SARS-CoV-2 Dynamics in the Mucus Layer of the Human Upper Respiratory Tract Based on Host–Cell Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. SARS-CoV-2 Dynamics Prediction Based on the Target-Cell-Limited Model
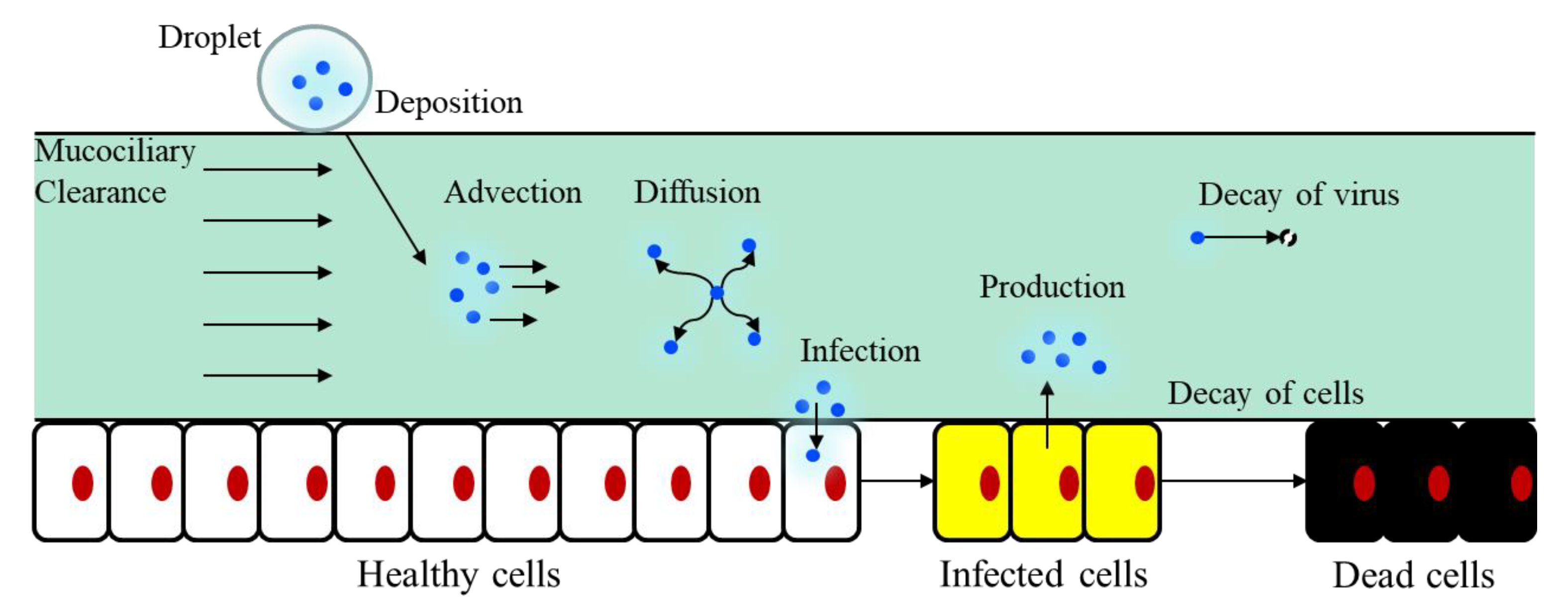
2.2. Coupling of Target-Cell Limited Model and Convection–Diffusion Model
2.3. Changes in the Infection Rate of the Target Cells β
3. Results
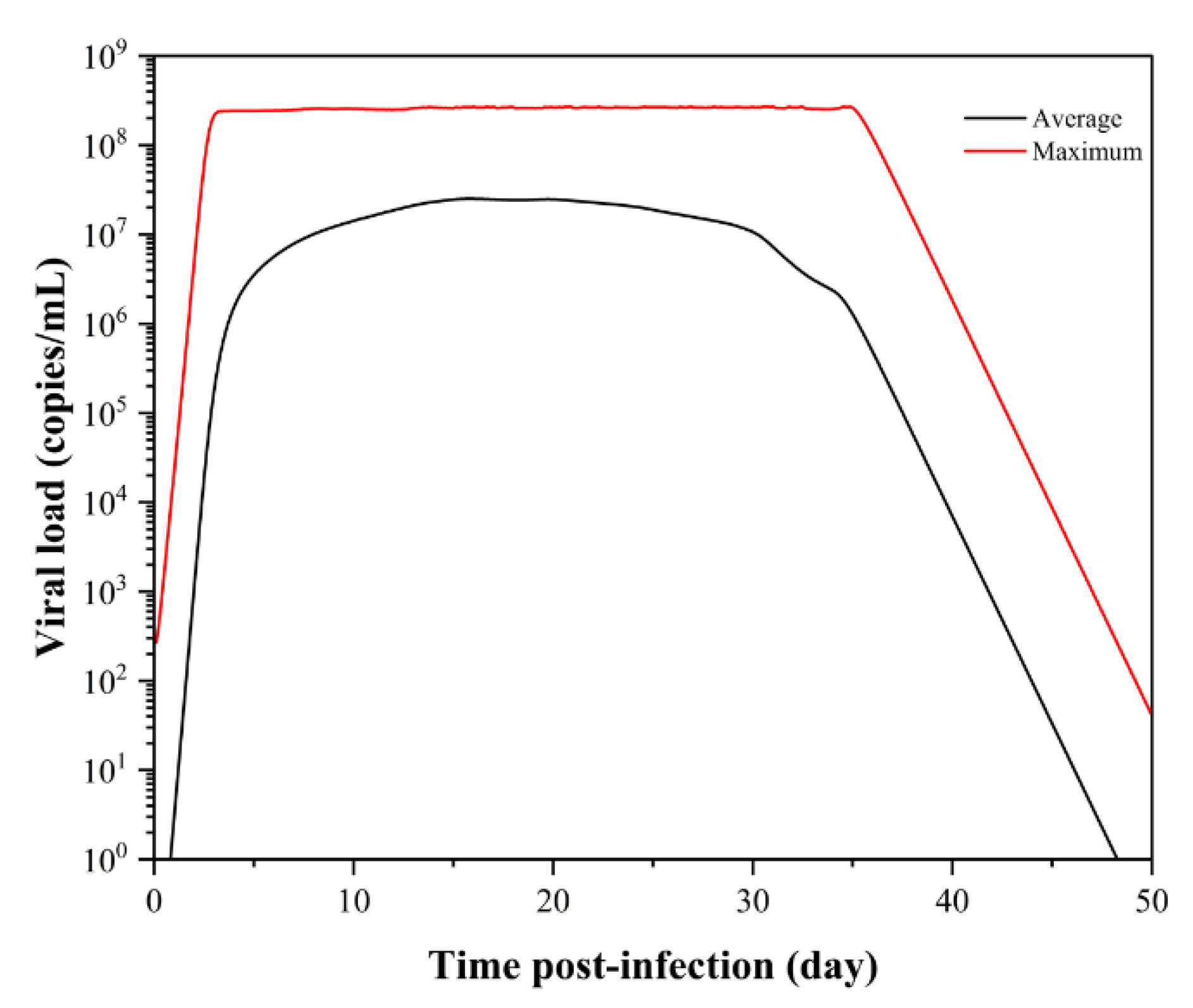
3.1. SARS-CoV-2 Dynamics Prediction of the Upper Respiratory Tract Based on the Target-Cell-Limited Model
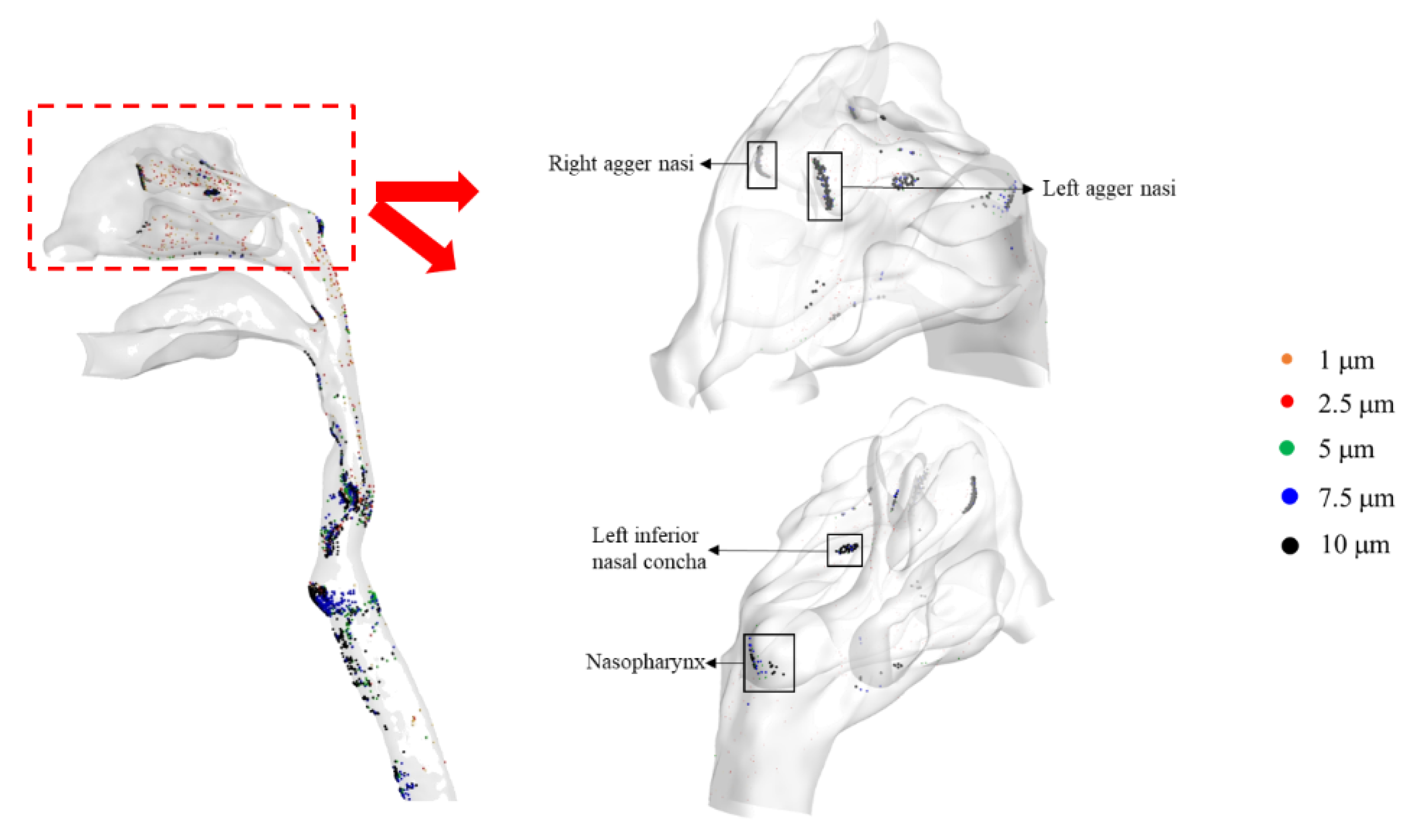
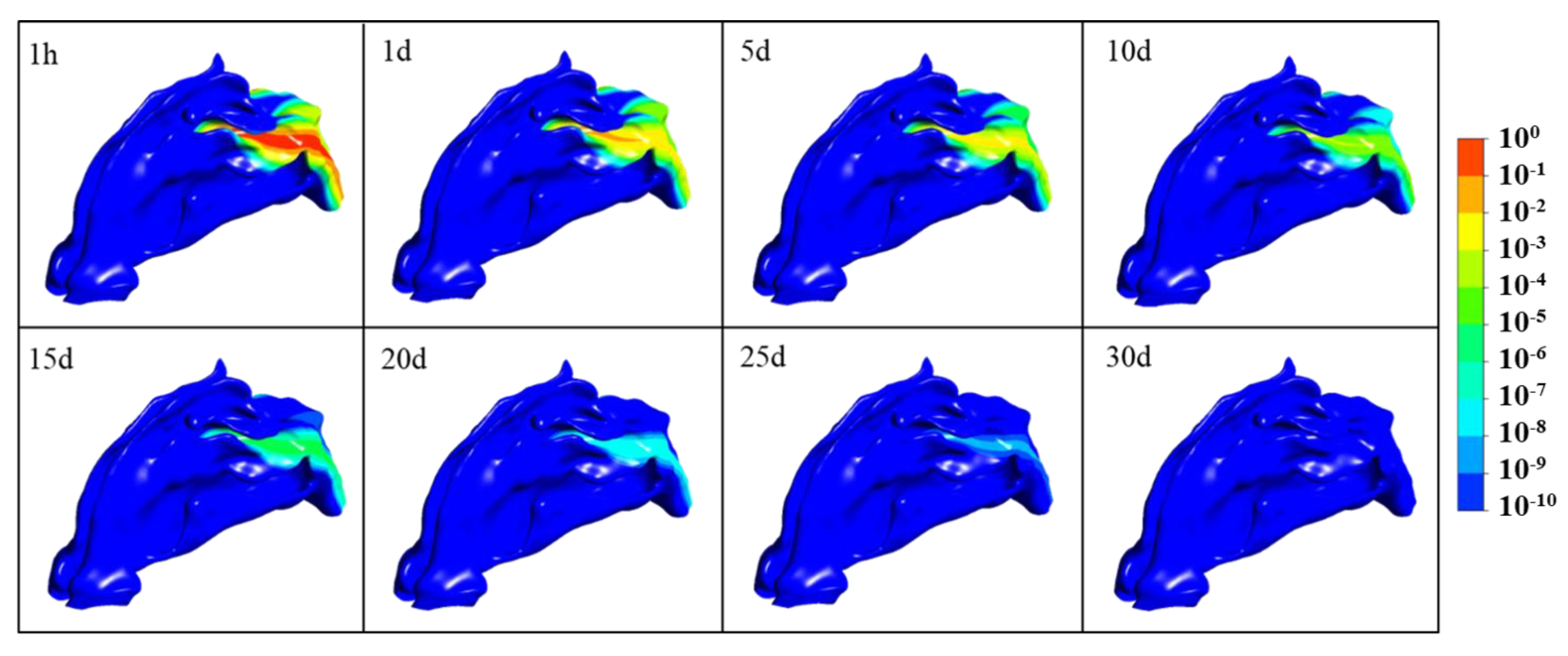
3.2. SARS-CoV-2 Dynamics Prediction of the Nasal Cavity–Nasopharynx with the Mucus Layer
3.3. Effects of the Infection Rate β on SARS-CoV-2 Dynamics Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 8 March 2022).
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Bar-on, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 ( COVID-19 ) by the Numbers. eLife 2020, 2, e57309. [Google Scholar] [CrossRef] [PubMed]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Vetter, P.; Eberhardt, C.S.; Meyer, B.; Martinez Murillo, P.A.; Torriani, G.; Pigny, F.; Lemeille, S.; Cordey, S.; Laubscher, F.; Vu, D.