Early Sowing of Quinoa Cultivars, Benefits from Rainy Season and Enhances Quinoa Development, Growth, and Yield under Arid Condition in Morocco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Measurements
2.2.1. Length of Plant Development Stages
2.2.2. Chlorophyll Content Index (CCI)
2.2.3. Leaf Stomatal Conductance
2.2.4. Plant Height and Morphological Parameters
2.2.5. Total Leaf Area
2.2.6. Yield and Its Components
2.3. Statistical Analysis
3. Results
3.1. Climatic Conditions of the Experiment Growing Season: Air Temperature, Rainfall, Radiation and Photoperiod
3.2. Probability Study of 40 Years Climatic Conditions of Rehamna Region
3.3. Development Stages Length
3.3.1. Days to Emergence
3.3.2. Days to Dicotyledonous Leaves
3.3.3. Days to Two True Leaves
3.3.4. Days to Four True Leaves
3.3.5. Days to Six True Leaves
3.3.6. Days to Panicle Emergence
3.3.7. Days to Flowering
3.3.8. Days to Maturity
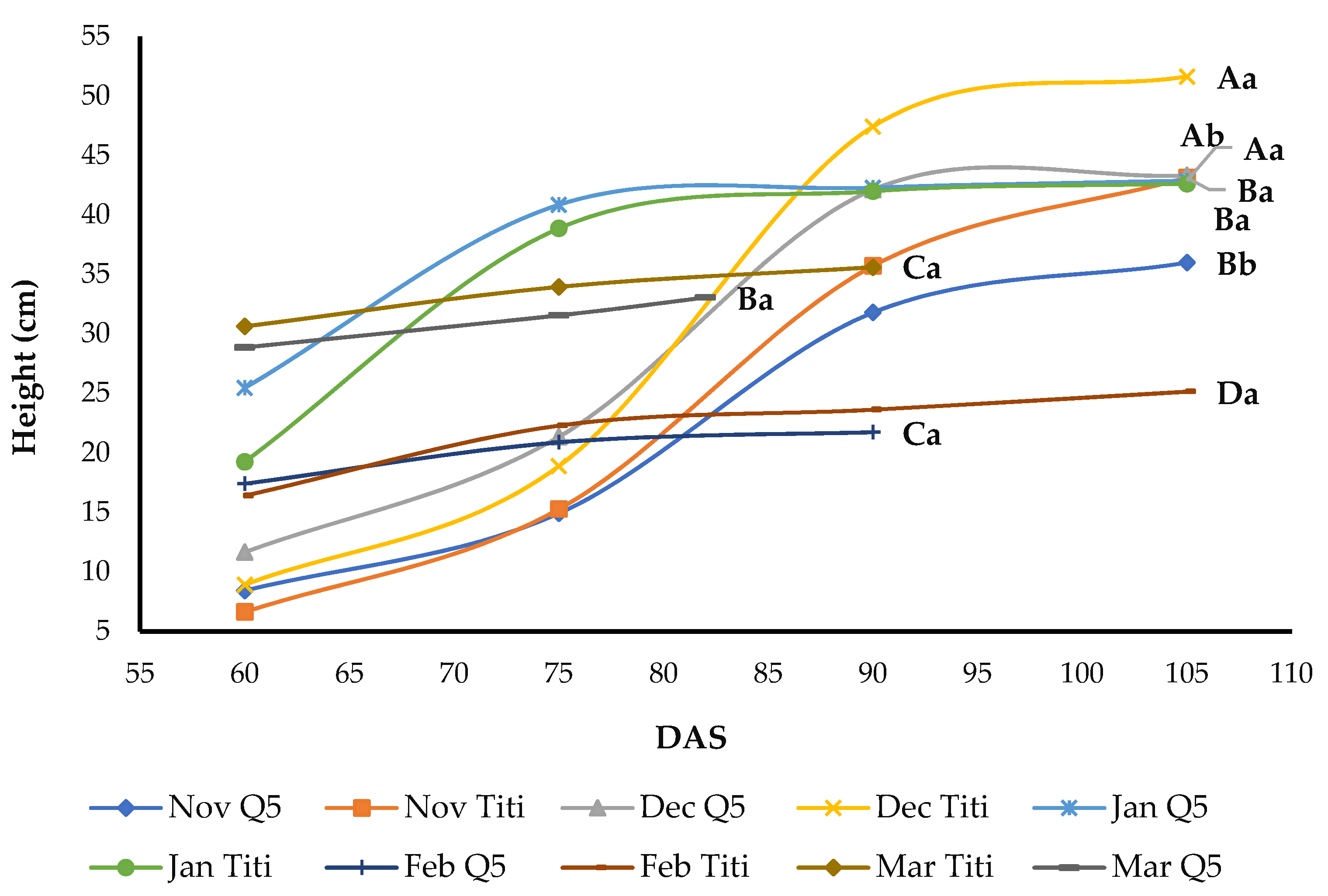
3.4. Plant Height
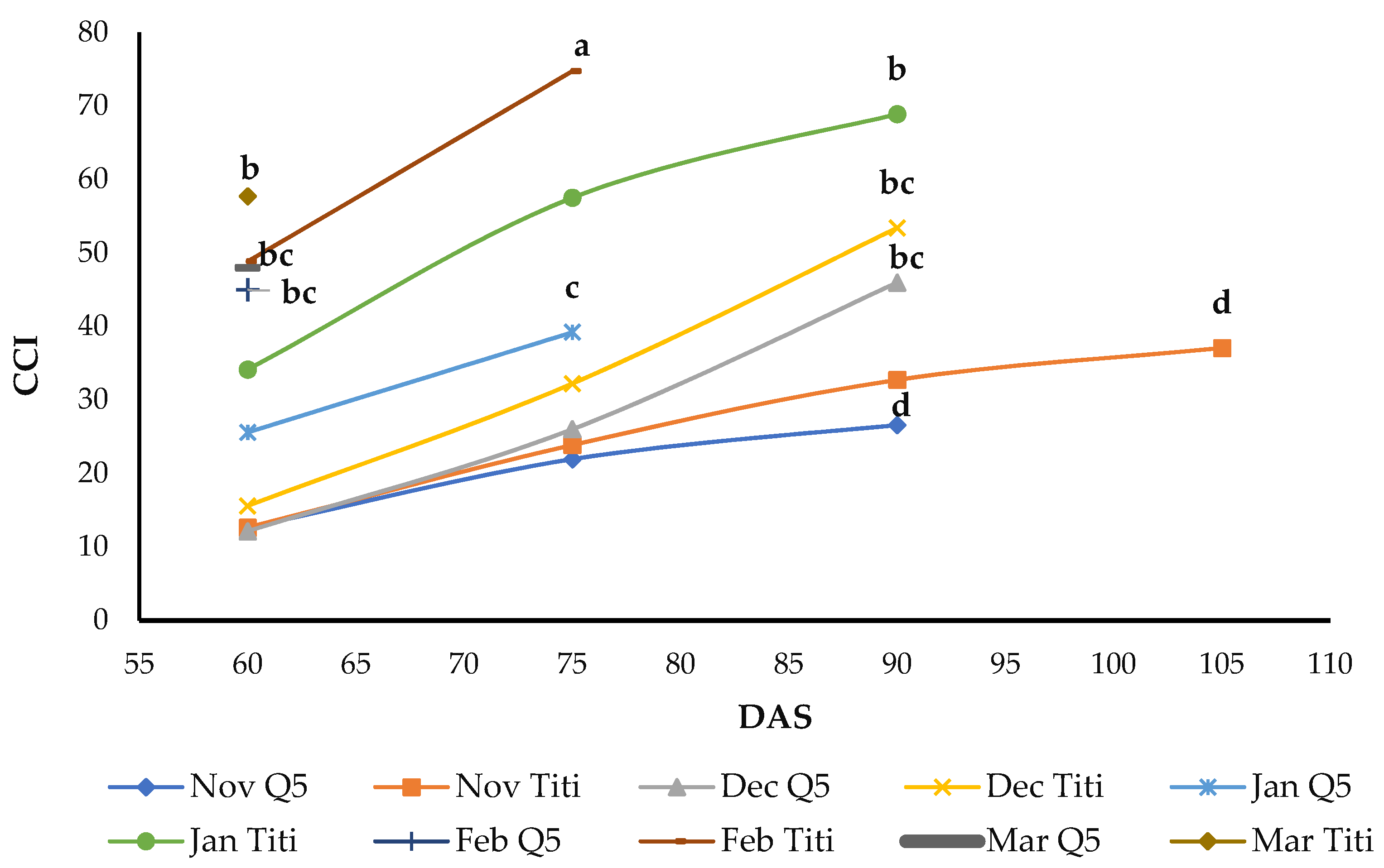
3.5. Chlorophyll Content Index (CCI)
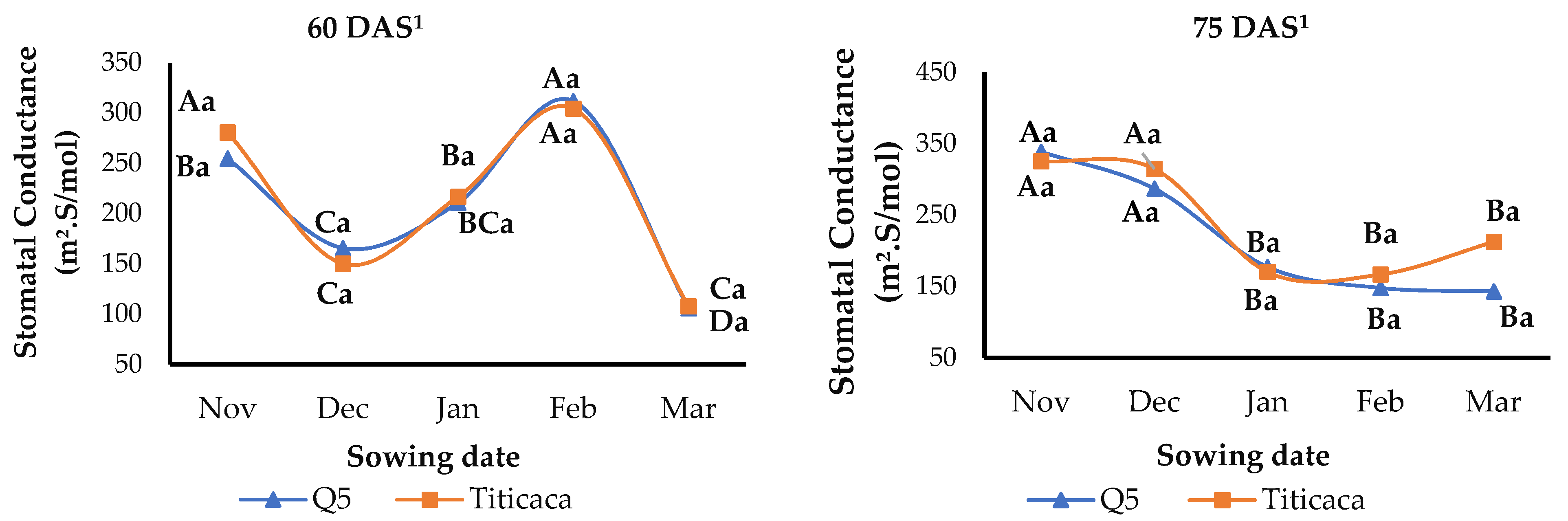
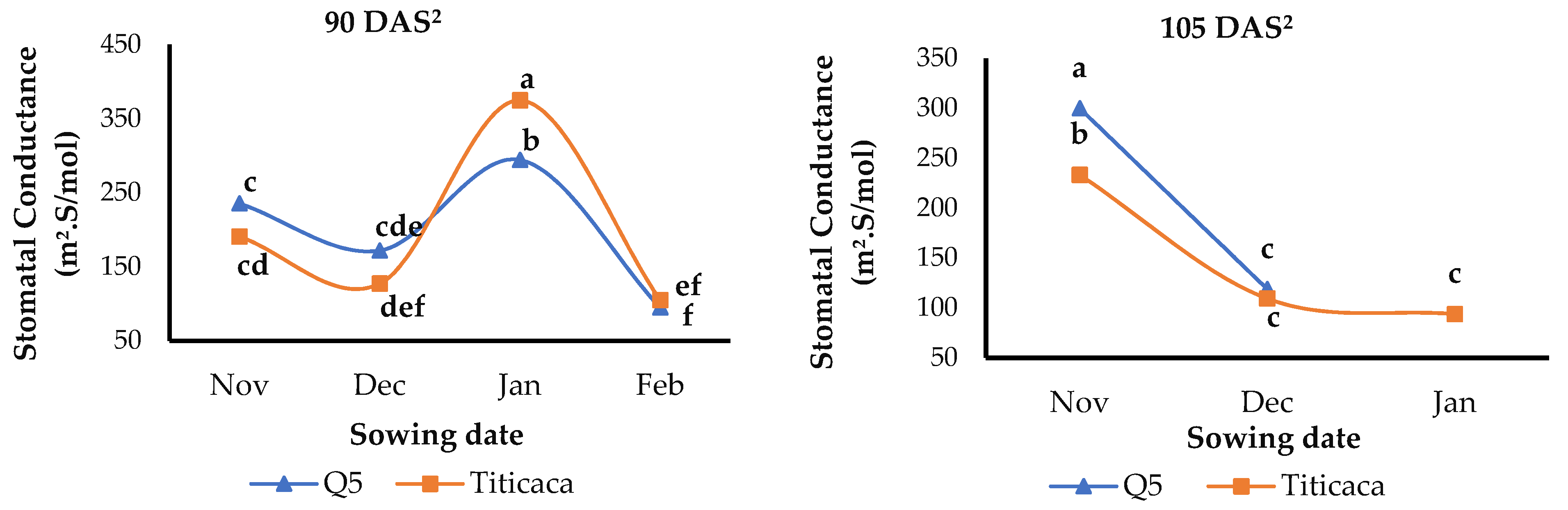
3.6. Stomatal Conductance
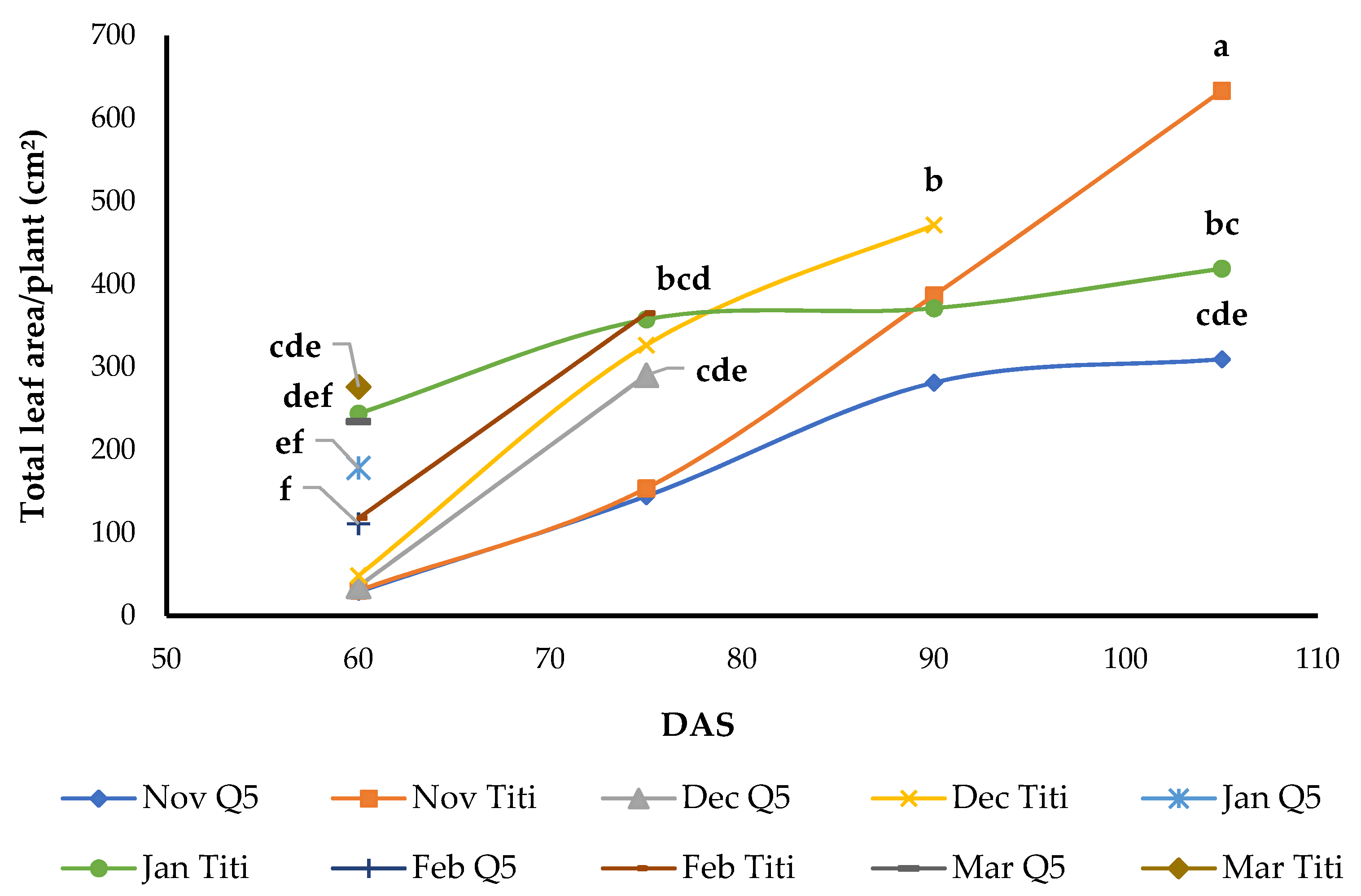
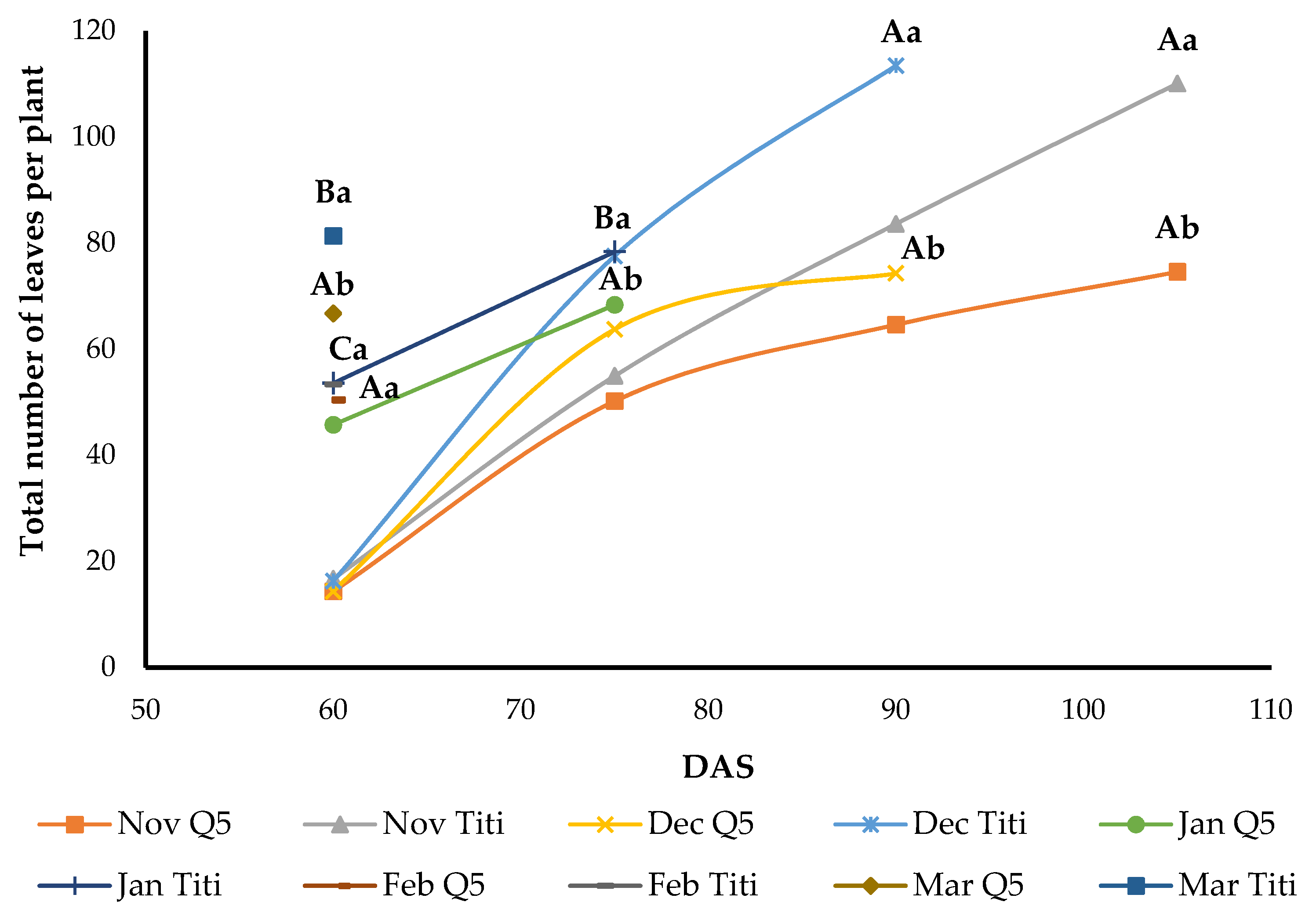
3.7. Total Leaf Area
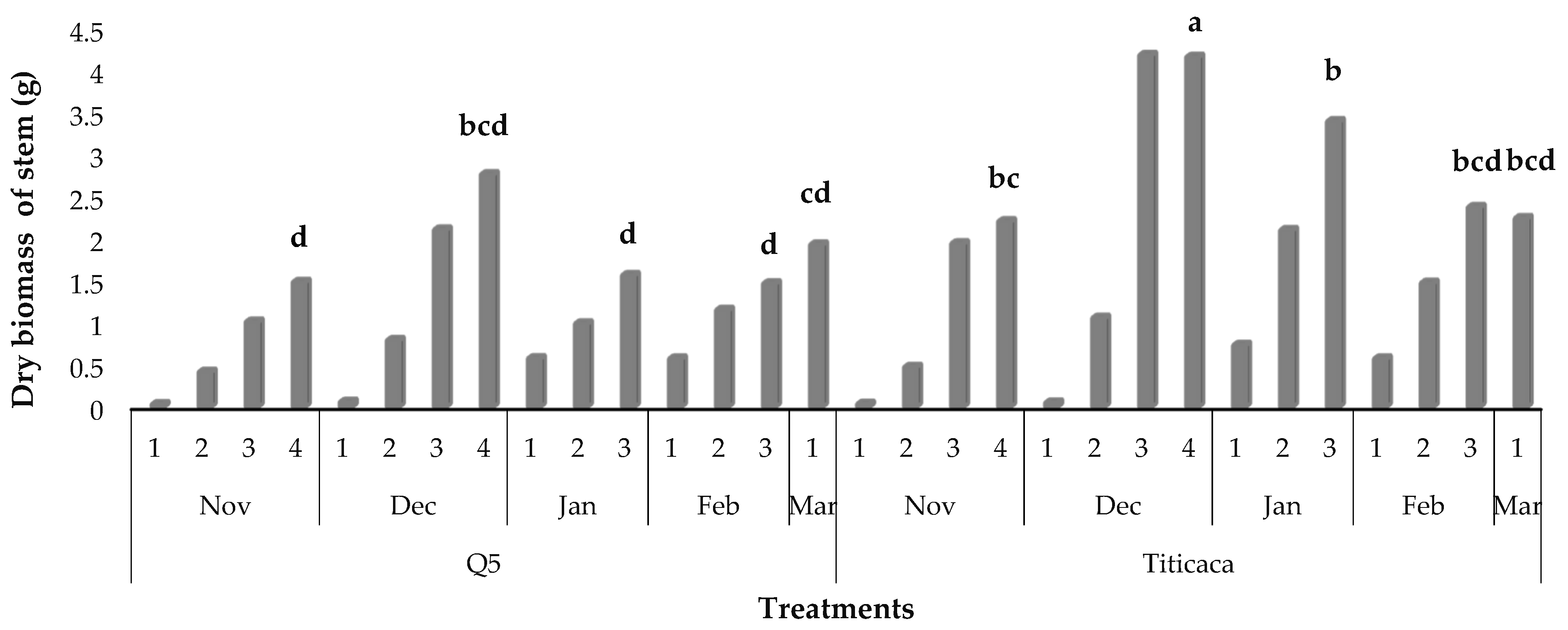
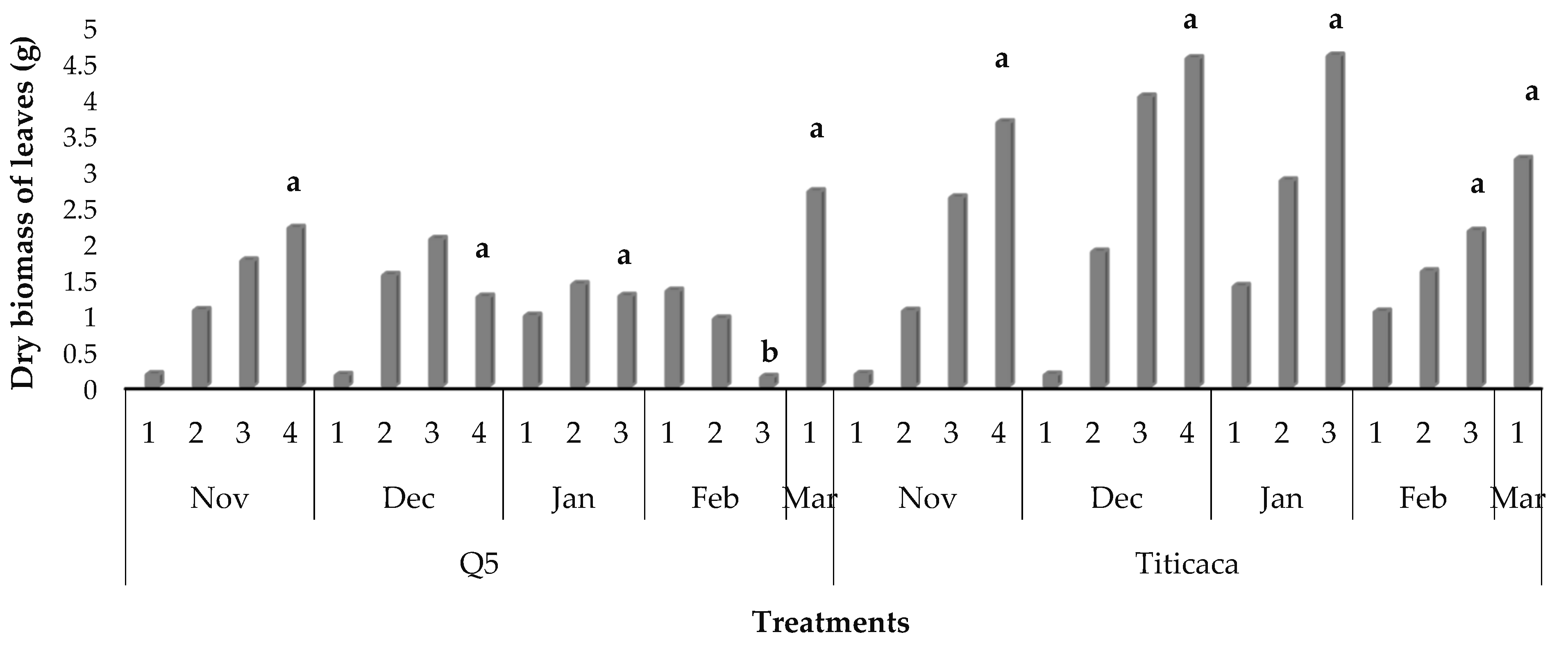
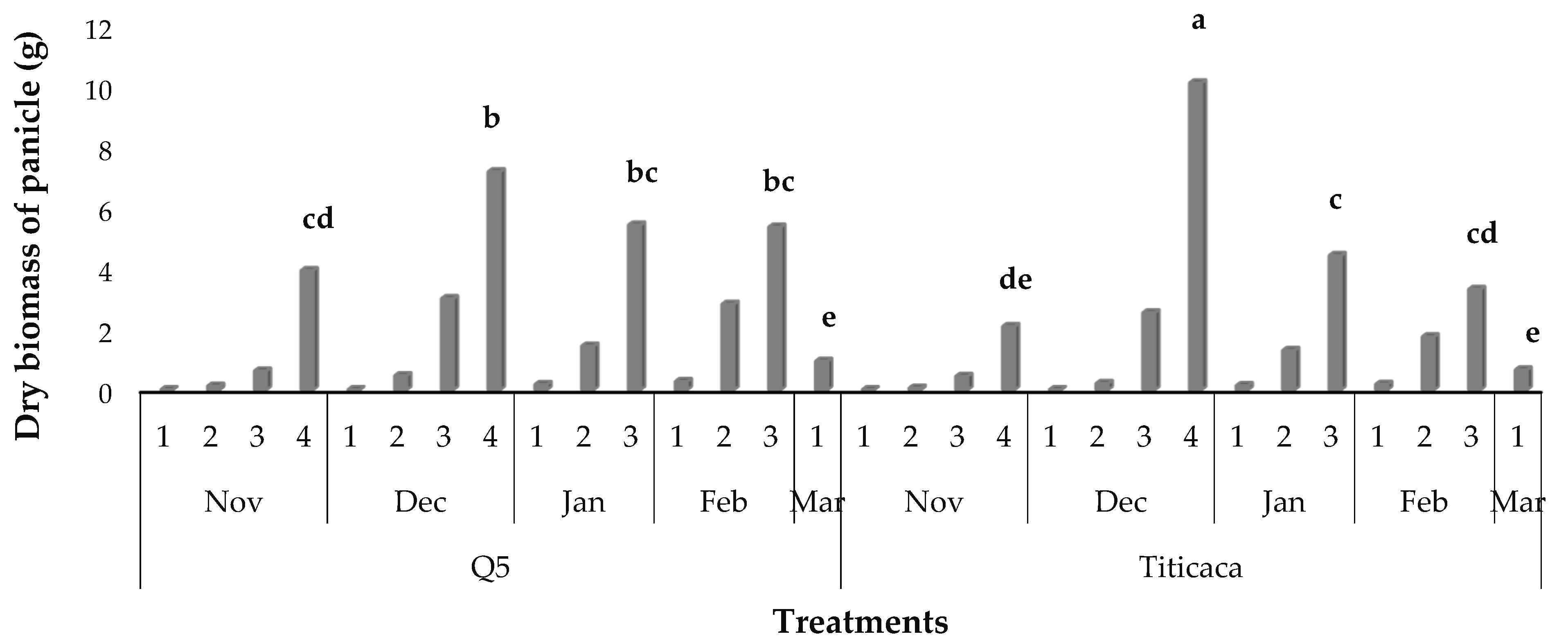
3.8. Dry Biomass Partitioning
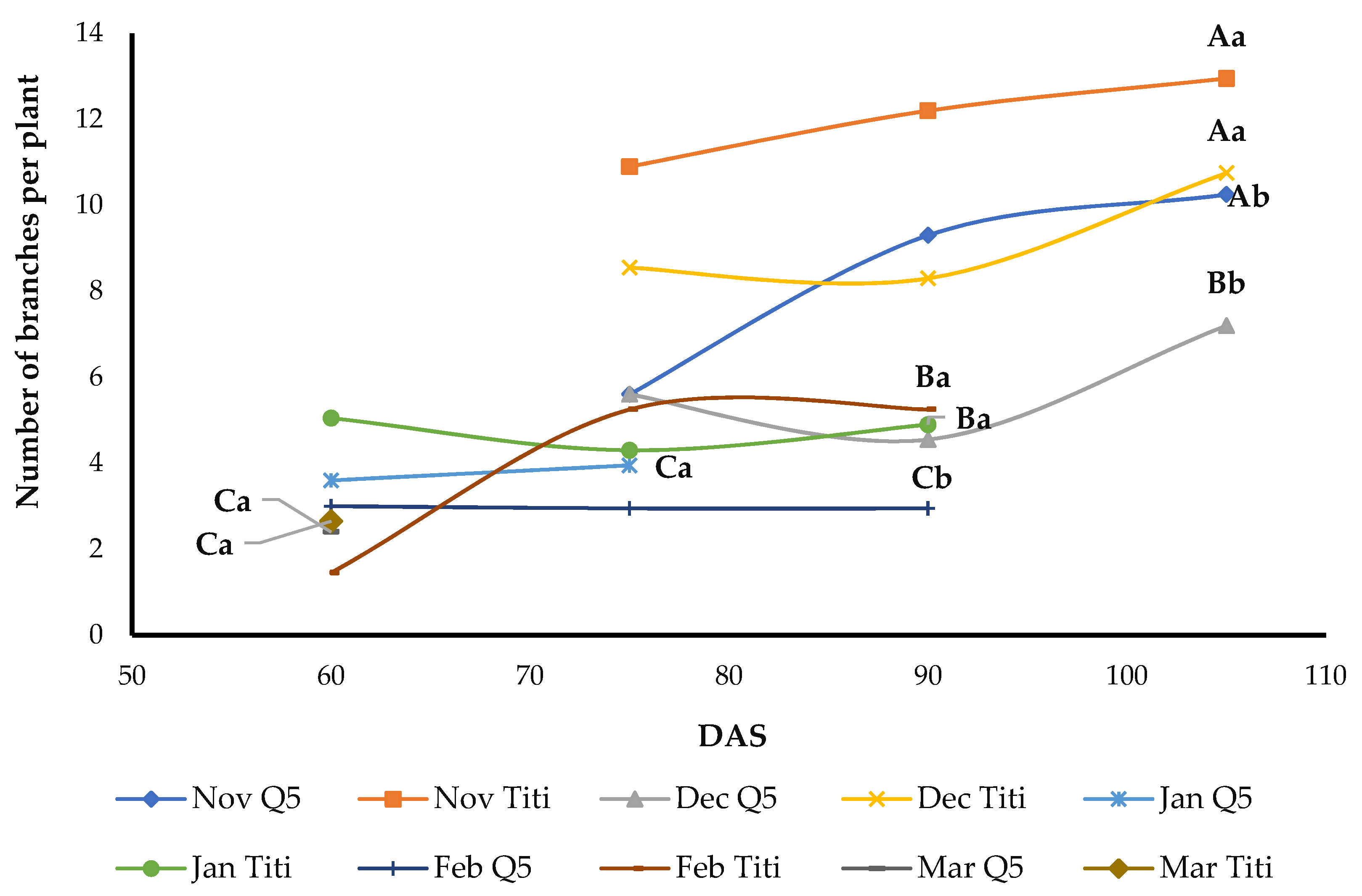
3.9. Number of Branches, Leaves, and Panicles per Plant
3.10. Yield Components and Panicle Length
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risi, J.C.; Galwey, N.W. Effects of Sowing Date and Sowing Rate on Plant Development and Grain Yield of Quinoa (Chenopodium Quinoa) in a Temperate Environment. J. Agric. Sci. 1991, 117, 325–332. [Google Scholar] [CrossRef]
