Nitrogen Uptake, Use Efficiency, and Productivity of Nigella sativa L. in Response to Fertilization and Plant Density
Abstract
:1. Introduction
2. Materials and Methods
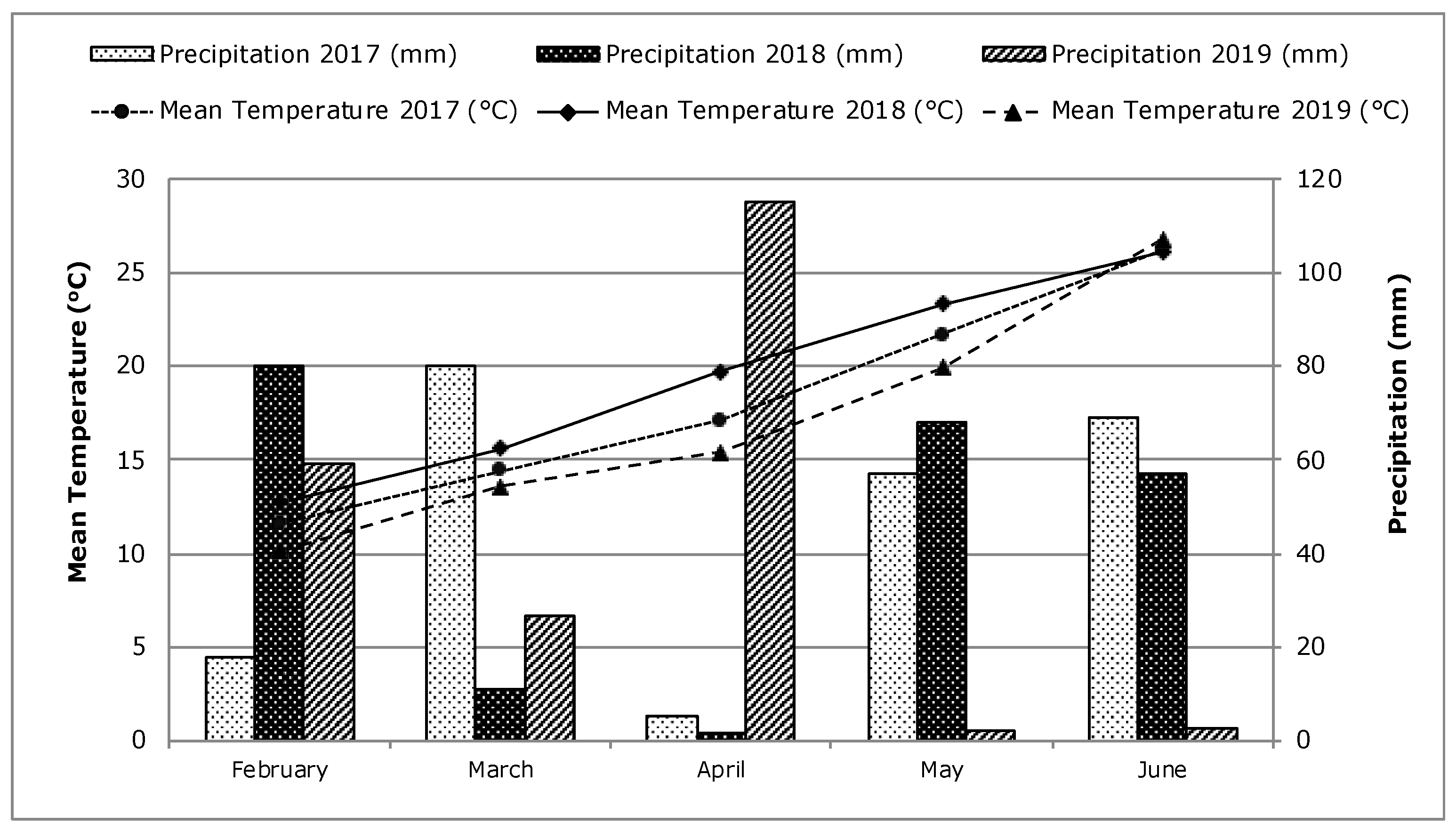
2.1. Site Description and Experimental Design
2.2. Sampling, Measurements, and Methods
2.3. Statistical Analysis
3. Results
3.1. Soil Properties
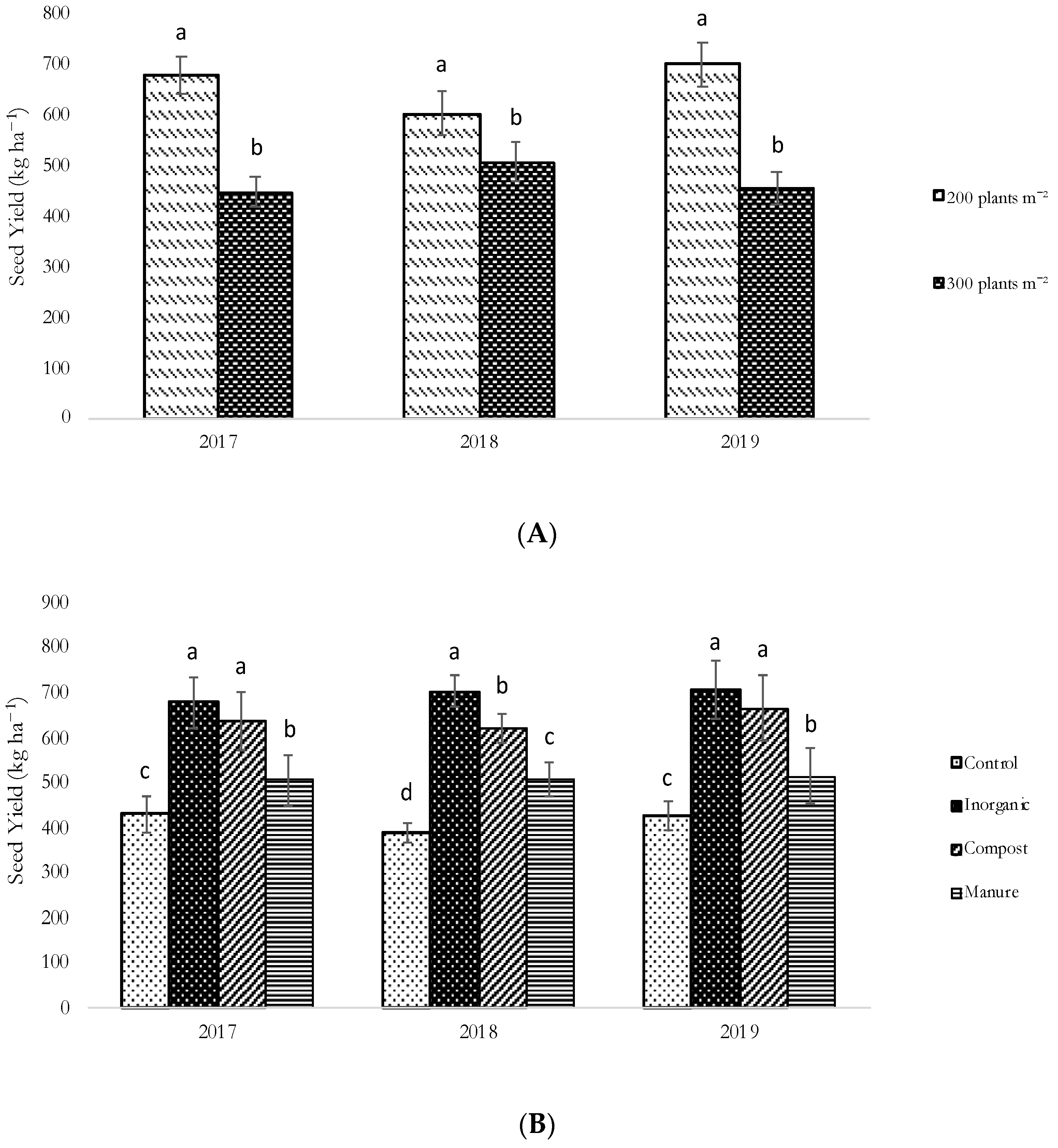
3.2. Growth, Seed Yield and Yield Components of N. sativa
3.3. Nitrogen Content and Uptake in the Aerial Components of N. sativa
3.4. Nitrogen Use Efficiency of N. sativa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iqbal, M.S.; Qureshi, A.S.; Ghafoor, A. Evaluation of Nigella sativa L. for genetic variation and ex-situ conservation. Pak. J. Bot. 2010, 42, 2489–2495. [Google Scholar]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [PubMed]
- Tuncturk, R.; Tuncturk, M.; Ciftci, V. The effects of varying nitrogen doses on yield and some yield components of black cumin (Nigella sativa L.). Adv. Environ. Biol. 2012, 6, 855–885. [Google Scholar]
- Riaz, M.; Syed, M.; Chaudhary, F.M. Chemistry of the medicinal plants of the genus Nigella. Hamdard Med. 1996, 39, 40–45. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar]
- Ashraf, M.; Ali, Q.; Iqbal, Z. Effect of nitrogen application rate on the content and composition of oil, essential oil and minerals in black cumin (Nigella sativa L.) seeds. J. Sci. Food Agric. 2006, 86, 871–876. [Google Scholar]
- Piras, A.; Rosa, A.; Marongiu, B.; Porcedda, S.; Falconieri, D.; Dessi, M.A.; Ozcelik, B.; Koca, U. Chemical composition and in vitro bioactivity of the volatile and fixed oils of Nigella sativa L. extracted by supercritical carbon dioxide. Ind. Crop. Prod. 2013, 46, 317–323. [Google Scholar]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar]
- Karna, S.K.L. Phytochemical screening and gas chromatography-mass spectrometry and analysis of grain extract of Nigella sativa. Int. J. Chem. Stud. 2013, 1, 183–188. [Google Scholar]
- Razavi, B.M.; Hosseinzadeh, H. A review of the effects of Nigella sativa L. and its constituent, thymoquinone, in metabolic syndrome. J. Endocrinol. Investig. 2014, 37, 1031–1040. [Google Scholar]
- European Commission (EC). EU Novel Food Catalogue (v.1.1). Available online: http://ec.europa.eu/food/safety/novel_food/catalogue/search/public/?event=home&seqfce=226&ascii=N (accessed on 18 February 2022).
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Roussis, I.; Katsenios, N.; Efthimiadou, A.; Papastylianou, P. Introduction of alternative crops in the Mediterranean to satisfy EU Green Deal goals. A review. Agron. Sustain. Dev. 2021, 41, 71. [Google Scholar]
- Nasr, A.S.; Attia, A.I.; Rashwan, A.A.; Abdine, A.M.M. Growth performance of New Zealand white rabbits as affected by partial replacement of diet with Nigella sativa or soybean meals. Egypt. J. Rabbit. Sci. 1996, 6, 129–141. [Google Scholar]
- Akhtar, M.S.; Nasir, Z.; Abid, A.R. Effect of feeding powdered Nigella sativa L. seeds on poultry egg production and their suitability for human consumption. Vet. Arh. 2003, 73, 181–190. [Google Scholar]
- Abo El-Nor, S.A.H.; Khattab, H.M.; Al-Alamy, H.A.; Salem, F.A.; Abdou, M.M. Effect of some medicinal plants seeds in the rations on the productive performance of lactating buffaloes. Int. J. Dairy Sci. 2007, 2, 348–355. [Google Scholar]
- El-Ghousein, S.S. Effects of some medicinal plants as feed additives on lactating awassi ewe performance, milk composition, lamb growth and relevant blood items. Egypt. J. Anim. Prod. 2010, 47, 37–49. [Google Scholar]
- Khan, S.H.; Ansari, J.; Haq, A.u.; Abbas, G. Black cumin seeds as phytogenic product in broiler diets and its effects on performance, blood constituents, immunity and caecal microbial population. Ital. J. Anim. Sci. 2012, 11, 438–444. [Google Scholar]
- Kumar, P.; Patra, A.K. Beneficial uses of black cumin (Nigella sativa L.) seeds as a feed additive in poultry nutrition. Worlds Poult. Sci. J. 2017, 73, 872–885. [Google Scholar]
- Roussis, I.; Kakabouki, I.; Tsiplakou, E.; Bilalis, D. Influence of plant density and fertilization on yield and crude protein of Nigella sativa L.: An alternative forage and feed source. In Nigella sativa: Properties, Uses and Effects; Berghuis, S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; pp. 145–180. [Google Scholar]
- Ju, X.; Christie, P. Calculation of theoretical nitrogen rate for simple nitrogen recommendations in intensive cropping systems: A case study on the North China Plain. Field Crop. Res. 2011, 124, 450–458. [Google Scholar]
- Li, X.; Lu, J.; Wu, L.; Chen, F. The difference of potassium dynamics between yellowish red soil and yellow cinnamon soil under rapeseed (Brassica napus L.)–rice (Oryza sativa L.) rotation. Plant Soil 2009, 320, 141–151. [Google Scholar]
