Various Fertilization Managements Influence the Flowering Attributes, Yield Response, Biochemical Activity and SoilNutrient Status of Chrysanthemum (Chrysanthemum morifolium Ramat.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Site
2.2. Inflorescence Attributes
2.3. Inflorescence Yield
2.4. Biochemical Attributes
2.4.1. Chlorophyll a (mg/g) of Fresh Leaves
2.4.2. Chlorophyll b (mg/g) of Fresh Leaves
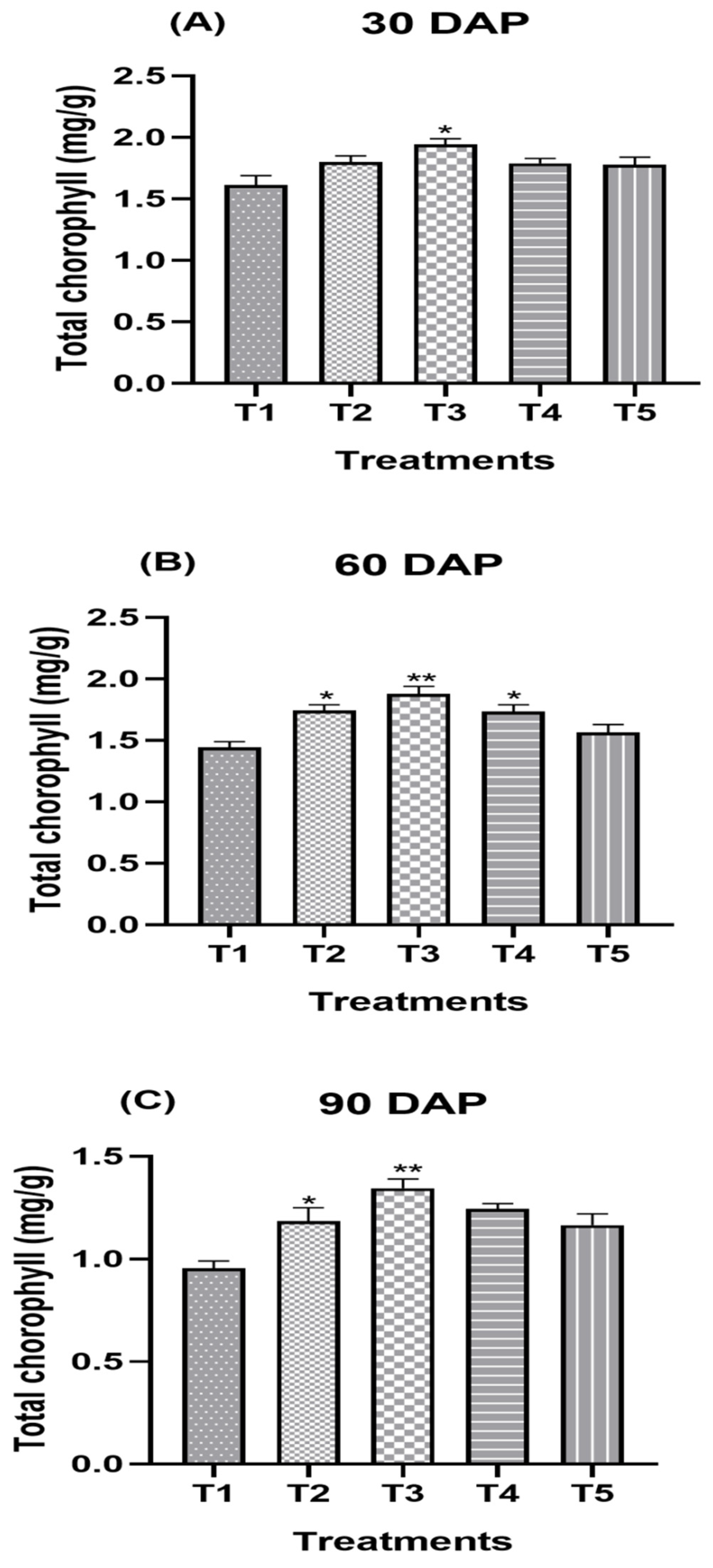
2.4.3. Total Chlorophyll (mg/g) of Fresh Leaves
2.4.4. Carotene Content (mg/g) of Inflorescence
2.5. Soil Nutrient Status
2.5.1. Organic Carbon (%)
2.5.2. Available Nitrogen, Available Phosphorus, Available Potassium (kg ha−1)
2.6. Experimental Design and Statistical Data Analysis
3. Results
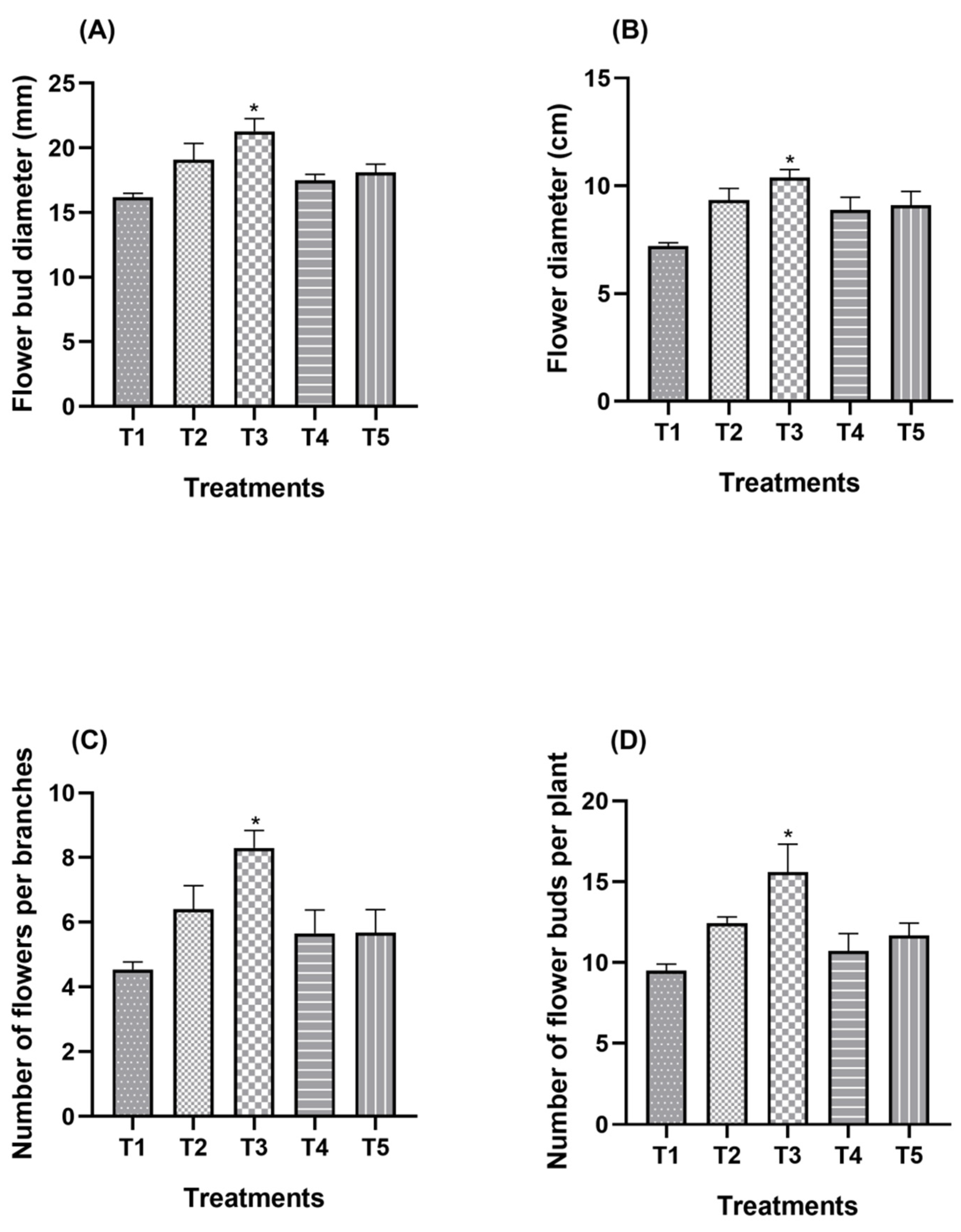