- FAO. Quinoa: An ancient crop to contribute to world food security. In Regional Office for Latin America and the Caribbean; Bojanic, A., Ed.; Food and Agriculture Organization: Rome, Italy, 2011; p. 63. [Google Scholar]
- Aguilar, P.; Jacobsen, S.E. Cultivation of quinoa on the Peruvian Altiplano. Food Rev. Int. 2003, 19, 31–41. [Google Scholar] [CrossRef]
- Bertero, H.D.; King, R.W.; Hall, A.J. Photoperiod-sensitive development phases in quinoa (Chenopodium quinoa Willd.). Field Crops Res. 1999, 60, 231–243. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.-E.; El Yousfi, L.; El Omari, H. Using deficit irrigation with treated wastewater in the production of quinoa (Chenopodium quinoa Willd.) in Morocco. Rev. Cient. UDO Agríc. 2012, 12, 570–583. [Google Scholar]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.-E.; Benlhabib, O. Could quinoa be an alternative crop of wheat in the Mediterranean region: Case of Morocco? Les Notes D’alerte Du CIHEAM 2012, 86, 1–8. [Google Scholar]
- Mujica, A.; Jacobsen, S.; Izquierdo, J.; Marathee, J. Agronomía del Cultivo de la Quinua. In Quinua (Chenopodium Quinoa Willd) Ancestral Cultivo Andino, Alimento del Presente y Futuro; Izquierdo, J., Ed.; Food and Agriculture Organization: Santiago, Chile, 2011. [Google Scholar]
- Hirich, A.; Choukr-Allah, R. Phenotyping the Combined Effect of Heat and Water Stress on Quinoa. In Emerging Research in Alternative Crops; Hirich, A., Choukr-Allah, R., Ragab, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Pando, L.G.; Castellanos, E.A. Guía de Cultivo de la Quinua; FAO y Univ.: Lima, Peru, 2016; p. 122. [Google Scholar]
