Study on the Sustainability Potential of Thyme, Oregano, and Coriander Essential Oils Used as Vapours for Antifungal Protection of Wheat and Wheat Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. EOs Chemical Composition and the Working Variants
2.2. Estimation of Antifungal Potential of EOs
- NOG = number of occurrences of the genus
- TNS = total number of fungi on sample
2.3. Estimation of the Phytotoxicity Effect of EOs on Wheat Seeds
2.4. Evaluation of Antimicotoxigenic Potential of EOs
- IMC—initial mycotoxin content (ppm)
- TMC—mycotoxin content at time T (ppm)
2.5. The Obtaining of Flouring Derivative Products
2.6. Sensory Evaluation of Bread Derivates from EOs Fumigated Wheat
2.7. Statistical Analysis
3. Results
3.1. GC-MS Composition of EOS
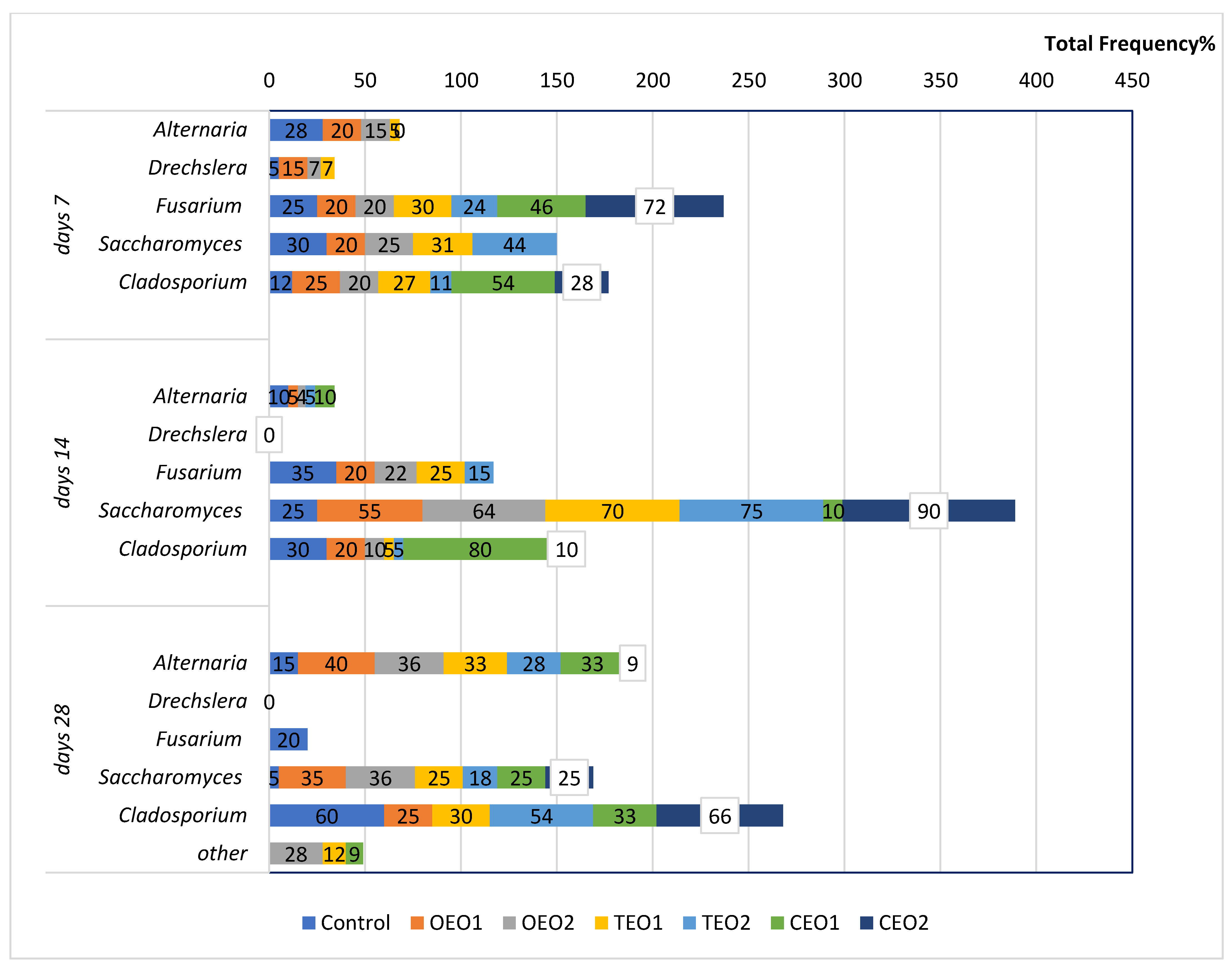
3.2. Estimation of Antifungal Potential of EOs
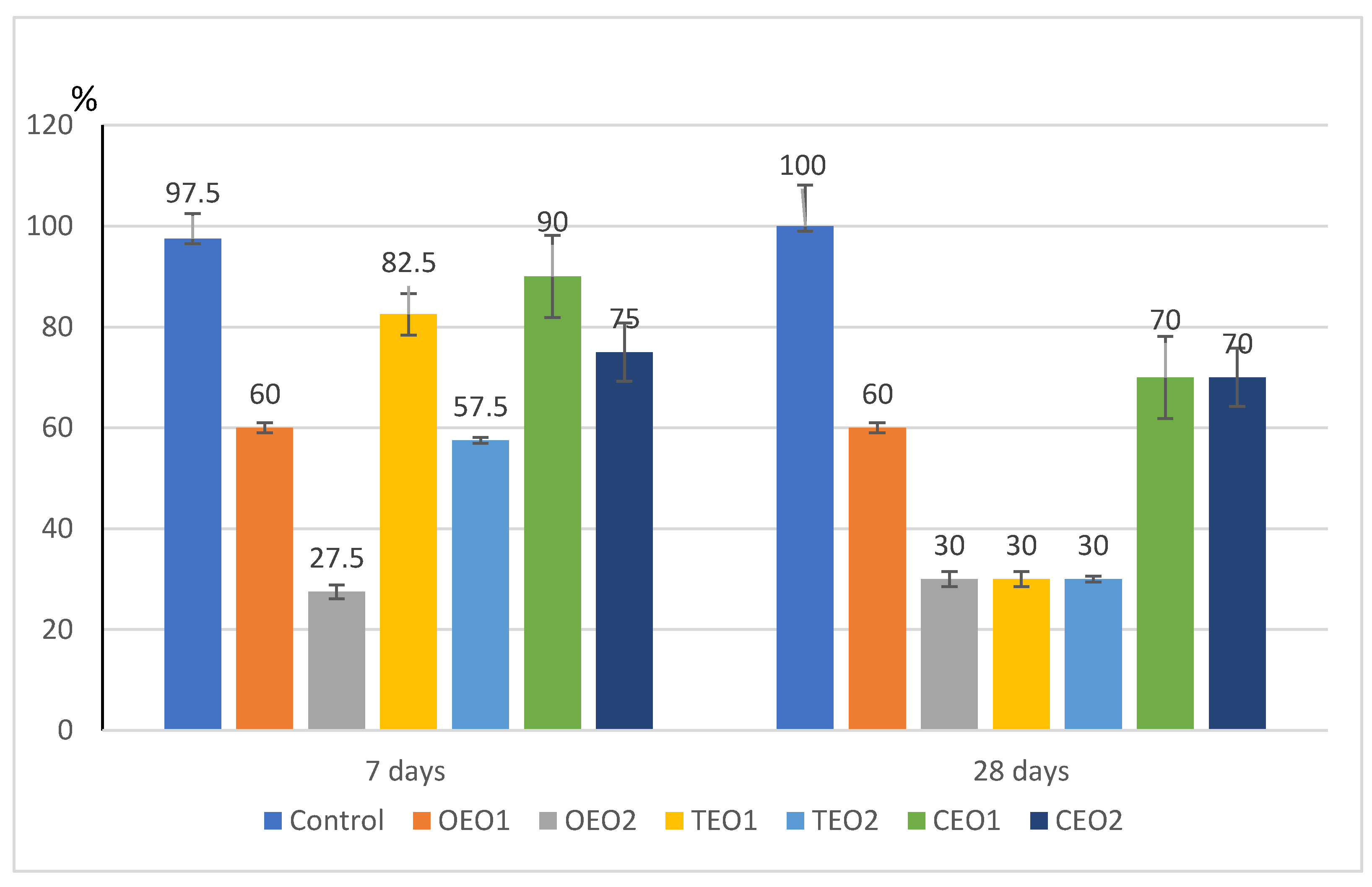
3.3. Estimation of the Phytotoxicity Effect of EOs on Wheat Seeds
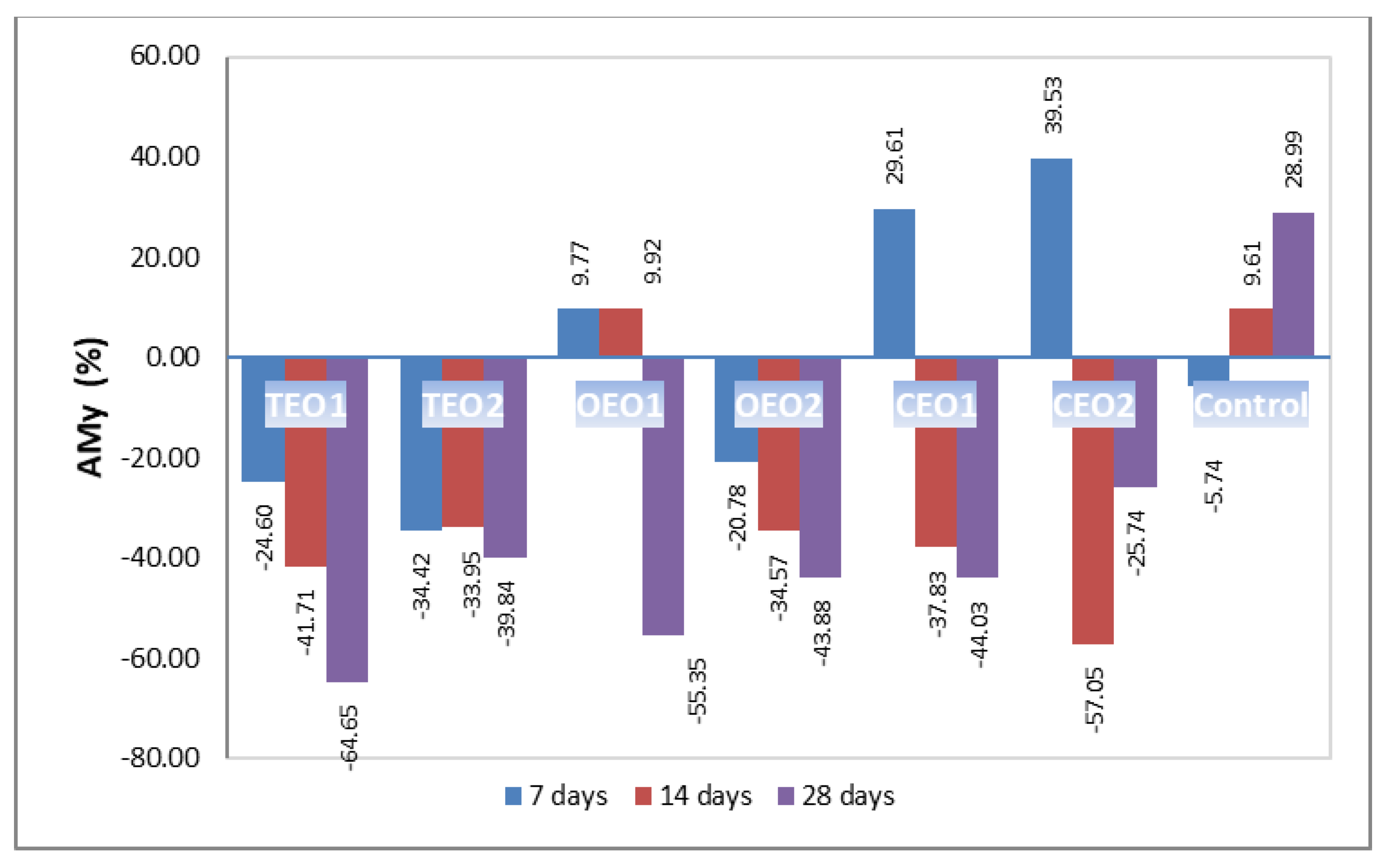
3.4. The Effect of EOs Treatment on Mycotoxins Contamination in Wheat Samples and Derivative Products
3.5. Results of the Sensory Evaluation of Bread Derivates from EOs Fumigated Wheat
4. Discussion
4.1. Chemical Composition of Essential Oils
4.2. Fungal Load of Wheat Seeds Kept in the Atmosphere Rich in Essential Oil Vapours
4.3. About Phytotoxicity Effect of EOs Vapours on Wheat
4.4. The Effect of EOs Treatment on Mycotoxins Contamination in Wheat Samples and Derivative Products
4.5. Sensorial Evaluation of Wheat Bread
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexa, E.; Poiana, M.-A.; Sumalan, R.-M. Mycoflora and Ochratoxin A Control in Wheat Grain Using Natural Extracts Obtained from Wine Industry By-Products. Int. J. Mol. Sci. 2012, 13, 4949–4967. [Google Scholar] [CrossRef] [PubMed]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal Effect of Five Essential Oils against Important Pathogenic Fungi of Cereals. Ind. Crops Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Negrea, M.; Pop, G.; Sumalan, R.; Alexa, E.; Poiana, M.