- Chowdhury, M.A.; Sultana, T.; Rahman, M.A.; Chowdhury, T.; Enyoh, C.E.; Saha, B.K.; Wang, Q. Nitrogen use efficiency and critical leaf N concentration of Aloe vera in urea and diammonium phosphate amended soil. Heliyon 2020, 6, e05718. [Google Scholar]
- Montemurro, F.; Diacono, M. Towards a better understanding of agronomic efficiency of nitrogen: Assessment and improvement strategies. Agronomy 2016, 6, 31. [Google Scholar]
- Delogu, G.; Cattivelli, L.; Pecchioni, N.; De Falcis, D.; Maggiore, T.; Stanca, A.M. Uptake and agronomic efficiency of nitrogen in winter barley and winter wheat. Eur. J. Agron. 1998, 9, 11–20. [Google Scholar]
- Sinclair, T.R.; de Wit, C.T. Photosynthate and nitrogen requirements for seed production by various crops. Science 1975, 18, 565–567. [Google Scholar]
- Sainju, U.M.; Senwo, Z.N.; Nyakatawa, E.Z.; Tazisong, I.A.; Reddy, K.C. Soil carbon and nitrogen sequestration as affected by long-term tillage, cropping systems, and nitrogen fertilizer sources. Agric. Ecosyst. Environ. 2008, 127, 234–240. [Google Scholar]
- Lin, H.-C.; Huber, J.A.; Gerl, G.; Hülsbergen, K.-J. Nitrogen balances and nitrogen–use efficiency of different organic and conventional farming systems. Nutr. Cycl. Agroecosystems 2016, 105, 1–23. [Google Scholar]
- Craswell, E.T.; Godwin, D.C. The efficiency of nitrogen fertilizers applied to cereals in different climates. Adv. Plant Nutr. 1984, 1, 1–55. [Google Scholar]
- Kakabouki, I.P.; Hela, D.; Roussis, I.; Papastylianou, P.; Sestras, A.; Bilalis, D.J. Influence of fertilization and soil tillage in quinoa crop (Chenopodium quinoa Willd.). Nitrogen uptake and utilization efficiency. Expression of nitrogen indices. J. Soil Sci. Plant Nutr. 2018, 18, 220–235. [Google Scholar]
- Mazzoncini, M.; Sapkota, T.B.; Bàrberi, P.; Antichi, D.; Risaliti, R. Long-term effect of tillage, nitrogen fertilization and cover crops on soil organic carbon and total nitrogen content. Soil Tillage Res. 2011, 114, 165–174. [Google Scholar]
- Ren, F.L.; Zhang, X.B.; Liu, J.; Sun, N.; Wu, L.H.; Li, Z.F.; Xu, M.G. A synthetic analysis of greenhouse gas emissions from manure amended agricultural soils in China. Sci. Rep. 2017, 7, 8123. [Google Scholar]
- Gong, W.; Yan, X.Y.; Wang, J.Y.; Hu, T.X.; Gong, Y.B. Long-term applications of chemical and organic fertilizers on plant-available nitrogen pools and nitrogen management index. Biol. Fertil. Soils 2011, 47, 767–775. [Google Scholar]
- Smith, L.E.D.; Siciliano, G. A comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric. Ecosyst. Environ. 2015, 209, 15–25. [Google Scholar]
- Hu, C.; Xia, X.G.; Chen, Y.F.; Qiao, Y.; Liu, D.H.; Fan, J.; Li, S.L. Yield, nitrogen use efficiency and balance response to thirty-five years of fertilization in paddy rice-upland wheat cropping system. Plant Soil Environ. 2019, 65, 55–62. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Malinas, A.; Vidican, R.; Rotar, I.; Malinas, C.; Moldovan, C.M.; Proorocu, M. Current Status and Future Prospective for Nitrogen Use Efficiency in Wheat (Triticum aestivum L.). Plants 2022, 11, 217. [Google Scholar] [PubMed]
- Zemenchik, R.A.; Albrecht, K.A. Nitrogen use efficiency and apparent nitrogen recovery of Kentucky bluegrass, smooth bromegrass, and orchardgrass. Agron. J. 2002, 94, 421–428. [Google Scholar]
- Zhang, Y.; Xu, Z.; Li, J.; Wang, R. Optimum planting density improves resource use efficiency and yield stability of rainfed maize in semiarid climate. Front. Plant Sci. 2021, 12, 752606. [Google Scholar]
- Mahler, R.L.; Koehler, F.E.; Lutcher, L.K. Soils. Nitrogen source, timing of application, and placement: Effects on winter wheat production. Agron. J. 1994, 86, 637–642. [Google Scholar]
- Fageria, N.K. Nitrogen harvest index and its association with crop yields. J. Plant Nutr. 2014, 37, 795–810. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Ye, Q.; Zhang, H.; Wei, H.; Zhang, Y.; Wang, B.; Xia, K.; Huo, Z.; Dai, Q.; Xu, K. Effects of nitrogen fertilizer on nitrogen use efficiency and yield of rice under different soil conditions. Front. Agric. China 2007, 1, 30–36. [Google Scholar]
- Roussis, I.; Kakabouki, I.; Bilalis, D. Comparison of growth indices of Nigella sativa L. under different plant densities and fertilization. Emir. J. Food Agric 2019, 31, 231–247. [Google Scholar]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar]
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [PubMed]
- González, M.; Gomez, E.; Comese, R.; Quesada, M.; Conti, M. Influence of organic amendments on soil quality potential indicators in an urban horticultural system. Bioresour. Technol. 2010, 101, 8897–8901. [Google Scholar]
- Craine, J.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar]
- Guo, P.; Wang, C.; Jia, Y.; Wang, Q.; Hang, G.; Tian, X. Responses of soil microbial biomass and enzymatic activities to fertilizations of mixed inorganic and organic nitrogen at a subtropical forest in East China. Plant Soil 2010, 338, 355–366. [Google Scholar]
