Changes in Mangrove Carbon Stocks and Exposure to Sea Level Rise (SLR) under Future Climate Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mangrove Data
2.2. Data for Predictors
2.3. Data Analysis for Quantifying Biomass Variation under the Different Scenarios
2.4. Sea Level Rises (SLR) under Future Climate Scenarios
3. Results
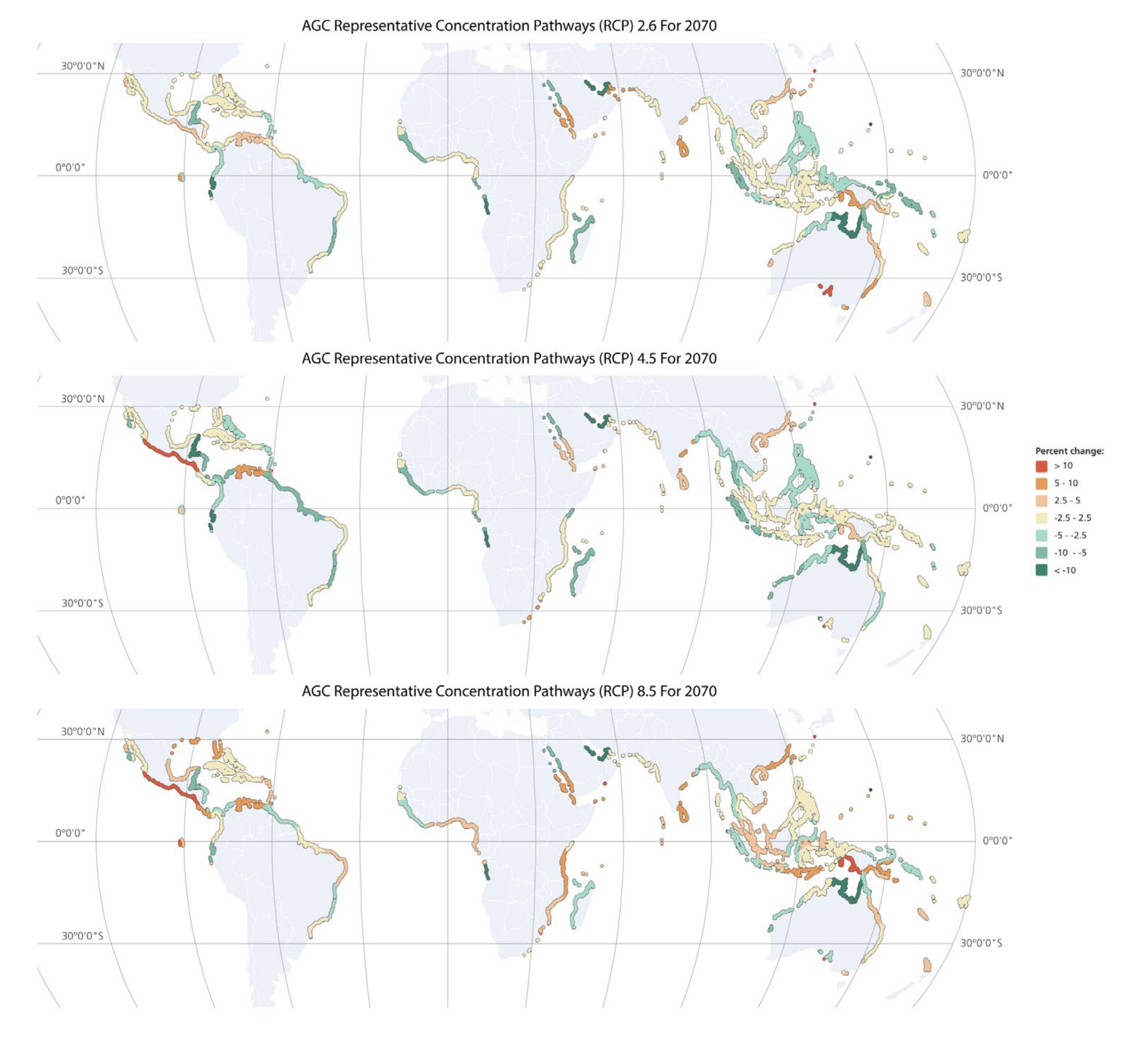
3.1. Modelling Mangrove AGC Variation under the Present and Future Climate Scenarios
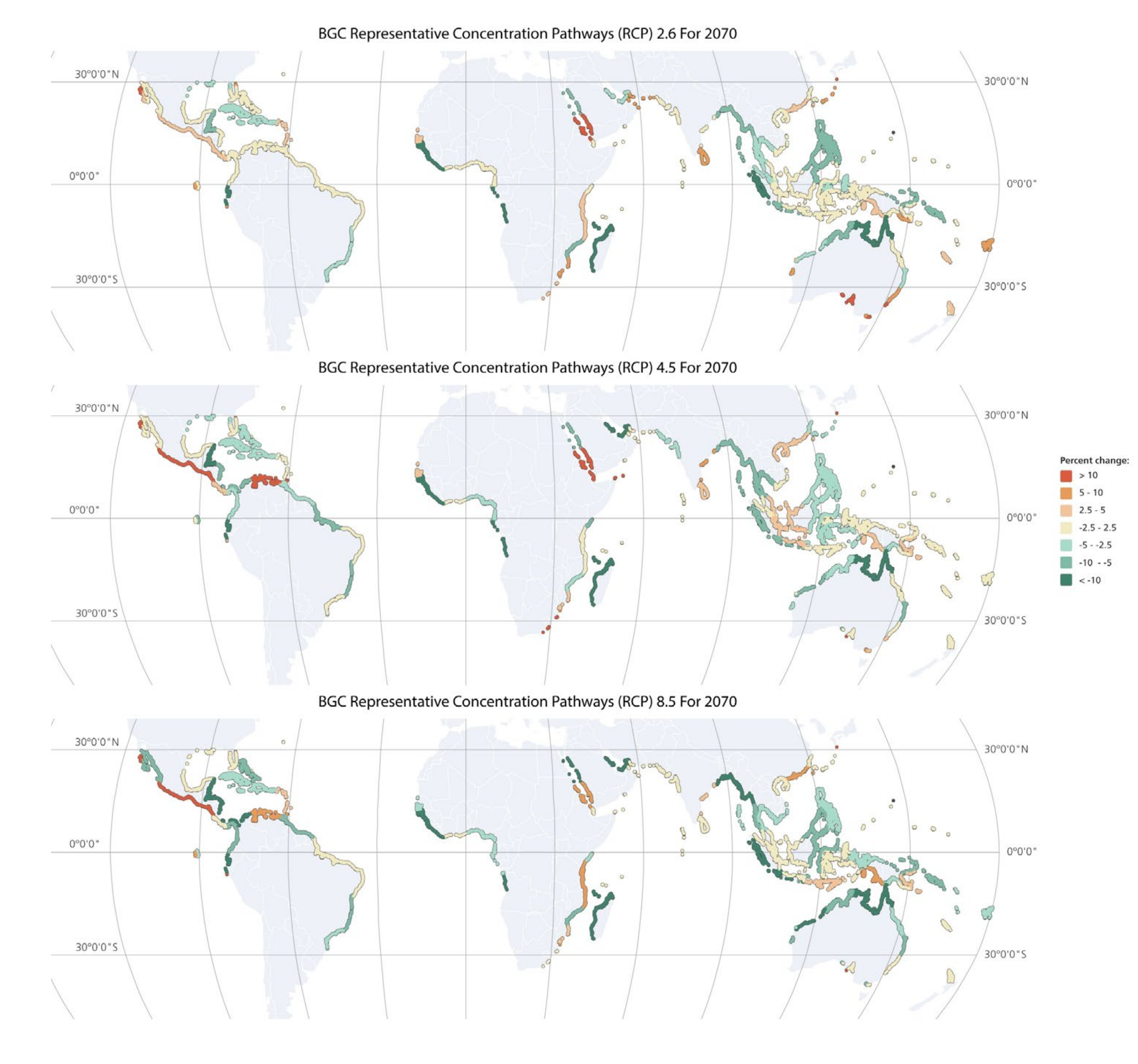
3.2. Modelling Mangrove BGC Stocks under the Present and Future Climate Scenarios
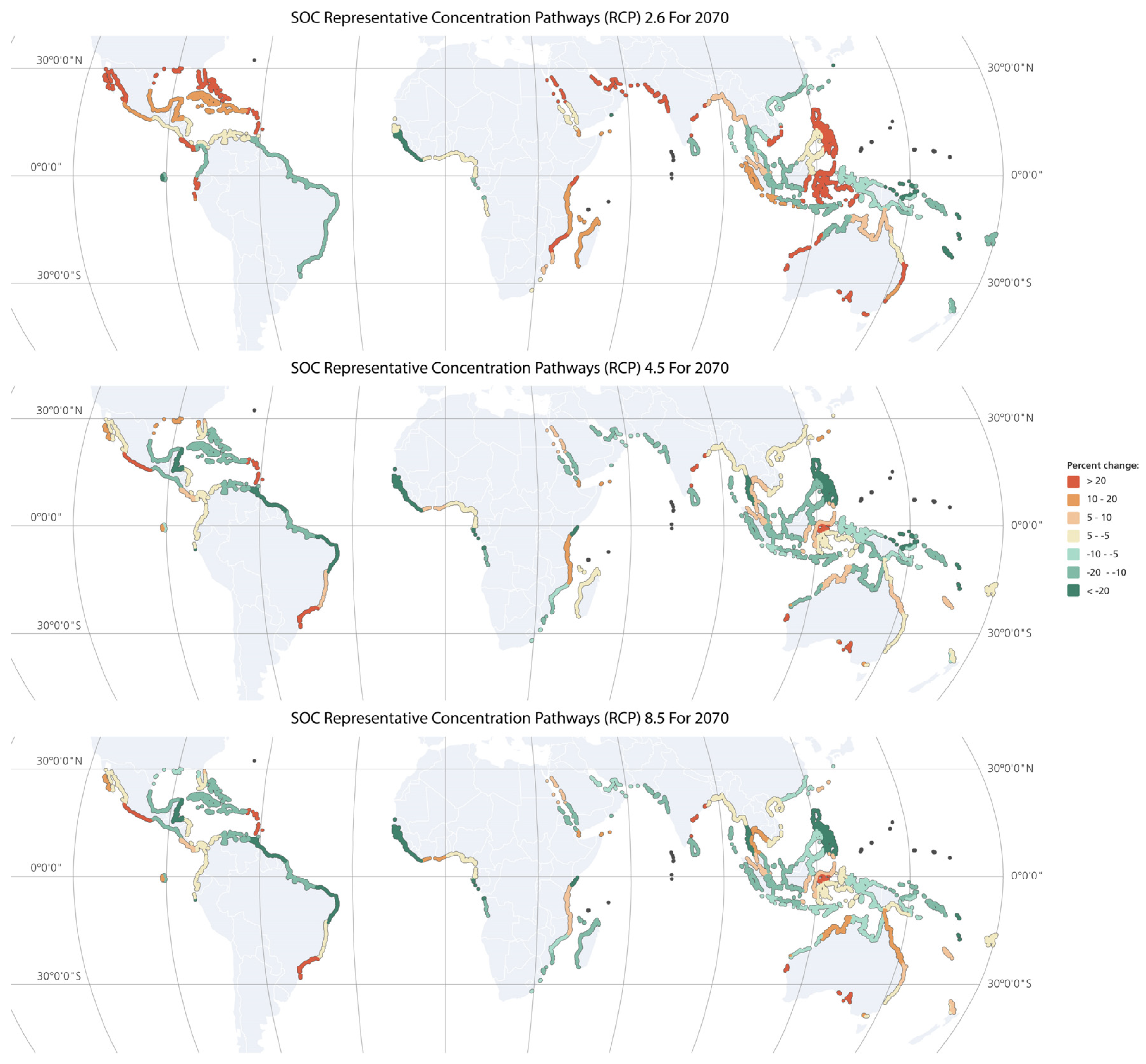
3.3. Modelling Mangrove SOC Stocks under the Present and Future Climate Scenarios
3.4. Modelling Mangrove Total Carbon Stocks under the Present and Future Climate Scenarios
3.5. Modelling Mangrove Sea-Level Rise Exposure under the Present and Future Climate Scenarios
4. Discussion
4.1. Drivers of Blue Carbon Stocks
4.2. Conservation Implications
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Brander, L.M.; Wagtendonk, A.J.; Hussain, S.S.; McVittie, A.; Verburg, P.H.; de Groot, R.S.; van der Ploeg, S. Ecosystem service values for mangroves in Southeast Asia: A meta-analysis and value transfer application. Ecosyst. Serv. 2012, 1, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Friess, D.A. Where the tallest mangroves are. Nat. Geosci. 2018, 12, 4–5. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Cycling and Storage in Mangrove Forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, J.B.; Bhomia, R.K. Ecosystem carbon stocks of mangroves across broad environmental gradients in West-Central Africa: Global and regional comparisons. PLoS ONE 2017, 12, e0187749. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.G.; Ratsimba, H.R.; Ravaoarinorotsihoarana, L.; Cripps, G.; Bey, A. Ecological Segregation of the Late Jurassic Stegosaurian and Iguanodontian Dinosaurs of the Morrison Formation in North America: Pronounced or Subtle? Forests 2014, 5, 177–205. [Google Scholar] [CrossRef] [Green Version]
- Sanders, C.J.; Maher, D.; Tait, D.; Williams, D.; Holloway, C.; Sippo, J.Z.; Santos, I. Are global mangrove carbon stocks driven by rainfall? J. Geophys. Res. Biogeosci. 2016, 121, 2600–2609. [Google Scholar] [CrossRef]
- Simard, M.; Fatoyinbo, L.; Smetanka, C.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Thomas, N.; Van Der Stocken, T. Mangrove canopy height globally related to precipitation, temperature and cyclone frequency. Nat. Geosci. 2019, 12, 40–45. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Castañeda-Moya, E.; Midway, S.R.; Friess, D.A.; Trettin, C.C.; Bukoski, J.J.; Stovall, A.E.; Pagliosa, P.R.; Fonseca, A.L.; et al. Macroecological patterns of forest structure and allometric scaling in mangrove forests. Glob. Ecol. Biogeogr. 2021, 30, 1000–1013. [Google Scholar] [CrossRef]