-A. Post-Harvest Fungi Production in Different Storage. In Proceedings of the 6th Central European Congress on Food-CEFood Congress, Novi Sad, Serbia, 23–26 May 2012. [Google Scholar]
- Fleurat-Lessard, F. Integrated Management of the Risks of Stored Grain Spoilage by Seedborne Fungi and Contamination by Storage Mould Mycotoxins—An Update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical Factors Responsible for Fungi Growth in Stored Food Grains and Non-Chemical Approaches for Their Control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Dawlal, P.; Barros, E.; Marais, G.J. Evaluation of Maize Cultivars for Their Susceptibility towards Mycotoxigenic Fungi under Storage Conditions. J. Stored Prod. Res. 2012, 48, 114–119. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enyiukwu, D.N.; Awurum, A.N.; Nwaneri, J.A. Mycotoxins in Stored Agricultural Products: Implications to Food Safety and Health and Prospects of Plant-Derived Pesticides as Novel Approach to Their Management. Gen. J. Microbiol. Antimicrob. 2014, 2, 32–48. [Google Scholar] [CrossRef]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life Cycle Impact Assessment of Pesticides on Human Health and Ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Pandey, P.K.; Kumar, P.; Singh, N.N.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservati. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [Green Version]
- Alexa, E.; Danciu, C.; Cocan, I.; Negrea, M.; Morar, A.; Obistioiu, D.; Dogaru, D.; Berbecea, A.; Radulov, I. Chemical Composition and Antimicrobial Potential of Satureja hortensis L. in Fresh Cow Cheese. J. Food Qual. 2018, 2018, 8424035. [Google Scholar] [CrossRef] [Green Version]
