Research Status and Trends of Underwater Photosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Methodology
2.3. Special Terms
3. Results and Discussion
3.1. Descriptive Analysis
3.1.1. Annual Variations in Publications
3.1.2. Subject Category Distribution
3.1.3. Journal Distribution
3.2. Research Power Analysis
3.2.1. Author Distribution and Collaboration
3.2.2. Institutional Distribution and Collaboration
3.2.3. International (Regional) Distribution and Collaboration
3.3. Intellectual Base Analysis
3.3.1. Reference Cocitation Network
3.3.2. Landmark References
3.4. Research Hotspots and Trend Analysis
3.4.1. Research Hotspot Analysis
3.4.2. Development Trend Analysis
Stable Development Stage (1994–2004)
Rapid Development Stage (2005–2015)
Deep Development Stage (2016–2021)
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morris, G.I. Photosynthetic Carboxylating Enzymes in Marine Phytoplankton. Limnol. Oceanogr. 1979, 24, 510–519. [Google Scholar] [CrossRef]
- Clevering, O.; Van Vierssen, W.; Blom, C. Growth, photosynthesis and carbohydrate utilization in submerged Scirpus maritimus L. during spring growth. New Phytol. 1995, 130, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.; Afreen-Zobayed, F.; Blyth, S.; Armstrong, W. Phragmites australis: Effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquat. Bot. 1999, 64, 275–289. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmer, T.; Voesenek, L. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Colmer, T.; Pierik, R.; Millenaar, F.; Peeters, A. How plants cope with complete submergence. New Phytol. 2006, 170, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Colmer, T.D.; Winkel, A.; Pedersen, O. A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 2011, 2011, plr030. [Google Scholar] [CrossRef] [Green Version]
- Mommer, L.; Pedersen, O.; Visser, E. Acclimation of a terrestrial plant to submergence facilitates gas exchange under water. Plant Cell Environ. 2004, 27, 1281–1287. [Google Scholar] [CrossRef]
- Setter, T.; Waters, I.; Wallace, I.; Bhekasut, P.; Greenway, H. Submergence of rice. I. Growth and photosynthetic response to CO2 enrichment of floodwater. Funct. Plant Biol. 1989, 16, 251–263. [Google Scholar] [CrossRef]
- Pedersen, O.; Rich, S.M.; Colmer, T.D. Surviving floods: Leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J. 2009, 58, 147–156. [Google Scholar] [CrossRef]
- Colmer, T.D.; Pedersen, O.; Wetson, A.M.; Flowers, T.J. Oxygen dynamics in a salt-marsh soil and in Suaeda maritima during tidal submergence. Environ. Exp. Bot. 2013, 92, 73–82. [Google Scholar] [CrossRef]
- Colmer, T.D.; Pedersen, O. Oxygen dynamics in submerged rice (Oryza sativa). New Phytol. 2008, 178, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Visser, E.J. Underwater photosynthesis in flooded terrestrial plants: A matter of leaf plasticity. Ann. Bot. 2005, 96, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Colmer, T.D. Underwater photosynthesis and internal aeration of submerged terrestrial wetland plants. In Low-Oxygen Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 315–327. ISBN 978-3-7091-1253-3. [Google Scholar]
- Vervuren, P.; Beurskens, S.; Blom, C. Light acclimation, CO2 response and long-term capacity of underwater photosynthesis in three terrestrial plant species. Plant Cell Environ. 1999, 22, 959–968. [Google Scholar] [CrossRef]