- Rafik, S.; Chaoui, M.; Assabban, Y.; Jazi, S.; Choukr-Allah, R.; El Gharouss, M.; Hirich, A. Quinoa value chain, adoption, and market assessment in Morocco. Environ. Sci. Pollut. Res. Int. 2021, 28, 46692–46703. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.; Rockström, J. Supplemental irrigation for dry-spell mitigation of rainfed agriculture in the Sahel. Agric. Water Manag. 2003, 61, 29–50. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Tekin, S.; Yildiz, M. Use of drainage water for irrigation of quinoa in a Mediterranean environment. In Proceedings of the 2nd World Irrigation Forum (WIF2), Chiang Mai, Thailand, 6–8 November 2016. [Google Scholar]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.-E. Growth and Physiological Responses of Quinoa to Drought and Temperature Stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D.; Garcia, M.; Vacher, J.; Mamani, R.; Mendoza, J.; Huanca, R.; Morales, B.; Miranda, R.; Cusicanqui, J.; et al. Introducing deficit irrigation to stabilize yields of quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2008, 28, 427–436. [Google Scholar] [CrossRef]
- Hirich, A.; Rafik, S.; Rahmani, M.; Fetouab, A.; Azaykou, F.; Filali, K.; Ahmadzai, H.; Jnaoui, Y.; Soulaimani, A.; Moussafir, M.; et al. Development of Quinoa Value Chain to Improve Food and Nutritional Security in Rural Communities in Rehamna, Morocco: Lessons Learned and Perspectives. Plants 2021, 10, 301. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.-E. Quinoa in Morocco—Effect of Sowing Dates on Development and Yield. J. Agron. Crop Sci. 2014, 200, 371–377. [Google Scholar] [CrossRef]
- Garcia, M.; Raes, D.; Jacobsen, S.-E. Evapotransporation analysis and irrigation requirements of quinoa (Chenopodium quinoa) in the Bolivian highlands. Agric. Water Manag. 2003, 60, 119–134. [Google Scholar] [CrossRef]
- Brouwer, C.; Prins, K.; Heibloem, M. Irrigation Water Management: Irrigation Scheduling; Training Manual No. 4; FAO: Rome, Italy, 1989; p. 43. [Google Scholar]