- Cocan, I.; Negrea, M.; Cozma, A.; Alexa, E.; Poiana, M.-A.; Raba, D.; Danciu, C.; Popescu, I.; Cadariu, A.I.; Obistioiu, D.; et al. Chili and Sweet Pepper Seed Oil Used as a Natural Antioxidant to Improve the Thermo-Oxidative Stability of Sunflower Oil. Agronomy 2021, 11, 2579. [Google Scholar] [CrossRef]
- Obistioiu, D.; Cocan, I.; Tîrziu, E.; Herman, V.; Negrea, M.; Cucerzan, A.; Neacsu, A.-G.; Cozma, A.L.; Nichita, I.; Hulea, A.; et al. Phytochemical Profile and Microbiological Activity of Some Plants Belonging to the Fabaceae Family. Antibiotics 2021, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.; Sumalan, R.; Poiana, M.; Pop, G.; et al. Phytochemical Screening and Biological Activity of Lamiaceae Family Plant Extracts. Exp. Ther. Med. 2018, 15, 1863–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexa, V.T.; Szuhanek, C.; Cozma, A.; Galuscan, A.; Borcan, F.; Obistioiu, D.; Dehelean, C.A.; Jumanca, D. Natural Preparations Based on Orange, Bergamot and Clove Essential Oils and Their Chemical Compounds as Antimicrobial Agents. Molecules 2020, 25, 5502. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme Oil to Control Alternaria Alternata In Vitro and In Vivo as Fumigant and Contact Treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [Green Version]
- Teneva, D.; Denkova-Kostova, R.; Goranov, B.; Hristova-Ivanova, Y.; Slavchev, A.; Denkova, Z.; Kostov, G. Chemical Composition, Antioxidant Activity and Antimicrobial Activity of Essential Oil from Citrus aurantium L. Zest against Some Pathogenic Microorganisms. Z. Nat. C 2019, 74, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal Activity of Selected Volatile Essential Oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef]