- Fernandez, M. Changes in photosynthesis and fluorescence in response to flooding in emerged and submerged leaves of Pouteria orinocoensis. Photosynthetica 2006, 44, 32–38. [Google Scholar] [CrossRef]
- Nielsen, S.R.L. A comparison of aerial and submerged photosynthesis in some Danish amphibious plants. Aquat. Bot. 1993, 45, 27–40. [Google Scholar] [CrossRef]
- Frost-Christensen, H.; Sand-Jensen, K. Comparative kinetics of photosynthesis in floating and submerged Potamogeton leaves. Aquat. Bot. 1995, 51, 121–134. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Sand-Jensen, K. Underwater photosynthesis of submerged plants–recent advances and methods. Front. Plant Sci. 2013, 4, 140. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Liu, X. Comparing photosynthetic characteristics of Isoetes sinensis Palmer under submerged and terrestrial conditions. Sci. Rep. 2015, 5, 17783. [Google Scholar] [CrossRef] [Green Version]
- Magnin, N.C.; Cooley, B.A.; Reiskind, J.B.; Bowes, G. Regulation and Localization of Key Enzymes during the Induction of Kranz-Less, C4-Type Photosynthesis in Hydrilla verticillata. Plant Physiol. 1998, 115, 1681–1689. [Google Scholar] [CrossRef] [Green Version]
- Spencer, W.E.; Wetzel, R.G.; Teeri, J. Photosynthetic phenotype plasticity and the role of phosphoenolpyruvate carboxylase in Hydrilla verticillata. Plant Sci. 1996, 118, 1–9. [Google Scholar] [CrossRef]
- Elzenga, J.T.M.; Prins, H.B.A. Light-Induced Polar pH Changes in Leaves of Elodea canadensis: I. Effects of Carbon Concentration and Light Intensity. Plant Physiol. 1989, 91, 62–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casati, P. Induction of a C4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiol. 2000, 123, 1611–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horiguchi, G.; Matsumoto, K.; Nemoto, K.; Inokuchi, M.; Hirotsu, N. Transition from Proto-Kranz-Type Photosynthesis to HCO3− Use Photosynthesis in the Amphibious Plant Hygrophila polysperma. Front. Plant Sci. 2021, 12, 675507. [Google Scholar] [CrossRef]
- Maberly, S.C.; Madsen, T.V. Freshwater angiosperm carbon concentrating mechanisms: Processes and patterns. Funct. Plant Biol. 2002, 29, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Prins, H.; Elzenga, J. Bicarbonate utilization: Function and mechanism. Aquat. Bot. 1989, 34, 59–83. [Google Scholar] [CrossRef]
- Winkel, A.; Pedersen, O.; Ella, E.; Ismail, A.M.; Colmer, T.D. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. J. Exp. Bot. 2014, 65, 3225–3233. [Google Scholar] [CrossRef] [Green Version]
- Molnár, A.; Toth, J.P.; Sramko, G.; Horvath, O.; Popiela, A.; Mesterházy, A.; Lukács, B.A. Flood induced phenotypic plasticity in amphibious genus Elatine (Elatinaceae). PeerJ 2015, 3, e1473. [Google Scholar] [CrossRef] [Green Version]
- Markovskaya, E.; Gulyaeva, E. Role of stomata in adaptation of Plantago maritima L. Plants to tidal dynamics on the White Sea coast. Russ. J. Plant Physiol. 2020, 67, 68–75. [Google Scholar] [CrossRef]