- Hutchison, J.; Manica, A.; Swetnam, R.; Balmford, A.; Spalding, M. Predicting Global Patterns in Mangrove Forest Biomass: Global patterns in mangrove biomass. Conserv. Lett. 2014, 7, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T. Blue Carbon in Shallow Coastal Ecosystems. Blue Carbon Shallow Coast. Ecosyst. 2019. [Google Scholar] [CrossRef]
- Walcker, R.; Gandois, L.; Proisy, C.; Corenblit, D.; Mougin, É.; Laplanche, C.; Ray, R.; Fromard, F. Control of “blue carbon” storage by mangrove ageing: Evidence from a 66-year chronosequence in French Guiana. Glob. Change Biol. 2018, 24, 2325–2338. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; MacKenzie, R.A. Impacts of climate change on mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 2018, 14, 20180251. [Google Scholar] [CrossRef] [PubMed]
- Rovai, A.S.; Coelho, C., Jr.; de Almeida, R.; Cunha-Lignon, M.; Menghini, R.P.; Twilley, R.R.; Cintrón-Molero, G.; Schaeffer-Novelli, Y. Ecosystem-level carbon stocks and sequestration rates in mangroves in the Cananéia-Iguape lagoon estuarine system, southeastern Brazil. For. Ecol. Manag. 2020, 479, 118553. [Google Scholar] [CrossRef]
- Thompson, B.S.; Clubbe, C.P.; Primavera, J.H.; Curnick, D.; Koldewey, H.J. Locally assessing the economic viability of blue carbon: A case study from Panay Island, the Philippines. Ecosyst. Serv. 2014, 8, 128–140. [Google Scholar] [CrossRef]
- Rovai, A.S.; Twilley, R.R.; Worthington, T.A.; Riul, P. Brazilian Mangroves: Blue Carbon Hotspots of National and Global Relevance to Natural Climate Solutions. Front. For. Glob. Chang. 2022, 4, 217. [Google Scholar] [CrossRef]
- Daniel, F.; Sidik, F. Dynamic Sedimentary Environments of Mangrove Coasts; Daniel, F., Sidik, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Qiu, P.; Wang, D.; Zou, X.; Yang, X.; Xie, G.; Xu, S.; Zhong, Z. Finer Resolution Estimation and Mapping of Mangrove Biomass Using UAV LiDAR and WorldView-2 Data. Forests 2019, 10, 871. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Evans, D.; Friess, D.A.; Tan, B.S.; Nin, C.S. Mapping Above-Ground Biomass in a Tropical Forest in Cambodia Using Canopy Textures Derived from Google Earth. Remote Sens. 2015, 7, 5057–5076. [Google Scholar] [CrossRef] [Green Version]
- Cleyndert, G.D.J.; Cuni-Sanchez, A.; Seki, H.A.; Shirima, D.D.; Munishi, P.K.T.; Burgess, N.; Calders, K.; Marchant, R. The effects of seaward distance on above and below ground carbon stocks in estuarine mangrove ecosystems. Carbon Balance Manag. 2020, 15, 27. [Google Scholar] [CrossRef]
- Wang, D.; Wan, B.; Qiu, P.; Zuo, Z.; Wang, R.; Wu, X. Mapping Height and Aboveground Biomass of Mangrove Forests on Hainan Island Using UAV-LiDAR Sampling. Remote Sens. 2019, 11, 2156. [Google Scholar] [CrossRef] [Green Version]
