Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications
Abstract
:1. Introduction
2. Methodology
3. Material Characterizations
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vo, D.H.; Vo, A.T. Renewable energy and population growth for sustainable development in the Southeast Asian countries. Energy Sustain. Soc. 2021, 11, 30. [Google Scholar] [CrossRef]
- Iqbal, S. Spatial Charge Separation and Transfer in L-Cysteine Capped NiCoP/CdS Nano-Heterojunction Activated with Intimate Covalent Bonding for High-Quantum-Yield Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2020, 274, 119097. [Google Scholar] [CrossRef]
- Irfan, R.M.; Tahir, M.H.; Nadeem, M.; Maqsood, M.; Bashir, T.; Iqbal, S. Fe3C/CdS as noble-metal-free composite photocatalyst for highly enhanced photocatalytic H2 production under visible light. Appl. Catal. A Gen. 2020, 603, 117768. [Google Scholar] [CrossRef]
- Muhammad Irfan, R.; Hussain Tahir, M.; Maqsood, M.; Lin, Y.; Bashir, T.; Iqbal, S.; Zhao, J.; Gao, L.; Haroon, M. CoSe as Non-Noble-Metal Cocatalyst Integrated with Heterojunction Photosensitizer for Inexpensive H2 Production under Visible Light. J. Catal. 2020, 390, 196–205. [Google Scholar] [CrossRef]
- Hussain, W.; Malik, H.; Hussain, R.A.; Hussain, H.; Green, I.R.; Marwat, S.; Bahadur, A.; Iqbal, S.; Farooq, M.U.; Li, H.; et al. Synthesis of MnS from Single- and Multi-Source Precursors for Photocatalytic and Battery Applications. J. Electron. Mater. 2019, 48, 2278–2288. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Saeed, A.; Zhou, K.; Shoaib, M.; Waqas, M. Electrochemical performance of 2D polyaniline anchored CuS/Graphene nano-active composite as anode material for lithium-ion battery. J. Colloid Interface Sci. 2017, 502, 16–23. [Google Scholar] [CrossRef]
- Sathiya Priya, A.; Geetha, D.; Henry, J. Effect of Cu and Sm doping on the ferroelectric character of bismuth ferrite thin films. Phosphorus Sulfur Silicon Relat. Elem. 2021, 197, 158–163. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Anwer, S.; Ali, S.; Irfan, R.M.; Li, H.; Shoaib, M.; Raheel, M.; Anjum, T.A.; Zulqarnain, M. Effect of temperature and reaction time on the morphology of l-cysteine surface capped chalcocite (Cu2S) snowflakes dendrites nanoleaves and photodegradation study of methyl orange dye under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124984. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyum, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef]
