007, 31, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mowry, R.W. Alcian blue techniques for the histochemical study of acidic carbohydrates. J. Histochem. Cytochem. 1956, 4, 403–407. [Google Scholar]
- Wang, Z.; Du, J.; Lam, S.H.; Mathavan, S.; Matsudaira, P.; Gong, Z. Morphological and molecular evidence for functional organization along the rostrocaudal axis of the adult zebrafish intestine. BMC Genom. 2010, 11, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsen, C.; Ytteborg, E.; Timmerhaus, G.; Høst, V.; Handeland, S.; Jørgensen, S.M.; Krasnov, A. Atlantic salmon skin barrier functions gradually enhance after seawater transfer. Sci. Rep. 2018, 8, 9510. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Pittman, K.; Sonne, C.; Hansson, S.; Bach, L.; Søndergaard, J.; Stride, M.; Nowak, B. Histological mucous cell quantification and mucosal mapping reveal different aspects of mucous cell responses in gills and skin of shorthorn sculpins (Myoxocephalus scorpius). Fish Shellfish Immunol. 2020, 100, 334–344. [Google Scholar] [CrossRef]
- Verdile, N.; Pasquariello, R.; Scolari, M.; Scirè, G.; Brevini, T.A.; Gandolfi, F. A detailed study of rainbow trout (Onchorhynchus mykiss) intestine revealed that digestive and absorptive functions are not linearly distributed along its length. Animals 2020, 10, 745. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Lukowicz, M.; Gerstner, P. Hältern und Wintern. In Lehrbuch der Teichwirtschaft, 1st ed.; Schäperclaus, W., Lukowicz, M., Eds.; Parey: Berlin, Germany, 1998; pp. 495–503. [Google Scholar]
- Geldhauser, F.; Gerstner, P. Der Teichwirt, 8th ed.; Ulmer: Stuttgart, Germany, 2003; pp. 1–288. [Google Scholar]
- Fernandes, T.; McMeans, B.C. Coping with the cold: Energy storage strategies for surviving winter in freshwater fish. Ecography 2019, 42, 2037–2052. [Google Scholar] [CrossRef] [Green Version]
- Ibarz, A.; Padrós, F.; Gallardo, M.Á.; Fernández-Borràs, J.; Blasco, J.; Tort, L. Low-temperature challenges to gilthead sea bream culture: Review of cold-induced alterations and ‘Winter Syndrome’. Rev. Fish Biol. Fish. 2010, 20, 539–556. [Google Scholar] [CrossRef]
- Horváth, L.; Tamás, G.; Seagrave, C. Carp and Pond Fish Culture, 2nd ed.; Fishing News Books; Blackwell Scientific Publications Ltd.: Oxford, UK, 1992; pp. 1–154. [Google Scholar]
- Pietsch, C.; Hirsch, P. Biology and Ecology of Carp, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–394. [Google Scholar]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773–825. [Google Scholar] [CrossRef] [PubMed]
- Hurst, T.P. Causes and consequences of winter mortality in fishes. J. Fish Biol. 2007, 71, 315–345. [Google Scholar] [CrossRef]
- Tabarrok, M.; Seyfabadi, J.; Salehi Jouzani, G.; Younesi, H. Comparison between recirculating aquaculture and biofloc systems for rearing juvenile common carp (Cyprinus carpio): Growth performance, haemato-immunological indices, water quality and microbial communities. Aquac. Res. 2020, 51, 4881–4892. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Zou, H.K.; Van Doan, H.; Kolangi Miandare, H.; Hoseini, S.M. Evaluation of some intestinal cytokines genes expression and serum innate immune parameters in common carp (Cyprinus carpio) fed dietary loquat (Eriobotrya japonica) leaf extract. Aquac. Res. 2018, 49, 120–127. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Steinum, T.; Olsen, A.B.; Lie, K.I.; Tavornpanich, S.; Colquhoun, D.J.; Gjevre, A.G. Histopathological investigation of complex gill disease in sea farmed Atlantic salmon. PLoS ONE 2019, 14, e0222926. [Google Scholar] [CrossRef] [Green Version]