- Shaaban, H.A. Essential Oil as Antimicrobial Agents: Efficacy, Stability, and Safety Issues for Food Application. In Essential Oils; de Oliveira, M.S., da Costa, W.A., Silva, S.G., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Sumalan, R.M.; Kuganov, R.; Obistioiu, D.; Popescu, I.; Radulov, I.; Alexa, E.; Negrea, M.; Salimzoda, A.F.; Sumalan, R.L.; Cocan, I. Assessment of Mint, Basil, and Lavender Essential Oil Vapor-Phase in Antifungal Protection and Lemon Fruit Quality. Molecules 2020, 25, 1831. [Google Scholar] [CrossRef]
- Harris, R. Synergism in the Essential Oil World. Int. J. Aromather. 2002, 12, 179–186. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liang, T.; Liu, Y.; Ding, G.; Zhang, F.; Ma, Z. Extraction, Structural Characterization, and Biological Functions of Lycium barbarum Polysaccharides: A Review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Liu, M.; An, W.; Yu, L.; Zhang, J.; Liu, Y.; Zhao, J.; Li, J. Lycium Barbarum Relieves Gut Microbiota Dysbiosis and Improves Colonic Barrier Function in Mice Following Antibiotic Perturbation. J. Funct. Foods 2020, 71, 103973. [Google Scholar] [CrossRef]
- Nguefack, J.; Leth, V.; Amvam Zollo, P.H.; Mathur, S.B. Evaluation of Five Essential Oils from Aromatic Plants of Cameroon for Controlling Food Spoilage and Mycotoxin Producing Fungi. Int. J. Food Microbiol. 2004, 94, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Chul, S. In Vitro and In Vivo Inhibion of Plant Pathogenic Fungi by Essential Oil and Extracts of Magnolia liliflora Desr. J. Agric. Sci. Technol. 2012, 14, 845–856. [Google Scholar]
- Sumalan, R.-M.; Alexa, E.; Poiana, M.-A. Assessment of Inhibitory Potential of Essential Oils on Natural Mycoflora and Fusarium Mycotoxins Production in Wheat. Chem. Cent. J. 2013, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2009; pp. 20–41. [Google Scholar]
- SR ISO 6658; 2017—Sensory Analysis—Methodology—General Guidance. ISO: Geneva, Switzerland, 2017.