- Mommer, L.; Pons, T.L.; Wolters-Arts, M.; Venema, J.H.; Visser, E.J. Submergence-induced morphological, anatomical, and biochemical responses in a terrestrial species affect gas diffusion resistance and photosynthetic performance. Plant Physiol. 2005, 139, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, G.; Nemoto, K.; Yokoyama, T.; Hirotsu, N. Photosynthetic acclimation of terrestrial and submerged leaves in the amphibious plant Hygrophila difformis. AoB Plants 2019, 11, plz009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Chen, B.; Liu, H.; Liu, Z.; Yan, H. Carbon sequestration and decreased CO2 emission caused by terrestrial aquatic photosynthesis: Insights from diel hydrochemical variations in an epikarst spring and two spring-fed ponds in different seasons. Appl. Geochem. J. Int. Assoc. Geochem. Cosmochem. 2015, 63, 248–260. [Google Scholar] [CrossRef]
- Yan, T.; Xue, J.; Zhou, Z.; Wu, Y. The Trends in Research on the Effects of Biochar on Soil. Sustainability 2020, 12, 7810. [Google Scholar] [CrossRef]
- Qin, F.; Zhu, Y.; Ao, T.; Chen, T. The Development Trend and Research Frontiers of Distributed Hydrological Models—Visual Bibliometric Analysis Based on Citespace. Water 2021, 13, 174. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Ji, X.; Yun, C.; Wang, M.; Luo, X. The knowledge domain and emerging trends in phytoremediation: A scientometric analysis with CiteSpace. Environ. Sci. Pollut. Res. 2020, 27, 15515–15536. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, X.; Zhang, J. Development trend and frontier of stormwater management (1980–2019): A bibliometric overview based on CiteSpace. Water 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yang, Z. Knowledge mapping of platform research: A visual analysis using VOSviewer and CiteSpace. Electron. Commer. Res. 2020, 4, 1–23. [Google Scholar] [CrossRef]
- Fang, J.; Pan, L.; Gu, Q.X.; Juengpanich, S.; Wang, Y.F. Scientometric analysis of mTOR signaling pathway in liver disease. Ann. Transl. Med. 2020, 8, 93. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Wu, L.; Zhang, H.; Chen, F. Visual analysis of influenza treated by traditional Chinese medicine based on CiteSpace. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 779–784. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Liu, Z.; Hu, Z.; Wang, X. The methodology function of CiteSpace mapping knowledge domains. Stud. Sci. Sci. 2015, 33, 242–253. [Google Scholar] [CrossRef]
- Xuyan, L.I. Analysis on International Scientific Cooperation of Mathematics by CiteSpace (2005–2015). Sci. Technol. Manag. Res. 2016, 36, 6. [Google Scholar] [CrossRef]
- Xu, Y. Digital Innovation Ecosystem: Research Context, Research Hotspot and Research Trends--Knowledge Mapping Analysis Using Citespace. J. Electron. Inf. Sci. 2020, 5, 72–80. [Google Scholar] [CrossRef]
- Xue, Y. The Research Status and Prospect of Global change Ecology in the Past 30 Years Based on CiteSpace. Adv. Environ. Prot. 2021, 11, 281–287. [Google Scholar] [CrossRef]
- Shao, H.; Kim, G.; Li, Q.; Newman, G. Web of Science-Based Green Infrastructure: A Bibliometric Analysis in CiteSpace. Land 2021, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Yu, L. The Scientific Development of Ecosystem Service Values. In Proceedings of the 2021 7th International Engineering Conference “Research & Innovation amid Global Pandemic” (IEC), Erbil, Iraq, 24–25 February 2021; pp. 128–133. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C. Visualization analysis of big data research based on Citespace. Soft Comput. 2020, 24, 8173–8186. [Google Scholar] [CrossRef]
- SStringer, M.J.; Sales-Pardo, M.; Amaral, L.A.N. Effectiveness of journal ranking schemes as a tool for locating information. PLoS ONE 2008, 3, e1683. [Google Scholar] [CrossRef]
