The Utilization of Recycled Sewage Sludge Ash as a Supplementary Cementitious Material in Mortar: A Review
Abstract
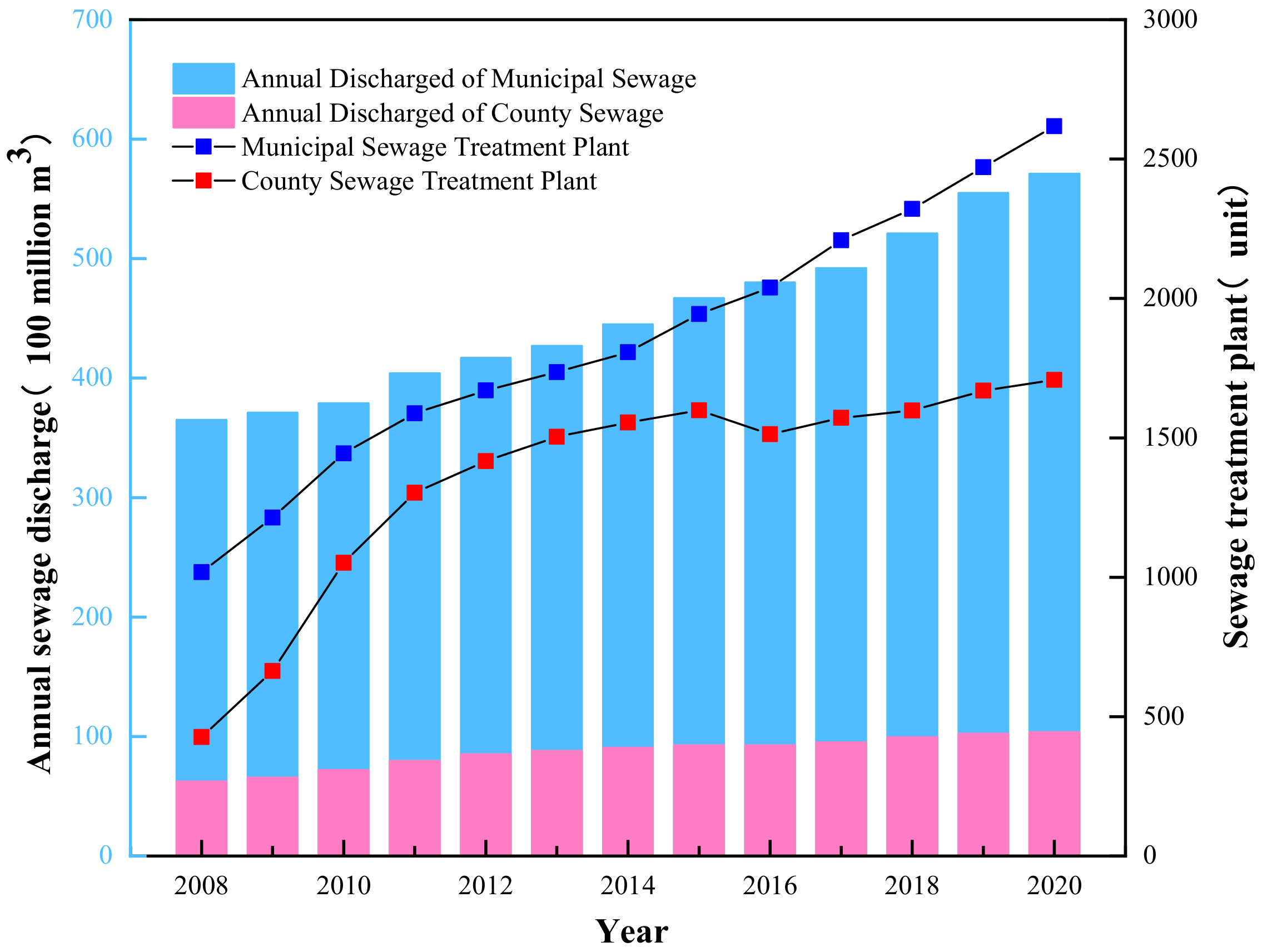
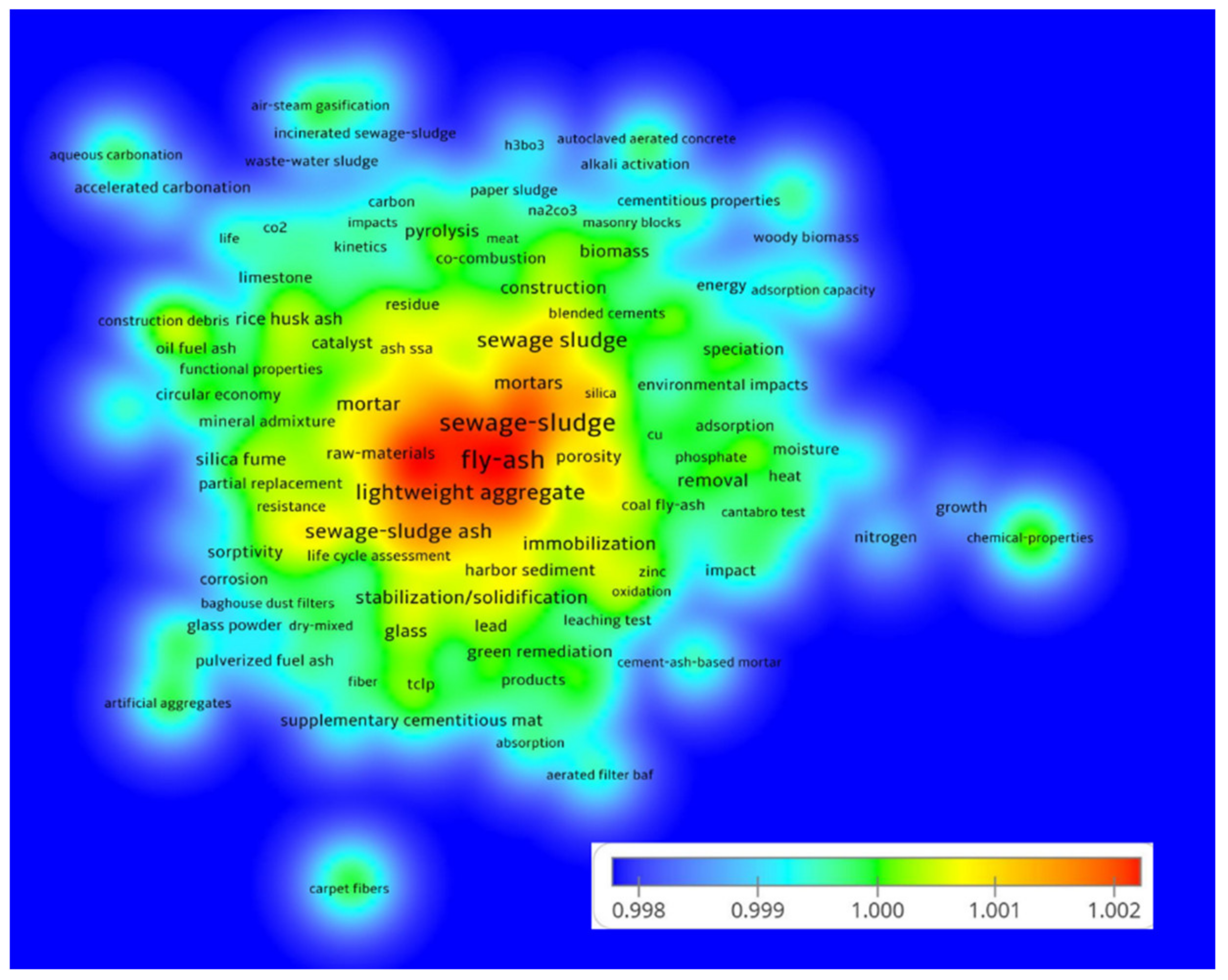
1. Introduction
2. Basic Characteristics of SSA
2.1. Specific Gravity and Density
2.2. Particle Size Distribution
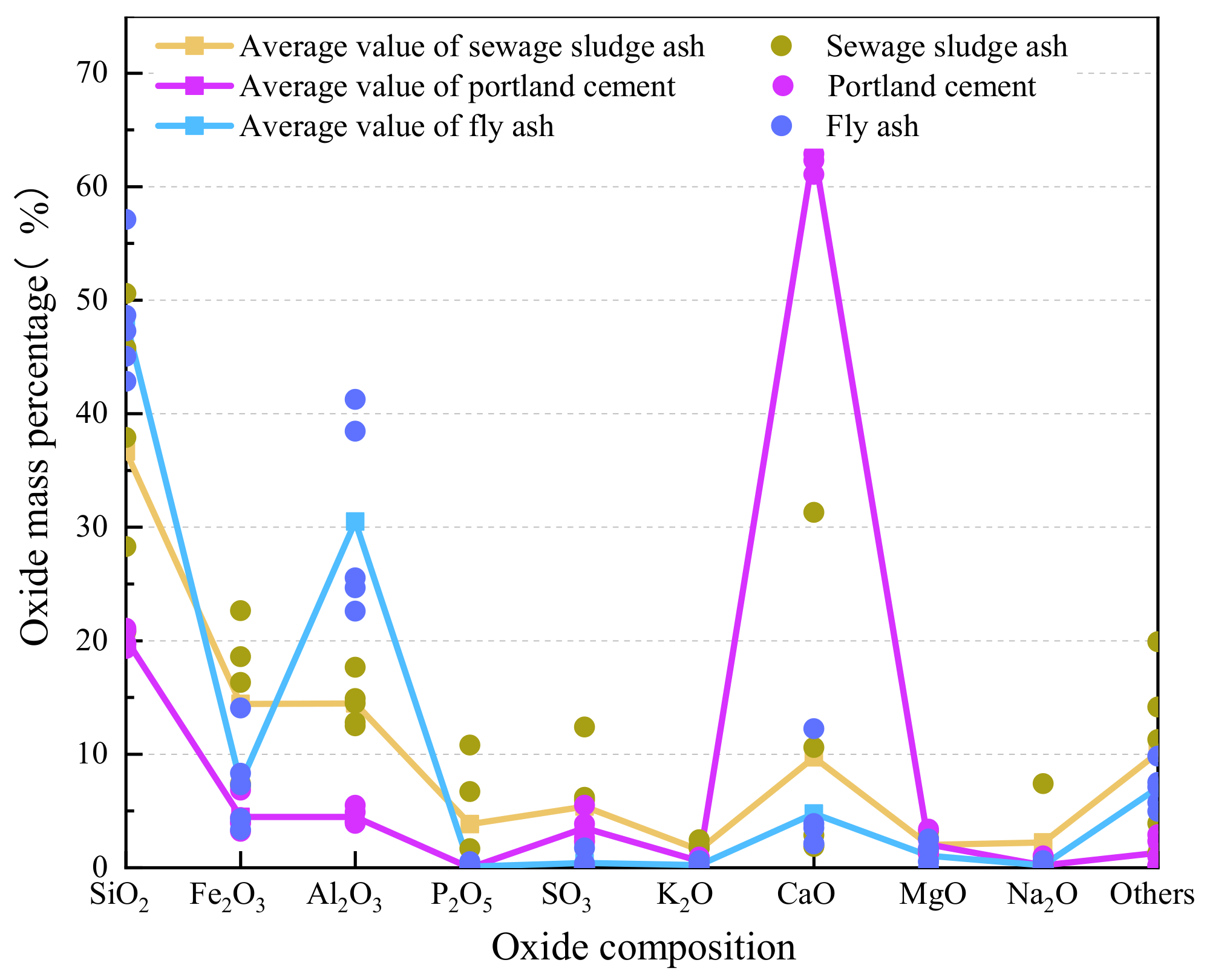
2.3. Chemical Composition
2.4. Heavy Metal Content