3.1. Effect of Split Supplication of NPK through Drip Fertigation on Inflorescence Attributes
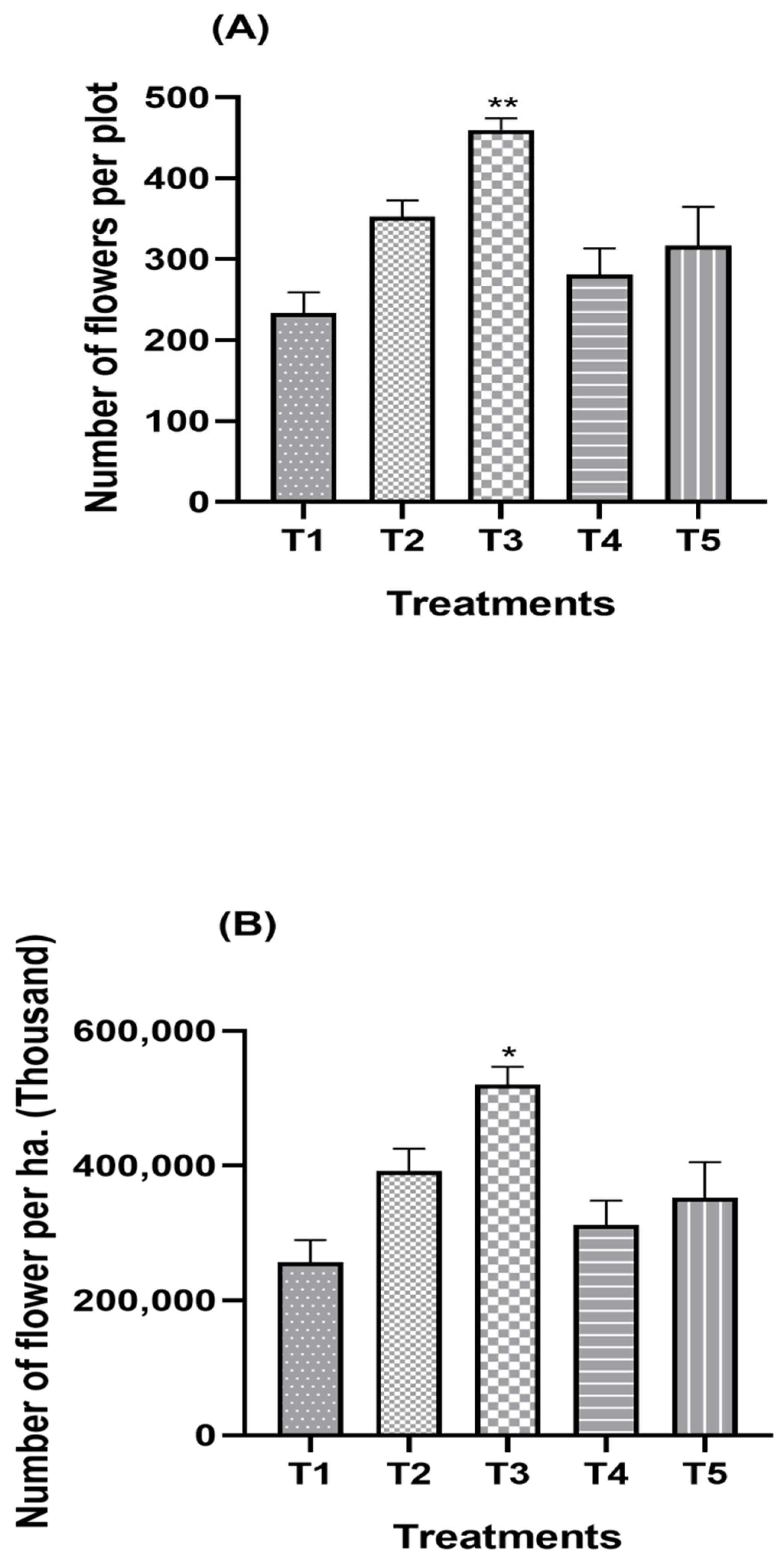
3.2. Effect of Split Supplication of NPK through Drip Fertigation on Yield Attributes
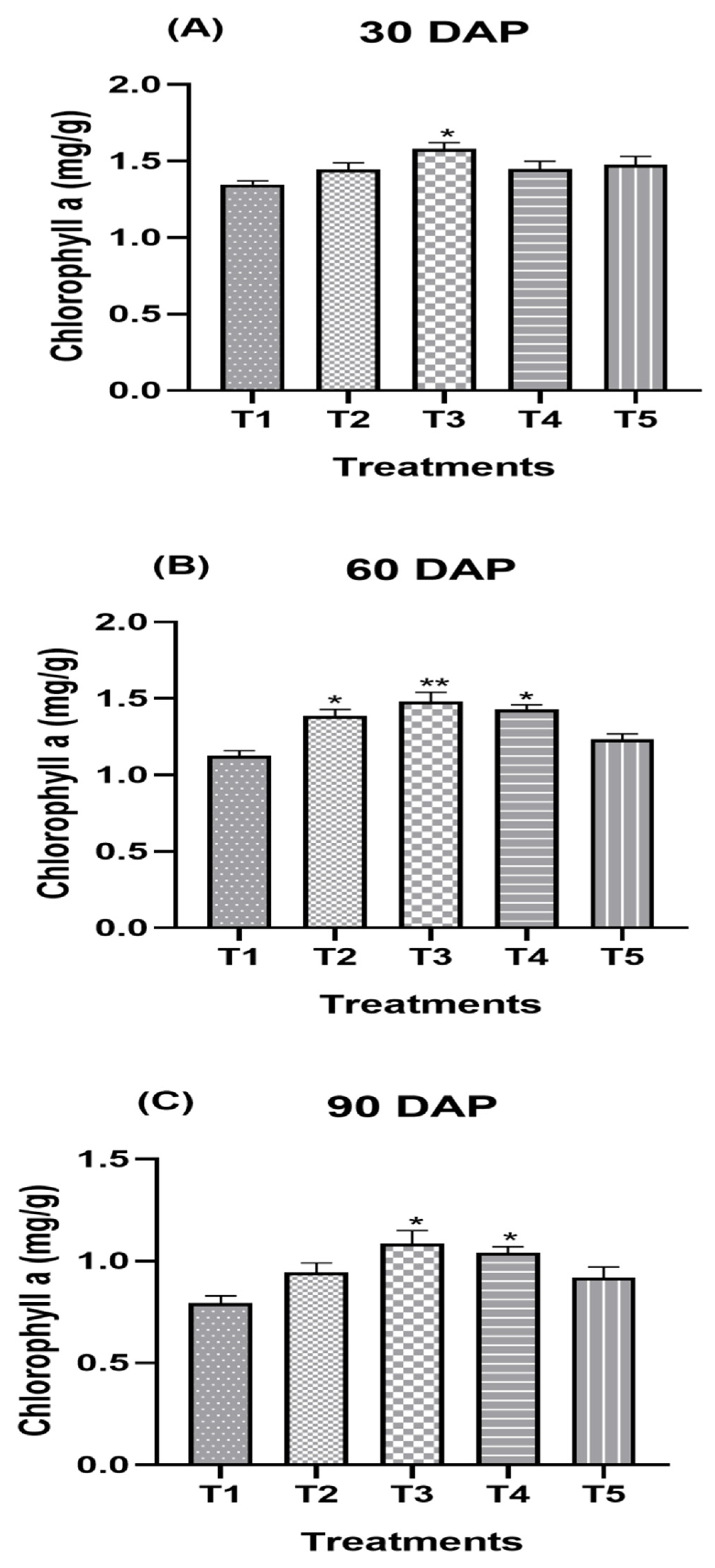
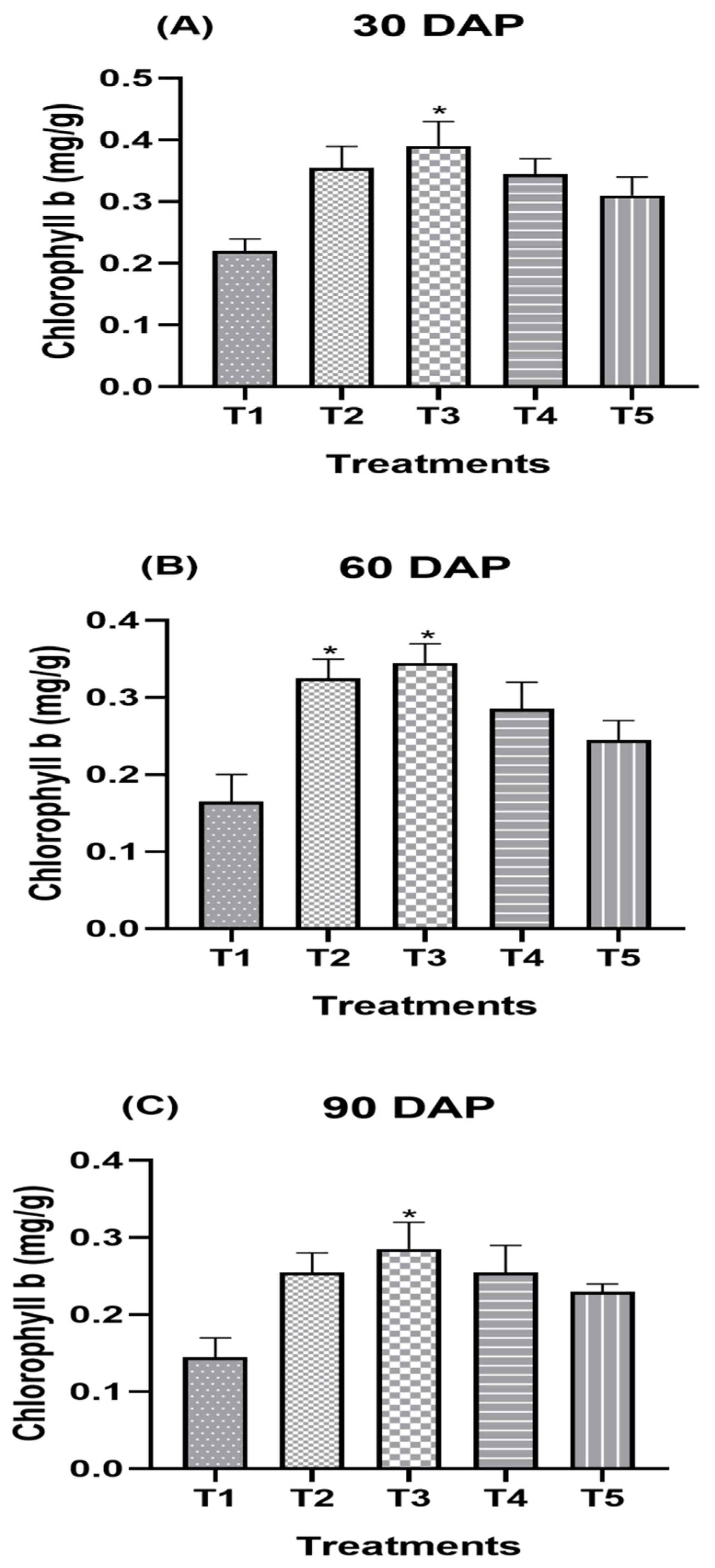
3.3. Effect of Split Supplication of NPK through Drip Fertigation on Biochemical Attributes
3.4. Effect of Split Supplication of NPK through Drip Fertigation on Soil Nutrient Status
4. Discussion
4.1. Effect of Split Supplication of NPK through Drip Fertigation on Inflorescence Attributes
4.2. Effect of Split Supplication of NPK through Drip Fertigation on Yield Attributes
4.3. Effect of Split Supplication of NPK through Drip Fertigation on Biochemical Attributes
4.4. Effect of Split Supplication of NPK through Drip Fertigation on Soil Nutrient Status
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huylenbroeck, J. Status of floriculture in Europe. In Protocols for In Vitro Propagation of Ornamental Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 365–376. [Google Scholar]
- Wani, M.A.; Nazki, I.T.; Din, A.; Iqbal, S.; Wani, S.A.; Khan, F.U. Floriculture sustainability initiative: The dawn of new era. In Sustainable Agriculture Reviews 27; Springer: Berlin/Heidelberg, Germany, 2018; pp. 91–127. [Google Scholar]
- Van Rijswick, C. World Floriculture Map 2015. Gearing up Stronger Compet Rabobank Ind Note. 2015, p. 475. Available online: https://research.rabobank.com/far/en/sectors/regional-foodagri/world_floriculture_map_2015.html (accessed on 12 March 2021).