- Ahire, R.; Deshpande, N.; Gudage, Y.; Sagade, A.; Chavhan, S.; Phase, D.; Sharma, R. A comparative study of the physical properties of CdS, Bi2S3 and composite CdS–Bi2S3 thin films for photosensor application. Sens. Actuators A Phys. 2007, 140, 207–214. [Google Scholar] [CrossRef]
- Rincón, M.; Campos, J.; Suárez, R. A comparison of the various thermal treatments of chemically deposited bismuth sulphide thin films and the effect on the structural and electrical properties. J. Phys. Chem. Solids 1999, 60, 385–392. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Nanocrystalline Bi2S3 thin films grown by thio-glycolic acid mediated successive ionic layer adsorption and reaction (SILAR) technique. Mater. Sci. Semicond. Process. 2013, 16, 43–50. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Onwudiwe, D.C. Bismuth sulphide based compounds: Properties, synthesis and applications. Results Chem. 2021, 3, 100151. [Google Scholar] [CrossRef]
- Su-juan, Z.; Ling-fang, Y.; Dan, S.; Ying-jie, W. Effect of Cs+, Ag+, Fe3+ Doping and Ag+, Fe3+ Co-Doping Contents on Photocatalytic Activity of TiO2 Films. In Proceedings of the 2011 Symposium on Photonics and Optoelectronics (SOPO), Wuhan, China, 16–18 May 2011; pp. 1–4. [Google Scholar]
- Chu, D.; Yuan, X.; Qin, G.; Xu, M.; Zheng, P.; Lu, J.; Zha, L. Efficient carbon-doped nanostructured TiO2 (anatase) film for photoelectrochemical solar cells. J. Nanopart. Res. 2008, 10, 357–363. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Javed, M.; Hakami, O.; Irfan, R.M.; Ahmad, Z.; AlObaid, A.; Al-Anazy, M.M.; Baghdadi, H.B.; Abd-Rabboh, H.S.M.; et al. Design Ag-doped ZnO heterostructure photocatalyst with sulfurized graphitic C3N4 showing enhanced photocatalytic activity. Mater. Sci. Eng. B 2021, 272, 115320. [Google Scholar] [CrossRef]
- Bahadur, A.; Iqbal, S.; Shoaib, M.; Saeed, A. Electrochemical study of specially designed graphene-Fe3O4-polyaniline nanocomposite as a high-performance anode for lithium-ion battery. Dalton Trans. 2018, 47, 15031–15037. [Google Scholar] [CrossRef]