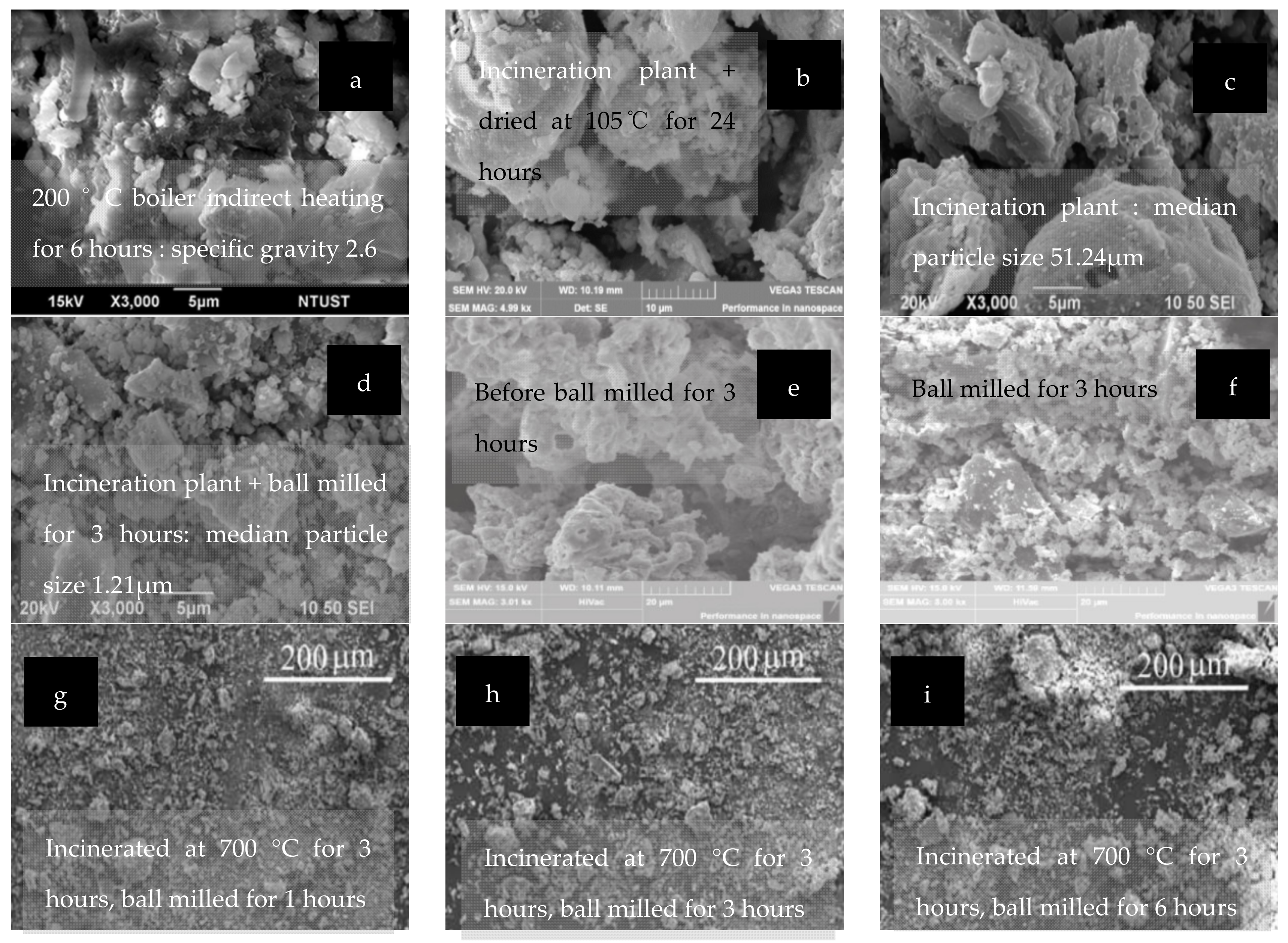
2.5. Appearance Morphology and Microstructure
2.6. Pozzolanic Activity
3. Effect of SSA on the Performance of Mortar
3.1. Workability
3.2. Setting Time
3.3. Water Absorption and Water Requirement
3.4. Compressive Strength
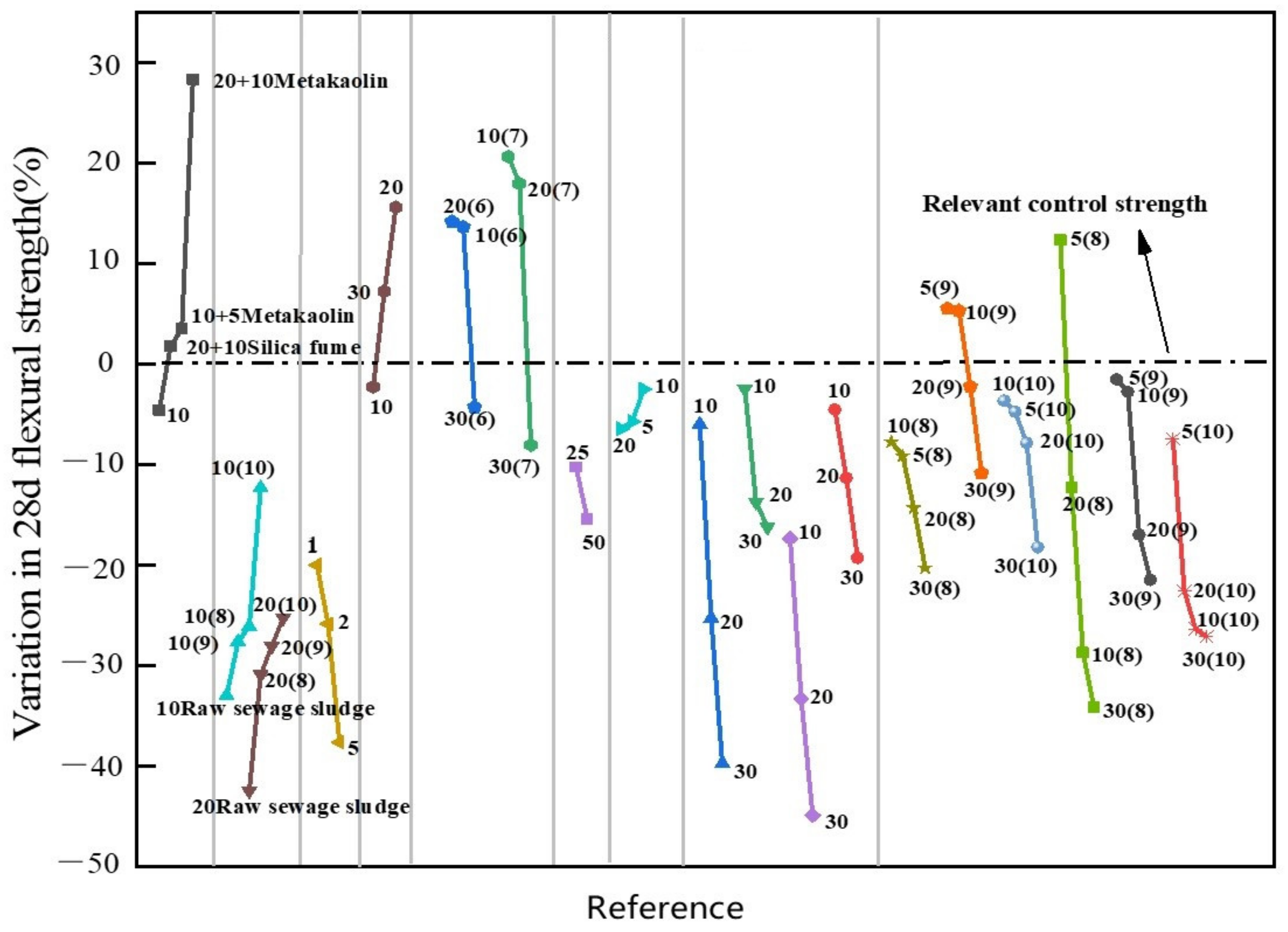
3.5. Flexural Strength
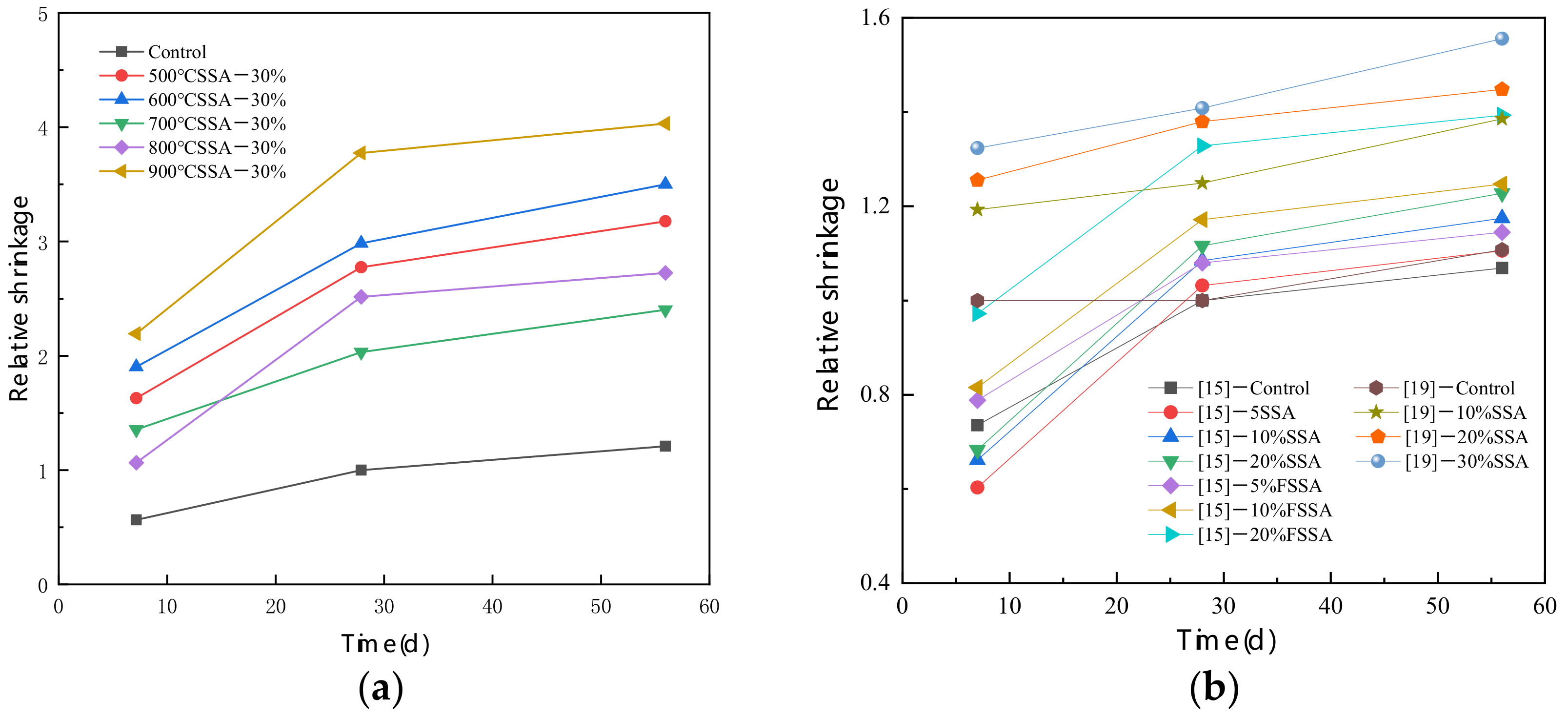
3.6. Durability
3.7. The Influence Mechanism of SSA on the Properties of Mortar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Yan, S.H.; Ye, Z.L.; Meng, H.J.; Zhu, Y.G. Utilization of urban sewage sludge: Chinese Perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Housing and Urban-Rural Development of the People’s Republic of China. Available online: http://www.mohurd.gov.cn/ (accessed on 24 November 2021).