- Adebayo, I.A.; Pam, V.K.; Arsad, H.; Samian, M.R. The Global Floriculture Industry: Status and Future Prospects. In The Global Floriculture Industry; Apple Academic Press: Waretown, NJ, USA, 2020; pp. 1–14. [Google Scholar]
- Darras, A.I. Implementation of sustainable practices to ornamental plant cultivation worldwide: A critical review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Anderson, N.O. Chrysanthemum. In Flower Breeding and Genetics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 389–437. [Google Scholar]
- Li, T.S.C. Chinese and Related North American Herbs: Phytopharmacology and Therapeutic Values; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Youssef, F.S.; Eid, S.Y.; Alshammari, E.; Ashour, M.L.; Wink, M.; El-Readi, M.Z. Chrysanthemum indicum and Chrysanthemum morifolium: Chemical composition of their essential oils and their potential use as natural preservatives with antimicrobial and antioxidant activities. Foods 2020, 9, 1460. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A. Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers. Breed Sci. 2018, 68, 17075. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Mitsuda, N.; Nashima, K.; Kishimoto, K.; Katayose, Y.; Kanamori, H.; Ohmiya, A. Generation of expressed sequence tags for discovery of genes responsible for floral traits of Chrysanthemum morifolium by next-generation sequencing technology. BMC Genom. 2017, 18, 683. [Google Scholar]
- Cheng, W.; Li, J.; You, T.; Hu, C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J. Ethnopharmacol. 2005, 101, 334–337. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Zandi, P.; Cheng, Q. A review of Chrysanthemum, the eastern queen in traditional Chinese medicine with healing power in modern pharmaceutical sciences. Appl. Ecol. Environ. Res. 2019, 17, 13355–13369. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Y.; Li, D.; Chen, Y. Chrysanthemum indicum L.: A comprehensive review of its botany, phytochemistry and pharmacology. Am. J. Chin. Med. 2020, 48, 871–897. [Google Scholar] [CrossRef]
- Cha, J.Y.; Nepali, S.; Lee, H.Y.; Hwang, S.W.; Choi, S.Y.; Yeon, J.M.; Song, B.-J.; Kim, D.-K.; Lee, Y.-M. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp. Ther. Med. 2018, 15, 5070–5076. [Google Scholar]
- Zhang, X.; Wu, J.Z.; Lin, Z.X.; Yuan, Q.J.; Li, Y.C.; Liang, J.L.; Zhan, J.Y.; Xie, Y.; Su, Z.; Liu, Y. Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnén against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2019, 234, 44–56. [Google Scholar] [CrossRef]
- De, L.C.; Bhattacharjee, S.K. Ornamental Crop Breeding; Aavishkar Publishers & Distributors: Jaipur, India, 2011. [Google Scholar]
- Malik, R.S.; Kumar, K.; Bhandari, A.R. Effect of urea application through drip irrigation system on nitrate distribution in loamy sand soils and pea yield. J. Indian Soc. Soil Sci. 1994, 42, 6–10. [Google Scholar]
- Dimri, D.C.; Lal, G. Effect of Nitrogen Fertilization, Spacing and Method of Planting on Yield Parameters and Quality of Tomato Cultivar Pant Bahar; Indian Society of Vegetable Science: New Delhi, India, 1988. [Google Scholar]
- Jeyabaskaran, K.J.; Shirgure, P.S.; Pandey, V.; Srivastava, A.K.; Uma, S. Fertigation in Horticulture: A Guarantee to Economized Quality Production. Indian J. Fertil. 2021, 17, 364–383. [Google Scholar]
- Rolaniya, M.K.; Khandelwal, S.K.; Choudhary, A.; Jat, P.K. Response of african marigold to NPK, biofertilizers and spacings. J. Appl. Nat. Sci. 2017, 9, 593–597. [Google Scholar] [CrossRef][Green Version]
- Kumar, R.; Pareek, N.K.; Rathore, V.S.; Nangiya, V.; Yadava, N.D.; Yadav, R.S. Effect of water and nitrogen levels on yield attributes, water productivity and economics of cluster bean (Cyamopsis tetragonoloba) in hot arid region. Legume Res. Int. J. 2020, 43, 702–705. [Google Scholar] [CrossRef]
- Kumar, R.; Pareek, N.K.; Rathore, V.S.; Nangiya, V. Effect of irrigation and nitrogen application on water productivity and performance of Cotton (Gossypium sp.). J. Soil Water Conserv. 2019, 18, 282–287. [Google Scholar] [CrossRef]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L. Soil fertility and fertilizers. Soil Sci. 1966, 101, 346. [Google Scholar] [CrossRef]
- Bergmann, W. Nutritional Disorders of Plants: Visual and Analytical Diagnosis (English, French, Spanish); Gustav Fischer Verlag: Jena, Germany, 1992. [Google Scholar]
- Luthra, K.L.; Saha, S.K.; Awasthi, P.K. Role of rock phosphate in present day agriculture. Indian J. Agric. Chem. 1983, 15, 13–27. [Google Scholar]
- Pattanaaik, S.K. Response of Drip Irrigatedbanana to Differentirrigationregimes. In Micro Irrigation Scheduling and Practices; Apple Academic Press: Oakville, ON, Canada, 2017; pp. 85–98. [Google Scholar]