-L.; et al. Daily Viral Kinetics and Innate and Adaptive Immune Response Assessment in COVID-19: A case series. mSphere 2020, 5, e00827-20. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, X.; Zhu, B.; Liang, H.; Fang, C.; Gong, Y.; Guo, Q.; Sun, X.; Zhao, D.; Shen, J.; et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020, 26, 502–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA—J. Am. Med. Assoc. 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, K.K.W.; Tsang, O.T.Y.; Yip, C.C.Y.; Chan, K.H.; Wu, T.C.; Chan, J.M.C.; Leung, W.S.; Chik, T.S.H.; Choi, C.Y.C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.G.; Yoon, J.; Song, J.Y.; Yoon, S.Y.; Lim, C.S.; Seong, H.; Noh, J.Y.; Cheong, H.J.; Kim, W.J. Clinical significance of a high SARS-CoV-2 viral load in the Saliva. J. Korean Med. Sci. 2020, 35, e195. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, J.; Xu, Y.; Chen, X. Viral dynamics of SARS-CoV-2 in Saliva from infected patients. J. Infect. 2020, 81, e48–e50. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, K.K.W.; Tsang, O.T.Y.; Leung, W.S.; Tam, A.R.; Wu, T.C.; Lung, D.C.; Yip, C.C.Y.; Cai, J.P.; Chan, J.M.C.; Chik, T.S.H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Kissler, S.M.; Fauver, J.R.; Mack, C.; Olesen, S.W.; Tai, C.; Shiue, K.Y.; Kalinich, C.C.; Jednak, S.; Ott, I.M.; Vogels, C.B.F.; et al. Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies. PLoS Biol. 2021, 19, e3001333. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, J.H.; Kim, Y.; Kim, Y.J.; Kim, J.M.; Chung, Y.S.; Kim, H.M.; Han, M.G.; Kim, S.Y.; Chin, B.S. Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea. J. Korean Med. Sci. 2020, 35, e86. [Google Scholar] [CrossRef]
- Hernandez-Vargas, E.A.; Velasco-Hernandez, J.X. In-host mathematical modelling of COVID-19 in humans. Annu. Rev. Control. 2020, 50, 448–456. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, F.; Bingham, R.J.; Dykeman, E.C.; Stockley, P.G.; Twarock, R. Comparing antiviral strategies against COVID-19 via multiscale within-host modelling. R. Soc. Open Sci. 2021, 8, 210082. [Google Scholar] [CrossRef]