- Dao, A.; Alvar-Beltrán, J.; Gnanda, A.; Guira, A.; Nebie, L.; Sanou, J. Effect of different planting techniques and sowing density rates on the development of quinoa. Afr. J. Agric. Res. 2020, 16, 1325–1333. [Google Scholar]
- Abendroth, L.; Elmore, R.W.; Boyer, M.J.; Marlay, S.K. Corn Growth and Development; Iowa State University Extension and Outreach: Ames, IA, USA, 2011. [Google Scholar]
- Barutcular, C.; Yıldırım, M.; Koc, M.; Akıncı, C.; Toptaş, I.; Albayrak, O.; El Sabagh, A. Evaluation of SPAD chlorophyll in spring wheat genotypes under different environments. Fresenius Environ. Bull. 2016, 25, 1258–1266. [Google Scholar]
- Xie, S.; Luo, X. Effect of leaf position and age on anatomical structure, photosynthesis, stomatal conductance and transpiration of Asian pear. Bot. Bull. Acad. Sin. 2003, 44, 297–303. [Google Scholar]
- Jacobsen, S.; Bach, A. The influence of temperature on seed germination rate in quinoa (Chenopodium quinoa Willd.). Seed Sci. Technol. 1998, 26, 515–523. [Google Scholar]
- Casini, P. Seed Yield of Two New Quinoa (Chenopodium quinoa Willd.) Breeding Lines as Affected by Sowing Date in Central Italy. Acta Agric. Slov. 2019, 113, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Sajjad, A.; Munir, H.; Anjum, S.A.; Tanveer, M.; Rehman, A. Growth and development of Chenopodium quinoa genotypes at different sowing dates. J. Agric. Res. 2014, 52, 535–546. [Google Scholar]
- Galwey, N.W. Exploited plants quinoa, grain amaranth. Crop yield: Physiology and processes. Agron. J. 1989, 85, 120–128. [Google Scholar]
- Pulvento, C.; Riccardi, M.; Lavini, A.; D’Andria, R.; Iafelice, G.; Marconi, E. Field Trial Evaluation of Two Chenopodium Quinoa Genotypes Grown Under Rain-Fed Conditions in a Typical Mediterranean Environment in South Italy: Quinoa in the Mediterranean. J. Agron. Crop Sci. 2010, 196, 407–411. [Google Scholar] [CrossRef]