- Estrada, G.C.D.; Soares, M.L.G. Global Patterns of Aboveground Carbon Stock and Sequestration in Mangroves. An. Acad. Bras. Cienc. 2017, 89, 973–989. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.M.; Murdiyarso, D.; Fourqurean, J.; Kauffman, J.B.; Hutahaean, A.; Crooks, S.; Lovelock, C.; Howard, J.B.; Herr, D.; Fortes, M.D.; et al. Indonesia’s blue carbon: A globally significant and vulnerable sink for seagrass and mangrove carbon. Wetl. Ecol. Manag. 2015, 24, 3–13. [Google Scholar] [CrossRef]
- Atwood, T.B.; Connolly, R.M.; Almahasheer, H.; Carnell, P.E.; Duarte, C.M.; Lewis, C.J.E.; Irigoien, X.; Kelleway, J.J.; Lavery, P.S.; Macreadie, P.I.; et al. Global patterns in mangrove soil carbon stocks and losses. Nat. Clim. Chang. 2017, 7, 523–528. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Van der Stocken, T.; Carroll, D.; Menemenlis, D.; Simard, M.; Koedam, N. Global-scale dispersal and connectivity in mangroves. Proc. Natl. Acad. Sci. USA 2018, 116, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adame, M.F.; Roberts, M.E.; Hamilton, D.P.; Ndehedehe, C.E.; Reis, V.; Lu, J.; Griffiths, M.; Curwen, G.; Ronan, M. Tropical Coastal Wetlands Ameliorate Nitrogen Export During Floods. Front. Mar. Sci. 2019, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Mafi-Gholami, D.; Zenner, E.K.; Jaafari, A. Mangrove regional feedback to sea level rise and drought intensity at the end of the 21st century. Ecol. Indic. 2019, 110, 105972. [Google Scholar] [CrossRef]
- Payo, A.; Mukhopadhyay, A.; Hazra, S.; Ghosh, T.; Ghosh, S.; Brown, S.; Nicholls, R.J.; Bricheno, L.; Wolf, J.; Kay, S.; et al. Projected changes in area of the Sundarban mangrove forest in Bangladesh due to SLR by 2100. Clim. Chang. 2016, 139, 279–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovelock, C.E.; Cahoon, D.R.; Friess, D.A.; Guntenspergen, G.R.; Krauss, K.W.; Reef, R.; Rogers, K.; Saunders, M.L.; Sidik, F.; Swales, A.; et al. The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 2015, 526, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Mpelasoka, F.; Awange, J.L.; Goncalves, R.M. Accounting for dynamics of mean precipitation in drought projections: A case study of Brazil for the 2050 and 2070 periods. Sci. Total Environ. 2018, 622–623, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Tramblay, Y.; Badi, W.; Driouech, F.; El Adlouni, S.; Neppel, L.; Servat, E. Climate change impacts on extreme precipitation in Morocco. Glob. Planet. Chang. 2012, 82, 104–114. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and distribution of mangrove forests of the world using earth observation satellite data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Center for International Forestry Research (CIFOR). The Sustainable Wetlands Adaptation and Mitigation Program (SWAMP). Available online: https://www2.cifor.org/swamp/ (accessed on 3 January 2022).