- Schmidt, E.; Wanner, J.; Höferl, M.; Jirovetz, L.; Buchbauer, G.; Gochev, V.; Girova, T.; Stoyanova, A.; Geissler, M. Chemical Composition, Olfactory Analysis and Antibacterial Activity of Thymus vulgaris Chemotypes Geraniol, 4-Thujanol/Terpinen-4-Ol, Thymol and Linalool Cultivated in Southern France. Nat. Prod. Commun. 2012, 7, 1934578X1200700. [Google Scholar] [CrossRef] [Green Version]
- Hudaib, M.; Speroni, E.; Di Pietra, A.M.; Cavrini, V. GC/MS Evaluation of Thyme (Thymus vulgaris L.) Oil Composition and Variations during the Vegetative Cycle. J. Pharm. Biomed. Anal. 2002, 29, 691–700. [Google Scholar] [CrossRef]
- Baranauskienė, R.; Venskutonis, P.R.; Viškelis, P.; Dambrauskienė, E. Influence of Nitrogen Fertilisers on the Yield and Composition of Thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef]
- Rus, C.; Rm, S.; Alexa, E.; Dm, C.; Pop, G.; Botau, D. Study on Chemical Composition and Antifungal Activity of Essential Oils Obtained from Representative Species Belonging to the Lamiaceae Family. Plant Soil Environ. 2016, 61, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Imelouane, B.; El Bachiri, A.; Wathelet, J.-P.; Dubois, J.H.A. Chemical Composition, Cytotoxic and Antioxydant Activity of The Essential Oil of Lavandula dentata. World J. Chem. 2010, 5, 103–110. [Google Scholar]
- Sumalan, R.M.; Alexa, E.; Popescu, I.; Negrea, M.; Radulov, I.; Obistioiu, D.; Cocan, I. Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules 2019, 24, 2040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Mandal, M. Coriander (Coriandrum sativum L.) Essential Oil: Chemistry and Biological Activity. Asian Pac. J. Trop. Biomed. 2015, 5, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Zamindar, N.; Sadrarhami, M.; Doudi, M. Antifungal Activity of Coriander (Coriandrum sativum L.) Essential Oil in Tomato Sauce. Food Meas. 2016, 10, 589–594. [Google Scholar] [CrossRef]
- Momin, A.H.; Acharya, S.S.; Gajjar, A.V. Coriandrum Sativum-Review of Advances in Phytopharmacology. Int. J. Pharm. Sci. Res. 2012, 3, 1233. [Google Scholar]
- Han, F.; Ma, G.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.; Zhao, Z.; Xu, H. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Leyva-López, N.; Gutiérrez-Grijalva, E.; Vazquez-Olivo, G.; Heredia, J. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality Characters, Chemical Composition and Biological Activities of Oregano (Origanum Spp.) Essential Oils from Central and Southern Argentina. Ind. Crops Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris Essential Oil: Chemical Composition and Antimicrobial Activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant Essential Oils as Food Preservatives to Control Moulds, Mycotoxin Contamination and Oxidative Deterioration of Agri-Food Commodities—Potentials and Challenges. Food Control 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Antih, J.; Houdkova, M.; Urbanova, K.; Kokoska, L. Antibacterial Activity of Thymus vulgaris L. Essential Oil Vapours and Their GC/MS Analysis Using Solid-Phase Microextraction and Syringe Headspace Sampling Techniques. Molecules 2021, 26, 6553. [Google Scholar] [CrossRef] [PubMed]
- Houdkova, M.; Kokoska, L. Volatile Antimicrobial Agents and In Vitro Methods for Evaluating Their Activity in the Vapour Phase: A Review. Planta Med. 2020, 86, 822–857. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felińczak, A.; Gołębiewska, J.; Górniak, A.; Junka, A. The Antimicrobial and Antibiofilm In Vitro Activity of Liquid and Vapour Phases of Selected Essential Oils against Staphylococcus aureus. Pathogens 2021, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Nostro, A.; Mandras, N.; Dugo, P.; Banche, G.; Cannatelli, M.A.; Cuffini, A.M.; Alonzo, V.; Carlone, N.A. Antifungal Activity of Essential Oils against Filamentous Fungi Determined by Broth Microdilution and Vapour Contact Methods. J. Appl. Microbiol. 2007, 102, 1544–1550. [Google Scholar] [CrossRef]