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, Y.; Huang, H.; Lai, F.; Chang, Y.; Pan, Z.; Xiao, X. Assessment optimization of the contamination degree and ecological risk of heavy metals in sewage sludge. Environ. Sci. Technol. 2019, 42, 202–209. (In Chinese) [Google Scholar]
- Wang, N.; Li, Y. Disposal status analysis and resource utilization of residual sludge in dingdao. Environ. Sci. Manag. 2010, 35, 82–84. (In Chinese) [Google Scholar]
- Jointly Issued by the Development and Reform Commission and the Ministry of Housing and Construction. The Implementation Plan for Strength and Weakness of Municipal Sewage Treatment Facilities. Available online: https://huanbao.bjx.com.cn/news/20200731/1093761.shtml (accessed on 28 July 2020).
- Yusuf, R.O.; Noor, Z.Z.; Moh’, N.A.; Moh’d, F.; Moh’d, D.; Abba, A.H. Use of sewage sludge ash (SSA) in the production of cement and concrete—A review. Int. J. Glob. Environ. Issues 2012, 12, 214–228. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage sludge ash characteristics and potential for use in concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, D.; Feng, P.; Hao, B.; Guo, Y.; Wang, S. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Khan, M.T.; Zubair, M.; Bilal, M.; Sajid, M. Removal of pharmaceuticals from water using sewage sludge-derived biochar: A review. Chemosphere 2022, 289, 133196. [Google Scholar] [CrossRef]
- Scrivener, K.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential Economically Viable Solutions for a Low-CO2 Cement-based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Gabel, K.; Tillman, A.M. Simulating operational alternatives for future cement production. J. Clean. Prod. 2005, 13, 1246–1257. [Google Scholar] [CrossRef]
- Pan, S.C.; Tseng, D.H.; Lee, C.C.; Lee, C. Influence of the fineness of sewage sludge ash on the mortar properties. Cem. Concr. Res. 2003, 33, 1749–1754. [Google Scholar] [CrossRef]
- Chen, Z.; Poon, C.S. Comparative studies on the effects of sewage sludge ash and fly ash on cement hydration and properties of cement mortars. Constr. Build. Mater. 2017, 154, 791–803. [Google Scholar] [CrossRef]
- Zhang, Q. Study on Mechanical Properties and Heavy Metal Curing Capacity of Cement-Based Materials Mixed with Sludge. Master’s Thesis, Engineering, Harbin Institute of Technology, Harbin, China, 2017. (In Chinese). [Google Scholar]
- Chen, Z.; Poon, C.S. Comparing the use of sewage sludge ash and glass powder in cement mortars. Environ. Technol. 2017, 38, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Monzó, J.; Payá, J.; Borrachero, M.V.; Córcoles, A. Use of sewage sludge ash (SSA)-cement admixtures in mortars. Cem. Concr. Res. 1996, 26, 1389–1398. [Google Scholar] [CrossRef]
- Li, J.S.; Guo, M.Z.; Xue, Q.; Poon, C.S. Recycling of incinerated sewage sludge ash and cathode ray tube funnel glass in cement mortars. J. Clean. Prod. 2017, 152, 142–149. [Google Scholar] [CrossRef]
- Smol, M.; Kulczycka, J.; Henclik, A. The possible use of sewage sludge ash (SSA) in the construction industry as a way towards a circular economy. J. Clean. Prod. 2015, 95, 45–54. [Google Scholar] [CrossRef]
- Świerczek, L.; Cieślik, B.M.; Konieczka, P. Challenges and opportunities related to the use of sewage sludge ash in cement-based building materials–A review. J. Clean. Prod. 2021, 287, 125054. [Google Scholar] [CrossRef]
- Tay, J.H.; Show, K.Y. Municipal wastewater sludge as cementitious and blended cement materials. Cem. Concr. Compos. 1994, 16, 39–48. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xue, Q.; Huang, X.; Liu, L.; Poon, C.S. Sludge biochar as a green additive in cement-based composites: Mechanical Properties and Hydration Kinetics. Constr. Build. Mater. 2020, 262, 120723. [Google Scholar] [CrossRef]
- Cong, X.; Lu, S.; Gao, Y.; Yao, Y.; Elchalakani, M.; Shi, X. Effects of microwave, thermomechanical and chemical treatments of sewage sludge ash on its early-age behavior as supplementary cementitious material. J. Clean. Prod. 2020, 258, 120647. [Google Scholar] [CrossRef]
- Kappel, A.; Ottosen, L.M.; Kirkelund, G.M. Colour, compressive strength and workability of mortars with an iron rich sewage sludge ash. Constr. Build. Mater. 2017, 157, 1199–1205. [Google Scholar] [CrossRef]
- Chen, M.; Blanc, D.; Gautier, M.; Mehu, J.; Gourdon, R. Environmental and technical assessments of the potential utilization of sewage sludge ashes (SSAs) as secondary raw materials in construction. Waste Manag. 2013, 33, 1268–1275. [Google Scholar] [CrossRef]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S. Environmental impacts of sewage sludge ash in construction: Leaching Assessment. Resour. Conserv. Recycl. 2018, 136, 306–314. [Google Scholar] [CrossRef]
- Classification of Medium, Coarse and Fine Sand, National Standard Size. Available online: https://www.docin.com/p-2295143164.html (accessed on 12 January 2020).