- Anwer, S.; Anjum, D.H.; Luo, S.; Abbas, Y.; Li, B.; Iqbal, S.; Liao, K. 2D Ti3C2Tx MXene nanosheets coated cellulose fibers based 3D nanostructures for efficient water desalination. Chem. Eng. J. 2021, 406, 126827. [Google Scholar] [CrossRef]
- Romanyuk, R. Optical properties of amorphous (GeS)1−x Bix (0 ≤ x ≤ 0.15) films and a tentative cluster model for their structure. Inorg. Mater. 2014, 50, 120–123. [Google Scholar] [CrossRef]
- Masumdar, E.; Gaikwad, V.; Pujari, V.; More, P.; Deshmukh, L. Some studies on chemically synthesized antimony-doped CdSe thin films. Mater. Chem. Phys. 2003, 77, 669–676. [Google Scholar] [CrossRef]
- Ismail, B.; Mushtaq, S.; Khan, A. Enhanced grain growth in the sn doped Sb2S3 thin film absorber materials for solar cell applications. Chalcogenide Lett. 2014, 11, 37–45. [Google Scholar]
- Tototzintle, M.Z.; Castilla, S.R.; Luevano, R.; Hernandez, K.B.; Sotarriba, J.G.; Yañez, A.C. PbS:Hg2+ Nanostructures Films by Chemical Bath. J. Mater. Sci. Eng. A 2013, 3, 407–414. [Google Scholar]
- Yang, Y.; Xiong, X.; Yin, H.; Zhao, M.; Han, J. Study of copper bismuth sulphide thin films for the photovoltaic application. J. Mater. Sci. Mater. Electron. 2019, 30, 1832–1837. [Google Scholar] [CrossRef]
- Mane, R.S.; Sankapal, B.R.; Lokhande, C.D. Thickness dependent properties of chemically deposited As2S3 thin films from thioacetamide bath. Mater. Chem. Phys. 2000, 64, 215–221. [Google Scholar] [CrossRef]
- Raoot, S.; Raoot, K. Selective complexometric determination of bismuth with mercaptans as masking agents, and its estimation in alloys. Talanta 1985, 32, 1011–1012. [Google Scholar] [CrossRef]
- Kishore, K.; Dwibedy, P.; Dey, G.; Naik, D.; Moorthy, P. Nature of the transient species formed during pulse radiolysis of thioacetamide in aqueous solutions. Res. Chem. Intermed. 1998, 24, 35–45. [Google Scholar] [CrossRef]
- Mane, R.; Sankapal, B.; Lokhande, C. Studies on chemically deposited nanocrystalline Bi2S3 thin films. Mater. Res. Bull. 2000, 35, 587–601. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (Version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Ginting, M.; Taslima, S.; Sebayang, K.; Aryanto, D.; Sudiro, T.; Sebayang, P. Preparation and characterization of zinc oxide doped with ferrite and chromium. AIP Conf. Proc. 2017, 1862, 030062. [Google Scholar]