- Casini, P. Possibilità di introdurre la Quinoa in ambienti mediterranei. Inf. Agrar. 2002, 27, 29–32. [Google Scholar]
- Jacobsen, S.-E. The Worldwide Potential for Quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Issa, A.O.; Fghire, R.; Anaya, F.; Benlhabib, O.; Wahbi, S. Physiological and Morphological Responses of two Quinoa Cultivars (Chenopodium quinoa Willd.) to Drought Stress. Gesunde Pflanz. 2019, 71, 123–133. [Google Scholar] [CrossRef]



| Soil Properties | Content (mg kg−1) |
|---|---|
| N-NH4 (SKALAR method) | 3.74 |
| N-NO3 (SKALAR method) | 32.54 |
| P2O5 (Olsen) | 32 |
| Exchangeable K (Ammonium Acetate extr.) | 431 |
| Exchangeable MgO (Ammonium Acetate extr.) | 742 |
| Exchangeable CaO (Ammonium Acetate extr.) | 11,471 |
| DTPA extractable Mn | 12.45 |
| DTPA extractable Fe | 9.28 |
| DTPA extractable Cu | 0.95 |
| DTPA extractable Zn | 0.41 |
| Sowing Date | Cultivars | Growing Stages | Rainfall (mm) | Supplemental Irrigation (mm) | Total Amount of Water per Stage (mm) | Total Amount of Water for the Growing Cycle (mm) |
|---|---|---|---|---|---|---|
| November | ICBA-Q5 | E 1 | 0.0 | 14 | 14.0 | 216.6 |
| E-STL 2 | 16.4 | 23 | 39.4 | |||
| STL-PE 3 | 12.8 | 3 | 15.8 | |||
| PE-F 4 | 20.6 | 10 | 30.6 | |||
| F-M 5 | 26.8 | 90 | 116.8 | |||
| Titicaca | E | 0.0 | 14 | 14.0 | 261 | |
| E-STL | 16.4 | 23 | 39.4 | |||
| STL-PE | 22.0 | 3 | 25.0 | |||
| PE-F | 11.6 | 4 | 15.6 | |||
| F-M | 69.0 | 98 | 167.0 | |||
| December | ICBA-Q5 | E | 9.0 | 7 | 16.0 | 250.2 |
| E-STL | 23.4 | 22 | 45.4 | |||
| STL-PE | 12.8 | 0 | 12.8 | |||
| PE-F | 14,4 | 9 | 23.4 | |||
| F-M | 48.6 | 104 | 152.6 | |||
| Titicaca | E | 9.0 | 7 | 16.0 | 249.2 | |
| E-STL | 23.4 | 22 | 45.4 | |||
| STL-PE | 26.8 | 0 | 26.8 | |||
| PE-F | 30.4 | 14 | 44.4 | |||
| F-M | 18.6 | 98 | 116.6 | |||
| January | ICBA-Q5 | E | 0.4 | 13 | 13.4 | 215.8 |