- Raina, J.N.; Thakur, B.C.; Suman, S.; Spehia, R.S. Effect of fertigation through drip system on nitrogen dynamics, growth, yield and quality of apricot. Acta Hortic. 2005, 696, 227–231. [Google Scholar] [CrossRef]
- Jat, R.A.; Wani, S.P.; Sahrawat, K.L.; Singh, P.; Dhaka, P.L. Fertigation in vegetable crops for higher productivity and resource use efficiency. Indian J. Fertil. 2011, 7, 22–37. [Google Scholar]
- Tu, J.C.; Liptay, A.; Tan, C.S.; Drury, C.F.; Reynolds, D. Effect of drip irrigation and drip fertigation on yield of processing tomato in South-Western Ontario. In Proceedings of the XXVI International Horticultural Congress: Managing Soil-Borne Pathogens: A Sound Rhizosphere to Improve Productivity in 635, Toronto, ON, Canada, 11–17 August 2002; pp. 195–200. [Google Scholar]
- Neilsen, G.; Neilsen, D. Strategies for nutrient and water management of high density apple orchards on coarse-textured soils. Ann. Wars. Agric. Univ. Hortic. Landsc. Archit. 2006, 27, 181–192. [Google Scholar]
- Neilsen, G.H.; Neilsen, D.; Peryea, F. Response of soil and irrigated fruit trees to fertigation or broadcast application of nitrogen, phosphorus, and potassium. Horttechnology 1999, 9, 393–401. [Google Scholar] [CrossRef]
- Gardenas, A.I.; Hopmans, J.W.; Hanson, B.R.; Simunek, J. Two-dimensional modeling of nitrate leaching for various fertigation scenarios under micro-irrigation. Agric. Water Manag. 2005, 74, 219–242. [Google Scholar] [CrossRef]
- William, M.; Shenker, M. Pulverized Tires as Soil Amendment for Plant Growth. Int. J. Sci. Eng. Res. 2016, 7, 161–167. [Google Scholar]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Wiley: Hoboken, NJ, USA, 1983; Volume 9, pp. 199–224. [Google Scholar]
- Singh, D.; Chhonkar, P.K.; Pande, R.N. Soil reaction in soil, plant, water analysis method: Manual. IARI ICAR New Delhi 1999, 1, 11–13. [Google Scholar]
- Bower, C.A.; Wilcox, L.V. Soluble salts. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Wiley: Hoboken, NJ, USA, 1965; Volume 9, pp. 933–951. [Google Scholar]
- Bache, B.W. The measurement of cation exchange capacity of soils. J. Sci. Food Agric. 1976, 27, 273–280. [Google Scholar] [CrossRef]
- Bauyoucens, G.J. The hydrometer as a new method for the mechanical analysis of soil. Soil Sci. 1927, 23, 343–353. [Google Scholar]
- Farooq, T.H.; Kumar, U.; Mo, J.; Shakoor, A.; Wang, J.; Rashid, M.H.U.; Tufail, M.A.; Chen, X.; Yan, W. Intercropping of peanut–Tea enhances soil enzymatic activity and soil nutrient status at different soil profiles in subtropical southern China. Plants 2021, 10, 881. [Google Scholar] [CrossRef]
- Subbiah, B.V.; Asija, G.L. A rapid method for the estimation of nitrogen in soil. Curr. Sci. 1956, 26, 259–260. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Nelson, L.B.; Heidel, H. Soil Analysis Methods as Used in the Iowa State College Soil Testing Laboratory; Iowa State College, Agronomy Department: Ames, IA, USA, 1952. [Google Scholar]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Wheatley, R.E.; MacDonald, R.; Smith, A.M. Extraction of nitrogen from soils. Biol. Fertil. Soils 1989, 8, 189–190. [Google Scholar] [CrossRef]
- Sundar, S.T.B.; Kannan, M.; Jawaharlal, M. Off season flower induction through fertigation and biostimulant spray in Jasminum sambac Ait. Asian J. Hortic. 2014, 9, 32–35. [Google Scholar]
- Ganesh, S.; Kannan, M.; Jawaharlal, M. Optimization of fertigation schedule for cut chrysanthemum (Dendranthema grandiflora Tzvelev). Hort Flora Res. Spectr. 2011, 3, 344–348. [Google Scholar]
- Phene, C.J.; Beale, O.W. High-frequency irrigation for water nutrient management in humid regions. Soil Sci. Soc. Am. J. 1976, 40, 430–436. [Google Scholar] [CrossRef]
- Sharma, A.K.; Chaudhary, S.V.S.; Gupta, Y.C. Effect of nitrogen and phosphorus on flowering and yield of African marigold (Tagetes erecta Linn.). Prog. Agric. 2010, 10, 158–160. [Google Scholar]
- Joshi, N.S.; Varu, D.K.; Barad, A.V.; Pathak, D.M. Performance of varieties and chemical fertilizers on growth and flowering in chrysanthemum. Int. J. Agric. Sci. 2013, 9, 182–188. [Google Scholar]
- Ganesh, S.; Kannan, M.; Jawaharlal, M.; Arulmozhiyan, R.; Jeyakumar, P. Enhancement of physiological response in spray chrysanthemum through fertigation. J. Ornam. Hortic. 2013, 16, 108–116. [Google Scholar]
- Jangir, R.P.; Jat, B.L.; Rathore, M.S. Comparative efficacy of sprinkler and surface methods of irrigation in cumin (Cuminum cyminum) under arid western Rajasthan conditions. Indian J. Agron. 2007, 52, 83–85. [Google Scholar]
- Gopinath, G.; Chandrashekar, S.Y. Yield of carnation as influenced by levels of fertigation and sources of nutrients of growing standard carnation cv. Trendy under cost greenhouse. J. Ornam. Hortic. 2009, 12, 251–255. [Google Scholar]