- Dogra, P.; Ruiz-Ramírez, J.; Sinha, K.; Butner, J.D.; Peláez, M.J.; Rawat, M.; Yellepeddi, V.K.; Pasqualini, R.; Arap, W.; Sostman, H.D.; et al. Innate immunity plays a key role in controlling viral load in COVID-19: Mechanistic insights from a whole-body infection dynamics model. ACS Pharmacol. Transl. Sci. 2021, 4, 248–265. [Google Scholar] [CrossRef]
- Sadria, M.; Layton, A.T. Modeling within-host Sars-Cov-2 infection dynamics and potential treatments. Viruses 2021, 13, 1141. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Bertrand, J.; Ke, R.; Comets, E.; de Lamballerie, X.; Malvy, D.; Pizzorno, A.; Terrier, O.; Rosa Calatrava, M.; Mentré, F.; et al. Timing of antiviral treatment initiation is critical to reduce SARS-CoV-2 viral load. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Czuppon, P.; Débarre, F.; Gonçalves, A.; Tenaillon, O.; Perelson, A.S.; Guedj, J.; Blanquart, F. Success of prophylactic antiviral therapy for SARS-CoV-2: Predicted critical efficacies and impact of different drug-specific mechanisms of action. PLoS Comput. Biol. 2021, 17, e1008752. [Google Scholar] [CrossRef] [PubMed]