| E-STL | 12.2 | 22 | 34.2 | |||
| STL-PE | 57.0 | 5 | 62.0 | |||
| PE-F | 4.2 | 30 | 34.2 | |||
| F-M | 2.0 | 70 | 72.0 | |||
| Titicaca | E | 0.4 | 13 | 13.4 | 215.6 | |
| E-STL | 12.4 | 24 | 36.4 | |||
| STL-PE | 56.8 | 3 | 59.8 | |||
| PE-F | 6.2 | 18 | 24.2 | |||
| F-M | 2.0 | 82 | 84.0 | |||
| February | ICBA-Q5 | E | 14.4 | 16 | 30.4 | 209 |
| E-STL | 42.2 | 9 | 51.2 | |||
| STL-PE | 4.8 | 0 | 4.8 | |||
| PE-F | 1.6 | 43 | 44.6 | |||
| F-M | 2.0 | 76 | 78.0 | |||
| Titicaca | E | 14.4 | 16 | 30.4 | 206 | |
| E-STL | 42.4 | 9 | 51.4 | |||
| STL-PE | 6.2 | 0 | 6.2 | |||
| PE-F | 0.0 | 23 | 23.0 | |||
| F-M | 2.0 | 93 | 95.0 | |||
| March | ICBA-Q5 | E | 4.2 | 18 | 22.2 | 157.2 |
| E-STL | 0.4 | 23 | 23.4 | |||
| STL-PE | 1.6 | 0 | 1.6 | |||
| PE-F | 0.6 | 35 | 35.6 | |||
| F-M | 1.4 | 73 | 74.4 | |||
| Titicaca | E | 4.2 | 18 | 22.2 | 153.8 | |
| E-STL | 2.0 | 23 | 25.0 | |||
| STL-PE | 0.4 | 0 | 0.4 | |||
| PE-F | 1.6 | 19 | 20.6 | |||
| F-M | 0.6 | 85 | 85.6 |
| Foliar Fertilizer Composition | Percentage (%) |
|---|---|
| B | 0.5 |
| Cu EDTA | 0.6 |
| Fe EDTA | 6 |
| Mn EDTA | 2.5 |
| Mo | 0.4 |
| Zn | 1.2 |
| Months | September | October | November | December | January | February | March | April | May | June | July | August | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rain | Average (mm) | 5.1 | 15.1 | 34.0 | 25.7 | 25.3 | 26.0 | 26.7 | 21.2 | 7.5 | 2.1 | 0.1 | 0.2 | 189.0 |
| CV 1 | 160.9 | 116.4 | 94.3 | 104.4 | 102.0 | 96.4 | 83.6 | 108.6 | 204.5 | 322.7 | 365.2 | 488.1 | 43.2 | |
| Prob 2 (p < 20 mm) | 0.95 | 0.7 | 0.43 | 0.49 | 0.54 | 0.46 | 0.49 | 0.43 | 0.93 | 1 | 1 | 1 | ||
| Tm | Average (°C) | 25.0 | 21.1 | 16.2 | 12.9 | 11.5 | 13.0 | 15.6 | 17.2 | 20.5 | 24.5 | 28.2 | 28.4 | 19.5 |
| CV | 5.6 | 7.9 | 8.7 | 10.2 | 10.0 | 13.3 | 8.5 | 8.4 | 8.3 | 7.2 | 5.5 | 5.6 | 2.8 | |