- Powell, M.D.; Yousaf, M.N.; Rasmussen, K.J.; Köllner, B.; Zou, J.; Secombes, C.; Speare, D.J. Immunohistochemical localization of inflammatory cells and cell cycle proteins in the gills of Loma salmonae infected rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2014, 40, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yao, C.L. Molecular and expression characterizations of interleukin-8 gene in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2013, 34, 799–809. [Google Scholar] [CrossRef]
- Püschel, F.; Favaro, F.; Redondo-Pedraza, J.; Lucendo, E.; Iurlaro, R.; Marchetti, S.; Majem, B.; Eldering, E.; Nadal, E.; Ricci, J.E.; et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9932–9941. [Google Scholar] [CrossRef] [Green Version]
- Shimon-Hophy, M.; Avtalion, R.R. Influence of chronic stress on the mechanism of the cytotoxic system in common carp (Cyprinus carpio). Immunology 2021, 164, 211–222. [Google Scholar] [CrossRef]
- Hoole, D.; Bucke, D.; Burgess, P.; Wellby, I. Diseases of Carp and Other Cyprinid Fishes; Wiley-Blackwell: Honoken, NJ, USA, 2001; p. 280. [Google Scholar]
- Yin, X.; Mu, L.; Fu, S.; Wu, L.; Han, K.; Wu, H.; Han, K.; Wu, H.; Bian, X.; Wei, X.; et al. Expression and characterization of Nile tilapia (Oreochromis niloticus) secretory and membrane-bound IgM in response to bacterial infection. Aquaculture 2019, 508, 214–222. [Google Scholar] [CrossRef]
- Leya, T.; Ahmad, I.; Valappil, R.K.; Kurcheti, P.P.; Tripathi, G.; Sharma, R.; Bedekar, M.K. Development of species-specific IgM antibodies and elevation of mucosal immune response in Labeo rohita using recombinant bicistronic nano DNA vaccine priming. Fish Shellfish Immunol. 2021, 113, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.E.; Crump, B.C.; Kling, G.W. Temperature controls on aquatic bacterial production and community dynamics in arctic lakes and streams. Environ. Microbiol. 2010, 12, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, H.; Shangguan, X.; Wang, L.; Liu, X. Identification, annotation of Mucin genes in channel catfish (Ictalurus punctatus) and their expression after bacterial infections revealed by RNA-Seq analysis. Aquac. Res. 2020, 51, 2020–2028. [Google Scholar] [CrossRef]
- Bayir, A.; Sirkecioglu, A.N.; Bayir, M.; Haliloglu, H.I.; Kocaman, E.M.; Aras, N.M. Metabolic responses to prolonged starvation, food restriction, and refeeding in the brown trout, Salmo trutta: Oxidative stress and antioxidant defenses. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 191–196. [Google Scholar] [CrossRef]
- Chen, L.; Feng, L.; Jiang, W.D.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary riboflavin deficiency decreases immunity and antioxidant capacity, and changes tight junction proteins and related signaling molecules mRNA expression in the gills of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 45, 307–320. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Jiang, W.-D.; Wu, P.; Kuang, S.-Y.; Jiang, J.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Liu, Y.; et al. Copper-induced tight junction mRNA expression changes, apoptosis and antioxidant responses via NF-rmkappaB, TOR and Nrf2 signaling molecules in the gills of fish: Preventive role of arginine. Aquat. Toxicol. 2014, 158, 125–137. [Google Scholar] [CrossRef]
- Castaldo, G.; Delahaut, V.; Slootmaekers, B.; Bervoets, L.; Town, R.M.; Blust, R.; De Boeck, G. A comparative study on the effects of three different metals (Cu, Zn and Cd) at similar toxicity levels in common carp, Cyprinus carpio. J. Appl. Toxicol. 2021, 41, 1400–1413. [Google Scholar] [CrossRef]
- Özaslan, M.S.; Demir, Y.; Küfrevioğlu, O.I.; Çiftci, M. Some metals inhibit the glutathione S-transferase from Van Lake fish gills. J. Biochem. Mol. Toxicol. 2017, 31, e21967. [Google Scholar] [CrossRef]