- Kolka, R.K.; Murdiyarso, D.; Kauffman, J.B.; Birdsey, R.A. Tropical wetlands, climate, and land-use change: Adaptation and mitigation opportunities. Wetl. Ecol. Manag. 2016, 24, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.E.; Friess, D.A. Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat. Clim. Chang. 2018, 8, 240–244. [Google Scholar] [CrossRef] [Green Version]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Mewded, B.; Lemessa, D. Factors affecting woody carbon stock in Sirso moist evergreen Afromontane forest, southern Ethiopia: Implications for climate change mitigation. Environ. Dev. Sustain. 2019, 22, 6363–6378. [Google Scholar] [CrossRef]
- Andriamananjara, A.; Ranaivoson, N.; Razafimbelo, T.M.; Hewson, J.; Ramifehiarivo, N.; Rasolohery, A.; Andrisoa, R.H.; Razafindrakoto, M.A.; Razafimanantsoa, M.-P.; Rabetokotany, N.; et al. Towards a better understanding of soil organic carbon variation in Madagascar. Eur. J. Soil Sci. 2017, 68, 930–940. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Yang, Y.; Javanroodi, K.; Nik, V.M. Climate Change and Renewable Energy Generation in Europe—Long-Term Impact Assessment on Solar and Wind Energy Using High-Resolution Future Climate Data and Considering Climate Uncertainties. Energies 2022, 15, 302. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.-F. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Cabrera, C.V.P.; Selvaraj, J.J. Geographic shifts in the bioclimatic suitability for Aedes aegypti under climate change scenarios in Colombia. Heliyon 2019, 6, e03101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Luis, M.; Álvarez-Jiménez, J.; Labarga, J.M.M.; Bartolomé, C. Four climate change scenarios for Gypsophila bermejoi G. López (Caryophyllaceae) to address whether bioclimatic and soil suitability will overlap in the future. PLoS ONE 2019, 14, e0218160. [Google Scholar] [CrossRef] [Green Version]
- Yigini, Y.; Panagos, P. Assessment of soil organic carbon stocks under future climate and land cover changes in Europe. Sci. Total Environ. 2016, 557–558, 838–850. [Google Scholar] [CrossRef]
- Dimobe, K.; Kouakou, J.L.N.; Tondoh, J.E.; Zoungrana, B.J.-B.; Forkuor, G.; Ouédraogo, K. Predicting the Potential Impact of Climate Change on Carbon Stock in Semi-Arid West African Savannas. Land 2018, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef]
- Wilson, R. Impacts of Climate Change on Fisheries in the Coastal and Marine Environments of Caribbean Small Island Developing States. Sci. Rev. 2017, 1994, 23–30. [Google Scholar]
- Chen, L.; Wang, Y.; Ren, C.; Zhang, B.; Wang, Z. Optimal Combination of Predictors and Algorithms for Forest Above-Ground Biomass Mapping from Sentinel and SRTM Data. Remote Sens. 2019, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Simard, M.; Zhang, K.; Rivera-Monroy, V.H.; Ross, M.S.; Ruiz, P.L.; Castañeda-Moya, E.; Twilley, R.R.; Rodriguez, E. Mapping Height and Biomass of Mangrove Forests in Everglades National Park with SRTM Elevation Data. Photogramm. Eng. Remote Sens. 2006, 72, 299–311. [Google Scholar] [CrossRef]