- Taziwa, R.; Meyer, E.; Katwire, D.; Ntozakhe, L. Influence of carbon modification on the morphological, structural, and optical properties of zinc oxide nanoparticles synthesized by pneumatic spray pyrolysis technique. J. Nanomater. 2017, 2017, 9095301. [Google Scholar] [CrossRef]
- Yilmaz, M.; Aydoğan, Ş. The effect of Pb doping on the characteristic properties of spin coated ZnO thin films: Wrinkle structures. Mater. Sci. Semicond. Process. 2015, 40, 162–170. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, K.K.; Sharma, A. Solar Cells: In Research and Applications—A Review. Mater. Sci. Appl. 2015, 6, 1145. [Google Scholar] [CrossRef] [Green Version]
- Barote, M.A.Y.; Masumdar, A.A. Synthesis, characterization and photoelectrochemical properties of n-CdS thin films. Physica B 2011, 406, 1865. [Google Scholar] [CrossRef]
- Podraza, N.J.; Qiu, W.; Hinojosa, B.B.; Xu, H.; Motyka, M.A.; Phillpot, S.R.; Baciak, J.E.; Trolier-McKinstry, S.; Nino, J.C. Band gap and structure of single crystal BiI3: Resolving discrepancies in literature. J. Appl. Phys. 2013, 114, 033110. [Google Scholar] [CrossRef] [Green Version]
- Roy, C.B.; Nandi, D.K.; Mahapatra, P.K. Photoelectrochemical cells with n-type ZnSe and n-type Sb2Se3 thin film semiconductor electrodes. Electrochim. Acta 1986, 31, 1227. [Google Scholar] [CrossRef]
- Linhart, W.M.; Zelewski, S.J.; Scharoch, P.; Dybała, F.; Kudrawiec, R. Nesting-like band gap in bismuth sulphide Bi2S3. J. Mater. Chem. C 2021, 9, 13733–13738. [Google Scholar] [CrossRef]
- Hankare, P.P.; Chate, P.A.; Sathe, D.J. Zinc sulphide semiconductor electrode synthesis and the photoelectrochemical application. J. Alloys Compd. 2009, 487, 367. [Google Scholar] [CrossRef]
- Deshmukh, L.P.; Rotti, C.B.; Garadkar, K.M. Cd1−xZnxS thin film electrode for photoelectrochemical (PEC) applications. Mater. Chem. Phys. 1997, 50, 45. [Google Scholar] [CrossRef]
- Rajpure, K.Y.; Bhosale, C.H. Sb2S3 semiconductor-septum rechargeable storage cell. Mater. Chem. Phys. 2000, 64, 14. [Google Scholar] [CrossRef]
- Bagdare, P.B.; Patil, S.B.; Singh, A.K. Phase evolution and PEC performance of ZnxCd1−xS nanocrystalline thin films deposited by CBD. J. Alloy. Compd. 2012, 506, 120–124. [Google Scholar]
- MacLachlan, A.J.; O’Mahony, F.T.F.; Sudlow, A.L.; Hill, M.S.; Molloy, K.C.; Nelson, J.; Haque, S.A. Solution-Processed Mesoscopic Bi2S3:Polymer Photoactive Layers. ChemPhysChem 2014, 15, 1019–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]