- Prabhu, P.A.J.; Kaushik, S.J.; Geurden, I.; Stouten, T.; Fontagne-Dicharry, S.; Veron, V.; Mariojouls, C.; Verreth, J.A.J.; Eding, E.H.; Schrama, J.W. Water exchange rate in RAS and dietary inclusion of micro-minerals influence growth, body composition and mineral metabolism in common carp. Aquaculture 2017, 471, 8–18. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Svobodová, Z.; Máchová, J.; Kroupová, H.; Smutná, M.; Groch, L. Ammonia autointoxication of common carp: Case studies. Aquacult. Int. 2007, 15, 277–286. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Liu, Q.; Sun, J.; He, K.; Adam, A.A.; Luo, J.; Li, Z.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; et al. Modulation of immune response, physical barrier and related signaling factors in the gills of juvenile grass carp (Ctenopharyngodon idella) fed supplemented diet with phospholipids. Fish Shellfish Immunol. 2016, 48, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Effects of dietary protein levels on the disease resistance, immune function and physical barrier function in the gill of grass carp (Ctenopharyngodon idella) after challenged with Flavobacterium columnare. Fish Shellfish Immunol. 2016, 57, 1–16. [Google Scholar] [CrossRef]
- Element, G.; Engel, K.; Neufeld, J.D.; Casselman, J.M.; van Coeverden de Groot, P.; Greer, C.W.; Walker, V.K. Seasonal habitat drives intestinal microbiome composition in anadromous Arctic char (Salvelinus alpinus). Environ. Microbiol. 2020, 22, 3112–3125. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Casadei, E.; Shibasaki, Y.; Ding, Y.; Sauters, T.; Yongyao, Y.U.; Salinas, J.; Sunyer, O. Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution. Sci. Immunol. 2020, 5, 3254. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef]
- Bisht, A.; Singh, U.P.; Pandey, N.N. Comparative study of seasonal variation in bacterial flora concomitant with farm raised fingerlings of Cyprinus carpio at tarai region of Uttarakhand. J. Environ. Biol. Mar. 2014, 35, 363–367. [Google Scholar]
- Eichmiller, J.J.; Hamilton, M.J.; Staley, C.; Sadowsky, M.J.; Sorensen, P.W. Environment shapes the fecal microbiome of invasive carp species. Microbiome 2016, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Tanck, M.W.T.; Booms, G.H.R.; Eding, E.H.; Wendellar Bonga, S.E.; Komen, J. Cold shocks: A stressor for common carp. J. Fish Biol. 2000, 57, 881–894. [Google Scholar] [CrossRef]
- Liang, L.; Chang, Y.; He, X.; Tang, R. Transcriptome analysis to identify cold-responsive genes in Amur carp (Cyprinus carpio haematopterus). PLoS ONE 2015, 10, e0130526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Secombes, C.J. The function of fish cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Baeverfjord, G.; Krogdahl, Å. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- van der Marel, M.; Adamek, M.; Gonzalez, S.F.; Frost, P.; Rombout, J.H.; Wiegertjes, G.F.; Savelkoul, H.F.; Steinhagen, D. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012, 32, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; O’Doherty, J.; Reilly, P.; Ryan, M.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedemeyer, G.A.; Barton, B.A.; McLeay, D.J. Stress and acclimation. In Methods for Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda, MD, USA, 1990; pp. 451–489. [Google Scholar]
- Li, Y.; Ding, W.; Li, X. Acute exposure of glyphosate-based herbicide induced damages on common carp organs via heat shock proteins-related immune response and oxidative stress. Toxin Rev. 2019, 40, 1071–1083. [Google Scholar] [CrossRef]
- Genest, O.; Wickner, S.; Doyle, S.M. Hsp90 and Hsp70 chaperones: Collaborators in protein remodelling. J. Biol. Chem. 2019, 29, 2109–2120. [Google Scholar] [CrossRef] [Green Version]