- Smith, D.R. A 30-year citation analysis of bibliometric trends at the Archives of Environmental Health, 1975–2004. Arch. Environ. Occup. Health 2009, 64 (Suppl. S1), 43–54. [Google Scholar] [CrossRef]
- Kim, J.; Perez, C. Co-Authorship Network Analysis in Industrial Ecology Research Community. J. Ind. Ecol. 2015, 19, 222–235. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Garcia-Robledo, E.; Revsbech, N.P. CO2 and O2 dynamics in leaves of aquatic plants with C3 or CAM photosynthesis–application of a novel CO2 microsensor. Ann. Bot. 2018, 122, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Aleixandre-Benavent, R.; Aleixandre-Tudó, J.L.; Castelló-Cogollos, L.; Aleixandre, J.L. Trends in scientific research on climate change in agriculture and forestry subject areas (2005–2014). J. Clean. Prod. 2017, 147, 406–418. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Du, J.; Long, H. A comparative study of Chinese and foreign green development from the perspective of mapping knowledge domains. Sustainability 2018, 10, 4357. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Li, C.; Ye, C.; Wang, J.; Wei, W.; Deng, Y. Research progress on ecological models in the field of water eutrophication: CiteSpace analysis based on data from the ISI web of science database. Ecol. Model. 2019, 410, 108779. [Google Scholar] [CrossRef]
- Sakagami, J.-I.; Joho, Y.; Sone, C. Complete submergence escape with shoot elongation ability by underwater photosynthesis in African rice, Oryza glaberrima Steud. Field Crops Res. 2013, 152, 17–26. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Van der Sman, A.; Harren, F.; Blom, C. An amalgamation between hormone physiology and plant ecology: A review on flooding resistance and ethylene. J. Plant Growth Regul. 1992, 11, 171–188. [Google Scholar] [CrossRef]
- Yan, L. Growth and morphological responses to water level variations in two Carex species from Sanjiang Plain, China. Afr. J. Agric. Res. 2011, 6, 28–34. [Google Scholar] [CrossRef]
- Pedersen, O.; Vos, H.; Colmer, T. Oxygen dynamics during submergence in the halophytic stem succulent Halosarcia pergranulata. Plant Cell Environ. 2006, 29, 1388–1399. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Häder, D.-P. Individual and interactive effects of ocean acidification, global warming, and UV radiation on phytoplankton. J. Appl. Phycol. 2018, 30, 743–759. [Google Scholar] [CrossRef]
- Montecino, V.; Pizarro, G. Phytoplankton acclimation and spectral penetration of UV irradiance off the central Chilean coast. Mar. Ecol. Prog. Ser. 1995, 121, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Küster, A.; Schaible, R.; Schubert, H. Light acclimation of the charophyte Lamprothamnium papulosum. Aquat. Bot. 2000, 68, 205–216. [Google Scholar] [CrossRef]
- Wolfstein, K.; Colijn, F.; Doerffer, R. Seasonal dynamics of microphytobenthos biomass and photosynthetic characteristics in the northern German Wadden Sea, obtained by the photosynthetic light dispensation system. Estuar. Coast. Shelf Sci. 2000, 51, 651–662. [Google Scholar] [CrossRef]