- Prentiss, M.; Chu, A.; Berggren, K.K. Superspreading events without superspreaders: Using high attack rate events to estimate N0 for airborne transmission of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Haghnegahdar, A.; Zhao, J.; Feng, Y. Lung aerosol dynamics of airborne influenza a virus-laden droplets and the resultant immune system responses: An in silico study. J. Aerosol Sci. 2019, 134, 34–55. [Google Scholar] [CrossRef]
- Rygg, A.; Longest, P.W. Absorption and Clearance of Pharmaceutical Aerosols in the Human Nose: Development of a CFD Model. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 416–431. [Google Scholar] [CrossRef]
- Shang, Y.; Inthavong, K.; Tu, J. Development of a computational fluid dynamics model for mucociliary clearance in the Nasal Cavity. J. Biomech. 2019, 85, 74–83. [Google Scholar] [CrossRef]
- Duguid, J.P. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J. Hyg. (Lond.) 1946, 44, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lee, G.W.M.; Chen, C.M.; Wu, C.C.; Yu, K.P. The size and concentration of droplets generated by coughing in human subjects. J. Aerosol Med. 2007, 20, 484–494. [Google Scholar] [CrossRef]
- Phuong, N.L.; Khoa, N.D.; Ito, K. Comparative numerical simulation of inhaled particle dispersion in upper human airway to analyse intersubject differences. Indoor Built Environ. 2020, 29, 793–809. [Google Scholar] [CrossRef]
- Fedoseev, G.B.; Geharev, S. Basic defense mechanisms of bronchio-lung system. Gen. Pulmonol. 1989, 1, 112–144. [Google Scholar]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, J. Mucus flows. Math. Biosci. 1973, 17, 301–313. [Google Scholar] [CrossRef]
- Shang, Y.; Inthavong, K.; Qiu, D.; Singh, N.; He, F.; Tu, J. Prediction of nasal spray drug absorption influenced by mucociliary clearance. PLoS ONE 2021, 16, e0246007. [Google Scholar] [CrossRef]
- Gelman, R.A.; Meyer, F.A. Mucociliary transference rate and mucus viscoelasticity dependence on dynamic storage and loss modulus. Am. Rev. Respir. Dis. 1979, 120, 553–557. [Google Scholar]