- Kucharik, C.J.; Brye, K.R.; Norman, J.M.; Foley, J.A.; Gower, S.T.; Bundy, L.G. Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern Wisconsin: Potential for SOC sequestration during the next 50 years. Ecosystems 2001, 4, 237–258. [Google Scholar]
- Sadej, W.; Przekwas, K. Fluctuations of nitrogen levels in soil profile under conditions of a long-term fertilization experiment. Plant Soil Environ. 2008, 54, 197–203. [Google Scholar]
- Zhengchao, Z.; Zhuoting, G.; Zhouping, S.; Fuping, Z. Effects of long-term repeated mineral and organic fertilizer applications on soil organic carbon and total nitrogen in a semi-arid cropland. Eur. J. Agron. 2013, 45, 20–26. [Google Scholar]
- Huang, S.; Peng, X.X.; Huang, Q.R.; Zhang, W.J. Soil aggregation and organic carbon fractions affected by long term fertilization in a red soil of subtropical China. Geoderma 2010, 154, 364–369. [Google Scholar]
- Kelley, K.R.; Stevenson, F.J. Forms and nature of organic N in soil. Fertil. Res. 1995, 42, 1–11. [Google Scholar]
- Mollafilabi, A.; Moodi, H.; Rashed, M.H.; Kafi, M. Effects of plant density and nitrogen on yield and yield components of black cumin (Nigella sativa L.). Acta Hortic. 2010, 853, 115–126. [Google Scholar]
- Toncer, O.; Kizil, S. Effect of seed rate on agronomic and technologic characters of Nigella sativa L. Int. J. Agric. Biol. 2004, 6, 529–532. [Google Scholar]
- Talafih, K.A.; Haddad, N.I.; Hattar, B.I.; Kharallah, K. Effect of some agricultural practices on the productivity of black cumin (Nigella sativa L.) grown under rainfed semi-arid conditions. Jordan J. Agric. Sci. 2007, 3, 385–397. [Google Scholar]
- Salisburry, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing: Belmont, CA, USA, 1992. [Google Scholar]
- Amin, M.E.-M.H. Effect of different nitrogen sources on growth, yield and quality of fodder maize (Zea mays L.). J. Saudi Soc. Agric. Sci. 2011, 10, 17–23. [Google Scholar]
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant Biol. 2015, 25, 10–16. [Google Scholar]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A review of the use of organic amendments and the risk to human health. Adv. Agron. 2013, 120, 275–379. [Google Scholar]
- Tittarelli, F.; Petruzzelli, G.; Pezzarossa, B.; Civilini, M.; Benedetti, A.; Sequi, P. Quality and agronomic use of compost. Waste Manag. Ser. 2007, 8, 119–157. [Google Scholar]
- Fabbri, D.; Chiavari, G.; Galletti, G.C. Characterization of soil humin by pyrolysis(/methylation)-gas chromatography/mass spectrometry: Structural relationships with humic acids. J. Anal. Appl. Pyrolysis 1996, 37, 161–172. [Google Scholar]
- Schmidt, W.; Santi, S.; Pinton, R.; Varanini, Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant Soil 2007, 300, 259–267. [Google Scholar]
- Tollenaar, M.; Dibo, A.A.; Aguilera, A.; Weise, S.F.; Swanton, C.J. Effect of crop density on weed interference in maize. Agron. J. 1994, 86, 591–595. [Google Scholar]
- Bayu, W.; Rethman, F.G.; Hammes, P.S. Growth and yield compensation in sorghum (Sorghum bicolor L. Moench) as a function of planting density and nitrogen fertilizer in semi-arid areas of northeastern Ethiopia. S. Afr. J. Plant Soil 2005, 22, 76–83. [Google Scholar]
- Özgüven, M.; Sekeroglu, N. Agricultural practices for high yield and quality of black cumin (Nigella sativa L.) cultivated in Turkey. Acta Hortic. 2007, 756, 329–338. [Google Scholar]
- Hera, C. The role of inorganic fertilizers and their management practices. In Fertilizers and Environment; Rodriguez-Barrueco, C., Ed.; Kluwer Academic Publishers: Norwell, MA, USA, 1996; pp. 131–149. [Google Scholar]
- Mengel, D.B.; Rehm, G.W. Fundamentals of fertilizer application. In Handbook of Soil Sciences, Resource Management and Environmental Impacts, 2nd ed.; Huang, P.M., Li, Y., Summer, M.E., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 14-1–14-15. [Google Scholar]
- Bilalis, D.; Krokida, M.; Roussis, I.; Papastylianou, P.; Travlos, I.; Cheimona, N.; Dede, A. Effects of organic and inorganic fertilization on yield and quality of processing tomato (Lycopersicon esculentum Mill.). Folia Hortic. 2018, 30, 321–332. [Google Scholar]
- Vega, C.R.C.; Sadras, V.O.; Andrade, F.H.; Uhart, S.A. Reproductive allometry in soybean, maize and sunflower. Ann. Bot. 2000, 85, 461–468. [Google Scholar]
- Yimam, E.; Nebiyu, A.; Mohammed, A.; Getachew, M. Effect of nitrogen and phosphorus fertilizers on growth, yield, and yield components of black cumin (Nigella sativa L.) at Konta District, South West Ethiopia. J. Agron. 2015, 14, 112–120. [Google Scholar]
- Das, A.K.; Sadhu, M.K.; Som, M.G.; Bose, T.K. Effects of spacings on growth and yield of black cumin. Indian Cocoa Arecanut Spices J. 1992, 16, 17–18. [Google Scholar]
- Vos, J.; Putten, P.E.L.; Birch, C.J. Effect of nitrogen supply on leaf appearance, leaf nitrogen economy and photosynthetic maize (Zea mays L.). Field Crop. Res. 2005, 93, 64–73. [Google Scholar]