- Caruso, G.; Maricchiolo, G.; Micale, V.; Genovese, L.; Caruso, R.; Denaro, M.G. Physiological responses to starvation in the European eel (Anguilla anguilla): Effects on haematological, biochemical, non-specific immune parameters and skin structures. Fish Physiol. Biochem. 2010, 36, 71–83. [Google Scholar] [CrossRef]
- Somejo, M.B.; Herrera, A.A.; Fabillo, M.D.; Abucay, J.S. The development of integumentary and skeletal systems of starved Nile Tilapia, Oreochromis niloticus L. In New Dimensions in Farmed Tilapia, Proceedings of the Sixth International Symposium on Tilapia in Aquaculture, Manila, Philippines, 12–16 September 2004; Bolivar, R., Mair, G., Fitzsimmons, K., Eds.; American Tilapia Association, Aquaculture CRSP, and Ministry of Agriculture: Manila, Philipines, 2004; pp. 733–740. [Google Scholar]
- Landeira-Dabarca, A.; Álvarez, M.; Molist, P. Food deprivation causes rapid changes in the abundance and glucidic composition of the cutaneous mucous cells of Atlantic salmon Salmo salar L. J. Fish Dis. 2014, 37, 899–909. [Google Scholar] [CrossRef]
- Elliott, D.G. Integumentary system. In The Laboratory Fish; Otrander, G.K., Ed.; Academic Press: New York, CA, USA, 2000; pp. 271–306. [Google Scholar]
- Iger, Y.; Jenner, H.A.; Bonga, S.W. Cellular responses of the skin of carp (Cyprinus carpio) exposed to acidified water. Cell Tissue Res. 1994, 275, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Iger, Y.; Jenner, H.A.; Bonga, S.W. Cellular responses in the skin of the trout (Oncorhynchus mykiss) exposed to temperature elevation. J. Fish Biol. 1994, 44, 921–935. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhang, J.L.; Jiao, J.G.; Du, X.X.; Limbu, S.M.; Qiao, F.; Zhang, M.L.; Li, D.L.; Du, Z.Y. Physiological and metabolic differences between visceral and subcutaneous adipose tissues in Nile tilapia (Oreochromis niloticus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 313, R608–R619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceballos-Francisco, D.; García-Carrillo, N.; Cuesta, A.; Esteban, M.Á. Radiological characterization of gilthead seabream (Sparus aurata) fat by X-ray micro-computed tomography. Sci. Rep. 2020, 10, 10527. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P. Pilgrimage to traditional carp pond culture in Central Europe. Aquac. Asia 2007, 12, 28–34. [Google Scholar]
- Kideys, A.E.; Hartnoll, G.H. Energetics of mucus production in the common whelk Buccinum undatum L. J. Exp. Mar. Biol. Ecol. 1991, 150, 91–105. [Google Scholar] [CrossRef]
- Sørensen, S.L.; Park, Y.; Gong, Y.; Vasanth, G.K.; Dahle, D.; Korsnes, K.; Phuong, T.H.; Kiron, V.; Øyen, S.; Pittman, K.; et al. Nutrient digestibility, growth, mucosal barrier status, and activity of leucocytes from head kidney of Atlantic salmon fed marine- or plant- derived protein and lipid Sources. Front. Immunol. 2021, 11, 623726. [Google Scholar] [CrossRef]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Ćirković, M.; Novakov, N.; Kartalović, B.; Pelić, M.; Jovanić, S.; Božić, B.; Đorđević, V. Ichthyophthiriosis–cause of significant losses of carp fingerlings. Arch. Vet. Med. 2015, 8, 3–12. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Lin, D.; Jia, J.; Cai, R.; Yu, Y.; Li, W. Innate immune response to fasting and refeeding in the zebrafish kidney. Biomolecules 2021, 11, 825. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, Y.X.; Song, X.H.; Wu, K.; Hu, B.; Sun, B.Y.; Liu, Z.J.; Fu, J.G. Characterization of interleukin-1β as a proinflammatory cytokine in grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Kalb, D.; Vo, H.D.; Adikari, S.; Hong-Geller, E.; Munsky, B.; Werner, J. Visualization and modeling of inhibition of IL-1β and TNFα mRNA transcription at the single-cell level. Sci. Rep. 2021, 11, 13692. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.V.M.; Mario, É.G.; Porto, L.C.J.; Andrade, S.P.; Botion, L.M. High-carbohydrate diet selectively induces tumor necrosis factor-α production in mice liver. Inflammation 2011, 34, 139–145. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Zhao, C.Y.; Guan, J.F.; Liu, X.C.; Li, X.F.; Xie, D.Z.; Xu, C. High-carbohydrate diet alleviates the oxidative stress, inflammation and apoptosis of Megalobrama amblycephala following dietary exposure to silver nanoparticles. Antioxidants 2021, 10, 1343. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Liu, W.B.; Remø, S.C.; Wang, B.K.; Shi, H.J.; Zhang, L.; Liu, J.D.; Li, X.F. Feeding restriction alleviates high carbohydrate diet-induced oxidative stress and inflammation of Megalobrama amblycephala by activating the AMPK-SIRT1 pathway. Fish Shellfish Immunol. 2019, 92, 637–648. [Google Scholar] [CrossRef]
- Zhou, C.P.; Ge, X.P.; Liu, B.; Xie, J.; Miao, L.H. Effect of high dietary carbohydrate on the growth performance and physiological responses of juvenile wuchang bream, Megalobrama Amblycephala. Asian-Australas. J. Anim. Sci. 2013, 26, 1598. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.H.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q. Dietary choline-enhanced skin immune response of juvenile grass carp might be related to the STAT3 and NF-kB signaling pathway (Ctenopharyngodon idella). Front. Nutr. 2021, 8, 652767. [Google Scholar] [CrossRef]
- Liu, X.W.; Zhang, J.X.; Feng, L.; Jiang, W.D.; Wu, P.; Kuang, S.Y.; Tang, L.; Shi, H.Q.; Zhou, X.Q.; Liu, Y. Protective effects and potential mechanisms of (2-Carboxyethyl) dimethylsulfonium Bromide (Br-DMPT) on gill health status of on-growing grass carp (Ctenopharyngodon idella) after infection with Flavobacterium columnare. Fish Shellfish Immunol. 2020, 106, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Krogdahl, Å.; Bakke-McKellep, A.M.; Røed, K.H.; Bæverfjord, G. Feeding Atlantic salmon Salmo salar L. soybean products: Effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquac. Nutr. 2000, 6, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Estensoro, I.; Calduch-Giner, J.A.; Kaushik, S.; Pérez-Sánchez, J.; Sitjà-Bobadilla, A. Modulation of the IgM gene expression and IgM immunoreactive cell distribution by the nutritional background in gilthead sea bream (Sparus aurata) challenged with Enteromyxum leei (Myxozoa). Fish Shellfish Immunol. 2012, 33, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppang, E.O.; Kvellestad, A.; Fischer, U. Fish mucosal immunity: Gill. In Mucosal Health in Aquaculture, 1st ed.; Beck, B., Peatman, E., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 93–133. [Google Scholar]
- Wu, J.; Shi, Y.H.; Zhang, X.H.; Li, C.H.; Li, M.Y.; Chen, J. Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection. Dong Wu Xue Yan Jiu = Zool. Res. 2015, 36, 133–141. [Google Scholar]
- Øvergård, A.C.; Nepstad, I.; Nerland, A.H.; Patel, S. Characterisation and expression analysis of the Atlantic halibut (Hippoglossus hippoglossus L.) cytokines: IL-1β, IL-6, IL-11, IL-12β and IFNγ. Mol. Biol. Rep. 2012, 39, 2201–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syahputra, K.; Kania, P.W.; Al-Jubury, A.; Marnis, H.; Setyawan, A.C.; Buchmann, K. Differential immune gene response in gills, skin, and spleen of rainbow trout Oncorhynchus mykiss infected by Ichthyophthirius multifiliis. PLoS ONE 2019, 14, e0218630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alix, M.; Bloundeau-Bidet, E.; Grousset, E.; Shirangui, A.; Vergnet, A.; Guinand, B.; Chatain, B.; Boulo, V.; Lignot, J.-H. Effects of fasting and re-alimentation on gill and intestine morphology and indicators of osmoregulatory capacity in genetically selected sea bass (Dicentrarchus labrax) populations with contrasting tolerance to fasting. Aquaculture 2017, 468, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Atarashi, K.; Tanoue, T.; Umesaki, Y.; Honda, K. Regulation of Th17 cell differentiation by intestinal commensal bacteria. Benef. Microbes 2010, 1, 327–334. [Google Scholar] [CrossRef]