- Bai, J.; Meng, Y.; Gou, R.; Lyu, J.; Dai, Z.; Diao, X.; Zhang, H.; Luo, Y.; Zhu, X.; Lin, G. Mangrove diversity enhances plant biomass production and carbon storage in Hainan island, China. Funct. Ecol. 2021, 35, 774–786. [Google Scholar] [CrossRef]
- Singh, M.; Friess, D.A.; Vilela, B.; De Alban, J.D.T.; Monzon, A.K.V.; Veridiano, R.K.A.; Tumaneng, R.D. Spatial relationships between above-ground biomass and bird species biodiversity in Palawan, Philippines. PLoS ONE 2017, 12, e0186742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wan, B.; Liu, J.; Su, Y.; Guo, Q.; Qiu, P.; Wu, X. Estimating aboveground biomass of the mangrove forests on northeast Hainan Island in China using an upscaling method from field plots, UAV-LiDAR data and Sentinel-2 imagery. Int. J. Appl. Earth Obs. Geoinf. 2019, 85, 101986. [Google Scholar] [CrossRef]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B. Caret: Classification and Regression Training. R Package Version 6.0-86; RStudio: Boston, MA, USA, 2020. [Google Scholar]
- Singh, M.; Evans, D.; Coomes, D.A.; Friess, D.A.; Tan, B.S.; Nin, C.S. Incorporating Canopy Cover for Airborne-Derived Assessments of Forest Biomass in the Tropical Forests of Cambodia. PLoS ONE 2016, 11, e0154307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, B.P.; Khan, N.S.; Cahill, N.; Lee, J.S.H.; Shaw, T.A.; Garner, A.J.; Kemp, A.C.; Engelhart, S.E.; Rahmstorf, S. Estimating global mean sea-level rise and its uncertainties by 2100 and 2300 from an expert survey. NPJ Clim. Atmos. Sci. 2020, 3, 18. [Google Scholar] [CrossRef]
- Dang, A.T.; Kumar, L.; Reid, M.; Anh, L.N.T. Modelling the susceptibility of wetland plant species under climate change in the Mekong Delta, Vietnam. Ecol. Inform. 2021, 64, 101358. [Google Scholar] [CrossRef]
- Gesch, D.B. Best Practices for Elevation-Based Assessments of Sea-Level Rise and Coastal Flooding Exposure. Front. Earth Sci. 2018, 6, 230. [Google Scholar] [CrossRef] [Green Version]
- Minderhoud, P.S.J.; Coumou, L.; Erkens, G.; Middelkoop, H.; Stouthamer, E. Mekong delta much lower than previously assumed in sea-level rise impact assessments. Nat. Commun. 2019, 10, 3847. [Google Scholar] [CrossRef]
- Rogers, K.; Saintilan, N.; Mazumder, D.; Kelleway, J. Mangrove dynamics and blue carbon sequestration. Biol. Lett. 2019, 15, 20180471. [Google Scholar] [CrossRef] [Green Version]
- Panpeng, J.; Ahmad, M.M. Vulnerability of Fishing Communities from Sea-Level Change: A Study of Laemsing District in Chanthaburi Province, Thailand. Sustainability 2017, 9, 1388. [Google Scholar] [CrossRef] [Green Version]
- Chatting, M.; Al-Maslamani, I.; Walton, M.; Skov, M.W.; Kennedy, H.; Husrevoglu, Y.S.; Le Vay, L. Future Mangrove Carbon Storage Under Climate Change and Deforestation. Front. Mar. Sci. 2022, 9, 781876. [Google Scholar] [CrossRef]
- Chatting, M.; LeVay, L.; Walton, M.; Skov, M.W.; Kennedy, H.; Wilson, S.; Al-Maslamani, I. Mangrove carbon stocks and biomass partitioning in an extreme environment. Estuar. Coast. Shelf Sci. 2020, 244, 106940. [Google Scholar] [CrossRef]