- Raymond, F.D.; Alley, M.M.; Parrish, D.J.; Thomason, W.E. Plant density and hybrid impacts on corn grain and forage yield and nutrient uptake. J. Plant Nutr. 2009, 32, 395–409. [Google Scholar]
- Mosanaei, H.; Ajamnorozi, H.; Dadashi, M.R.; Faraji, A.; Pessarakli, M. Improvement effect of nitrogen fertilizer and plant density on wheat (Triticum aestivum L.) seed deterioration and yield. Emir. J. Food Agric. 2017, 29, 899–910. [Google Scholar]
- Johnson, E.N.; Malhi, S.S.; Hall, L.M.; Phelps, S. Effects of nitrogen fertilizer application on seed yield, N uptake, N use efficiency, and seed quality of Brassica carinata. Can. J. Plant Sci. 2013, 93, 1073–1081. [Google Scholar]
- Dordas, C. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source–sink relation. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar]
- Bulman, P.; Smith, D.L. Post-heading nitrogen uptake, retranslocation, and partitioning in spring barley. Crop Sci. 1994, 34, 977–984. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops, 2nd ed.; Marcel Dekker: New York, NY, USA, 1997. [Google Scholar]
- Li, Y.; Chen, Y.; Wu, C.-Y.; Tang, X.; Ji, X.-J. Determination of optimum nitrogen application rates in Zhejiang Province, China, based on rice yields and ecological security. J. Integr. Agric. 2015, 14, 2426–2433. [Google Scholar]
- Li, P.; Dong, H.; Zheng, C.; Sun, M.; Liu, A.; Wang, G.; Liu, S.; Zhang, S.; Chen, J.; Li, Y.; et al. Optimizing nitrogen application rate and plant density for improving cotton yield and nitrogen use efficiency in the North China Plain. PLoS ONE 2017, 12, e0185550. [Google Scholar]
- Ayadi, S.; Karmous, C.; Chamekh, Z.; Hammami, Z.; Baraket, M.; Esposito, S.; Rezgui, S.; Trifa, Y. Effects of nitrogen rates on grain yield and nitrogen agronomic efficiency of durum wheat genotypes under different environments. Ann. Appl. Biol. 2016, 168, 264–273. [Google Scholar]
- Abebe, Z.; Feyisa, H. Effects of nitrogen rates and time of application on yield of maize: Rainfall variability influenced time of N application. Int. J. Agron. 2017, 2017, 1545280. [Google Scholar]
- Yan, P.; Pan, J.; Zhang, W.; Shi, J.; Chen, X.; Cui, Z. A high plant density reduces the availability of maize to use soil nitrogen. PLoS ONE 2017, 12, e0172717. [Google Scholar]
| Soil Type | Clay Loam |
|---|---|
| Clay | 29.1% |
| Silt | 35.3% |
| Sand | 35.6% |
| pH (1:1 H2O) | 7.43 |
| Organic matter | 1.82% |
| CaCO3 | 15.93% |
| Total Nitrogen | 0.121% |
| Phosphorus–Olsen P | 13.4 mg kg−1 soil |
| Potassium | 206 mg kg−1 soil |
| Plant Density | Fertilization Treatment | Fertilization Amount | N Content | N Application Rate |
|---|---|---|---|---|
| 200 plants m−2 | Control | No fertilizer | - | - |
| Seaweed Compost | 2000 kg ha−1 | 1.98% | 40 kg N ha−1 | |
| 300 plants m−2 | Farmyard Manure | 2000 kg ha−1 | 1.52% | 30.5 kg N ha−1 |
| Inorganic Fertilizer | 300 kg ha−1 | 15% | 45 kg N ha−1 |
| Source of Variance | Df | Soil Organic Matter (SOM) | Soil Total Nitrogen (STN) | Plant Height | Leaf Area Index (LAI) | Seed Yield | Harvest Index (HI) |
| Year (Y) | 2 | 0.0351 ns | 13.961 *** | 4.0073 * | 0.7294 ns | 1.0164 ns | 1.4479 ns |
| Plant Density (PD) | 1 | 0.8345 ns | 1.3599 ns | 21.074 *** | 15.560 *** | 192.62 *** | 141.19 *** |
| Fertilization (F) | 3 | 41.816 *** | 24.680 *** | 14.559 *** | 49.391 *** | 86.882 *** | 3.8504 * |
| Y × PD | 2 | 0.0121 ns | 0.0186 ns | 0.2291 ns | 0.0003 ns | 11.977 *** | 1.9516 ns |
| Y × F | 6 | 1.3296 ns | 0.4647 ns | 0.1068 ns | 0.0284 ns | 0.4720 ns | 0.2259 ns |
| PD × F | 3 | 0.6242 ns | 0.7007 ns | 1.2897 ns | 1.4720 ns | 3.4119 ns | 0.0958 ns |
| Y × PD × F | 6 | 0.0860 ns | 0.1276 ns | 0.0808 ns | 0.0410 ns | 0.5430 ns | 0.1046 ns |
| Source of Variance | Df | 1000 Seed Weight | Biomass N Content 75 DAS | Biomass N Uptake | Total Plant N Uptake | Seed N Content | |
| Year (Y) | 2 | 0.0583 ns | 2.0454 ns | 2.0973 ns | 2.1456 ns | 3.3859 * | |
| Plant Density (PD) | 1 | 0.3819 ns | 35.589 *** | 32.151 *** | 5.4115 * | 14.410 *** | |
| Fertilization (F) | 3 | 2.3924 ns | 82.643 *** | 17.876 *** | 39.993 *** | 57.611 *** | |
| Y × PD | 2 | 0.0125 ns | 0.0944 ns | 0.5695 ns | 0.1464 ns | 1.6664 ns | |
| Y × F | 6 | 0.0861 ns | 0.3605 ns | 0.3183 ns | 0.1639 ns | 1.7254 ns | |
| PD × F | 3 | 0.2194 ns | 3.4300 * | 1.3991 ns | 1.3836 ns | 1.5031 ns | |
| Y × PD × F | 6 | 0.0013 ns | 0.4569 ns | 0.3112 ns | 0.4115 ns | 0.6907 ns | |
| Source of Variance | Df | Seed N Uptake | N Harvest Index (NHI) | Apparent N Recovery Efficiency (ANRE) | N Utilization Efficiency (NUtE) | N Agronomic Efficiency (NAE) | |
| Year (Y) | 2 | 0.5149 ns | 0.5615 ns | 0.4057 ns | 6.0298 ** | 1.2989 ns | |
| Plant Density (PD) | 1 | 161.89 *** | 184.12 *** | 3.1052 ns | 171.58 *** | 16.651 *** | |
| Fertilization (F) | 3 | 135.41 *** | 10.162 *** | 5.2455 * | 7.2316 *** | 9.6107 *** | |
| Y × PD | 2 | 3.9989 * | 2.3714 ns | 0.0808 ns | 6.7993 ** | 0.2620 ns | |