- Inouye, S.; Nishiyama, Y.; Hasumi, Y.; Yamaguchi, H.; Abe, S.; Uchida, K.; Yamaguchi, H. The Vapor Activity of Oregano, Perilla, Tea Tree, Lavender, Clove, and Geranium Oils against a Trichophyton Mentagrophytes in a Closed Box. J. Infect. Chemother. 2006, 12, 349–354. [Google Scholar] [CrossRef]
- Inouye, S.; Uchida, K.; Maruyama, N.; Yamaguchi, H.; Abe, S. A Novel Method to Estimate the Contribution of the Vapor Activity of Essential Oils in Agar Diffusion Assay. Nippon Ishinkin Gakkai Zasshi 2006, 47, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The Role of Structure and Molecular Properties of Terpenoids in Determining Their Antimicrobial Activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Ait Haddou, L.; Belabess, Z.; Merah, O.; Lahlali, R. In Vitro and In Vivo Antifungal Activities of Nine Commercial Essential Oils against Brown Rot in Apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Prashanth, N.S.; Marchal, B.; Devadasan, N.; Kegels, G.; Criel, B. Advancing the Application of Systems Thinking in Health: A Realist Evaluation of a Capacity Building Programme for District Managers in Tumkur, India. Health Res. Policy Syst. 2014, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Saleh, N.B.; Chambers, B.; Aich, N.; Plazas-Tuttle, J.; Phung-Ngoc, H.N.; Kirisits, M.J. Mechanistic Lessons Learned from Studies of Planktonic Bacteria with Metallic Nanomaterials: Implications for Interactions between Nanomaterials and Biofilm Bacteria. Front. Microbiol. 2015, 6, 677. [Google Scholar] [CrossRef] [Green Version]
- Chiș, M.S.; Muste, S.; Păucean, A.; Man, S.; Sturza, A.; Petruț, G.S.; Mureșan, A. A Comprehensive Review about Antimicrobial Effects of Herb and Oil Oregano. Hop Med. Plants 2017, 25, 17–27. [Google Scholar]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus Essential Oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnieszka, S.; Magdalena, R.; Jan, B.; Katarzyna, W.; Malgorzata, B.; Krzysztof, H.; Danuta, K. Phytotoxic Effect of Fiber Hemp Essential Oil on Germination of Some Weeds and Crops. J. Essent. Oil Bear. Plants 2016, 19, 262–276. [Google Scholar] [CrossRef]
- Koiou, K.; Vasilakoglou, I.; Dhima, K. Herbicidal Potential of Lavender (Lavandula angustifolia Mill.) Essential Oil Components on Bristly Foxtail (Setaria verticillata (L.) P. Beauv.): Comparison with Carvacrol, Carvone, Thymol and Eugenol. Arch. Biol. Sci. 2020, 72, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Benarab, H.; Fenni, M.; Louadj, Y.; Boukhabti, H.; Ramdani, M. Allelopathic Activity of Essential Oil Extracts from Artemisia Herba-Alba Asso. on Seed and Seedling Germination of Weed and Wheat Crops. Acta Sci. Nat. 2020, 7, 86–97. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic Potential of Essential Oils from Temperate Climate Plants against the Germination of Selected Weeds and Crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential Oils from Mediterranean Lamiaceae as Weed Germination Inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef]
- Rahman, A. (Ed.) Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1988; ISBN 978-0-444-42970-4. [Google Scholar]
- Poonpaiboonpipat, T.; Pangnakorn, U.; Suvunnamek, U.; Teerarak, M.; Charoenying, P.; Laosinwattana, C. Phytotoxic Effects of Essential Oil from Cymbopogon citratus and Its Physiological Mechanisms on Barnyardgrass (Echinochloa crus-galli). Ind. Crops Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R.B. Hormesis and Plant Biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Rentzsch, S.; Podzimska, D.; Voegele, A.; Imbeck, M.; Müller, K.; Linkies, A.; Leubner-Metzger, G. Dose- and Tissue-Specific Interaction of Monoterpenes with the Gibberellin-Mediated Release of Potato Tuber Bud Dormancy, Sprout Growth and Induction of α-Amylases and β-Amylases. Planta 2012, 235, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Możdżeń, K.; Krajewska, A.; Bocianowski, J.; Jop, B.; Synowiec, A. Microencapsulated Caraway Essential Oil Affects Initial Growth of Maize Cultivars. Molecules 2021, 26, 5059. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic Activities of Mediterranean Essential Oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Garcia, S.N.; Garcia-Mier, L.; Garcia-Trejo, J.F.; Ramirez-Gomez, X.S.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Fusarium Mycotoxins and Metabolites That Modulate Their Production. In Fusarium—Plant Diseases, Pathogen Diversity, Genetic Diversity, Resistance and Molecular Markers; Askun, T., Ed.; InTech: Rijeka, Croatia, 2018; ISBN 978-1-78923-318-6. [Google Scholar]