- Abrol, A. Effect of Integrated Nutrient Management Studies and Planting Dates on Commercial Flower Production in China Aster (Callistephus chinensis (l.) Nees). Trends Biosci. 2014, 7, 1217–1221. [Google Scholar]
- Teja, P.R.; Bhaskar, V.V.; Dorajeerao, A.V.D.; Subbaramamma, P. Effect of Graded Levels of Nitrogen and Potassium on Growth and Flower Yield of Annual Chrysanthemum (Chrysanthemum coronariuml.). Plant Arch. 2017, 17, 1371–1376. [Google Scholar]
- Loganathan, V.; Latha, K.R. Effect of drip fertigation on nutrient uptake and seed yield of pigeonpea [Cajanus Cajan (L.) Millsp.] under westeren agroclimatic zones of Tamil Nadu. Legume Res. Int. J. 2016, 39, 780–785. [Google Scholar] [CrossRef]
- Harisha, C.B.; Diwakar, Y.; Aishwath, O.P.; Singh, R.; Asangi, H. Soil fertility and micronutrient uptake by fennel (Foeniculum vulgare Mill.) as influenced by micronutrients fertilization. Environ. Ecol. 2017, 35, 514–518. [Google Scholar]
- Mozafariyan, M.; Saghafi, K.; Bayat, A.E.; Bakhtiari, S. The effects of different sodium Chloride concentrations on the growth and photosynthesis parameters of tomato (Lycopersicum esculentum cv. Foria). Int. J. Agric. Crop Sci. 2013, 6, 203. [Google Scholar]
- Teixeira, L.A.J.; Natale, W.; Bettiol Neto, J.E.; Martins, A.L.M. Nitrogen and potassium application on banana plant by fertirrigation and conventional fertilization-soil chemical properties. Rev. Bras. Frutic. 2007, 29, 143–152. [Google Scholar] [CrossRef]
- Sheela, V.L. Flowers for Trade; New India Publishing: Delhi, India, 2008; Volume 10. [Google Scholar]
- Yadav, P.K. Effect of N and FYM on chlorophyll and nutrient content in leaf of African marigold (Tagetes erecta L.) at flower bud initiation stage. Environ. Ecol. 1999, 17, 188–190. [Google Scholar]
- Chandra Kumar, S.S. Growth and Productivity of Robusta Banana under Nitrogen and Potassium Fertigation. Master’s Thesis, Indian Institute of Horticultural Research, Bagalore, India, 1998. [Google Scholar]
- Sainju, U.M.; Singh, B.P.; Yaffa, S. Soil organic matter and tomato yield following tillage, cover cropping, and nitrogen fertilization. Agron. J. 2002, 94, 594–602. [Google Scholar] [CrossRef]
- Yoshida, C.; Iwasaki, Y.; Makino, A.; Ikeda, H. Effects of irrigation management on the growth and fruit yield of tomato under drip fertigation. Hortic. Res. 2011, 10, 325–331. [Google Scholar] [CrossRef]
- Meena, A.K.; Singh, D.K.; Pandey, P.C.; Nanda, G. Growth, yield, economics, and nitrogen use efficiency of transplanted rice (Oryza sativa L.) as influenced by different nitrogen management practices through neem (Azadirachta indica) coated urea. Int. J. Chem. Stud. 2018, 6, 1388–1395. [Google Scholar]
- Farooq, T.H.; Kumar, U.; Shakoor, A.; Albasher, G.; Alkahtani, S.; Rizwana, H.; Tayyab, M.; Dobaria, J.; Hussain, M.I.; Wu, P. Influence of Intraspecific Competition Stress on Soil Fungal Diversity and Composition in Relation to Tree Growth and Soil Fertility in Sub-Tropical Soils under Chinese Fir Monoculture. Sustainability 2021, 13, 10688. [Google Scholar] [CrossRef]
- Hazarika, T.K.; Nautiyal, B.P.; Bhattacharya, R.K. Effect of INM on productivity and soil characteristics of tissue cultured banana cv. Grand Naine in Mizoram, India. Prog. Hortic. 2011, 43, 30–35. [Google Scholar]
- Sujatha, K.; Gowda, J.V.N.; Khan, M.M. Effects of different fertigation levels on gerbera under low cost greenhouse. J. Ornam. Hortic. 2002, 5, 54–59. [Google Scholar]
- Ahmad, I.; Asif, M.; Amjad, A.; Ahmad, S. Fertilization enhances growth, yield, and xanthophyll contents of marigold. Turk. J. Agric. For. 2011, 35, 641–648. [Google Scholar]
- Angadi, A.P. Effect of integrated nutrient management on yield, economics and nutrient uptake of garland chrysanthemum (Chrysanthemum coronarium L.). Asian J. Hortic. 2014, 9, 132–135. [Google Scholar]
- Laishram, N.; Dhiman, S.R.; Gupta, Y.C.; Bhardwaj, S.K.; Singh, A. Microbial dynamics and physico-chemical properties of soil in the rhizosphere of chrysanthemum (Dendranthema grandiflora) as influenced by integrated nutrient management. Indian J. Agric. Sci. 2013, 83, 447–455. [Google Scholar]
- Singh, A.; Laishram, N.; Gupta, Y.C.; Sharma, B.P.; Dilta, B.S.; Bhardwaj, S.K. Influence of NPK fertigation and foliar application on flower quality, media physico-chemical properties and foliar nutrient content in carnation (Dianthus caryophyllus) cv. Master Indian J. Agric. Sci. 2015, 85, 1461–1465. [Google Scholar]


| Soil Property | Method | Soil Status | Category | Reference |
|---|---|---|---|---|
| pH (1:2 soil water suspension) | Digital pH meter | 6.95 | Neutral | McLean, 1983 [35] Singh, 1999 [36] |