| Y × F | 6 | 0.7091 ns | 1.0662 ns | 0.1224 ns | 1.0731 ns | 0.1332 ns | |
| PD × F | 3 | 5.3673 ** | 0.4542 ns | 1.9750 ns | 3.2337 * | 0.3123 ns | |
| Y × PD × F | 6 | 0.9550 ns | 0.1338 ns | 0.3889 ns | 0.5864 ns | 0.3835 ns | |
| Plant Density (Plants m−2) | ||||||
|---|---|---|---|---|---|---|
| Fertilization | 200 | 300 | 200 | 300 | ||
| Soil Organic Matter (SOM) (%) | Soil Total Nitrogen (STN) (%N) | |||||
| 2017 | Mean | Mean | ||||
| Control | 1.644 | 1.686 | 1.665 b | 0.124 | 0.126 | 0.125 b |
| Manure | 2.033 | 1.941 | 1.987 a | 0.156 | 0.150 | 0.153 a |
| Compost | 1.903 | 1.972 | 1.938 a | 0.150 | 0.149 | 0.150 a |
| Inorganic | 1.786 | 1.692 | 1.739 b | 0.148 | 0.142 | 0.145 a |
| Mean | 1.842 A | 1.823 A | 0.145 A | 0.142 A | ||
| FPlant Density | 0.3748 ns | 0.3907 ns | ||||
| FFertilization | 7.0649 ** (Tukey = 0.1274) | 6.4801 ** (Tukey = 0.0132) | ||||
| FPlant Density × Fertilization | 0.0940 ns | 0.0932 ns | ||||
| 2018 | ||||||
| Control | 1.594 | 1.608 | 1.601 b | 0.138 | 0.137 | 0.138 c |
| Manure | 2.104 | 2.033 | 2.069 a | 0.178 | 0.169 | 0.174 a |
| Compost | 2.030 | 2.013 | 2.022 a | 0.163 | 0.170 | 0.167 ab |
| Inorganic | 1.739 | 1.627 | 1.683 b | 0.160 | 0.146 | 0.153 bc |
| Mean | 1.867 A | 1.820 A | 0.160 A | 0.156 A | ||
| FPlant Density | 0.1974 ns | 0.6661 ns | ||||
| FFertilization | 6.8596 ** (Tukey = 0.1557) | 8.2321 ** (Tukey = 0.0083) | ||||
| FPlant Density × Fertilization | 0.6888 ns | 0.7344 ns | ||||
| 2019 | ||||||
| Control | 1.577 | 1.549 | 1.563 b | 0.139 | 0.137 | 0.138 c |
| Manure | 2.154 | 2.075 | 2.115 a | 0.181 | 0.175 | 0.178 a |
| Compost | 2.071 | 2.055 | 2.064 a | 0.176 | 0.173 | 0.175 ab |
| Inorganic | 1.664 | 1.564 | 1.614 b | 0.158 | 0.155 | 0.157 b |
| Mean | 1.867 A | 1.811 A | 0.164 A | 0.160 A | ||
| FPlant Density | 0.2963 ns | 0.3367 ns | ||||
| FFertilization | 10.8148 *** (Tukey = 0.1276) | 10.4148 *** (Tukey = 0.0112) | ||||
| FPlant Density × Fertilization | 0.0999 ns | 0.1053 ns | ||||
| Fertilization | Plant Density (Plants m−2) | |||||
|---|---|---|---|---|---|---|
| 200 | 300 | 200 | 300 | |||
| Plant Height (cm) | Leaf Area Index (LAI) (m2 m−2) | |||||
| 2017 | Mean | Mean | ||||
| Control | 45.33 | 41.98 | 43.66 b | 1.146 | 1.527 | 1.337 b |
| Manure | 55.89 | 45.99 | 50.94 ab | 1.702 | 1.757 | 1.730 b |
| Compost | 66.56 | 53.90 | 60.23 a | 2.173 | 2.532 | 2.353 a |
| Inorganic | 67.29 | 56.09 | 61.69 a | 2.378 | 2.964 | 2.671 a |
| Mean | 58.77 A | 49.49 B | 1.850 B | 2.195 A | ||
| FPlant Density | 4.9897 * (Tukey = 9.253) | 5.8572 * (Tukey = 0.1866) | ||||
| FFertilization | 4.1390 * (Tukey = 12.773) | 17.7728 *** (Tukey = 0.4575) | ||||
| FPlant Density × Fertilization | 0.2445 ns | 0.5864 ns | ||||
| 2018 | ||||||
| Control | 38.02 | 34.92 | 36.47 b | 1.232 | 1.617 | 1.425 b |
| Manure | 49.54 | 39.96 | 44.75 ab | 1.834 | 1.860 | 1.847 b |
| Compost | 56.93 | 44.77 | 50.85 a | 2.169 | 2.682 | 2.426 a |
| Inorganic | 62.04 | 48.36 | 55.20 a | 2.606 | 3.081 | 2.844 a |
| Mean | 51.63 A | 42.00 B | 1.961 A | 2.310 A | ||
| FPlant Density | 6.9992 * (Tukey = 9.024) | 4.2587 ns | ||||
| FFertilization | 4.9831 * (Tukey = 11.812) | 13.6067 *** (Tukey = 0.5193) | ||||
| FPlant Density × Fertilization | 0.4121 ns | 0.4295 ns | ||||
| 2019 | ||||||
| Control | 42.68 | 38.59 | 40.64 c | 1.225 | 1.630 | 1.428 b |
| Manure | 55.91 | 46.08 | 51.00 bc | 1.795 | 1.842 | 1.818 b |
| Compost | 68.11 | 47.41 | 57.76 ab | 2.290 | 2.656 | 2.473 a |
| Inorganic | 71.67 | 55.34 | 63.51 a | 2.537 | 3.102 | 2.820 a |
| Mean | 59.59 A | 46.86 B | 1.962 B | 2.308 A | ||
| FPlant Density | 9.5346 ** (Tukey = 10.847) | 5.8230 * (Tukey = 0.2043) | ||||
| FFertilization | 5.6748 ** (Tukey = 14.368) | 19.2348 *** (Tukey = 0.4584) | ||||
| FPlant Density × Fertilization | 0.7822 ns | 0.5742 ns | ||||
| Plant Density (Plants m−2) | ||||||
|---|---|---|---|---|---|---|
| Fertilization | 200 | 300 | 200 | 300 | ||
| Harvest Index (HI) | 1000 Seed Weight (g) | |||||
| 2017 | Mean | Mean | ||||
| Control | 0.225 | 0.106 | 0.166 a | 1.496 | 1.512 | 1.504 a |
| Manure | 0.217 | 0.122 | 0.170 a | 1.545 | 1.487 | 1.516 a |
| Compost | 0.231 | 0.119 | 0.175 a | 1.540 | 1.535 | 1.538 a |
| Inorganic | 0.251 | 0.130 | 0.191 a | 1.655 | 1.596 | 1.626 a |
| Mean | 0.231 A | 0.119 B | 1.559 A | 1.533 A | ||
| FPlant Density | 47.5421 *** (Tukey = 0.0302) | 0.1714 ns | ||||
| FFertilization | 0.4673 ns | 0.7616 ns | ||||
| FPlant Density × Fertilization | 0.1382 ns | 0.0910 ns | ||||
| 2018 | ||||||
| Control | 0.196 | 0.129 | 0.163 a | 1.502 | 1.524 | 1.513 a |
| Manure | 0.233 | 0.148 | 0.191 a | 1.532 | 1.483 | 1.508 a |
| Compost | 0.232 | 0.154 | 0.193 a | 1.527 | 1.529 | 1.528 a |
| Inorganic | 0.257 | 0.179 | 0.218 a | 1.621 | 1.587 | 1.604 a |
| Mean | 0.229 A | 0.153 B | 1.546 A | 1.531 A | ||
| FPlant Density | 24.5912 *** (Tukey = 0.0325) | 0.0591 ns | ||||