- Magan, N. Mycotoxin Contamination of Food in Europe: Early Detection and Prevention Strategies. Mycopathologia 2006, 162, 245–253. [Google Scholar] [CrossRef]
- Mshelia, L.P.; Selamat, J.; Iskandar Putra Samsudin, N.; Rafii, M.Y.; Abdul Mutalib, N.-A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef]
- Kyprianou, M. Commission Regulation (EC) No 1126/2007 of 28 September 2007. Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs As Regards Fusarium Toxins in Maize and Maize Products; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Current Sights for Mechanisms of Deoxynivalenol-Induced Hepatotoxicity and Prospective Views for Future Scientific Research: A Mini Review: DON-Induced Hepatotoxicity and Prospective Views. J. Appl. Toxicol. 2017, 37, 518–529. [Google Scholar] [CrossRef]
- Cenkowski, S.; Pronyk, C.; Zmidzinska, D.; Muir, W.E. Decontamination of Food Products with Superheated Steam. J. Food Eng. 2007, 83, 68–75. [Google Scholar] [CrossRef]
- Passarinho, A.T.P.; Dias, N.F.; Camilloto, G.P.; Cruz, R.S.; Otoni, C.G.; Moraes, A.R.F.; Soares, N.D.F.F. Sliced Bread Preservation through Oregano Essential Oil-Containing Sachet: Bread Preservation through Antimicrobial Sachet. J. Food Process Eng. 2014, 37, 53–62. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Sikkhamondhol, C.; Teanpook, C.; Boonbumrung, S.; Chittrepol, S. Quality of Bread with Added Turmeric (Curcuma longa): Powder, Essential Oil and Extracted Residues. Asian J. Food Agro-Ind. 2009, 2, 690–701. [Google Scholar]
- Khalil, P.; Masood, S.; Rehman, A.; Zafar, A.; Islam, Z.; Javaid, N.; Ilyas, A.; Qamar, S.; Zeb, A. Proximate and Sensory Analysis of Wheat Bread Supplemented with Nigella Sativa Oil and Nigella Sativa Extract. Pure Appl. Biol. 2021, 10, 1158–1165. [Google Scholar] [CrossRef]
- Dhillon, G.K.; Amarjeet, K. Quality Evaluation of Bread Incorporated with Different Levels Cinnamon Powder. Int. J. Food Sci. 2013, 2, 70–74. [Google Scholar]
- Ávila Sosa Sánchez, R.; Portillo-Ruiz, M.C.; Viramontes-Ramos, S.; Muñoz-Castellanos, L.N.; Nevárez-Moorillón, G.V. Effect of Mexican Oregano (Lippia berlandieri Schauer) Essential Oil Fractions on the Growth of Aspergillus Spp. in a Bread Model System: Modeling Aw and Mexican Oregano on Aspergillus. J. Food Process. Preserv. 2015, 39, 776–783. [Google Scholar] [CrossRef]
- Muresan, C.; Stan, L.; Man, S.; Scrob, S.; Muste, S. Sensory Evaluation of Bakery Products and Its Role in Determining of the consumer preferences. J. Agroaliment. Processes Technol. 2012, 18, 304–306. [Google Scholar]



| EOs | µL | % * | |
|---|---|---|---|
| OEO1 | Oreganum sativum EOs | 800 | 0.2 |
| OEO2 | Oreganum sativum EOs | 1600 | 0.4 |
| TEO1 | Thymus vulgaris EOs | 800 | 0.2 |
| TEO2 | Thymus vulgaris EOs | 1600 | 0.4 |
| CEO1 | Coriandrum sativum EOs | 800 | 0.2 |
| CEO2 | Coriandrum sativum EOs | 1600 | 0.4 |
| Mycotoxins | Calibration Curve Parameters (r2) | MDL * |
|---|---|---|
| DON | 0.9959 | 0.08 ppm |
| ZEA | 0.9884 | 5 ppm |
| FUMO | 0.9946 | 0.015 ppm |
| Compounds | Type | TR | EOs | ||
|---|---|---|---|---|---|
| OEO | TEO | CEO | |||
| α-Pinene | MH | 11.314 | 2.403 | 1.918 | 6.071 |
| Camphene | MH | 12.641 | - | 1.675 | 0.308 |
| β-Pinene | MH | 13.468 | 0.594 | 3.477 | 0.143 |
| Β-Myrcene | MH | 14.549 | 4.243 | 1.773 | 0.213 |
| α-Phellandrene | MH | 14.800 | 0.255 | - | - |
| α-Terpinene | MH | 15.159 | 2.143 | 0.725 | - |
| D-Limonene | MH | 15.245 | 3.713 | 2.388 | 4.766 |
| γ-Terpinene | MH | 16.504 | 4.121 | - | 1.335 |
| cis- β-Ocimene | MH | 16.850 | - | - | 0.237 |