- Banerjee, K.; Sahoo, C.K.; Bal, G.; Mallik, K.; Paul, R.; Mitra, A. High blue carbon stock in mangrove forests of Eastern India. Trop. Ecol. 2020, 61, 150–167. [Google Scholar] [CrossRef]
- Atsbha, T.; Desta, A.B.; Zewdu, T. Carbon sequestration potential of natural vegetation under grazing influence in Southern Tigray, Ethiopia: Implication for climate change mitigation. Heliyon 2019, 5, e02329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Gandhi, S.; Jones, T.G. Identifying Mangrove Deforestation Hotspots in South Asia, Southeast Asia and Asia-Pacific. Remote Sens. 2019, 11, 728. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; MacKenzie, R.A.; Tieng, T.; Soben, K.; Tulyasuwan, N.; Resanond, A.; Blate, G.; Litton, C.M. The impacts of degradation, deforestation and restoration on mangrove ecosystem carbon stocks across Cambodia. Sci. Total Environ. 2019, 706, 135416. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.F.; Estrada, E.A.; Ortiz, L.F.; Urrego, L.E. Ecosystem-Wide Impacts of Deforestation in Mangroves: The Urabá Gulf (Colombian Caribbean) Case Study. ISRN Ecol. 2012, 2012, 958709. [Google Scholar] [CrossRef] [Green Version]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Saura, S.; Bastin, L.; Battistella, L.; Mandrici, A.; Dubois, G. Protected areas in the world’s ecoregions: How well connected are they? Ecol. Indic. 2017, 76, 144–158. [Google Scholar] [CrossRef]
- Saenger, P.; Ragavan, P.; Sheue, C.-R.; López-Portillo, J.; Yong, J.W.H.; Mageswaran, T. Mangrove Biogeography of the Indo-Pacific. In Sabkha Ecosystems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 379–400. [Google Scholar] [CrossRef]
- Banerjee, K.; Gatti, R.C.; Mitra, A. Climate change-induced salinity variation impacts on a stenoecious mangrove species in the Indian Sundarbans. Ambio 2016, 46, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasmito, S.D.; Murdiyarso, D.; Friess, D.A.; Kurnianto, S. Can mangroves keep pace with contemporary sea level rise? A global data review. Wetl. Ecol. Manag. 2015, 24, 263–278. [Google Scholar] [CrossRef]
- Di Nitto, D.; Neukermans, G.; Koedam, N.; Defever, H.; Pattyn, F.; Kairo, J.G.; Dahdouh-Guebas, F. Mangroves facing climate change: Landward migration potential in response to projected scenarios of sea level rise. Biogeosciences 2014, 11, 857–871. [Google Scholar] [CrossRef] [Green Version]
- Suyadi; Gao, J.; Lundquist, C.J.; Schwendenmann, L. Land-based and climatic stressors of mangrove cover change in the Auckland Region, New Zealand. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1466–1483. [Google Scholar] [CrossRef]
- Komiyama, A.; Kato, S.; Poungparn, S.; Sangtiean, T.; Maknual, C.; Piriyayotha, S.; Jintana, V.; Prawiroatmodjo, S.; Sastrosuwondo, P.; Ogino, K. Comprehensive dataset of mangrove tree weights in Southeast Asia. Ecol. Res. 2016, 32, 3. [Google Scholar] [CrossRef]