- Du, S.Q.; Yuan, W. Mathematical modeling of interaction between innate and adaptive immune responses in COVID-19 and implications for viral pathogenesis. J. Med. Virol. 2020, 92, 1615–1628. [Google Scholar] [CrossRef]
- Pawelek, K.A.; Huynh, G.T.; Quinlivan, M.; Cullinane, A.; Rong, L.; Perelson, A.S. Modeling within-host dynamics of influenza virus infection including immune responses. PLoS Comput. Biol. 2012, 8, e1002588. [Google Scholar] [CrossRef] [Green Version]
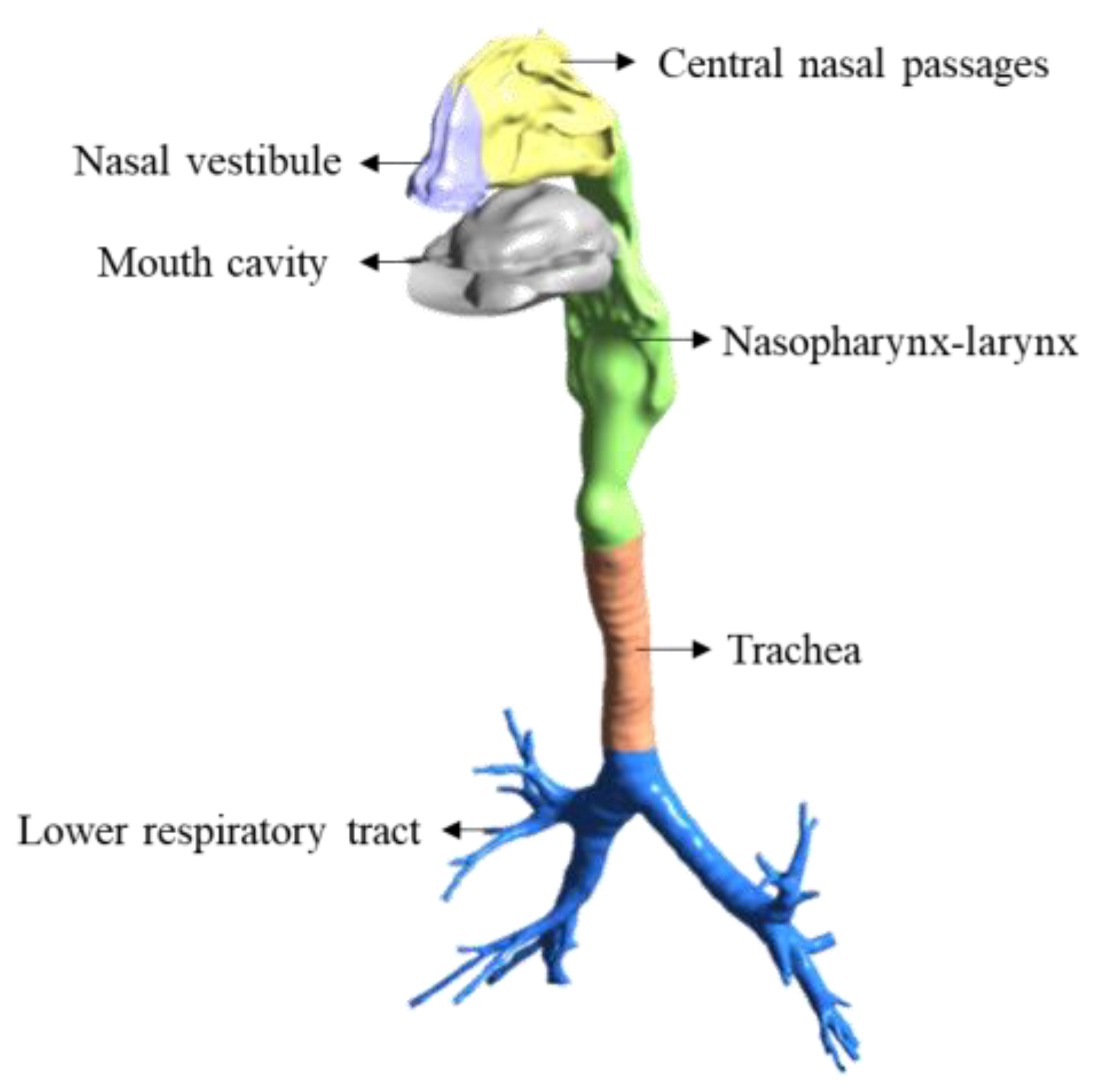

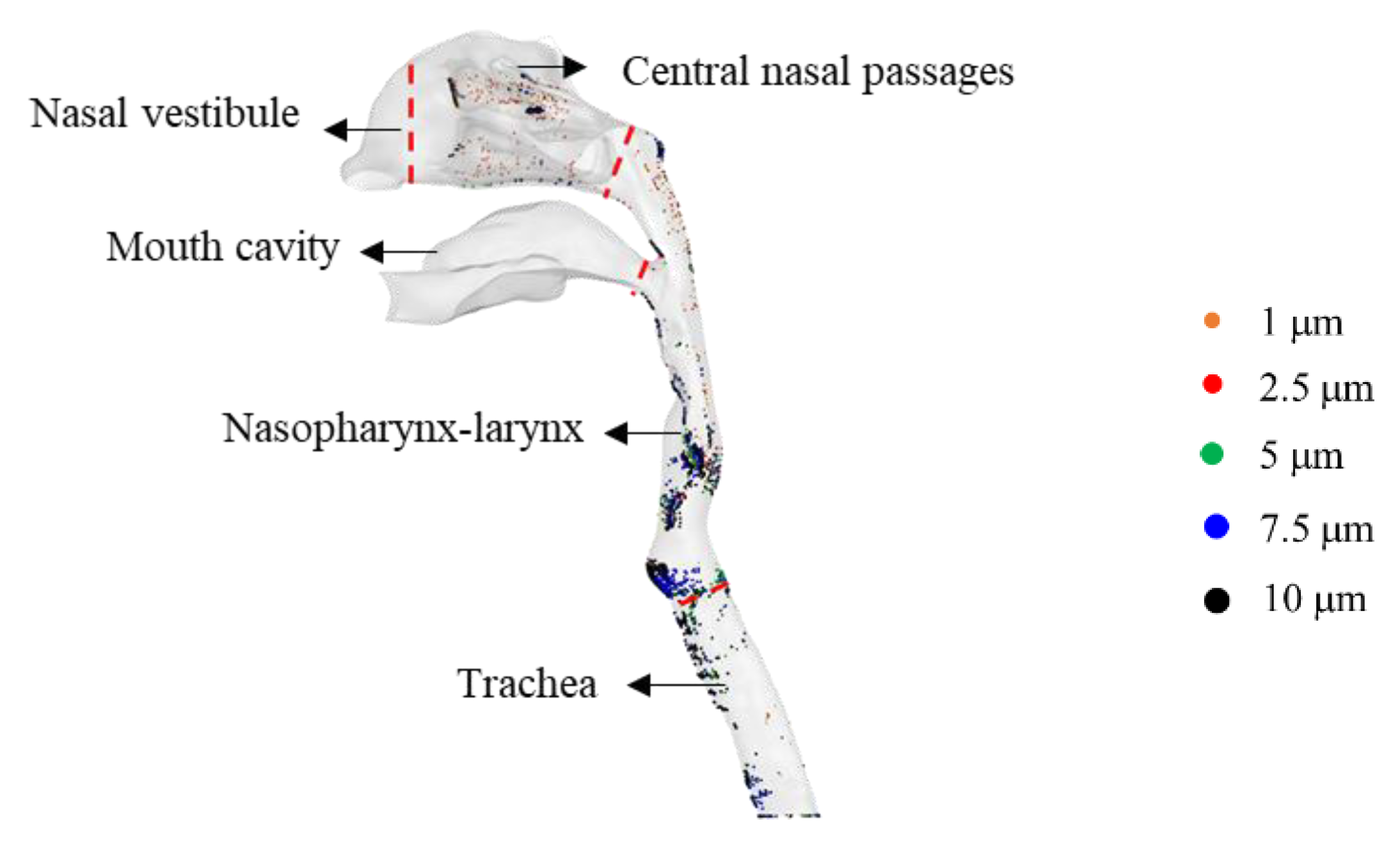











| Regions | Vestibule | Central Nasal Passages | Nasopharynx-Larynx | Trachea | Total | |
|---|---|---|---|---|---|---|
| Surface area (m2) | 3.71 × 10−3 | 1.55 × 10−2 | 9.88 × 10−3 | 4.72 × 10−3 | 3.38 × 10−2 | |
| Vmucus (mL) | 0.06 | 0.23 | 0.15 | 0.07 | 0.51 | |
| T(0) [cells] | 9.27 × 107 | 3.88 × 108 | 2.47 × 108 | 1.18 × 108 | 8.46 × 108 | |
| Particles | 1 μm | 0 | 8 | 5 | 1 | 14 |
| 2.5 μm | 0 | 16 | 10 | 2 | 28 | |
| 5 μm | 0 | 14 | 14 | 18 | 46 | |
| 7.5 μm | 0 | 42 | 49 | 72 | 163 | |
| 10 μm | 0 | 88 | 71 | 84 | 243 | |
| Percentage | 0.00% | 1.68% | 1.49% | 1.77% | 4.94% | |