| Tmin | Average (°C) | 14.1 | 10.7 | 6.5 | 4.2 | 2.6 | 3.6 | 4.5 | 6.2 | 8.5 | 12.5 | 15.3 | 16.1 | 1.5 |
| CV | 10.7 | 17.9 | 26.5 | 49.3 | 77.1 | 65.3 | 36.5 | 28.9 | 18.0 | 8.9 | 8.6 | 6.8 | 122.5 | |
| Min (°C) | 10.2 | 5.4 | 3.0 | −0.9 | −3.4 | −2.3 | 1.4 | 2.8 | 5.1 | 9.7 | 11.5 | 13.6 | −3.4 | |
| Tmax | Average (°C) | 40.6 | 35.3 | 30.1 | 25.3 | 24.4 | 26.9 | 31.2 | 33.5 | 37.1 | 41.1 | 45.2 | 44.4 | 45.7 |
| CV | 5.7 | 6.7 | 7.7 | 8.8 | 8.8 | 9.0 | 7.3 | 7.0 | 9.7 | 7.4 | 3.9 | 4.6 | 2.8 | |
| Max (°C) | 44.4 | 39.8 | 34.8 | 30.0 | 29.2 | 30.1 | 36.6 | 37.6 | 43.6 | 46.5 | 47.5 | 47.1 | 47.5 | |
| Prob (T > 35 °C) | 1 | 0.55 | 0 | 0 | 0 | 0 | 5 | 0.25 | 0.7 | 0.95 | 1 | 1 | ||
| Days to Emergence 1 | Days to Dicotyledonous Leaves 1 | Days to Two True Leaves 1 | Days to Four True Leaves 2 | |||||
|---|---|---|---|---|---|---|---|---|
| ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | |
| November | 6 Ca | 6 Ca | 7.56 Ca | 7.38 Ca | 16.50 Ca | 16.44 Ca | 18.69 c | 18.69 c |
| December | 7 Ba | 7 Ba | 8.81 Ba | 9.31 Ba | 20.00 Ba | 19.94 Ba | 23.94 a | 23.63 a |
| January | 10 Aa | 10 Aa | 11.19 Ab | 11.75 Aa | 21.19 Ab | 22.31 Aa | 22.31 b | 23.56 a |
| February | 10 Aa | 10 Aa | 11.5 Aa | 11.69 Aa | 14.06 Da | 14.38 Da | 16.75e | 17.50 d |
| March | 6 Ca | 6 Ca | 7.13 Cb | 7.69 Ca | 10.13 Eb | 10.81 Ea | 12.44 g | 13.44 f |
| Days to six true leaves 2 | Days to panicle emergence 2 | Days to flowering 2 | Days to maturity | |||||
| ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | |
| November | 21.44 d | 21.25 d | 50.63 e | 52.50 d | 72.63 c | 76.56 b | 101 | 119 |
| December | 32.19 a | 32.38 a | 61.00 b | 70.38 a | 71.31 d | 78.63 a | 112 | 125 |
| January | 25.25 c | 26.63 b | 52.00 d | 58.56 c | 68.44 e | 78.19 a | 103 | 127 |
| February | 19.75 e | 21.00 d | 40.69 h | 49.50 f | 54.75 h | 64.50 f | 98 | 111 |