| Electrical conductivity (1:2 soil water suspension) | Conductivity meter | 0.38 dS/m | - | Bower and Wilcox, 1965 [37] |
| Cation-exchange capacity | Ammonium acetate method | 32.49(cmol (p+) kg−1 soil) | - | Bache, 1976 [38] |
| Sand | Hydrometer method | 49.12% | - | Bauyoucens, 1927 [39] |
| Silt | Hydrometer method | 25.72% | - | Bauyoucens, 1927 [39] |
| Clay | Hydrometer method | 25.16% | - | Bauyoucens, 1927 [39] |
| Organic carbon | Walkley–Black Modified method | 0.52% | - | Farooq, 2021 [40] |
| Available nitrogen | Alkaline KMnO4 | 216.65 kg ha−1 | Low | Subbiah and Asija, 1956 [41] |
| Available phosphorus | Olsen’s extraction method | 21.03 kg ha−1 | Medium | Olsen et al., 1954 [42] |
| Available potassium | Flame photometer | 112.05 kg ha−1 | Medium | Nelson and Heidel, 1952 [43] |
| Treatments | Fertilizer Dose | Dose | Stage |
|---|---|---|---|
| T1 (Control) | 100:150:100 kg NPK/ha/year | 100% P and K | Full dose of P and K as basal |
| Split application @ 33.3% each | N in three equal splits—first dose as basal, second dose at first pinching and third dose at one month after pinching | ||
| T2 | 100:150:100 kg NPK/ha/year | 33.3:33.3:33.3% | Vegetative stage |
| 33.3:33.3:33.3% | Bud stage | ||
| 33.3:33.3:33.3% | Inflorescence stage | ||
| T3 | 100:150:100 kg NPK/ha/year | 40:20:20% | Vegetative stage |
| 30:40:40% | Bud stage | ||
| 30:40:40% | Inflorescence stage | ||
| T4 | 75:112.5:75 kg NPK/ha/year (75% RDF) | 33.3:33.3:33.3% | Vegetative stage |
| 33.3:33.3:33.3% | Bud stage | ||
| 33.3:33.3:33.3% | Inflorescence stage | ||
| T5 | 75:112.5:75 kg NPK/ha/year (75% RDF) | 40:20:20% | Vegetative stage |
| 30:40:40% | Bud stage | ||
| 30:40:40% | Inflorescence stage |
| Treatment Combination | Inflorescence Bud Diameter (mm) | Inflorescence Diameter (cm) | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 16.49 c | 15.86 b | 16.18 d | 7.04 b | 8.36 c | 7.70 c |
| T2 | 20.35 ab | 17.85 b | 19.10 b | 8.80 a | 9.88 ab | 9.34 ab |
| T3 | 22.26 a | 20.29 a | 21.27 a | 9.02 a | 10.75 a | 9.89 a |
| T4 | 17.26 c | 17.24 b | 17.25 cd | 8.28 a | 9.47 bc | 8.88 b |
| T5 | 18.75 bc | 17.49 | 18.12 bc | 8.46 a | 9.74 ab | 9.10 b |
| Treatment Combination | Number of Inflorescences per Branch | Number of Inflorescence Buds per Plant | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 6.30 b | 4.77 b | 6.30 c | 9.17 c | 9.51 b | 9.42 c |
| T2 | 7.13 ab | 5.67 ab | 7.13 b | 12.66 b | 12.42 ab | 12.54 b |
| T3 | 7.84 a | 5.75 a | 7.84 a | 17.32 a | 13.88 a | 15.60 a |
| T4 | 6.38 b | 4.92 ab | 6.38 c | 11.79 bc | 9.67 b | 10.65 bc |
| T5 | 6.39 b | 4.97 b | 6.39 c | 12.44 bc | 10.92 ab | 11.68 bc |
| Treatment Combination | Weight of Inflorescence (g) | Number of Inflorescences per Plant | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 10.79 b | 10.04 b | 10.41 b | 7.12 c | 5.31 c | 6.21 c |
| T2 | 11.88 ab | 12.09 ab | 11.41 bc | 8.90 b | 7.50 a | 7.40 ab |
| T3 | 12.99 a | 12.90 a | 12.94 | 11.03 a | 8.03 a | 9.53 a |
| T4 | 11.50 b | 10.93 ab | 11.79 ab | 7.29 c | 5.79 bc | 7.14 bc |
| T5 | 11.65 b | 11.97 ab | 11.81 ab | 8.48 bc | 6.26 ab | 7.58 ab |
| Treatment Combination | Inflorescence Stem Diameter (mm) | Inflorescence Stem Length (cm) | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 4.23 a | 4.09 a | 4.29 b | 5.57 b | 4.72 a | 5.14 b |
| T2 | 4.52 a | 4.41 a | 4.40 b | 5.85 ab | 5.12 a | 5.39 ab |
| T3 | 4.92 a | 4.87 a | 4.89 a | 6.04 a | 5.16 a | 5.60 a |
| T4 | 4.40 a | 4.36 a | 4.25 b | 5.71 ab | 4.93 a | 5.42 ab |
| T5 | 4.41 a | 4.39 a | 4.45 b | 5.74 ab | 5.10 a | 5.42 ab |
| Treatment Combination | Number of Inflorescences per Plot | Number of Inflorescences per ha. (Thousand) | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 305.95 c | 228.12 e | 267.03 d | 339,940.00 c | 253,460.00 c | 296,700.00 d |
| T2 | 382.70 b | 322.50 b | 352.60 b | 425,220.00 b | 358,330.00 a | 391,775.00 b |
| T3 | 474.29 a | 345.29 a | 409.79 a | 526,990.00 a | 383,660.00 a | 455,325.00 a |
| T4 | 313.47 c | 248.97 d | 281.22 d | 348,300.00 c | 276,630.00 bc | 312,465.00 dc |
| T5 | 364.65 bc | 269.28 c | 316.96 | 405,170.00 bc | 299,200.00 b | 352,185.00 c |
| Treatment Combination | Chlorophyll a (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 DAP | 60 DAP | 90 DAP | |||||||
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 1.35 c | 1.40 c | 1.37 c | 1.20 c | 1.26 d | 1.23 b | 0.81 c | 0.79 d | 0.80 c |
| T2 | 1.47 b | 1.45 bc | 1.46 b | 1.44 a | 1.40 bc | 1.42 a | 0.95 abc | 0.93 c | 0.94 b |
| T3 | 1.55 a | 1.54 a | 1.55 a | 1.47 a | 1.51 a | 1.49 a | 1.08 a | 1.10 a | 1.09 a |
| T4 | 1.43 bc | 1.50 ab | 1.47 b | 1.43 a | 1.43 ab | 1.43 a | 1.01 ab | 1.01 b | 1.01 b |
| T5 | 1.48 ab | 1.492 b | 1.492 b | 1.313 b | 1.32 cd | 1.32 | 0.90 | 0.97 bc | 0.94 b |