- Valls, S.; Yagüe, A.; Vázquez, E.; Mariscal, C. Physical and mechanical properties of concrete with added dry sludge from a sewage treatment plant. Cem. Concr. Res. 2004, 34, 2203–2208. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, S.Y. Application of water treatment sludge in the manufacturing of lightweight aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Li, J.; Cheeseman, C.; Poon, C.S. Effect of NaCl and MgCl2 on the hydration of lime-pozzolan blend by recycling sewage sludge ash. J. Clean. Prod. 2021, 313, 127759. [Google Scholar] [CrossRef]
- Lin, K.L.; Chang, W.C.; Lin, D.F.; Luo, H.L.; Tsai, M.C. Effects of nano-SiO2 and different ash particle sizes on sludge ash-cement mortar. J. Environ. Manag. 2008, 88, 708–714. [Google Scholar] [CrossRef]
- Li, X.G.; He, C.H.; Lv, Y.; Jian, S.W.; Liu, G.; Jiang, W.G.; Jiang, D.B. Utilization of municipal sewage sludge and waste glass powder in production of lightweight aggregates. Constr. Build. Mater. 2020, 256, 119413. [Google Scholar] [CrossRef]
- Monzó, J.; Payá, J.; Borrachero, M.V.; Girbés, I. Reuse of sewage sludge ashes (SSA) in cement mixtures: The Effect of SSA on the Workability of Cement Mortars. Waste Manag. 2003, 23, 373–381. [Google Scholar] [CrossRef]
- Pan, L. Application of fly ash in ceramic plate. Ceramics 2015, 41, 36–39. (In Chinese) [Google Scholar]
- Zhu, B.; Dai, R.; Hu, C.Y. Experimental study of application of fiy ash polypropylene fiber and other materials in sludge solidification. Fly Ash. 2016, 28, 5–7. (In Chinese) [Google Scholar]
- Zhang, Y.; Wang, Z.; Wang, Z.Y.; Sun, H.Q.; Wang, L.L.; Wen, C.M.; Zhang, Y.T.; Tang, Z.D. Effect of denitrification in thermal power plant on properties of fiy ash. Non-Met. Mines. 2015, 38, 9–12. (In Chinese) [Google Scholar]
- Li, C.C.; Xu, Z.R.; Chen, T.D. Effect of fly ash on thaumasite form of sulfate attack. J. Build. Mater. 2014, 17, 685–689. [Google Scholar]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Kazberuk, M.K. Application of SSA as partial replacement of aggregate in concrete. Pol. J. Environ. Stud. 2011, 20, 365–370. [Google Scholar]
- Balonis, M. Thermodynamic modelling of temperature effects on the mineralogy of Portland cement systems containing chloride. Cem. Concr. Res. 2019, 120, 66–76. [Google Scholar] [CrossRef]
- Cheng, M.; Wu, L.; Huang, Y.; Luo, Y.; Christie, P. Total concentrations of heavy metals and occurrence of antibiotics in sewage sludges from cities throughout China. J. Soils Sediments 2014, 14, 1123–1135. [Google Scholar] [CrossRef]
- Tella, M.; Doelsch, E.; Letourmy, P.; Chataing, S.; Cuoq, F.; Bravin, M.N.; Saint Macary, H. Investigation of potentially toxic heavy metals in different organic wastes used to fertilize market garden crops. Waste Manag. 2013, 33, 184–192. [Google Scholar] [CrossRef]
- Ščančar, J.; Milačič, R.; Stražar, M.; Burica, O. Total metal concentrations and partitioning of Cd, Cr, Cu, Fe, Ni and Zn in sewage sludge. Sci. Total Environ. 2000, 250, 9–19. [Google Scholar] [CrossRef]
- Cyprowski, M.; Krajewski, J.A. Harmful agents in municipal wastewater treatment plants. Med. Pr. 2003, 54, 73–80. [Google Scholar] [PubMed]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Kendir, E.; Kentel, E.; Sanin, F.D. Evaluation of heavy metals and associated health risks in a metropolitan wastewater treatment plant’s sludge for its land application. Hum. Ecol. Risk Assess. 2019, 21, 1631–1643. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.L.; Stoffella, P.J. Land application of biosolids in the USA: A Review. Appl. Environ. Soil Sci. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Antunes, E.; Schumann, J.; Brodie, G.; Jacob, M.V.; Schneider, P.A. Biochar produced from biosolids using a single-mode microwave: Characterisation and its potential for phosphorus removal. J. Environ. Manag. 2017, 196, 119–126. [Google Scholar] [CrossRef]
- Coutand, M.; Cyr, M.; Clastres, P. Use of sewage sludge ash as mineral admixture in mortars. Constr. Mater. 2006, 159, 153–162. [Google Scholar] [CrossRef]
- Franz, M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef]
- Chiou, I.J.; Wang, K.S.; Chen, C.H.; Lin, Y.T. Lightweight aggregate made from sewage sludge and incinerated ash. Waste Manag. 2006, 26, 1453–1461. [Google Scholar] [CrossRef]
- Morais, L.C.; Dweck, J.; Goncalves, E.M.; Buchler, P.M. An experimental study of sewage sludge incineration. Environ. Technol. 2005, 27, 1047–1051. [Google Scholar] [CrossRef]
- Adam, C.; Peplinski, B.; Michaelis, M.; Kley, G.; Simon, F.G. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009, 29, 1122–1128. [Google Scholar] [CrossRef]
- Lin, K.L.; Lin, C.Y. Hydration properties of eco-cement pastes from waste sludge ash clinkers. J. Air Waste Manag. Assoc. 2004, 54, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Lin, C.Y. Hydration characteristics of waste sludge ash utilized as raw cement material. Cem. Concr. Res. 2005, 35, 1999–2007. [Google Scholar] [CrossRef]
- Lin, K.L.; Chiang, K.Y.; Lin, D.F. Effect of heating temperature on the sintering characteristics of sewage sludge ash. J. Hazard. Mater. 2006, 128, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Huang, W.J.; Chen, K.C.; Chow, J.D.; Chen, H.J. Behaviour of heavy metals immobilized by co-melting treatment of sewage sludge ash and municipal solid waste incinerator fly ash. Waste Manag. Res. 2009, 27, 660–667. [Google Scholar] [CrossRef]
- Saikia, N.; Kato, S.; Kojima, T. Compositions and leaching behaviours of combustion residues. Fuel 2006, 85, 264–271. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yin, Y.; Liang, X.; Li, A. The recycling of incinerated sewage sludge ash as a raw material for CaO–Al2O3–SiO2–P2O5 glass ceramic production. Environ. Technol. 2015, 36, 1098–1103. [Google Scholar] [CrossRef]
- Fraissler, G.; Joller, M.; Mattenberger, H.; Brunner, T.; Obernberger, I. Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination. Chem. Eng. Process 2009, 48, 152–164. [Google Scholar] [CrossRef]
- Mattenberger, H.; Fraissler, G.; Brunner, T.; Herk, P.; Hermann, L.; Obernberger, I. Sewage sludge ash to phosphorus fertiliser: Variables Influencing Heavy Metal Removal During Thermochemical Treatment. Waste Manag. 2008, 28, 2709–2722. [Google Scholar] [CrossRef]
- Vogel, C.; Exner, R.M.; Adam, C. Heavy metal removal from sewage sludge ash by thermochemical treatment with polyvinylchloride. Environ. Sci. Technol. 2013, 47, 563–567. [Google Scholar] [CrossRef]