| March | 15.25 g | 16.44 f | 34.06 i | 45.69 g | 47.56 i | 57.31 g | 83 | 109 |
| Grain Yield (t h−1) | Straw Yield (t h−1) | HI (%) | 1000 Seed Weight (g) | Panicle Length (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | ICBA-Q5 | Titicaca | |
| November | 0.39 c | 0.15 d | 0.58 d | 1.76 b | 0.43 a | 0.08 d | 2.25 b | 1.6 e | 19.69 bc | 25.21 a |
| December | 0.84 a | 0.8 a | 2.19 a | 2.08 a | 0.29 b | 0.33 b | 2.44 a | 1.99 c | 26.22 a | 21.43 b |
| January | 0.74 a | 0.5 b | 1.59 b | 2.25 a | 0.35 b | 0.18 c | 2.36 ab | 1.76 d | 16.93 c | 17.95 c |
| February | 0.22 d | 0.18 d | 2.14 a | 2.22 a | 0.11 d | 0.08 d | 1.69 de | 1.4 f | 12.79 d | 10.75 d |
| March | 0.08 d | 0.09 d | 0.96 c | 0.77 cd | 0.09 d | 0.13 cd | 1.28 f | 1.04 g | 12.3 d | 13.54 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taaime, N.; El Mejahed, K.; Moussafir, M.; Bouabid, R.; Oukarroum, A.; Choukr-Allah, R.; El Gharous, M. Early Sowing of Quinoa Cultivars, Benefits from Rainy Season and Enhances Quinoa Development, Growth, and Yield under Arid Condition in Morocco. Sustainability 2022, 14, 4010. https://doi.org/10.3390/su14074010
Taaime N, El Mejahed K, Moussafir M, Bouabid R, Oukarroum A, Choukr-Allah R, El Gharous M. Early Sowing of Quinoa Cultivars, Benefits from Rainy Season and Enhances Quinoa Development, Growth, and Yield under Arid Condition in Morocco. Sustainability. 2022; 14(7):4010. https://doi.org/10.3390/su14074010
Chicago/Turabian StyleTaaime, Nawal, Khalil El Mejahed, Mariam Moussafir, Rachid Bouabid, Abdallah Oukarroum, Redouane Choukr-Allah, and Mohamed El Gharous. 2022. "Early Sowing of Quinoa Cultivars, Benefits from Rainy Season and Enhances Quinoa Development, Growth, and Yield under Arid Condition in Morocco" Sustainability 14, no. 7: 4010. https://doi.org/10.3390/su14074010