- McEntee, C.P.; Finlay, C.M.; Lavelle, E.C. Divergent roles for the IL-1 family in gastrointestinal homeostasis and inflammation. Front. Immunol. 2019, 10, 1266. [Google Scholar] [CrossRef]






| Gene | Primer Sequence 5′ -> 3′ | Tm (°C) | Function | Reference | |
|---|---|---|---|---|---|
| IgM 1 | F | TCGTATTAGCACCCCCAGAG | 53.8 | First line of host defence against infections | [18] |
| R | TCATCAGCAAGCCAAGACACA | 52.4 | |||
| il-1β2 | F | CCTGAAGAAGAGGAGGCTGTCA | 56.7 | Mediator of inflammatory response | [19] |
| R | AAGGAGGCCAGTGGCTCTGT | 55.9 | |||
| il-6 3 | F | CCGCACATGAAGACAGTGAT | 51.8 | Stimulating acute phase protein synthesis | [20] |
| R | GGGTATATTTGGCTGCAGGA | 51.8 | |||
| il-8 4 | F | TGGAGCTCTTCCCTCCAAG | 53.2 | Attracting and activating neutrophils | [20] |
| R | AGGGTGCAGTAGGGTCCAG | 55.4 | |||
| tnf-α5 | F | CCTTGGAAGTGACATTTGCTTTT | 51.7 | Signalling events within cells | [19] |
| R | GCTGTCTGCTTCACGCTCAA | 53.8 | |||
| muc5b 6 | F | CAGCCCTCTTCCTCTTTCATC | 54.4 | Ensure normal mucus clearance | [20] |
| R | CCACTCATCTTTCCTTTCTCTTC | 53.5 | |||
| hsp707 | F | TGAGAACATCAACGAGCCCA | 51.8 | Protein maturation, re-folding and degradation | [21] |
| R | TTGTCAAAGTCCTCCCCACC | 53.8 | |||
| hsp90 8 | F | AAAGACCAGGTCGCCCACTC | 55.9 | Protein maturation, re-folding and degradation | [22] |
| R | AGTACTCGTCGATGGGCTCG | 55.9 | |||
| sod1 9 | F | TGGTCCACCGTGAGCTTTATT | 52.4 | Antioxidant enzyme | [23] |
| R | GACAACACAAACGGCGGCAT | 53.8 | |||
| gpx 10 | F | TGCAACCAGTTCGGACATCA | 51.8 | Catalyses the reduction of hydrogen peroxide | [24] |
| R | GAAGCCATTTCCAGGACGGA | 53.8 | |||
| cat11 | F | CTGGAAGTGGAATCCGTTTG | 51.8 | Maintaining the cellular redox homeostasis | [25] |
| R | CGACCTCAGCGAAATAGTTG | 51.8 | |||
| gst 12 | F | TACAATACTTTCACGCTTTCCC | 51.1 | Protect cellular macromolecules | [26] |
| R | GGCTCAACACCTCCTTCAC | 53.2 | |||
| rpl813 | F | CTCCGTCTTCAAAGCCCATGT | 54.4 | Ribosomal protein coding | [27] |
| R | TCCTTCACGATCCCCTTGATG | 54.4 | |||
| 40sRNA14 | F | CCGTGGGTGACATCGTTACA | 53.8 | Ribosomal RNA gene | [28] |
| R | TCAGGACATTGAACCTCACTGTCT | 55.7 | |||
| Parameter/Fish Group | MCF | AFC | ABF |
|---|---|---|---|
| SNV (µm) | ND | 23.2 ± 3.13 a | 18.8 ± 1.67 b |
| LP (µm) | 42.4 ± 11.69 a | 19.3 ± 3.93 b | 24.5 ± 5.16 c |
| GC (n) | 28.0 ± 2.45 a | 57.6 ± 3.44 b | 73.5 ± 7.77 c |
| SM (µm) | 72.9 ± 9.68 a | 45.1 ± 8.31 b | 58.9 ± 4.56 c |
| GC (µm2) | 50.7 ± 13.54 a | 50.7 ± 9.95 a | 41.7 ± 10.63 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eljasik, P.; Panicz, R.; Sobczak, M.; Sadowski, J. Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes. Sustainability 2022, 14, 3724. https://doi.org/10.3390/su14073724
Eljasik P, Panicz R, Sobczak M, Sadowski J. Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes. Sustainability. 2022; 14(7):3724. https://doi.org/10.3390/su14073724
Chicago/Turabian StyleEljasik, Piotr, Remigiusz Panicz, Małgorzata Sobczak, and Jacek Sadowski. 2022. "Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes" Sustainability 14, no. 7: 3724. https://doi.org/10.3390/su14073724
APA StyleEljasik, P., Panicz, R., Sobczak, M., & Sadowski, J. (2022). Key Performance Indicators of Common Carp (Cyprinus carpio L.) Wintering in a Pond and RAS under Different Feeding Schemes. Sustainability, 14(7), 3724. https://doi.org/10.3390/su14073724