| V(0) (copies/mL) | 0 | 5.64 × 10−3 | 4.90 × 10−3 | 6.11 × 10−3 | 1.66 × 10−2 | |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| β ((copies/mL) day−1) | 1 × 10−2 | 1 × 10−3 | 1 × 10−4 | 1 × 10−5 | 1 × 10−6 | 1 × 10−7 | 1 × 10−8 |
| Virus No. | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Sites | Right Agger Nasi | Left Agger Nasi | Left Inferior Nasal Concha | Nasopharynx |
| Initial Virus Counting (copies) | 2.71 × 10−4 | 1.17 × 10−4 | 2.21 × 10−5 | 2.95 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Kuga, K.; Ito, K. SARS-CoV-2 Dynamics in the Mucus Layer of the Human Upper Respiratory Tract Based on Host–Cell Dynamics. Sustainability 2022, 14, 3896. https://doi.org/10.3390/su14073896
Li H, Kuga K, Ito K. SARS-CoV-2 Dynamics in the Mucus Layer of the Human Upper Respiratory Tract Based on Host–Cell Dynamics. Sustainability. 2022; 14(7):3896. https://doi.org/10.3390/su14073896
Chicago/Turabian StyleLi, Hanyu, Kazuki Kuga, and Kazuhide Ito. 2022. "SARS-CoV-2 Dynamics in the Mucus Layer of the Human Upper Respiratory Tract Based on Host–Cell Dynamics" Sustainability 14, no. 7: 3896. https://doi.org/10.3390/su14073896