| Treatment Combination | Chlorophyll b (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 DAP | 60 DAP | 90 DAP | |||||||
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 0.20 c | 0.28 ± 0.01 d | 0.24 d | 0.21 c | 0.21 e | 0.21 d | 0.17 c | 0.17 d | 0.17 d |
| T2 | 0.35 a | 0.36 ± 0.01 b | 0.35 b | 0.33 ab | 0.37 b | 0.35 a | 0.25 ab | 0.26 b | 0.26 ab |
| T3 | 0.37 a | 0.40 ± 0.01 a | 0.38 a | 0.35 a | 0.38 a | 0.37 a | 0.28 a | 0.28 a | 0.28 a |
| T4 | 0.34 a | 0.33 ± 0.01 c | 0.33 b | 0.30 ab | 0.32 c | 0.31 b | 0.25 ab | 0.25 bc | 0.25 bc |
| T5 | 0.29 b | 0.30 d | 0.29 c | 0.27 bc | 0.27 d | 0.27 c | 0.22 bc | 0.24 c | 0.23 c |
| Treatment Combination | Total Chlorophyll (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 DAP | 60 DAP | 90 DAP | |||||||
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 1.54 ± 0.02 c | 1.69 ± 0.02 c | 1.61 ± 0.01 d | 1.41 ± 0.03 c | 1.47 ± 0.03 d | 1.44 ± 0.02 d | 0.97 ± 0.04 d | 0.97 ± 0.01 d | 0.97 ± 0.02 d |
| T2 | 1.81 ± 0.02 b | 1.80 ± 0.02 b | 1.81 ± 0.01 b | 1.78 ± 0.03 a | 1.76 ± 0.03 b | 1.77 ± 0.02 b | 1.20 ± 0.04 bc | 1.20 ± 0.01 c | 1.20 ± 0.02 bc |
| T3 | 1.92 ± 0.02 a | 1.94 ± 0.02 a | 1.93 ± 0.01 a | 1.83 ± 0.03 a | 1.90 ± 0.03 a | 1.87 ± 0.02 a | 1.36 ± 0.04 a | 1.37 ± 0.01 a | 1.36 ± 0.02 a |
| T4 | 1.77 ± 0.02 b | 1.83 ± 0.02 b | 1.80 ± 0.01 bc | 1.73 ± 0.03 | 1.76 ± 0.03 b | 1.74 ± 0.02 b | 1.26 ± 0.04 ab | 1.27 ± 0.01 b | 1.26 ± 0.02 b |
| T5 | 1.76 ± 0.02 b | 1.80 ± 0.02 b | 1.78 ± 0.01 c | 1.59 ± 0.03 b | 1.59 ± 0.03 c | 1.59 ± 0.02 c | 1.11 ± 0.04 c | 1.22 ± 0.01 c | 1.17 ± 0.02 c |
| Treatments Combination | Carotene Content of Inflorescence (mg/g) | ||
|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 0.24 c | 0.22 d | 0.23 c |
| T2 | 0.35 a | 0.39 c | 0.32 b |
| T3 | 0.37 a | 0.41 a | 0.39 a |
| T4 | 0.28 bc | 0.29 a | 0.34 b |
| T5 | 0.31 ab | 0.36 b | 0.33 b |
| Treatment Combination | Organic Carbon (%) | Available Soil N Content (kg/ha) | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 0.56 c | 0.58 d | 0.57 b | 265.54 b | 275.07 ± 6.48 b | 270.31 b |
| T2 | 0.65 ab | 0.68 ab | 0.67 | 281.21 ab | 295.76 ± 6.48 ab | 288.48 a |
| T3 | 0.68 a | 0.72 a | 0.70 a | 299.42 a | 313.32 ± 6.48 b | 290.49 a |
| T4 | 0.58 bc | 0.62 cd | 0.61 b | 240.14 c | 277.54 ± 6.48 a | 276.73 ab |
| T5 | 0.61 c | 0.65 bc | 0.61 b | 266.16 b | 281.56 ± 6.48 b | 271.85 b |
| Initial | 0.52 | 216.65 | ||||
| Treatment Combination | Available Soil P Content (kg/ha) | Available Soil K Content (kg/ha) | ||||
|---|---|---|---|---|---|---|
| 2019–2020 | 2020–2021 | Pooled Mean | 2019–2020 | 2020–2021 | Pooled Mean | |
| T1 (Control) | 26.79 c | 29.76 d | 28.28 c | 131.07 c | 134.51 c | 132.79 c |
| T2 | 32.39 b | 34.15 c | 32.25 b | 142.65 b | 147.54 b | 143.94 b |
| T3 | 36.99 a | 38.98 d | 33.78 a | 156.67 a | 162.54 b | 152.11 a |
| T4 | 28.56 c | 30.56 a | 33.77 a | 140.26 bc | 141.10 a | 151.40 a |
| T5 | 30.56 b | 32.11 b | 32.36 b | 142.04 b | 145.23 bc | 141.57 b |
| Initial | 21.03 | 112.05 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, A.; Kumar, A.; Kumar, U.; Choudhary, R.; Kumar, R.; Jat, R.; Nidhibahen, P.; Hatamleh, A.A.; Al-Dosary, M.A.; Al-Wasel, Y.A.; et al. Various Fertilization Managements Influence the Flowering Attributes, Yield Response, Biochemical Activity and SoilNutrient Status of Chrysanthemum (Chrysanthemum morifolium Ramat.). Sustainability 2022, 14, 4561. https://doi.org/10.3390/su14084561
Choudhary A, Kumar A, Kumar U, Choudhary R, Kumar R, Jat R, Nidhibahen P, Hatamleh AA, Al-Dosary MA, Al-Wasel YA, et al. Various Fertilization Managements Influence the Flowering Attributes, Yield Response, Biochemical Activity and SoilNutrient Status of Chrysanthemum (Chrysanthemum morifolium Ramat.). Sustainability. 2022; 14(8):4561. https://doi.org/10.3390/su14084561
Chicago/Turabian StyleChoudhary, Ashok, Ajit Kumar, Uttam Kumar, Rajesh Choudhary, Rakesh Kumar, Rajkumar Jat, Patel Nidhibahen, Ashraf Atef Hatamleh, Munirah Abdullah Al-Dosary, Yasmeen Abdualrhman Al-Wasel, and et al. 2022. "Various Fertilization Managements Influence the Flowering Attributes, Yield Response, Biochemical Activity and SoilNutrient Status of Chrysanthemum (Chrysanthemum morifolium Ramat.)" Sustainability 14, no. 8: 4561. https://doi.org/10.3390/su14084561
APA StyleChoudhary, A., Kumar, A., Kumar, U., Choudhary, R., Kumar, R., Jat, R., Nidhibahen, P., Hatamleh, A. A., Al-Dosary, M. A., Al-Wasel, Y. A., Rajagopal, R., & Ravindran, B. (2022). Various Fertilization Managements Influence the Flowering Attributes, Yield Response, Biochemical Activity and SoilNutrient Status of Chrysanthemum (Chrysanthemum morifolium Ramat.). Sustainability, 14(8), 4561. https://doi.org/10.3390/su14084561