- Zhang, Q.; Visser, E.J.; de Kroon, H.; Huber, H. Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Ann. Bot. 2015, 116, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkel, A.; Visser, E.J.; Colmer, T.D.; Brodersen, K.P.; Voesenek, L.A.; Sand-Jensen, K.; Pedersen, O. Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant Cell Environ. 2016, 39, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Huang, X.P.; Zhang, J.P. Effects of CO2 enrichment on photosynthesis, growth, and biochemical composition of seagrass Thalassia hemprichii (Ehrenb.) Aschers. J. Integr. Plant Biol. 2010, 52, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Jin, Q.; Yin, L.; Li, W. Responses of CO2—concentrating mechanisms and photosynthetic characteristics in aquatic plant Ottelia alismoides following cadmium stress under low CO2. Ecotoxicol. Environ. Saf. 2020, 202, 110955. [Google Scholar] [CrossRef] [PubMed]
- Striker, G.G.; Izaguirre, R.; Manzur, M.E.; Grimoldi, A.A. Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biol. 2012, 14, 50–55. [Google Scholar] [CrossRef]
- Manzur, M.; Grimoldi, A.; Insausti, P.; Striker, G. Escape from water or remain quiescent? Lotus tenuis changes its strategy depending on depth of submergence. Ann. Bot. 2009, 104, 1163–1169. [Google Scholar] [CrossRef]
- Rich, S.M.; Ludwig, M.; Pedersen, O.; Colmer, T.D. Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: Implications for root function during flooding. New Phytol. 2011, 190, 311–319. [Google Scholar] [CrossRef]
- Rich, S.M.; Ludwig, M.; Colmer, T.D. Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant Cell Environ. 2008, 31, 1007–1016. [Google Scholar] [CrossRef]
- Striker, G.G.; Kotula, L.; Colmer, T.D. Tolerance to partial and complete submergence in the forage legume Melilotus siculus: An evaluation of 15 accessions for petiole hyponastic response and gas-filled spaces, leaf hydrophobicity and gas films, and root phellem. Ann. Bot. 2019, 123, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Sou, H.-D.; Masumori, M.; Ezaki, G.; Tange, T. Source of Oxygen Fed to Adventitious Roots of Syzygium kunstleri (King) Bahadur and RC Gaur Grown in Hypoxic Conditions. Plants 2020, 9, 1433. [Google Scholar] [CrossRef] [PubMed]
- Mommer, L.; Pons, T.L.; Visser, E.J.W. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J. Exp. Bot. 2006, 57, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Teakle, N.L.; Colmer, T.D.; Pedersen, O. Leaf gas films delay salt entry and enhance underwater photosynthesis and internal aeration of M elilotus siculus submerged in saline water. Plant Cell Environ. 2014, 37, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Pedersen, O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: Gas films improve C and O2 exchange. New Phytol. 2008, 177, 918–926. [Google Scholar] [CrossRef]
- Winkel, A.; Colmer, T.D.; Pedersen, O. Leaf gas films of Spartina anglica enhance rhizome and root oxygen during tidal submergence. Plant Cell Environ. 2011, 34, 2083–2092. [Google Scholar] [CrossRef]
- Winkel, A.; Colmer, T.D.; Ismail, A.M.; Pedersen, O. Internal aeration of paddy field rice (O ryza sativa) during complete submergence–importance of light and floodwater O2. New Phytol. 2013, 197, 1193–1203. [Google Scholar] [CrossRef]
- Yang, H.; Shao, X.; Wu, M. A review on ecosystem health research: A visualization based on CiteSpace. Sustainability 2019, 11, 4908. [Google Scholar] [CrossRef] [Green Version]
- Best, E.P.; Buzzelli, C.P.; Bartell, S.M.; Wetzel, R.L.; Boyd, W.A.; Doyle, R.D.; Campbell, K.R. Modeling submersed macrophyte growth in relation to underwater light climate: Modeling approaches and application potential. Hydrobiologia 2001, 444, 43–70. [Google Scholar] [CrossRef]