| FFertilization | 2.1250 ns | 0.5433 ns | ||||
| FPlant Density × Fertilization | 0.0579 ns | 0.0723 ns | ||||
| 2019 | ||||||
| Control | 0.213 | 0.107 | 0.160 a | 1.470 | 1.481 | 1.476 a |
| Manure | 0.221 | 0.113 | 0.167 a | 1.499 | 1.437 | 1.468 a |
| Compost | 0.238 | 0.124 | 0.181 a | 1.553 | 1.543 | 1.548 a |
| Inorganic | 0.260 | 0.137 | 0.199 a | 1.656 | 1.604 | 1.630 a |
| Mean | 0.233 A | 0.120 B | 1.545 A | 1.516 A | ||
| FPlant Density | 90.3770 *** (Tukey = 0.0248) | 0.1651 ns | ||||
| FFertilization | 2.0547 ns | 1.1697 ns | ||||
| FPlant Density × Fertilization | 0.1083 ns | 0.0615 ns | ||||
| Fertilization | Plant Density (Plants m−2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 | 300 | 200 | 300 | 200 | 300 | ||||
| Biomass N Content (%N) | |||||||||
| 2017 | 45 DAS | 60 DAS | 75 DAS | ||||||
| Mean | Mean | Mean | |||||||
| Control | 1.13 | 1.01 | 1.07 b | 1.72 | 1.13 | 1.43 b | 2.39 | 2.15 | 2.27 c |
| Manure | 1.53 | 1.20 | 1.37 ab | 2.34 | 1.53 | 1.94 ab | 3.12 | 2.50 | 2.81 b |
| Compost | 1.67 | 1.52 | 1.60 a | 2.49 | 1.67 | 2.08 a | 3.22 | 2.95 | 3.09 b |
| Inorganic | 1.68 | 1.56 | 1.62 a | 2.44 | 1.68 | 2.06 a | 3.59 | 3.34 | 3.47 a |
| Mean | 1.50 A | 1.32 A | 2.25 A | 1.49 B | 3.08 A | 2.74 B | |||
| FPlant Density | 3.0759 ns | 7.7270 * (Tukey = 0.347) | 14.1810 ** (Tukey = 0.424) | ||||||
| FFertilization | 6.3472 ** (Tukey = 0.298) | 6.3375 ** (Tukey = 0.432) | 30.0160 *** (Tukey = 0.349) | ||||||
| FPlant Density × Fertilization | 0.2714 ns | 0.7434 ns | 1.0235 ns | ||||||
| 2018 | |||||||||
| Control | 1.21 | 1.07 | 1.14 b | 1.89 | 1.71 | 1.80 b | 2.56 | 2.28 | 2.42 c |
| Manure | 1.64 | 1.25 | 1.45 ab | 2.56 | 1.87 | 2.22 ab | 3.36 | 2.59 | 2.98 b |
| Compost | 1.70 | 1.61 | 1.66 a | 2.59 | 2.31 | 2.45 a | 3.20 | 3.21 | 3.21 b |
| Inorganic | 1.80 | 1.62 | 1.71 a | 2.74 | 2.47 | 2.61 a | 3.87 | 3.36 | 3.62 a |
| Mean | 1.59 A | 1.39 A | 2.45 A | 2.09 B | 3.25 A | 2.86 B | |||
| FPlant Density | 3.1729 ns | 5.4360 * (Tukey = 0.392) | 11.3631 ** (Tukey = 0.448) | ||||||
| FFertilization | 5.3518 ** (Tukey = 0.333) | 4.9055 * (Tukey = 0.494) | 18.9643 *** (Tukey = 0.436) | ||||||
| FPlant Density × Fertilization | 0.3535 ns | 0.5351 ns | 2.1428 ns | ||||||
| 2019 | |||||||||
| Control | 1.09 | 0.98 | 1.04 b | 1.65 | 1.50 | 1.58 b | 2.29 | 2.10 | 2.20 d |
| Manure | 1.56 | 1.24 | 1.40 a | 2.37 | 1.75 | 2.06 a | 3.17 | 2.57 | 2.86 c |
| Compost | 1.71 | 1.58 | 1.65 a | 2.56 | 2.14 | 2.35 a | 3.31 | 3.05 | 3.18 b |
| Inorganic | 1.75 | 1.64 | 1.70 a | 2.54 | 2.37 | 2.46 a | 3.75 | 3.52 | 3.64 a |
| Mean | 1.53 A | 1.36 A | 2.28 A | 1.94 B | 3.13 A | 2.81 B | |||
| FPlant Density | 2.1809 ns | 6.2529 * (Tukey = 0.296) | 10.7108 ** (Tukey = 0.498) | ||||||
| FFertilization | 7.4514 ** (Tukey = 0.316) | 8.2270 ** (Tukey = 0.445) | 37.6659 *** (Tukey = 0.351) | ||||||
| FPlant Density × Fertilization | 0.2151 ns | 0.6731 ns | 0.8870 ns | ||||||
| Fertilization | Plant Density (Plants m−2) | ||||||||
| 200 | 300 | 200 | 300 | 200 | 300 | ||||
| Biomass N Content (%N) | |||||||||
| 2017 | 85 DAS | 100 DAS | 115 DAS | ||||||
| Mean | Mean | Mean | |||||||
| Control | 2.03 | 1.82 | 1.93 c | 1.81 | 1.63 | 1.72 c | 1.68 | 1.59 | 1.64 b |
| Manure | 2.64 | 2.17 | 2.41 b | 2.47 | 1.98 | 2.23 b | 2.29 | 1.85 | 2.07 a |
| Compost | 2.73 | 2.50 | 2.62 b | 2.61 | 2.23 | 2.42 ab | 2.42 | 2.08 | 2.25 a |
| Inorganic | 3.10 | 2.71 | 2.91 a | 2.65 | 2.39 | 2.52 a | 2.40 | 2.21 | 2.31 a |
| Mean | 2.63 A | 2.30 B | 2.39 A | 2.06 B | 2.20 A | 1.93 B | |||
| FPlant Density | 14.3382 ** (Tukey = 0.354) | 12.0698 ** (Tukey = 0.324) | 5.6917 * (Tukey = 0.308) | ||||||
| FFertilization | 22.8282 *** (Tukey= 0.321) | 13.9822 *** (Tukey = 0.316) | 7.2337 ** (Tukey = 0.354) | ||||||
| FPlant Density × Fertilization | 0.5520 ns | 0.4812 ns | 0.4797 ns | ||||||
| 2018 | |||||||||
| Control | 2.17 | 1.90 | 2.04 c | 1.86 | 1.74 | 1.80 b | 1.90 | 1.70 | 1.80 b |
| Manure | 2.82 | 2.29 | 2.56 b | 2.63 | 2.08 | 2.36 a | 2.44 | 1.91 | 2.18 a |
| Compost | 2.74 | 2.68 | 2.71 b | 2.62 | 2.41 | 2.52 a | 2.39 | 2.27 | 2.33 a |
| Inorganic | 3.30 | 2.82 | 3.06 a | 2.81 | 2.47 | 2.64 a | 2.64 | 2.29 | 2.47 a |
| Mean | 2.76 A | 2.42 B | 2.48 A | 2.18 B | 2.34 A | 2.04 B | |||
| FPlant Density | 8.2015 * (Tukey = 0.393) | 7.5441 * (Tukey = 0.301) | 6.2373 * (Tukey = 0.271) | ||||||
| FFertilization | 13.2084 *** (Tukey = 0.398) | 11.0254 *** (Tukey = 0.370) | 5.8531 ** (Tukey = 0.383) | ||||||
| FPlant Density × Fertilization | 0.8515 ns | 0.6719 ns | 0.5636 ns | ||||||
| 2019 | |||||||||
| Control | 1.95 | 1.78 | 1.87 c | 1.74 | 1.59 | 1.67 c | 1.43 | 1.37 | 1.40 c |
| Manure | 2.69 | 2.24 | 2.47 b | 2.51 | 2.04 | 2.28 b | 1.98 | 1.57 | 1.78 b |
| Compost | 2.80 | 2.59 | 2.70 b | 2.69 | 2.31 | 2.50 ab | 2.15 | 1.73 | 1.94 b |
| Inorganic | 3.24 | 2.84 | 3.04 a | 2.77 | 2.51 | 2.64 a | 2.29 | 2.18 | 2.24 a |