- Vogel, C.; Adam, C.; Kappen, P.; Schiller, T.; Lipiec, E.; McNaughton, D. Chemical state of chromium in sewage sludge ash based phosphorus fertilisers. Chemosphere 2014, 103, 250–255. [Google Scholar] [CrossRef]
- Erich Schmitt. Merkblatt Entsorgung von Abfällen aus Verbrennungsanlagen für Siedlungsabfälle; Beschlossen durch die Länder-Arbeitsgemeinschaft Abfall (LAGA): Berlin, Germany, 1994. [Google Scholar]
- Lin, D.F.; Lin, K.L.; Luo, H.L.; Cai, M.Q. Improvements of nano-SiO2 on sludge/fly ash mortar. Waste Manag. 2008, 28, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Long, G.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A Review. Resour. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Bhatty, J.I.; Malisci, A.; Iwasaki, I.; Reid, K.J. Sludge ash pellets as coarse aggregate in concrete. J. Cem. Concr. Aggreg. 1992, 14, 55–61. [Google Scholar] [CrossRef]
- Chen, L.; Lin, D.F. Applications of sewage sludge ash and nano-SiO2 to manufacture tile as construction material. Constr. Build. Mater. 2009, 23, 3312–3320. [Google Scholar] [CrossRef]
- Chen, C.H.; Chiou, I.J.; Wang, K.S. Sintering effect on cement bonded sewage sludge ash. Cem. Concr. Res. 2006, 28, 26–32. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Poon, C.S. Combined use of sewage sludge ash and recycled glasewage sludge cullet for the production of concrete blocks. J. Clean. Prod. 2018, 171, 1447–1459. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, K.C. Effect of reducing conditions on sludge melting process. Chemosphere 2003, 50, 1063–1068. [Google Scholar] [CrossRef]
- Khanbilvardi, R.; Afshari, S. Sludge ash as fine aggregate for concrete mix. J. Environ. Eng. 1995, 121, 633–638. [Google Scholar] [CrossRef]
- Latosinska, J. The evaluation of the impact of sewage sludge ash modification on leaching of heavy metals. Adv. Civ. Environ. Eng. 2014, 1, 27–42. [Google Scholar]
- Li, J.S.; Xue, Q.; Fang, L.; Poon, C.S. Characteristics and metal leachability of incinerated sewage sludge ash and air pollution control residues from Honk Kong evaluated by different methods. Waste Manag 2017, 64, 161–170. [Google Scholar] [CrossRef]
- Li, J.S.; Tsang, D.C.W.; Wang, Q.M.; Fang, L.; Xue, Q.; Poon, C.S. Fate of metals before and after chemical extraction of incinerated sewage sludge ash. Chemosphere 2017, 186, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L. Mineralogy and microstructure of sintered sewage sludge ash as lightweight aggregates. J. Ind. Eng. Chem. 2006, 12, 425–429. [Google Scholar]
- Lin, D.F.; Weng, C.H. Use of sewage sludge ash as brick material. J. Environ. Eng. 2001, 127, 922–927. [Google Scholar] [CrossRef]
- Lin, D.F.; Luo, H.L.; Sheen, Y.N. Glazed tiles manufactured from incinerated sludge ash and clay. J. Air Waste Manag. Assoc. 2005, 55, 163–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.F.; Luo, H.L.; Halao, D.H.; Yang, C.C. The effects of sludge ash on the strength of soft subgrade soil. J. Chin. Inst. Environ. Eng. 2005, 15, 1–10. [Google Scholar]
- Tsai, C.C.; Wang, K.S.; Chiou, I.J. Effect of SiO2–Al2O3–flux ratio change on the bloating characteristics of lightweight aggregate material produced from recycled sewage sludge. J. Hazard. Mater. 2006, 134, 87–93. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Yuan, H. Cement-based solidification of incinerated sewage sludge ash by the addition of a novel solidifying aid. Int. J. Waste Resour. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Weng, C.H. Removal of Nickel(II) from dilute aqueous solution by sludge-ash. J. Environ. Eng. 2002, 128, 716–722. [Google Scholar] [CrossRef]
- Chang, F.C.; Lin, J.D.; Tsai, C.C.; Wang, K.S. Study on cement mortar and concrete made with sewage sludge ash. Water Sci. Technol. 2010, 62, 1689–1693. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chen, C.T. Strength development of cement pastes with alkali-activated dehydrated sewage sludge. Constr. Build. Mater. 2020, 255, 119243. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Lu, J.; Lu, J.; Cheeseman, C.; Poon, C.S. Recycling incinerated sewage sludge ash (ISSA) as a cementitious binder by lime activation. J. Clean. Prod. 2020, 244, 118856. [Google Scholar] [CrossRef]
- Wang, S. The Effect of Sludge Ash Treated at Different Calcination Temperatures on the Performance of Cement Mortar. Full-time. Master’s Thesis, Engineerring, Harbin Institute of Technology, Harbin, China, 2015. (In Chinese). [Google Scholar]
- De Carvalho Gomes, S.; Zhou, J.L.; Li, W.; Long, G. Progress in manufacture and properties of construction materials incorporating water treatment sludge: A Review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Res. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- ASTM C618-17a. Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM Int’L: West Conshohocken, PA, USA, 2017.
- IS 1344. Specification for Calcined Clay Pozzolana. Bureau of Indian Standards (BIS): New Delhi, India, 1981.
- Pan, S. Use of sewage sludge ash as fine aggregate and pozzolan in portland cement mortar. J. Solid Waste Technol. Manag. 2002, 28, 121–130. [Google Scholar]
- Pan, S.C.; Tseng, D.H. Sewage sludge ash characteristics and its potential applications. Water Sci. Technol. 2001, 44, 261–267. [Google Scholar] [CrossRef]
- Quarcioni, V.A.; Chotoli, F.F.; Coelho, A.C.V.; Cincotto, M.A. Indirect and direct Chapelle’s methods for the determination of lime consumption in pozzolani materials. IBRACON Struct. Mater. J. 2015, 8, 1–7. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Alam, M. Investigating calcined filter backwash solids as supplementary cementitious material for recycling in construction practices. Constr. Build. Mater. 2018, 175, 664–671. [Google Scholar] [CrossRef]
- Donatello, S.; Tong, D.; Cheeseman, C.R. Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manag. 2010, 30, 1634–1642. [Google Scholar] [CrossRef]
- Basto, P.A.; Junior, H.S.; Neto, A.A.M. Characterization and pozzolanic properties of sewage sludge ashes (SSA) by electrical conductivity. Cem. Concr. Compos. 2019, 104, 103410. [Google Scholar] [CrossRef]
- Naamane, S.; Rais, Z.; Taleb, M. The effectiveness of the incineration of sewage sludge on the evolution of physicochemical and mechanical properties of Portland cement. Constr. Build. Mater. 2016, 112, 783–789. [Google Scholar] [CrossRef]
- Vouk, D.; Serdar, M.; Vučinić, A.A. Use of incinerated sewage sludge ash in cement mortars: Case Sudy in Croatia. Tech. Gaz. 2017, 24, 43–51. [Google Scholar]