- Clark, D.R.; Flynn, K.J. The relationship between the dissolved inorganic carbon concentration and growth rate in marine phytoplankton. Proc. R. Soc. London Ser. B Biol. Sci. 2000, 267, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.; Pulido, C.; Rich, S.M.; Colmer, T.D. In situ O2 dynamics in submerged Isoetes australis: Varied leaf gas permeability influences underwater photosynthesis and internal O2. J. Exp. Bot. 2011, 62, 4691–4700. [Google Scholar] [CrossRef]
- Konnerup, D.; Pedersen, O. Flood tolerance of Glyceria fluitans: The importance of cuticle hydrophobicity, permeability and leaf gas films for underwater gas exchange. Ann. Bot. 2017, 120, 521–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant, N.; Ballesteros, E. Photosynthetic activity of macroalgae along a bathymetric gradient: Interspecific and seasonal variability. Sci. Mar. 2020, 84, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhou, M.; Lv, J.; Chen, K. Trends in global research in forest carbon sequestration: A bibliometric analysis. J. Clean. Prod. 2020, 252, 119908. [Google Scholar] [CrossRef]
- Post, A.; Larkum, A. UV-absorbing pigments, photosynthesis and UV exposure in Antarctica: Comparison of terrestrial and marine algae. Aquat. Bot. 1993, 45, 231–243. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Sand-Jensen, K. Light attenuation and photosynthesis of aquatic plant communities. Limnol. Oceanogr. 1998, 43, 396–407. [Google Scholar] [CrossRef]
- Schwarz, A.M.; Howard-Williams, C.; Clayton, J. Analysis of relationships between maximum depth limits of aquatic plants and underwater light in 63 New Zealand lakes. N. Z. J. Mar. Freshw. Res. 2000, 34, 157–174. [Google Scholar] [CrossRef]
- Tamang, B.G.; Fukao, T. Plant adaptation to multiple stresses during submergence and following desubmergence. Int. J. Mol. Sci. 2015, 16, 30164–30180. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Cieraad, E.; Clarkson, B.R.; Colmer, T.D.; Pedersen, O.; Visser, E.J.; Voesenek, L.A.; van Bodegom, P.M. Drivers of plant traits that allow survival in wetlands. Funct. Ecol. 2020, 34, 956–967. [Google Scholar] [CrossRef]
- Chakraborty, K.; Guru, A.; Jena, P.; Ray, S.; Guhey, A.; Chattopadhyay, K.; Sarkar, R.K. Rice with SUB1 QTL possesses greater initial leaf gas film thickness leading to delayed perception of submergence stress. Ann. Bot. 2021, 127, 251–265. [Google Scholar] [CrossRef]
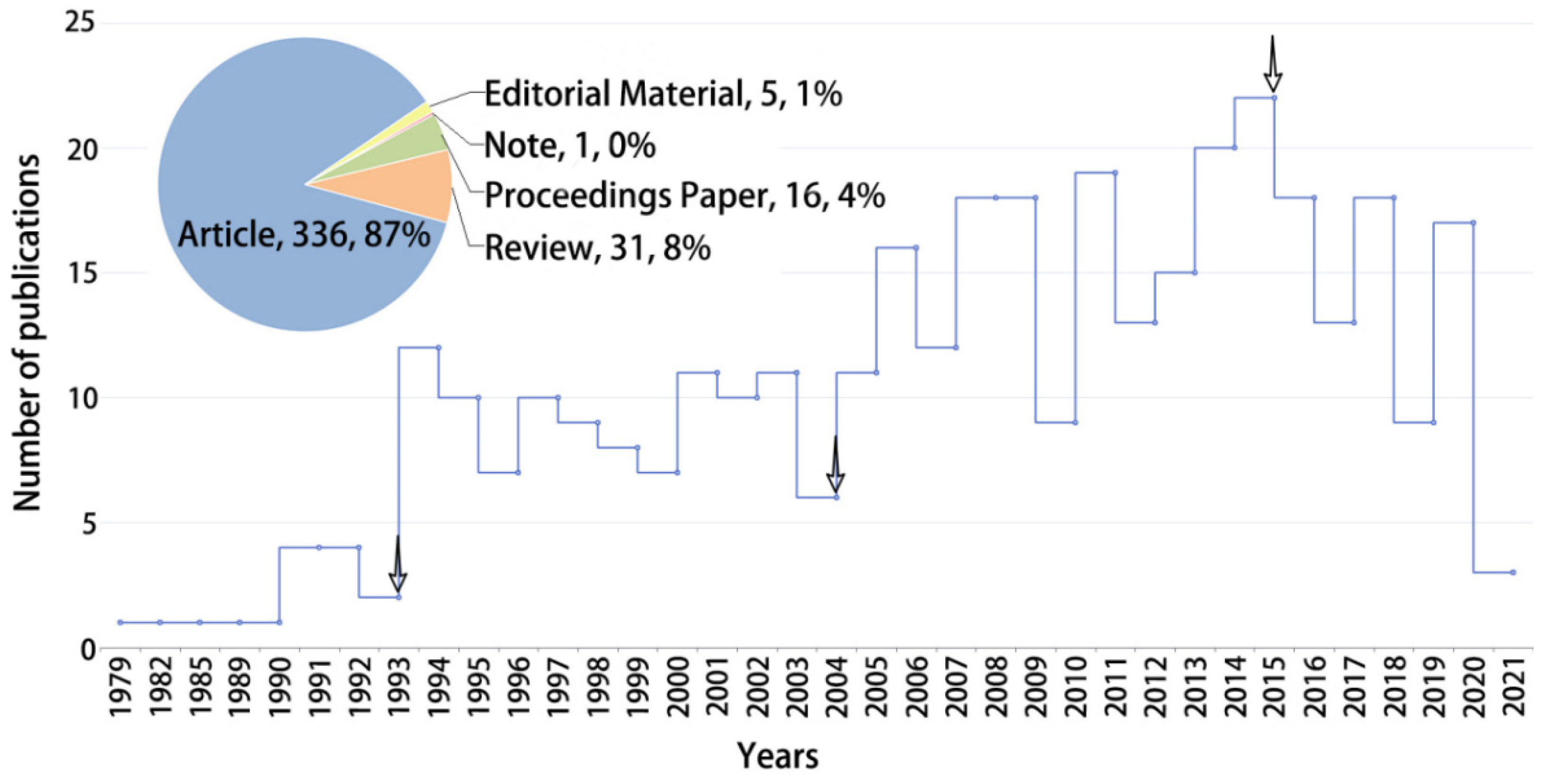
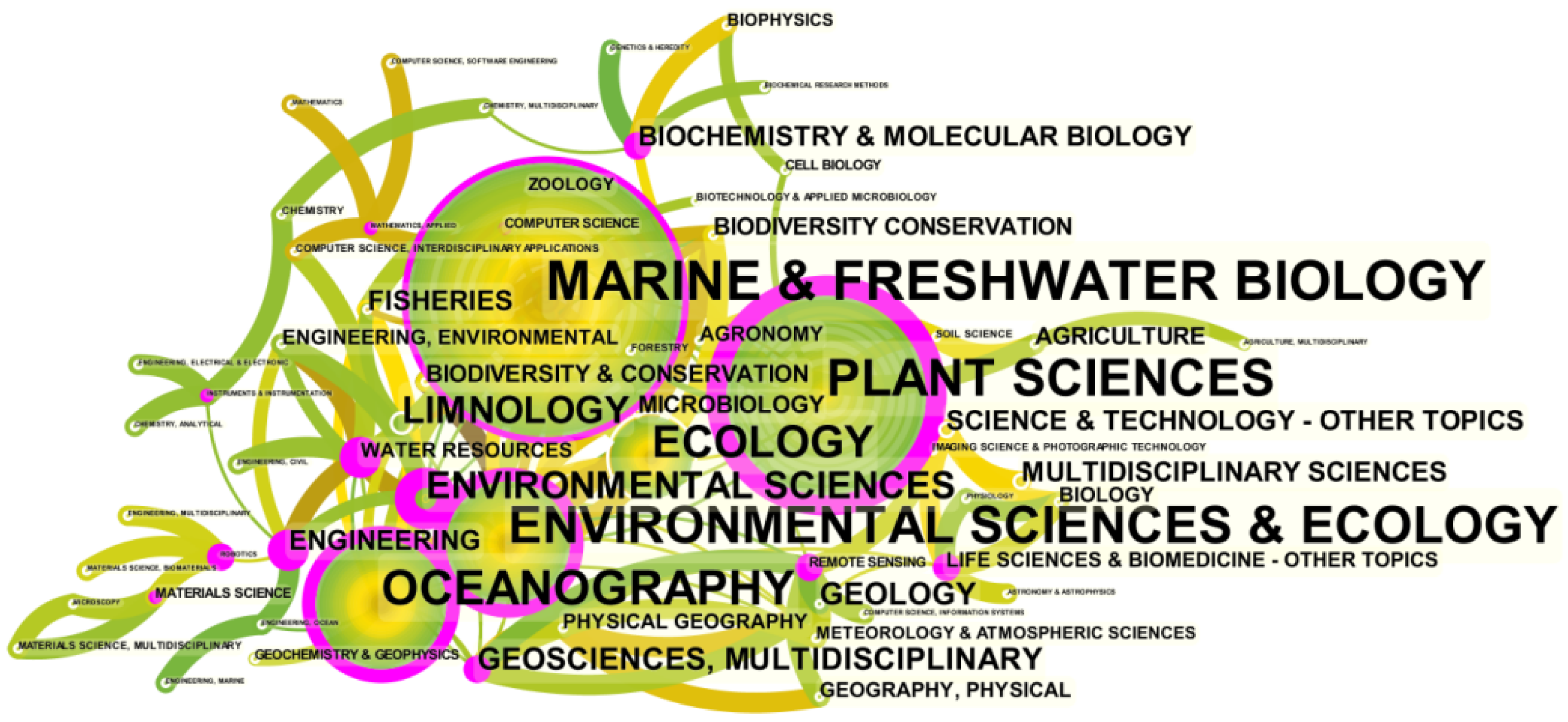
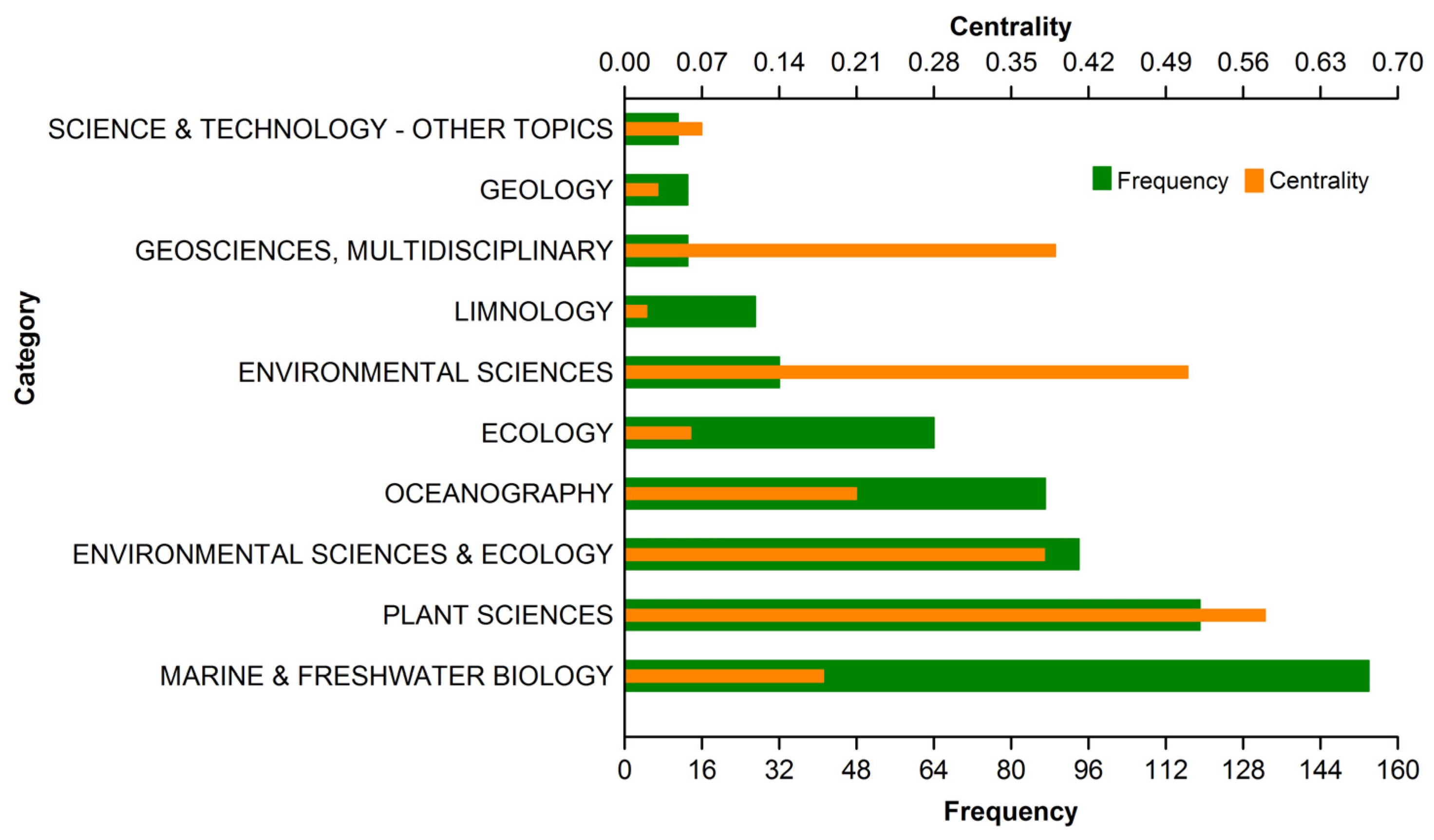


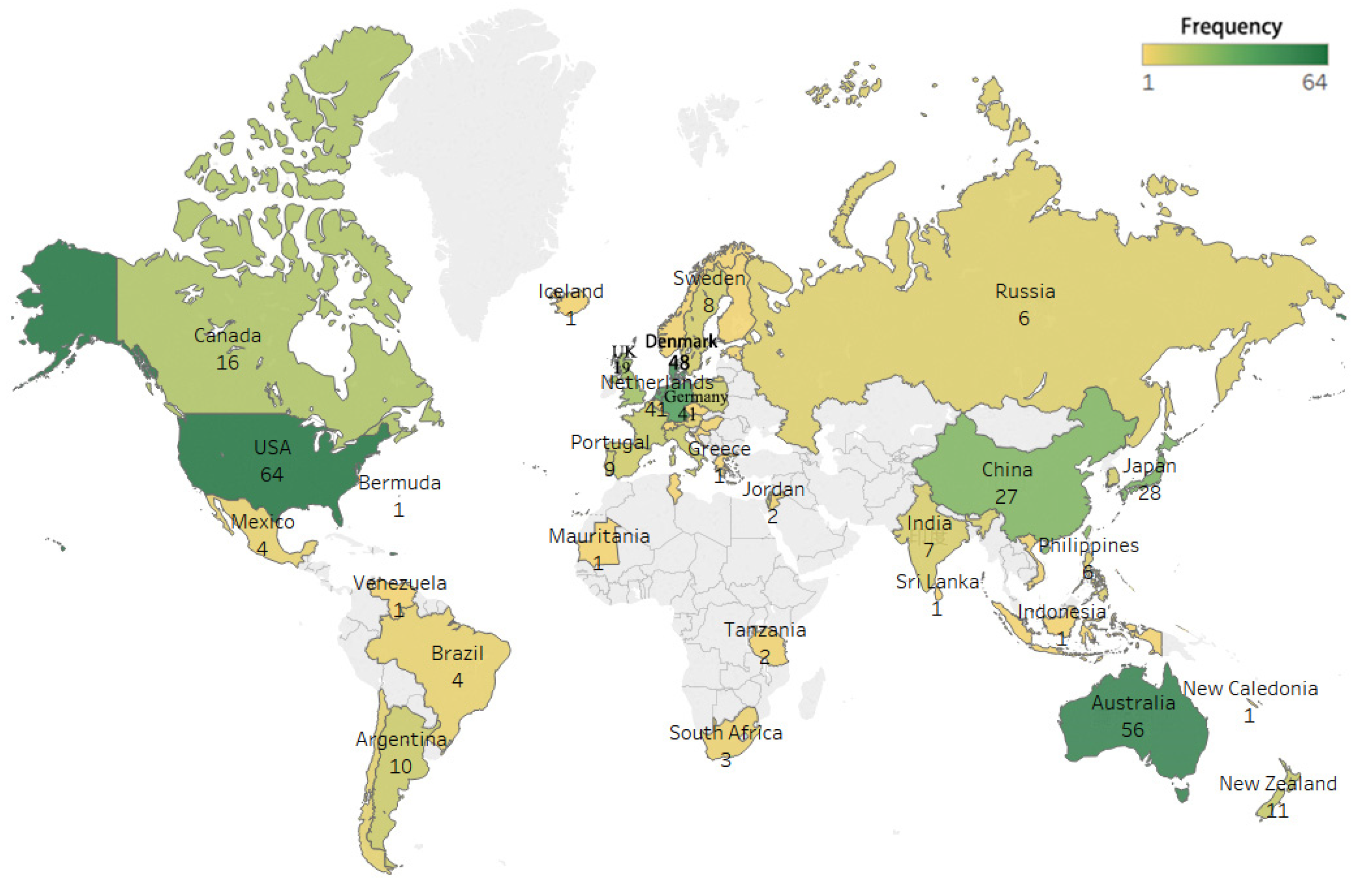

| Journal | Number of Documents | Total Citation Frequency | Average Citation Frequency per Paper | Impact Factor | First Cited Year |
|---|---|---|---|---|---|
| Limnology and Oceanography | 227 | 8273 | 36.44 | 3.589 | 1979 |
| Marine Ecology Progress Series | 171 | 6881 | 40.24 | 2.210 | 1991 |
| New Phytologist | 148 | 6016 | 40.65 | 8.086 | 1992 |
| Aquatic Botany | 147 | 6086 | 41.40 | 1.625 | 1990 |
| Plant Physiology | 143 | 6491 | 45.39 | 6.557 | 1985 |
| Nature | 142 | 7156 | 50.39 | 40.639 | 1995 |
| Plant Cell and Environment | 140 | 6036 | 43.11 | 6.044 | 1985 |
| Annals of Botany | 125 | 5042 | 40.34 | 3.805 | 1990 |
| Marine Biology | 124 | 5002 | 40.34 | 1.948 | 1979 |
| Journal of Experimental Botany | 123 | 5300 | 43.09 | 5.613 | 1990 |