- Gao, J; Lundquist, C.J.; Schwendenmann, L. Aboveground Carbon Stocks in Rapidly Expanding Mangroves in New Zealand: Regional Assessment and Economic Valuation of Blue Carbon. Estuaries Coasts 2020, 43, 1456–1469. [Google Scholar] [CrossRef]
- Thormann, I.; Reeves, P.; Reilley, A.; Engels, J.M.M.; Lohwasser, U.; Börner, A.; Pillen, K.; Richards, C. Geography of Genetic Structure in Barley Wild Relative Hordeum vulgare subsp. spontaneum in Jordan. PLoS ONE 2016, 11, e0160745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Realm | Province | Ecoregion |
|---|---|---|
| Central Indo-Pacific | Eastern Coral Triangle | Bismarck Sea |
| Solomon Archipelago | ||
| Solomon Sea | ||
| Southeast Papua New Guinea | ||
| Java Transitional | Southern Java | |
| Northern Australian Shelf | Central and Southern Great Barrier Reef | |
| Torres Strait Northern Great Barrier Reef | ||
| Northwest Australian Shelf | Exmouth to Broome | |
| Ningaloo | ||
| Sahul Shelf | Arafura Sea | |
| Arnhem Coast to Gulf of Carpenteria | ||
| Bonaparte Coast | ||
| Gulf of Papua | ||
| South China Sea | Gulf of Tonkin | |
| Southern China | ||
| South Kuroshio | South Kuroshio | |
| Sunda Shelf | Gulf of Thailand | |
| Malacca Strait | ||
| Southern Vietnam | ||
| Sunda Shelf/Java Sea | ||
| Tropical Southwestern Pacific | Fiji Islands | |
| New Caledonia | ||
| Tonga Islands | ||
| Vanuatu | ||
| Western Coral Triangle | Banda Sea | |
| Eastern Philippines | ||
| Halmahera | ||
| Lesser Sunda | ||
| Northeast Sulawesi | ||
| Palawan/North Borneo | ||
| Papua | ||
| Sulawesi Sea/Makassar Strait | ||
| Eastern Indo-Pacific | Central Polynesia | Samoa Islands |
| Hawaii | Hawaii | |
| Temperate Australasia | East Central Australian Shelf | Manning-Hawkesbury |
| Tweed-Moreton | ||
| Northern New Zealand | Northeastern New Zealand | |
| Southeast Australian Shelf | Bassian | |
| Cape Howe | ||
| Southwest Australian Shelf | Great Australian Bight | |
| South Australian Gulfs | ||
| West Central Australian Shelf | Shark Bay | |
| Temperate Northern Atlantic | Warm Temperate Northwest Atlantic | Carolinian |
| Northern Gulf of Mexico | ||
| Temperate Northern Pacific | Warm Temperate Northeast Pacific | Cortezian |
| Magdalena Transition | ||
| Southern California Bight | ||
| Warm Temperate Northwest Pacific | Central Kuroshio Current | |
| East China Sea | ||
| Temperate South America | Warm Temperate Southeastern Pacific | Central Peru |
| Warm Temperate Southwestern Atlantic | Southeastern Brazil | |
| Temperate Southern Africa | Agulhas | Natal |
| Tropical Atlantic | Gulf of Guinea | Angolan |
| Gulf of Guinea Central | ||
| Gulf of Guinea South | ||
| Gulf of Guinea Upwelling | ||
| Gulf of Guinea West | ||
| North Brazil Shelf | Amazonia | |
| Guianan | ||
| Tropical Northwestern Atlantic | Bahamian | |