- Monzo, J.; Paya, J.; Borrachero, M.V.; Bellver, A.; Peris-Mora, E. Study of cement-based mortars containing Spanish ground sewage sludge ash. Waste Mater. Constr. 1997, 38, 349–354. [Google Scholar]
- Rutkowska, G.; Wichowski, P.; Fronczyk, J.; Franus, M.; Chalecki, M. Use of fly ashes from municipal sewage sludge combustion in production of ash concretes. Constr. Build. Mater. 2018, 188, 874–883. [Google Scholar] [CrossRef]
- Chakraborty, S.; Jo, B.W.; Jo, J.H.; Baloch, Z. Effectiveness of sewage sludge ash combined with waste pozzolanic minerals in developing sustainable construction material: An Alternative Approach for Waste Management. J. Clean. Prod. 2017, 153, 253–263. [Google Scholar] [CrossRef]
- Pinarli, V. Sustainable waste management—Studies on the use of sewage sludge ash in construction industry as concrete material. In Sustainable Construction: Use Incinerator Ash; Thomas Telford Publishing: London, UK, 2000; pp. 415–425. [Google Scholar]
- Mejdi, M.; Saillio, M.; Chaussadent, T.; Divet, L.; Tagnit-Hamou, A. Hydration mechanisms of sewage sludge ashes used as cement replacement. Cem. Concr. Res. 2020, 135, 106115. [Google Scholar] [CrossRef]
- Piasta, W.; Lukawska, M. The effect of sewage sludge ash on properties of cement composites. Procedia Eng. 2016, 161, 1018–1024. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, M.M.R.; Uddin, M.T.; Islam, M.A. Textile Effluent Treatment Plant Sludge: Characterization and Utilization in Building Materials. Arab. J. Sci. Eng. 2017, 42, 1435–1442. [Google Scholar] [CrossRef]
- Pan, S.C.; Lin, C.C.; Tseng, D.H. Reusing sewage sludge ash as adsorbent for copper removal from wastewater. Resour. Conserv. Recycl. 2003, 39, 79–90. [Google Scholar] [CrossRef]
- Monzó, J.; Paya, M.V.; Borrachero, E.P.M. Mechanical behavior of mortar containing sewage sludge ash (SSA) and Portland cements with different tricalcium aluminate content. Cem. Concr. Res. 1999, 29, 87–94. [Google Scholar] [CrossRef]
- Federico, L.M.; Chidiac, S.E. Waste glasewage sludge as a supplementary cementitious material in concrete—Critical review of treatment methods. Cem. Concr. Compos. 2009, 31, 606–610. [Google Scholar] [CrossRef]
- Wang, K.S.; Chiou, I.J.; Chen, C.H.; Wang, D. Lightweight properties and pore structure of foamed material made from sewage sludge ash. Constr. Build. Mater. 2005, 19, 627–633. [Google Scholar] [CrossRef]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C.R. Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cem. Concr. Res. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Pinarli, V.; Kaymal, G. An innovative sludge disposal option—Reuse of sludge ash by incorporation in construction materials. Environ. Technol. 1994, 15, 843–852. [Google Scholar] [CrossRef]
- Baeza-Brotons, F.; Garcés, P.; Payá, J.; Saval, J.M. Portland cement systems with addition of sewage sludge ash. Application in concretes for the manufacture of blocks. J. Clean. Prod. 2014, 82, 112–124. [Google Scholar] [CrossRef]
- Garcés, P.; Pérez Carrión, M.; García-Alcocel, E.; Payá, J.; Monzó, J.; Borrachero, M.V. Mechanical and physical properties of cement blended with sewage sludge ash. Waste Manag. 2008, 28, 2495–2502. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Effect of fly ash characteristics on delayed high-strength development of geopolymers. Constr. Build. Mater. 2016, 102, 260–269. [Google Scholar] [CrossRef]
- Richardson, I.G.; Girão, A.V.; Taylor, R.; Jia, S. Hydration of water- and alkali activated white Portland cement pastes and blends with low-calcium pulverized fuel ash. Cem. Concr. Res. 2016, 83, 1–18. [Google Scholar] [CrossRef]
- Suksiripattanapong, C.; Horpibulsuk, S.; Boongrasan, S.; Udomchai, A.; Chinkulkijniwat, A.; Arulrajah, A. Unit weight, strength and microstructure of a water treatment sludge-fly ash lightweight cellular geopolymer. Constr. Build. Mater. 2015, 94, 807–816. [Google Scholar] [CrossRef]
- Da Cunha Oliveira, J.V.; Chagas, L.S.V.B.; de Andrade Meira, F.F.D.; Carneiro, A.M.P.; de Melo Neto, A.A. Study of the Potential of Adhesion to the Substrate of Masonry and Tensile in the Flexion in Mortars of Coating with Gray of the Sewage Sludge. Mater. Sci. Forum. 2020, 1012, 256–261. [Google Scholar] [CrossRef]
- Pinarli, V.; Emre, N.K. Constructive sludge management—Reutilization of municipal sewage sludge in Portland cement mortars. Environ. Technol. 1994, 15, 833–841. [Google Scholar] [CrossRef]
- Nakic, D.; Vouk, D.; Serdar, M.; Cheeseman, C.R. Use of MID-MIX® treated sewage sludge in cement mortars and concrete. Eur. J.Environ. Civ. Eng. 2018, 24, 1–16. [Google Scholar] [CrossRef]
- Cyr, M.; Coutand, M.; Clastres, P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cem. Concr. Res. 2007, 37, 1278–1289. [Google Scholar] [CrossRef]
- Vouk, D.; Nakic, D.; Stirmer, N.; Cheeseman, C. Influence of combustion temperature on the performance of sewage sludge ash as a supplementary cementitious material. J. Mater. Cycles Waste Manag. 2018, 20, 1458–1467. [Google Scholar] [CrossRef]
- Pavlík, Z.; Fořt, J.; Záleská, M.; Pavlíková, M.; Trník, A.; Igor, M.; Keppert, M.; Koutsoukos, P.G.; Černýa, R. Energy-efficient thermal treatment of sewage sludge for its application in blended cements. J. Clean. Prod. 2016, 112, 409–419. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Y.; Yu, Z. Effects of sewage sludge ash produced at different calcining temperatures on pore structure and durability of cement mortars. J. Mater. Cycles Waste Manag. 2021, 23, 755–763. [Google Scholar] [CrossRef]
- Sasaoka, N.; Yokoi, K.; Yamahaka, T. Basic study of concrete made using ash derived from the incinerating sewage sludge. Int. J. Mod. Phys. B 2006, 20, 3716–3721. [Google Scholar] [CrossRef]
- Pittman, D.W.; Ragan, S.A. Drying shrinkage of roller-compacted concrete for pavement applications. ACI Mater. J. 1998, 95, 19–26. [Google Scholar]
- Farzadnia, N.; Noorvand, H.; Yasin, A.M.; Aziz, F.N.A. The efect of nano silica on short term drying shrinkage of POFA cement mortars. Constr. Build. Mater. 2015, 95, 636–646. [Google Scholar] [CrossRef]
- Zhang, W.; Zakaria, M.; Hama, Y. Influence of aggregate materials characteristics on the drying shrinkage properties of mortar and concrete. Constr. Build. Mater. 2013, 49, 500–510. [Google Scholar] [CrossRef]
- Uygunoglu, T.; Topçu, I.B. The role of scrap rubber particles on the drying shrinkage and mechanical properties of self-consolidating mortars. Constr. Build. Mater. 2010, 24, 1141–1150. [Google Scholar] [CrossRef]