| Hydrobiologia | 116 | 4501 | 38.80 | 2.266 | 1991 |
| Journal of Phycology | 105 | 4841 | 46.10 | 2.212 | 1985 |
| Science | 100 | 4992 | 49.92 | 39.753 | 1995 |
| Author | Number of Documents | Total Citation Frequency | Average Citation Frequency per Paper | Time |
|---|---|---|---|---|
| Ole Pedersen | 29 | 772 | 26.62 | 2004–2019 |
| Timothy David Colmer | 21 | 1028 | 48.95 | 2009–2020 |
| Eric J. W. Visser | 14 | 732 | 52.29 | 2004–2020 |
| Anders Winkel | 10 | 260 | 26.00 | 2011–2018 |
| C.W.P.M. Blom | 9 | 535 | 59.44 | 1990–2006 |
| Hendrik Schubert | 8 | 224 | 28.00 | 1995–2006 |
| Dennis Konnerup | 7 | 40 | 5.71 | 2015–2018 |
| R.M. Forster | 5 | 451 | 90.20 | 1994–1997 |
| L. A. C. J. Voesenek | 5 | 216 | 43.20 | 1992–2003 |
| Warwick F. Vincent | 5 | 114 | 22.80 | 1989–2000 |
| Anne-Maree Schwarz | 5 | 119 | 23.80 | 1995–2003 |
| Institution | Number of Documents | Total Citation Frequency | Average Citation Frequency per Paper | Centrality |
|---|---|---|---|---|
| The University of Western Australia | 39 | 2041 | 52.33 | 0.08 |
| University of Copenhagen | 38 | 1311 | 34.50 | 0.06 |
| Radboud University Nijmegen | 16 | 736 | 46.00 | 0.1 |
| Utrecht University | 10 | 1241 | 124.10 | 0.08 |
| Chinese Academy of Sciences | 8 | 135 | 16.88 | 0.06 |
| Polish Academy of Sciences | 8 | 133 | 16.63 | 0.00 |
| Tel Aviv University | 8 | 397 | 49.63 | 0.00 |
| Alfred Wegener Institute for Polar and Marine Research | 8 | 628 | 78.50 | 0.03 |
| Kyushu University | 7 | 101 | 14.43 | 0.05 |
| University of Rostock | 5 | 215 | 43.00 | 0.05 |
| Pusan National University | 5 | 406 | 81.20 | 0.00 |
| Country | Number of Documents | Total Citation Frequency | Average Citation Frequency per Paper | Centrality |
|---|---|---|---|---|
| United States of America | 64 | 2797 | 43.70 | 0.56 |
| Australia | 56 | 2588 | 46.21 | 0.23 |
| Denmark | 48 | 1576 | 32.83 | 0.36 |
| Germany | 41 | 2485 | 60.61 | 0.36 |
| The Netherlands | 41 | 3125 | 76.22 | 0.21 |
| Japan | 28 | 338 | 12.07 | 0.18 |
| China | 27 | 361 | 13.37 | 0.09 |
| England | 19 | 416 | 21.89 | 0.31 |
| Canada | 16 | 580 | 36.25 | 0.09 |
| Israel | 12 | 517 | 43.08 | 0.05 |
| Funding Agencies | Record Count | % of 367 | Country |
|---|---|---|---|
| National Natural Science Foundation of China (NSFC) | 13 | 3.54% | China |
| Villum Fonden | 8 | 2.18% | UK |
| Natural Environment Research Council UK Research and Innovation (UKRI) | 5 | 1.36% | UK |
| Danish Council for Independent Research Det Frie Forskningsrad (DFF) | 5 | 1.36% | Denmark |
| Australian Research Council | 4 | 1.09% | Australia |
| Fundamental Research Funds for the Central Universities | 4 | 1.09% | China |
| Grants-in-Aid for Scientific Research Ministry of Education Culture Sports Science and Technology Japan (MEXT) | 3 | 0.82% | Japan |
| Research Council of Norway | 3 | 0.82% | Norway |
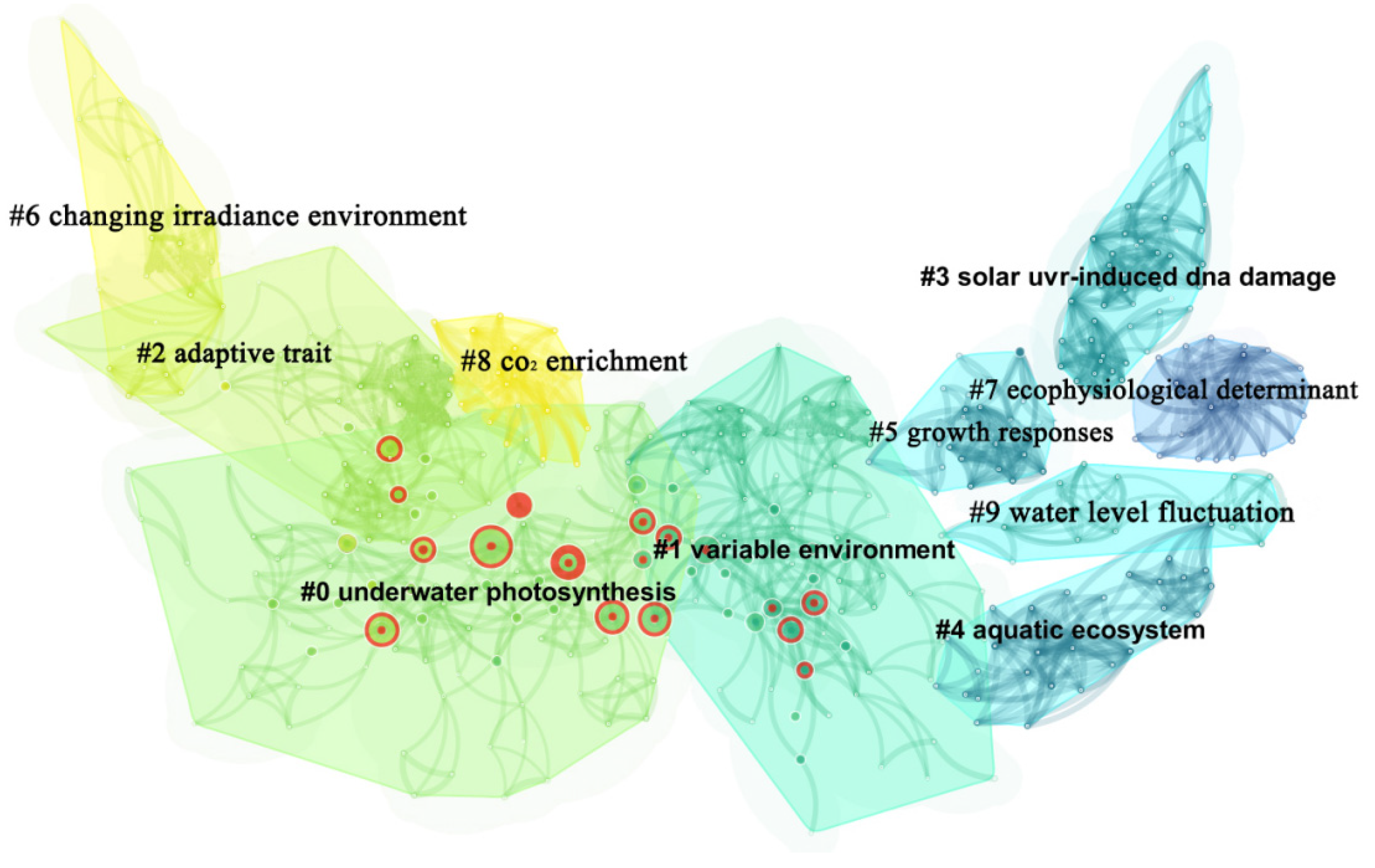
| Cluster ID | Size | Silhouette | Mean Cited Year | Label (LLR) |
|---|---|---|---|---|
| 0 | 83 | 0.906 | 2011 | underwater photosynthesis |
| 1 | 74 | 0.902 | 2004 | variable environment |
| 2 | 49 | 0.956 | 2012 | adaptive trait |
| 3 | 46 | 0.985 | 1998 | solar UV-induced DNA damage |
| 4 | 39 | 0.989 | 1994 | aquatic ecosystem |
| 5 | 37 | 0.955 | 2016 | growth responses |
| 6 | 26 | 0.973 | 1995 | changing irradiance environment |
| 7 | 21 | 1.000 | 1988 | eco-physiological determinant |