| Eastern Caribbean | ||
| Floridian | ||
| Greater Antilles | ||
| Southern Caribbean | ||
| Southern Gulf of Mexico | ||
| Southwestern Caribbean | ||
| Western Caribbean | ||
| Tropical Southwestern Atlantic | Eastern Brazil | |
| Northeastern Brazil | ||
| West African Transition | Sahelian Upwelling | |
| Tropical Eastern Pacific | Galapagos | Eastern Galapagos Islands |
| Western Galapagos Islands | ||
| Tropical East Pacific | Chiapas-Nicaragua | |
| Guayaquil | ||
| Mexican Tropical Pacific | ||
| Nicoya | ||
| Panama Bight | ||
| Western Indo-Pacific | Andaman | Andaman and Nicobar Islands |
| Andaman Sea Coral Coast | ||
| Western Sumatra | ||
| Bay of Bengal | Eastern India | |
| Northern Bay of Bengal | ||
| Red Sea and Gulf of Aden | Gulf of Aden | |
| Northern and Central Red Sea | ||
| Southern Red Sea | ||
| Somali/Arabian | Arabian (Persian) Gulf | |
| Gulf of Oman | ||
| Western Arabian Sea | ||
| West and South Indian Shelf | South India and Sri Lanka | |
| Western India | ||
| Western Indian Ocean | Bight of Sofala/Swamp Coast | |
| Delagoa | ||
| East African Coral Coast | ||
| Northern Monsoon Current Coast | ||
| Western and Northern Madagascar |
| Variable Categories | Variables |
|---|---|
| Bioclimatic variables | Annual temperature (BIO1), temperature seasonality (BIO4), annual precipitation (BIO12), and precipitation seasonality (BIO15) |
| Species | Mangrove species richness |
| Topography | Elevation, soil erosion by water |
| Ecoregions | Countries |
|---|---|
| Gulf of Papua | Oceania (Papua New Guinea) |
| Vanuatu | Oceania (Vanuatu) |
| Palawan/North Borneo | SE Asia (Borneo and the Palawan Islands, Philippines) |
| Papua | Oceania (Papua New Guinea) |
| Central Kuroshio Current | East Asia (Japan) |
| Angolan | SW Africa (Angola) |
| Gulf of Guinea West | NW Africa |
| Amazonia | South America (Brazil) |
| Guianan | South America (French Guiana, Guyana, Suriname) |
| Northeastern Brazil | South America (Brazil) |
| Andaman and Nicobar Islands | South Asia (Southeastern India) |
| Andaman Sea Coral Coast | SE Asia (Thailand) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Schwendenmann, L.; Wang, G.; Adame, M.F.; Mandlate, L.J.C. Changes in Mangrove Carbon Stocks and Exposure to Sea Level Rise (SLR) under Future Climate Scenarios. Sustainability 2022, 14, 3873. https://doi.org/10.3390/su14073873
Singh M, Schwendenmann L, Wang G, Adame MF, Mandlate LJC. Changes in Mangrove Carbon Stocks and Exposure to Sea Level Rise (SLR) under Future Climate Scenarios. Sustainability. 2022; 14(7):3873. https://doi.org/10.3390/su14073873
Chicago/Turabian StyleSingh, Minerva, Luitgard Schwendenmann, Gang Wang, Maria Fernanda Adame, and Luís Junior Comissario Mandlate. 2022. "Changes in Mangrove Carbon Stocks and Exposure to Sea Level Rise (SLR) under Future Climate Scenarios" Sustainability 14, no. 7: 3873. https://doi.org/10.3390/su14073873