- Krejcirikova, B.; Ottosen, L.M.; Kirkelund, G.M.; Rode, C.; Peuhkuri, R. Characterization of sewage sludge ash and its effect on moisture physics of mortar. J. Build. Eng. 2019, 21, 396–403. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Krejcirikova, B.; Rode, C.; Peuhkuri, R. Determination of hygrothermal properties of cementitious mortar: The Effect of Partial Replacement of Cement by Incinerated Sewage Sludge Ash. J. Build. Phys. 2019, 42, 771–787. [Google Scholar] [CrossRef]
- Powers, T.C. A working hypothesis for further studied of frost resistance of concrete. J. Am. Concr. Inst. 1945, 41, 245–272. [Google Scholar]
- Salihoglu, G.; Mardani-Aghabaglou, A. Characterization of sewage sludge incineration ashes from multi-cyclones and baghouse dust filters as possible cement substitutes. Environ. Sci. Pollut. Res. 2020, 28, 645–663. [Google Scholar] [CrossRef]
- Rutkowska, G.; Chalecki, M.; Żółtowski, M. Fly ash from thermal conversion of sludge as a cement substitute in concrete manufacturing. Sustainability 2021, 13, 4182. [Google Scholar] [CrossRef]
- Chen, Y. Effect of Activated Water Treatment Sludge on Carbonation of Mortar. Key Eng. Mater. 2013, 539, 120–123. [Google Scholar] [CrossRef]
- Nasir, M.; Aziz, M.A.; Zubair, M.; Manzar, M.S.; Ashraf, N.; Mu’azu, N.D.; Al-Harthi, M. Recent review on synthesis, evaluation, and SWOT analysis of nanostructured cellulose in construction applications. J. Build. Eng. 2022, 46, 103747. [Google Scholar] [CrossRef]
- Luo, H.L.; Lin, D.F.; Shieh, S.I.; You, Y.F. Micro-observations of different types of nano-Al2O3 on the hydration of cement paste with sludge ash replacement. Environ. Technol. 2014, 36, 2967–2976. [Google Scholar] [CrossRef]
- Feldman, R.F. Significance of porosity measurements on blended cement performance. In Fly Ash Silica Fume, Slag and Other Mineral By-Products in Concrete; Special Publication: Farmington, CT, USA, 1983; Volume 79, pp. 415–434. [Google Scholar]
- Manmohan, D.; Mehta, P.K. Influence of pozzolana, slag and chemical admixtures on pore size distribution and permeability of hardened cement pastes. ASTM Cem. Concr. Aggreg. 1981, 3, 63–67. [Google Scholar]
- Feldman, R.F. Durability of blended cements to high concentration of chloride solutions. In Proceedings of the 5th International Symp. On Concrete Technology, Building Energy Efficiency, Shenyang, China, 21 March 1981; pp. 262–288. [Google Scholar]
- Hu, X.; Shi, C.; Shi, Z.; Zhang, L. Compressive strength, pore structure and chloride transport properties of alkali-activated slag/fly ash mortars. Cem. Concr. Compos. 2019, 104, 103392. [Google Scholar] [CrossRef]
- Pandey, S.; Sharma, R. The influence of mineral additives on the strength and porosity of OPC mortar. Cem. Concr. Res. 2000, 30, 19–23. [Google Scholar] [CrossRef]
- Zelić, J.; Krstulović, R.; Tkalčec, E.; Krolo, P. The properties of Portland cement-limestone-silica fume mortars. Cem. Concr. Res. 2000, 30, 145–152. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Alcocel, E.G.; Garcés, P.; Martínez, J.J.; Payá, J.; Andión, L.G. Effect of sewage sludge ash (SSA) on the mechanical performance and corrosion levels of reinforced Portland cement mortars. Mater. Constr. 2006, 56, 31–43. [Google Scholar]


| Elements | Sample Number | Range | Average Value | Standard Deviations | Coefficient of Variation | LAGA Limits | Swiss Regulation Limits |
|---|---|---|---|---|---|---|---|
| Ba | 5 | 245–1663 | 1159.20 | 597.40 | 51.54 | - | - |
| Zn | 58 | 19.08–7103 | 1724.96 | 1384.81 | 80.28 | 10,000 | 500 |
| Cu | 54 | 33.32–8325 | 841.38 | 1327.20 | 157.74 | 7000 | 250 |
| Cr | 57 | 1.2–2636 | 302.46 | 466.88 | 154.36 | 2000 | 250 |
| Pb | 58 | 0.28–4254 | 284.88 | 559.27 | 196.32 | 6000 | 250 |
| Hg | 30 | 0.05–11 | 1.45 | 2.49 | 171.85 | - | - |
| As | 31 | 0.27–270 | 35.57 | 60.58 | 170.30 | - | 40 |
| Cd | 50 | 0.1–130 | 8.63 | 19.60 | 227.17 | 20 | 5 |
| Ni | 49 | 4–720 | 137.44 | 180.78 | 131.54 | 500 | 250 |
| Sb | 17 | 3.9–183 | 41.42 | 48.19 | 116.35 | - | - |
| Se | 16 | 0.5–57 | 6.49 | 13.60 | 209.62 | - | - |
| Elements | Sample Number | Range | Average Value | Standard Deviations | Coefficient of Variation | TCLP Limits (Us EPA) | GB 5085.3-2007 Limits |
|---|---|---|---|---|---|---|---|
| Ba | 9 | 0.004–0.75 | 0.221 | 0.28 | 125.66 | 100 | 100 |
| Zn | 22 | 0.019–17.66 | 5.64 | 5.51 | 97.66 | 25 | 100 |
| Cu | 24 | 0.008–11.9 | 4.028 | 3.87 | 96.19 | 15 | 100 |
| Cr | 24 | 0.009–15.2 | 1.523 | 4.24 | 278.37 | 5 | 15 |
| Pb | 27 | 0.012–2.43 | 0.262 | 0.46 | 176.86 | 5 | 5 |
| Hg | 5 | 0.0002–0.2 | 0.041 | 0.09 | 216.96 | 0.2 | - |
| As | 12 | 0.01–0.77 | 0.265 | 0.26 | 98.15 | 5 | - |
| Cd | 25 | 0.009–1.05 | 0.145 | 0.29 | 197.09 | 1 | 1 |
| Ni | 14 | 0.033–6.52 | 0.986 | 1.73 | 175.40 | 25 | 5 |
| References | SiO2 | Al2O3 | Fe2O3 | CaO | CaO/SiO2 | SiO2/Al2O3 |
|---|---|---|---|---|---|---|
| [14] | 50.6 | 12.8 | 7.21 | 1.93 | 0.04 | 3.95 |
| [15] | 28.3 | 12.5 | 18.6 | 10.6 | 0.37 | 2.26 |
| [16] | 37.895 | 14.503 | 22.646 | 1.863 | 0.05 | 2.61 |
| [17] | 27.78 | 12.2 | 18.23 | 10.42 | 0.38 | 2.28 |
| [17] | 27.91 | 12.26 | 18.32 | 10.47 | 0.38 | 2.28 |
| [18] | 20.8 | 14.9 | 7.4 | 31.3 | 1.50 | 1.40 |
| [18] | 30.1 | 11.9 | 7.1 | 25.9 | 0.86 | 2.53 |
| [18] | 16.2 | 14.7 | 8.2 | 32.9 | 2.03 | 1.10 |
| [19] | 27.24 | 14.44 | 27.35 | 6.34 | 0.23 | 1.89 |
| [25] | 17.1 | 5.1 | 15.7 | 23.8 | 1.39 | 3.35 |
| [66] | 64.6 | 23.1 | 1.31 | 8.6 | 0.13 | 2.80 |
| [112] | 28.8 | 7.8 | 3.9 | 21.9 | 0.76 | 3.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Le, X.; Fang, W.; Zhao, J.; Fang, L.; Hou, S. The Utilization of Recycled Sewage Sludge Ash as a Supplementary Cementitious Material in Mortar: A Review. Sustainability 2022, 14, 4432. https://doi.org/10.3390/su14084432
Liang C, Le X, Fang W, Zhao J, Fang L, Hou S. The Utilization of Recycled Sewage Sludge Ash as a Supplementary Cementitious Material in Mortar: A Review. Sustainability. 2022; 14(8):4432. https://doi.org/10.3390/su14084432
Chicago/Turabian StyleLiang, Chaofeng, Xinqian Le, Weijiong Fang, Jianming Zhao, Liuji Fang, and Shaodan Hou. 2022. "The Utilization of Recycled Sewage Sludge Ash as a Supplementary Cementitious Material in Mortar: A Review" Sustainability 14, no. 8: 4432. https://doi.org/10.3390/su14084432
APA StyleLiang, C., Le, X., Fang, W., Zhao, J., Fang, L., & Hou, S. (2022). The Utilization of Recycled Sewage Sludge Ash as a Supplementary Cementitious Material in Mortar: A Review. Sustainability, 14(8), 4432. https://doi.org/10.3390/su14084432