| 8 | 83 | 0.906 | 2011 | CO2 enrichment |
| 9 | 74 | 0.902 | 2004 | water level fluctuation |
| Frequency | Author | Title | Source | Year | Burst | Centrality | Cluster ID |
|---|---|---|---|---|---|---|---|
| 21 | Colmer et al. | Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange | New Phytologist | 2008 | 10.25 | 0.01 | 0 |
| 19 | Bailey-Serres et al. | Flooding Stress: Acclimations and Genetic Diversity | Annual Review of Plant Biology | 2008 | 9.25 | 0.02 | 0 |
| 18 | Colmer et al. | Flooding tolerance: suites of plant traits in variable environments | Functional Plant Biology | 2009 | 8.85 | 0.01 | 0 |
| 18 | Pedersen et al. | Underwater photosynthesis of submerged plants—recent advances and methods | Frontiers in Plant Science | 2013 | 7.96 | 0.01 | 0 |
| 18 | Colmer et al. | A perspective on underwater photosynthesis in submerged terrestrial wetland plants | AoB Plant | 2011 | 8.45 | 0.03 | 0 |
| 17 | Pedersen et al. | Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice | Plant Journal | 2009 | 8.35 | 0.02 | 0 |
| 16 | Winkel et al. | Internal aeration of paddy field rice (Oryza sativa) during complete submergence—the importance of light and floodwater O2 | New Phytologist | 2013 | 7.06 | 0.01 | 0 |
| 15 | Winkel et al. | Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes | Journal of Experimental Botany | 2014 | 7.83 | 0.01 | 2 |
| 15 | Mommer et al. | Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity | Annals of Botany | 2005 | 7.74 | 0.01 | 1 |
| 14 | Winkel et al. | Leaf gas films of Spartina anglica enhance rhizome and root oxygen during tidal submergence | Plant, Cell and Environment | 2011 | 6.56 | 0.01 | 0 |
| Cluster ID | Size | Silhouette | Mean Cited Year | Label (LSI) |
|---|---|---|---|---|
| 0 | 120 | 0.855 | 2009 | underwater photosynthesis |
| 1 | 75 | 0.847 | 2001 | light absorption |
| 2 | 55 | 0.842 | 2001 | phytoplankton productivity |
| 3 | 54 | 0.889 | 2003 | chlorophyll-a distribution |
| 4 | 52 | 0.854 | 2003 | in situ photosynthesis |
| 5 | 37 | 0.951 | 2007 | HCO3− use photosynthesis |
| 6 | 32 | 0.979 | 2003 | microphytobenthos community production |
| 7 | 32 | 0.917 | 2005 | carbohydrate utilization |
| 8 | 31 | 0.956 | 1998 | coastal area |
| 9 | 28 | 0.953 | 2002 | photosynthetic efficiency |
| Keywords | Strength | Begin | End | 1979–2021 |
|---|---|---|---|---|
| Light | 4.02 | 1993 | 1998 |  |
| Ultraviolet radiation | 5.15 | 1997 | 2002 |  |
| Phytoplankton | 4.40 | 1999 | 2001 |  |
| Plant | 4.73 | 2008 | 2018 |  |
| Submergence tolerance | 5.97 | 2011 | 2016 |  |
| Flooding tolerance | 5.82 | 2012 | 2021 | 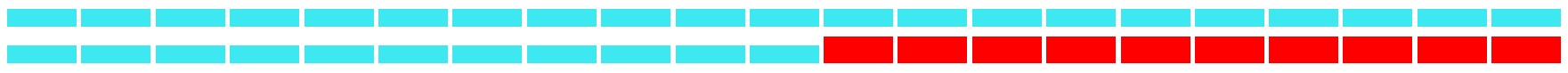 |
| Underwater photosynthesis | 10.39 | 2014 | 2021 |  |
| Response | 6.31 | 2016 | 2021 | 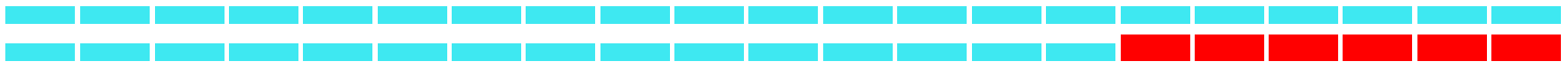 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xue, J.; Hua, J.; Xuan, L.; Yin, Y. Research Status and Trends of Underwater Photosynthesis. Sustainability 2022, 14, 4644. https://doi.org/10.3390/su14084644
Guo J, Xue J, Hua J, Xuan L, Yin Y. Research Status and Trends of Underwater Photosynthesis. Sustainability. 2022; 14(8):4644. https://doi.org/10.3390/su14084644
Chicago/Turabian StyleGuo, Jinbo, Jianhui Xue, Jianfeng Hua, Lei Xuan, and Yunlong Yin. 2022. "Research Status and Trends of Underwater Photosynthesis" Sustainability 14, no. 8: 4644. https://doi.org/10.3390/su14084644