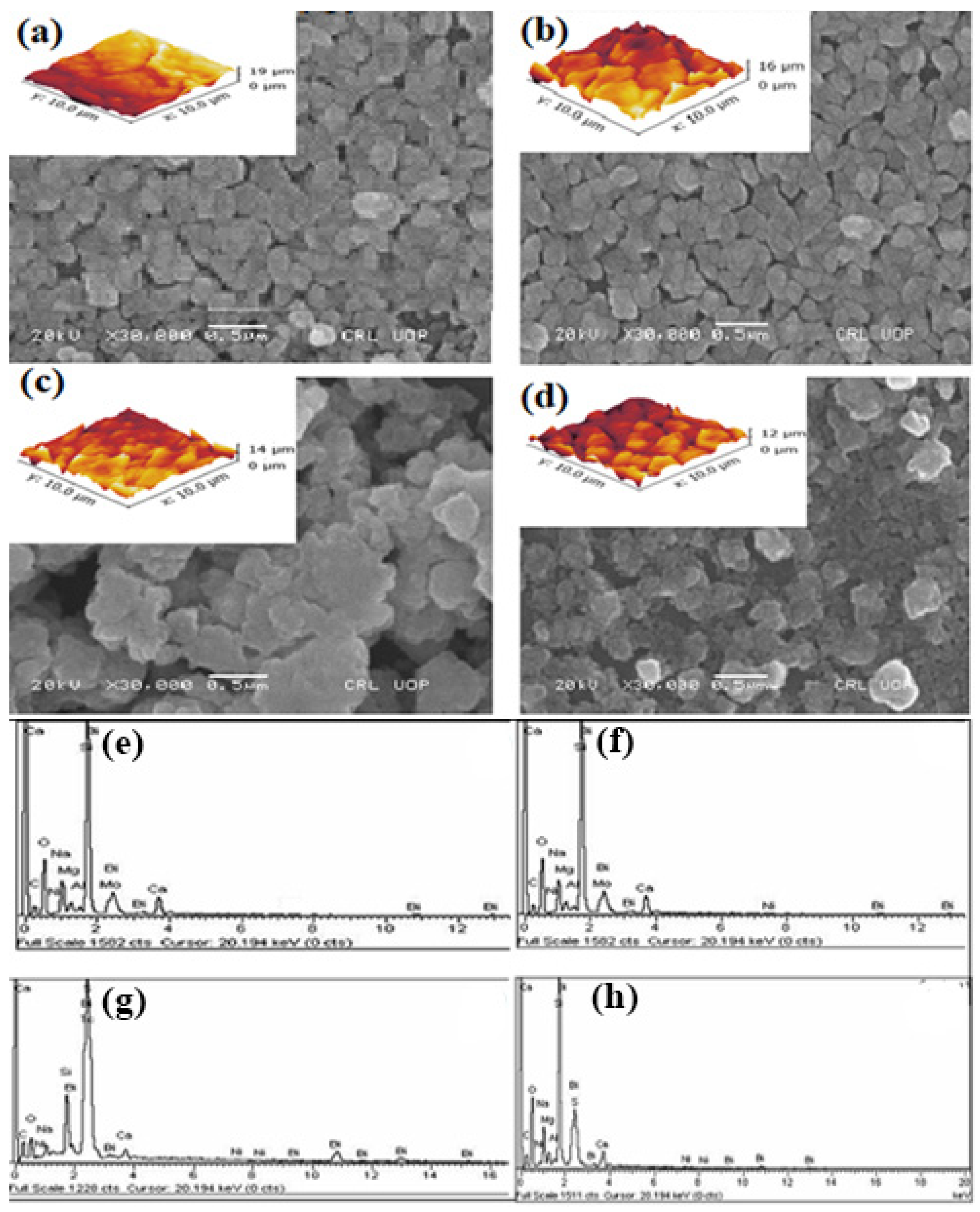
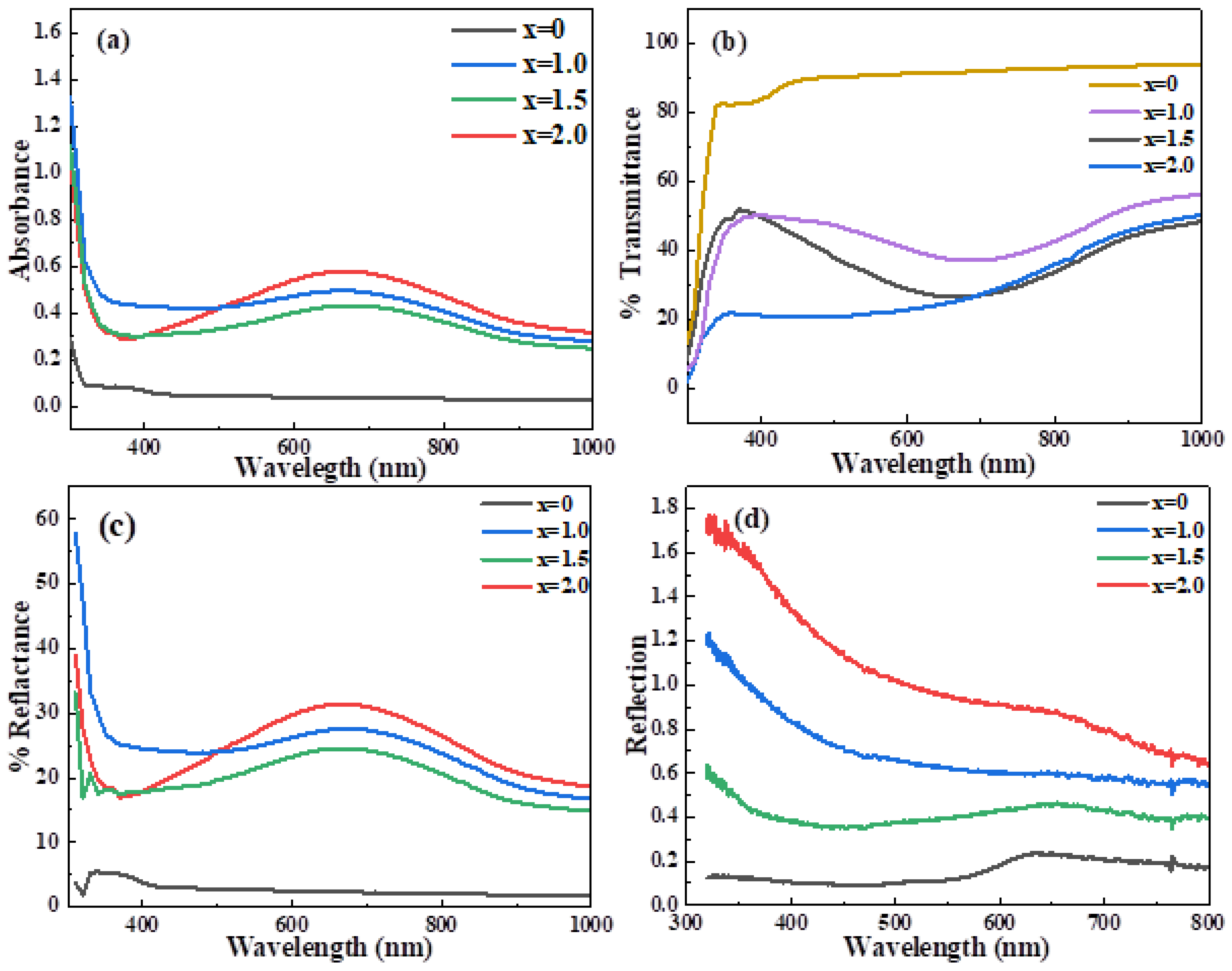


| Ni Content (at. %) | Calculated Lattice Constants | Volume of Cell (Å3) | Average Crystallite Size (nm) | X-ray Density (gcm−3) | ||
|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | ||||
| 0 | 11.11 | 11.71 | 3.52 | 458 | 288 | 7.46 |
| 1.0 | 11.10 | 11.68 | 3.89 | 509 | 235 | 6.71 |
| 1.5 | 11.12 | 11.22 | 4.16 | 519 | 211 | 6.58 |
| 2.0 | 11.65 | 11.16 | 4.02 | 522 | 159 | 6.54 |
| Parameters | Conc. of Ni (at. %) | |||||
|---|---|---|---|---|---|---|
| 0 | 1.0 | 1.25 | 1.5 | 1.75 | 2.0 | |
| α × 104 (cm−1) | 0.42 | 1.07 | 1.69 | 2.16 | 2.74 | 3.74 |
| ε | 0.08 | 4.56 | 5.00 | 6.81 | 7.30 | 8.76 |
| σe × 103 (Ω cm−1) | 0.345 | 0.010 | 0.042 | 0.044 | 0.063 | 0.079 |
| σo × 1012 (s−1) | 1.33 | 1.06 | 1.33 | 1.85 | 2.01 | 2.9 |
| σt × 10−4 (Ω cm/K) | 0.0063 | 1.03 | 1.15 | 1.72 | 1.68 | 2.08 |
| Ni Conc. (at. %) | I (µA) | Resistivity (Ω cm) × 102 | Conductivity (Ω−1 cm−1) × 102 | Carrier Conc. (cm−2) × 1011 | Sheet Carrier Mobility (cm2/Vs) × 102 |
|---|---|---|---|---|---|
| 0 | 0.1 | 2.99 | 0.393 | −0.016 | 4.77 |
| 1.0 | 0.1 | 0.188 | 5.4 | −2.62 | 3.14 |
| 1.5 | 0.1 | 0.276 | 3.62 | −2.71 | 0.35 |
| 2.0 | 0.1 | 0.431 | 2.32 | −11.8 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazal, T.; Ismail, B.; Shah, M.; Iqbal, S.; Elkaeed, E.B.; Awwad, N.S.; Ibrahium, H.A. Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications. Sustainability 2022, 14, 4603. https://doi.org/10.3390/su14084603
Fazal T, Ismail B, Shah M, Iqbal S, Elkaeed EB, Awwad NS, Ibrahium HA. Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications. Sustainability. 2022; 14(8):4603. https://doi.org/10.3390/su14084603
Chicago/Turabian StyleFazal, Tanzeela, Bushra Ismail, Mazloom Shah, Shahid Iqbal, Eslam B. Elkaeed, Nasser S. Awwad, and Hala A. Ibrahium. 2022. "Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications" Sustainability 14, no. 8: 4603. https://doi.org/10.3390/su14084603
APA StyleFazal, T., Ismail, B., Shah, M., Iqbal, S., Elkaeed, E. B., Awwad, N. S., & Ibrahium, H. A. (2022). Simplified Route for Deposition of Binary and Ternary Bismuth Sulphide Thin Films for Solar Cell Applications. Sustainability, 14(8), 4603. https://doi.org/10.3390/su14084603