| Mean | 2.67 A | 2.36 B | 2.43 A | 2.11 B | 1.96 A | 1.71 B | |||
| FPlant Density | 11.6009 ** (Tukey = 0.295) | 9.6582 ** (Tukey = 0.237) | 6.6947 * (Tukey = 0.229) | ||||||
| FFertilization | 29.9780 *** (Tukey = 0.322) | 18.0244 *** (Tukey = 0.346) | 12.816 *** (Tukey = 0.265) | ||||||
| FPlant Density × Fertilization | 0.5447 ns | 0.4465 ns | 0.9498 ns | ||||||
| Fertilization | Plant Density (Plants m−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 300 | 200 | 300 | 200 | 300 | 200 | 300 | |||||
| Biomass N Uptake (kg N ha−1) | Total Plant N Uptake (kg N ha−1) | Seed N Content (%N) | Seed N Uptake (kg N ha−1) | |||||||||
| 2017 | Mean | Mean | Mean | Mean | ||||||||
| Control | 23.88 | 42.03 | 32.96 b | 38.71 | 51.71 | 45.21 c | 2.88 | 2.80 | 2.84 c | 14.83 | 9.67 | 12.25 d |
| Manure | 41.89 | 52.77 | 47.33 ab | 64.96 | 64.24 | 64.60 b | 3.76 | 3.00 | 3.38 b | 23.06 | 11.47 | 17.27 c |
| Compost | 51.72 | 68.74 | 60.23 a | 81.88 | 86.27 | 84.08 a | 3.87 | 3.54 | 3.71 b | 30.16 | 17.54 | 23.85 b |
| Inorganic | 43.35 | 74.75 | 59.05 a | 77.95 | 97.51 | 87.73 a | 4.31 | 4.09 | 4.20 a | 34.60 | 22.76 | 28.68 a |
| Mean | 40.21 B | 59.57 A | 65.88 A | 74.93 A | 3.71 A | 3.36 B | 25.66 A | 15.36 B | ||||
| FPlant Density | 12.2542 ** (Tukey = 14.188) | 2.2639 ns | 7.9482 * (Tukey = 0.499) | 88.9179 *** (Tukey = 6.009) | ||||||||
| FFertilization | 5.2763 * (Tukey = 20.011) | 10.6237 *** (Tukey = 17.738) | 21.7320 *** (Tukey = 0.427) | 43.7798 *** (Tukey = 3.639) | ||||||||
| FPlant Density × Fertilization | 0.6103 ns | 0.5589 ns | 1.4044 ns | 2.5077 ns | ||||||||
| 2018 | ||||||||||||
| Control | 26.61 | 39.22 | 32.92 b | 38.87 | 48.84 | 43.86 c | 3.05 | 2.59 | 2.82 c | 12.27 | 9.62 | 10.95 d |
| Manure | 39.38 | 45.40 | 42.39 ab | 61.54 | 58.03 | 56.79 b | 3.82 | 2.94 | 3.38 bc | 22.16 | 12.63 | 17.40 c |
| Compost | 45.16 | 64.52 | 54.84 a | 69.30 | 85.20 | 77.25 a | 3.63 | 3.65 | 3.64 ab | 24.14 | 20.68 | 22.41 b |
| Inorganic | 46.84 | 59.41 | 53.13 a | 79.89 | 84.65 | 82.27 a | 4.93 | 3.81 | 4.37 a | 33.05 | 25.25 | 29.15 a |
| Mean | 39.50 B | 52.14 A | 62.40 A | 69.18 A | 3.86 A | 3.25 B | 22.91 A | 17.05 B | ||||
| FPlant Density | 9.1053 ** (Tukey = 10.975) | 1.7885 ns | 6.0353 * (Tukey = 0.551) | 16.4998 *** (Tukey = 6.556) | ||||||||
| FFertilization | 5.9493 ** (Tukey = 14.189) | 11.9203 *** (Tukey= 16.442) | 7.6640 ** (Tukey = 0.634) | 28.5723 *** (Tukey = 3.748) | ||||||||
| FPlant Density × Fertilization | 0.4223 ns | 0.6585 ns | 0.9271 ns | 1.3379 ns | ||||||||
| 2019 | ||||||||||||
| Control | 22.68 | 37.31 | 30.00 c | 33.72 | 44.70 | 39.21 c | 2.20 | 2.12 | 2.16 c | 11.04 | 7.39 | 9.22 d |
| Manure | 33.87 | 39.62 | 36.74 b | 56.27 | 52.87 | 54.57 b | 3.55 | 3.36 | 3.46 b | 22.40 | 13.26 | 17.83 c |
| Compost | 44.65 | 54.84 | 49.75 a | 74.81 | 72.31 | 73.56 a | 3.65 | 3.46 | 3.56 b | 30.16 | 17.48 | 23.82 b |
| Inorganic | 43.22 | 68.96 | 56.09 a | 76.41 | 91.72 | 84.07 a | 3.97 | 3.98 | 3.98 a | 33.19 | 22.76 | 27.98 a |
| Mean | 36.11 B | 50.18 A | 60.30 A | 65.40 A | 3.34 A | 3.23 A | 24.20 A | 15.22 B | ||||
| FPlant Density | 11.0706 ** (Tukey = 12.232) | 1.3639 ns | 1.5880 ns | 128.0487 *** (Tukey = 6.559) | ||||||||
| FFertilization | 7.9176 ** (Tukey = 16.335) | 20.8526 *** (Tukey = 13.162) | 76.5050 *** (Tukey = 0.254) | 105.3903 *** (Tukey = 3.652) | ||||||||
| FPlant Density × Fertilization | 1.0293 ns | 1.1758 ns | 0.3006 ns | 5.8659 ns | ||||||||
| Fertilization | Plant Density (Plants m−2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 300 | 200 | 300 | 200 | 300 | 200 | 300 | |||||
| N Harvest Index (NHI) | Apparent N Recovery Efficiency (ANRE) (%) | N Utilization Efficiency (NUtE) (kg kg−1) | N Agronomic Efficiency (NAE) (kg kg−1) | |||||||||
| 2017 | Mean | Mean | Mean | Mean | ||||||||
| Control | 0.383 | 0.188 | 0.286 b | - | - | - | 13.29 | 6.68 | 9.99 a | - | - | - |
| Manure | 0.357 | 0.184 | 0.271 b | 86.35 | 41.23 | 63.79 a | 9.49 | 6.39 | 7.94 b | 3.27 | 1.65 | 2.46 b |
| Compost | 0.369 | 0.204 | 0.287 b | 107.94 | 86.42 | 97.18 a | 9.52 | 5.76 | 7.64 b | 6.61 | 3.72 | 5.17 a |
| Inorganic | 0.452 | 0.243 | 0.348 a | 87.20 | 101.79 | 94.50 a | 10.56 | 5.97 | 8.27 b | 6.34 | 4.68 | 5.51 a |
| Mean | 0.390 A | 0.205 B | 93.83 A | 76.48 A | 10.72 A | 6.20 B | 5.41 A | 3.35 A | ||||
| FPlant Density | 97.9952 *** (Tukey = 0.0429) | 0.7851 ns | 63.8975 *** (Tukey = 1.409) | 4.6058 ns | ||||||||
| FFertilization | 3.3397 * (Tukey = 0.0323) | 1.1973 ns | 3.4509 * (Tukey = 1.621) | 4.0459 * (Tukey = 2.318) | ||||||||
| FPlant Density × Fertilization | 0.2970 ns | 0.7864 ns | 1.8166 ns | 0.1899 ns | ||||||||
| 2018 | ||||||||||||
| Control | 0.317 | 0.197 | 0.257 b | - | - | - | 10.42 | 7.59 | 9.01 a | - | - | |
| Manure | 0.363 | 0.223 | 0.293 b | 74.57 | 30.21 | 52.39 a | 9.52 | 7.65 | 8.58 a | 6.06 | 1.85 | 3.96 a |
| Compost | 0.352 | 0.242 | 0.297 b | 76.08 | 90.88 | 83.48 a | 9.70 | 6.78 | 8.24 a | 6.70 | 4.94 | 5.82 a |