| p-Mentha-1,4(8)-diene | MH | 17.224 | - | - | 0.281 |
| Terpinolene | MH | 17.225 | 1.048 | - | - |
| Eucalyptol | MO | 17.338 | 0.129 | 0.698 | - |
| o-Cymene | MH | 18.491 | 28.750 | 31.957 | - |
| o-Cymol | MH | 18.551 | - | - | 5.386 |
| 1-Octen-3-ol * | 22.141 | - | 0.239 | - | |
| cis-Linalyl Oxide | MO | 23.520 | - | - | 1.375 |
| Trans-Linalool oxide | MO | 24.441 | - | - | 1.162 |
| β- Linalool | MO | 24.583 | 4.786 | 6.301 | 72.231 |
| Caryophyllene | SH | 25.121 | 6.210 | 1.425 | - |
| Thymol methyl ether | MO | 25.701 | - | 1.041 | - |
| Benzene, 1-methoxy-4-methyl-2-(1-methylethyl) | MO | 26.341 | - | 0.734 | - |
| Bornyl acetate | MO | 26.484 | - | 0.513 | - |
| 4-Terpinenol | MO | 26.903 | - | 1.761 | - |
| Camphor | MO | 27,913 | 0.206 | 0.319 | 3.643 |
| Geranyl acetate | MO | 28.93 | - | - | 0.901 |
| Borneol | MO | 29.277 | - | 1.725 | - |
| trans-Geraniol | MO | 30.96 | - | - | 0.077 |
| p-Propenylanisole | MO | 32.462 | - | - | 0.948 |
| p-Cymen-8-ol | MO | 32.887 | - | - | 0.091 |
| 3,7-Octadiene-2,6-diol, 2,6-dimethyl- | MO | 35.316 | - | - | 0.679 |
| Caryophyllene oxide | SO | 36.09 | 0.236 | 0.555 | - |
| Caryophyllene | SH | 36.419 | 3.573 | 3.380 | - |
| Thymol | MO | 37.378 | - | 35.862 | - |
| Carvacrol | MO | 38.198 | 37.349 | 1.524 | - |
| 1,7-Octadiene-3,6-diol, 2,6-dimethyl- | MO | 38.275 | - | - | 0.1456 |
| Viridiflorol | SO | 40.635 | 0.234 | - | - |
| Total major compounds ** | 98.343 | 96.213 | 95.969 | ||
| Monoterpene hidrocarbonates (MH) | 46.424 | 43.193 | 17.559 | ||
| Monoterpenes oxygenate (MO) | 42.136 | 48.215 | 78.410 | ||
| Sesquiterpene hidrocarbonates (SH) | 9.783 | 4.805 | - | ||
| Sesquiterpene oxygenate (SO) | 0.471 | 0.556 | - | ||
| Treatment | Radicles (mm) | Seedling (mm) | ||
|---|---|---|---|---|
| 7 Days | 28 Days | 7 Days | 28 Days | |
| Control | 11.50 ± 0.22 a | 10.94 ± 0.55 a | 4.29 ± 0.41 a | 4.29 ± 0.41 a |
| OEO1 | 9.64 ± 1.52 b | 5.52 ± 0.66 d | 3.64 ± 0.66 b | 3.65 ± 0.76 b |
| OEO2 | 2.97 ± 0.40 e | 3.33 ± 0.00 e | 2.17 ± 0.41 d | 2.00 ± 0.00 e |
| TEO1 | 9.14 ± 0.62 c | 9.07 ± 0.81 b | 4.00 ± 0.86 a | 3.84 ± 1.00 b |
| TEO2 | 2.84 ± 0.22 e | 3.00 ± 0.00 e | 2.75 ± 0.88 c | 2.67 ± 1.15 c |
| CEO1 | 11.09 ± 0.44 a | 11.03 ± 0.47 a | 3.00 ± 0.50 c | 3.00 ± 0.58 c |
| CEO2 | 6.19 ± 1.05 d | 5.99 ± 1.07 d | 2.38 ± 0.52 d | 2.43 ± 0.53 d |
| DON Content (ppm) | ||||
|---|---|---|---|---|
| EOs Treatment | Initial | 7 Days | 14 Days | 28 Days |
| CONTROL | 6.45 ± 0.04 a | 6.08 ± 0.06 f | 7.07 ± 0.05 c | 8.32 ± 0.10 g |
| OEO1 | 6.45 ± 0.07 a | 7.08 ± 0.08 c | 7.09 ± 0.08 c | 2.88 ± 0.05 c |
| OEO2 | 6.45 ± 0.06 a | 5.11 ± 0.09 a | 4.22 ± 0.04 bd | 3.62 ± 0.07 be |
| TEO1 | 6.45 ± 0.07 a | 4.86 ± 0.06 a | 3.76 ± 0.05 a | 2.28 ± 0.04 a |
| TEO2 | 6.45 ± 0.08 a | 4.23 ± 0.05 b | 4.26 ± 0.06 b | 3.88 ± 0.6 b |
| CEO1 | 6.45 ± 0.08 a | 8.36 ± 0.07 d | 4.01 ± 0.06 d | 3.61 ± 0.06 de |
| CEO2 | 6.45 ± 0.07 a | 9.00 ± 0.08 e | 2.77 ± 0.03 e | 4.79 ± 0.05 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bota, V.; Sumalan, R.M.; Obistioiu, D.; Negrea, M.; Cocan, I.; Popescu, I.; Alexa, E. Study on the Sustainability Potential of Thyme, Oregano, and Coriander Essential Oils Used as Vapours for Antifungal Protection of Wheat and Wheat Products. Sustainability 2022, 14, 4298. https://doi.org/10.3390/su14074298
Bota V, Sumalan RM, Obistioiu D, Negrea M, Cocan I, Popescu I, Alexa E. Study on the Sustainability Potential of Thyme, Oregano, and Coriander Essential Oils Used as Vapours for Antifungal Protection of Wheat and Wheat Products. Sustainability. 2022; 14(7):4298. https://doi.org/10.3390/su14074298
Chicago/Turabian StyleBota, Voichita, Renata Maria Sumalan, Diana Obistioiu, Monica Negrea, Ileana Cocan, Iuliana Popescu, and Ersilia Alexa. 2022. "Study on the Sustainability Potential of Thyme, Oregano, and Coriander Essential Oils Used as Vapours for Antifungal Protection of Wheat and Wheat Products" Sustainability 14, no. 7: 4298. https://doi.org/10.3390/su14074298