| Inorganic | 0.425 | 0.296 | 0.361 a | 91.15 | 79.59 | 85.37 a | 9.79 | 7.78 | 8.79 a | 7.71 | 6.37 | 7.04 a |
| Mean | 0.364 A | 0.240 B | 80.60 A | 66.89 A | 9.86 A | 7.45 B | 6.82 A | 4.39 A | ||||
| FPlant Density | 43.4772 *** (Tukey = 0.0472) | 0.5093 ns | 24.2701 *** (Tukey = 0.923) | 3.7986 ns | ||||||||
| FFertilization | 5.2374 * (Tukey = 0.0563) | 1.2400 ns | 0.4454 ns | 2.0503 ns | ||||||||
| FPlant Density × Fertilization | 0.1118 ns | 0.7942 ns | 0.3020 ns | 0.5102 ns | ||||||||
| 2019 | ||||||||||||
| Control | 0.328 | 0.167 | 0.248 b | - | - | - | 14.90 | 7.88 | 11.39 a | - | - | |
| Manure | 0.399 | 0.247 | 0.323 a | 74.18 | 26.88 | 50.53 b | 11.23 | 7.37 | 9.30 b | 4.22 | 1.56 | 2.89 b |
| Compost | 0.403 | 0.248 | 0.326 a | 102.73 | 69.03 | 85.88 ab | 11.04 | 7.18 | 9.11 b | 8.10 | 3.88 | 5.99 a |
| Inorganic | 0.456 | 0.250 | 0.353 a | 94.86 | 104.48 | 99.67 a | 11.39 | 6.27 | 8.83 b | 7.51 | 4.95 | 6.23 a |
| Mean | 0.397 A | 0.228 B | 90.59 A | 66.80 A | 12.14 A | 7.18 B | 6.61 A | 3.46 B | ||||
| FPlant Density | 52.9955 *** (Tukey = 0.0544) | 2.6135 ns | 92.7315 *** (Tukey = 1.401) | 9.6704 ** (Tukey = 2.493) | ||||||||
| FFertilization | 3.8349 * (Tukey = 0.0975) | 3.9543 * (Tukey = 41.301) | 5.1415 * (Tukey = 1.343) | 4.5135 * (Tukey = 2.975) | ||||||||
| FPlant Density × Fertilization | 0.2896 ns | 1.3600 ns | 2.0984 ns | 0.2822 ns | ||||||||
| Trait | Coefficient of Correlation (r) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Organic Matter (SOM) | Soil Total Nitrogen (STN) | Plant Height | Leaf Area Index (LAI) | Seed Yield | Harvest Index (HI) | 1000 Seed Weight | Biomass N Content 75 DAS | Biomass N Uptake | Total Plant N Uptake | Seed N Content | Seed N Uptake | N Harvest Index (NHI) | Apparent N Recovery Efficiency (ANRE) | Ν Utilization Efficiency (NUtE) | |
| Soil Total Nitrogen (STN) | 0.5515 *** | ||||||||||||||
| Plant Height | 0.2150 ns | 0.2958 * | |||||||||||||
| Leaf Area Index (LAI) | 0.0893 ns | 0.2836 * | 0.6263 *** | ||||||||||||
| Seed Yield | 0.1639 ns | 0.3130 ** | 0.7711 *** | 0.4676 *** | |||||||||||
| Harvest Index (HI) | 0.0162 ns | 0.1017 ns | 0.1987 ns | −0.2077 ns | 0.6806 *** | ||||||||||
| 1000 Seed Weight | −0.3074 ns | −0.0077 ns | 0.1965 ns | 0.2333 * | 0.2360 * | 0.1363 ns | |||||||||
| Biomass N Content 75 DAS | 0.1686 ns | 0.4463 *** | 0.6613 *** | 0.6839 *** | 0.7717 *** | 0.4066 *** | 0.2554 * | ||||||||
| Biomass N Uptake | 0.1430 ns | 0.1567 ns | 0.3949 *** | 0.7980 *** | 0.1791 ns | −0.4090 *** | 0.1317 ns | 0.4134 *** | |||||||
| Total Plant N Uptake | 0.1819 ns | 0.2633 * | 0.6469 *** | 0.8865 *** | 0.5307 *** | −0.0969 ns | 0.2121 ns | 0.7002 *** | 0.9189 *** | ||||||
| Seed N Content | 0.2215 ns | 0.3467 ** | 0.6493 *** | 0.6730 *** | 0.6319 *** | 0.2659 * | 0.2214 ns | 0.8910 *** | 0.4115 *** | 0.6775 *** | |||||
| Seed N Uptake | 0.1631 ns | 0.3344 ** | 0.8019 *** | 0.5969 *** | 0.9413 *** | 0.5637 *** | 0.2587 * | 0.8956 *** | 0.2808 * | 0.6366 *** | 0.8438 *** | ||||
| N Harvest Index (NHI) | 0.0582 ns | 0.1990 ns | 0.4549 *** | −0.0113 ns | 0.7344 *** | 0.8391 *** | 0.1481 ns | 0.5441 *** | −0.4296 *** | −0.0515 ns | 0.5086 *** | 0.7146 *** | |||
| Apparent N Recovery Efficiency (ANRE) | −0.1835 ns | −0.0217 ns | 0.6979 *** | 0.6321 *** | 0.5394 *** | 0.0088 ns | 0.1447 ns | 0.5964 *** | 0.6667 *** | 0.8550 *** | 0.4750 *** | 0.5731 *** | 0.0338ns | ||
| N Utilization Efficiency (NUtE) | −0.1343 ns | −0.0616 ns | 0.0492 ns | −0.4813 *** | 0.3714 ** | 0.7525 *** | −0.0026 ns | −0.0257 ns | −0.7494 *** | −0.5257 *** | −0.1526 ns | 0.1860ns | 0.7516 *** | -0.1671ns | |
| N Agronomic Efficiency (NAE) | −0.2682 ns | 0.1042 ns | 0.5535 *** | 0.3699 ** | 0.8375 *** | 0.4921 *** | 0.2129 ns | 0.6015 *** | 0.1539 ns | 0.4541 *** | 0.2810 * | 0.7316 *** | 0.4428 *** | 0.6105 *** | 0.4081 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussis, I.; Kakabouki, I.; Beslemes, D.; Tigka, E.; Kosma, C.; Triantafyllidis, V.; Mavroeidis, A.; Zotos, A.; Bilalis, D. Nitrogen Uptake, Use Efficiency, and Productivity of Nigella sativa L. in Response to Fertilization and Plant Density. Sustainability 2022, 14, 3842. https://doi.org/10.3390/su14073842
Roussis I, Kakabouki I, Beslemes D, Tigka E, Kosma C, Triantafyllidis V, Mavroeidis A, Zotos A, Bilalis D. Nitrogen Uptake, Use Efficiency, and Productivity of Nigella sativa L. in Response to Fertilization and Plant Density. Sustainability. 2022; 14(7):3842. https://doi.org/10.3390/su14073842
Chicago/Turabian StyleRoussis, Ioannis, Ioanna Kakabouki, Dimitrios Beslemes, Evangelia Tigka, Chariklia Kosma, Vassilios Triantafyllidis, Antonios Mavroeidis, Anastasios Zotos, and Dimitrios Bilalis. 2022. "Nitrogen Uptake, Use Efficiency, and Productivity of Nigella sativa L. in Response to Fertilization and Plant Density" Sustainability 14, no. 7: 3842. https://doi.org/10.3390/su14073842






