Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift-Valley Landscapes, Ethiopia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sites
2.2. Characteristics of the Three Indigenous Agroforestry Systems That Relate to the Study
2.2.1. Enset Based Agroforestry System
2.2.2. Coffee–Enset Based Agroforestry System
2.2.3. Coffee–Fruit-Tree–Enset Based Agroforestry System
2.3. Sampling Design and Data Collection
2.3.1. Inventory for Biomass Estimation
2.3.2. Biomass and Determination of Biomass C Stocks
2.3.3. Soil Sampling and Determination of Soil Organic Carbon Stocks
2.4. Statistical Analysis
3. Results
3.1. Biomass and Biomass Carbon Stocks
3.2. Soil Organic Carbon Stock
3.3. Ecosystem Carbon Stocks
3.4. Correlation between Biomass Carbon and SOC of Agroforestry Systems
3.5. Relationship between Biomass C and Abundance, Shannon Diversity, Marglef’s Richness
3.6. Soil Organic Carbon Stocks of AF Systems versus Their Adjacent Monocrop Farms
4. Discussion
4.1. Biomass, Biomass Carbon, Soil Organic Carbon, and Ecosystem Carbon Stocks
4.2. Correlation between Biomass Carbon and SOC of Agroforestry Systems
4.3. Relationship between Biomass C and Abundance, Shannon Diversity, Marglef’s Richness
4.4. Soil Organic Carbon Stocks of AF Systems versus Their Adjacent Monocrop Farms
5. Conclusion and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| BC Stock of C–Ft–E Based AF | SOC Stock of C–Ft–E Based AF | BC Stock of Enset Based AF | SOC Stock of Enset Based AF | BC Stock of C–E Based AF | SOC Stock of C–E Based AF | ||
|---|---|---|---|---|---|---|---|
| BC stock of C–Ft–E based AF | Pearson Correlation | 1 | |||||
| Sig. (2-tailed) | |||||||
| N | 10 | ||||||
| SOC stock of C–Ft–E based AF | Pearson Correlation | −0.005 | 1 | ||||
| Sig. (2-tailed) | 0.989 | ||||||
| N | 10 | 10 | |||||
| BC stock of Enset based AF | Pearson Correlation | 0.184 | −0.186 | 1 | |||
| Sig. (2-tailed) | 0.612 | 0.606 | |||||
| N | 10 | 10 | 10 | ||||
| SOC stock of Ensetbased AF | Pearson Correlation | 0.080 | 0.055 | 0.458 | 1 | ||
| Sig. (2-tailed) | 0.825 | 0.881 | 0.183 | ||||
| N | 10 | 10 | 10 | 10 | |||
| BC stock of C–E based AF | Pearson Correlation | −0.088 | −0.069 | 0.029 | −0.527 | 1 | |
| Sig. (2-tailed) | 0.810 | 0.850 | 0.937 | 0.117 | |||
| N | 10 | 10 | 10 | 10 | 10 | ||
| SOC stock of C–E based AF | Pearson Correlation | −0.552 | .008 | −0.412 | 0.415 | −0.246 | 1 |
| Sig. (2-tailed) N | 0.098 10 | 0.983 10 | 0.237 10 | 0.233 10 | 0.493 10 | 10 |
References
- Gebremeskel, D.; Birhane, E.; Rannestad, M.M.; Gebre, S.; Tesfay, G. Biomass and soil carbon stocks of Rhamnus prinoides based agroforestry practice with varied density in the drylands of Northern Ethiopia. Agrofor. Syst. 2021, 95, 1275–1293. [Google Scholar] [CrossRef]
- Morgan, J.A.; Follett, R.F.; Allen, L.H.; Del Grosso, S.; Derner, J.D.; Dijkstra, F.; Franzluebbers, A.; Fry, R.; Paustian, K.; Schoeneberger, M.M. Carbon sequestration in agricultural lands of the United States. J. Soil Water Conserv. 2010, 65, 6A–13A. [Google Scholar] [CrossRef] [Green Version]
- Schoeneberger, M.M. Agroforestry: Working trees for sequestering carbon on agricultural lands. Agrofor. Syst. 2009, 75, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Kirby, K.R.; Potvin, C. Variation in carbon storage among tree species: Implications for the management of a small-scale carbon sink project. For. Ecol. Manag. 2007, 246, 208–221. [Google Scholar] [CrossRef]
- Nair, P.R.; Nair, V.D.; Kumar, B.M.; Haile, S.G. Soil carbon sequestration in tropical agroforestry systems: A feasibility appraisal. Environ. Sci. Policy 2009, 12, 1099–1111. [Google Scholar] [CrossRef]
- Sharrow, S.; Ismail, S. Carbon and nitrogen storage in agroforests, tree plantations, and pastures in western Oregon, USA. Agrofor. Syst. 2004, 60, 123–130. [Google Scholar] [CrossRef]
- Haile, S.G.; Nair, P.K.R.; Nair, V.D. Carbon Storage of Different Soil-Size Fractions in Florida Silvopastoral Systems. J. Environ. Qual. 2008, 37, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Lal, R. Soil organic carbon sequestration in agroforestry systems. A review. Agron. Sustain. Dev. 2014, 34, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Zomer, R.; Neufeldt, H.; Xu, J.; Ahrends, A.; Bossio, D.; Trabucco, A.; Van Noordwijk, M.; Wang, M. Global Tree Cover and Biomass Carbon on Agricultural Land: The contribution of agroforestry to global and national carbon budgets. Sci. Rep. 2016, 6, 29987. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Nair, P.K.R.; Kumar, B.M.; Nair, V.D. Agroforestry as a strategy for carbon sequestration. J. Plant Nutr. Soil Sci. 2009, 172, 10–23. [Google Scholar] [CrossRef]
- Montagnini, F.; Nair, P.K.R. Carbon sequestration: An underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 2004, 61–62, 281–295. [Google Scholar] [CrossRef]
- Luedeling, E.; Neufeldt, H. Carbon sequestration potential of parkland agroforestry in the Sahel. Clim. Chang. 2012, 115, 443–461. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Dossa, E.L.; Fernandes, E.C.M.; Reid, W.S.; Ezui, K. Above- and belowground biomass, nutrient and carbon stocks contrasting an open-grown and a shaded coffee plantation. Agrofor. Syst. 2007, 72, 103–115. [Google Scholar] [CrossRef]
- Siyum, G.E.; Tassew, T. The Use of Homegarden Agroforestry Systems for Climate Change Mitigation in Lowlands of Southern Tigray, Northern Ethiopia. Asian Soil Res. J. 2019, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Van Noordwijk, M.; Rahayu, S.; Hairiah, K.; Wulan, Y.C.; Farida, A.; Verbist, B. Carbon Stock Assessment for a Forest to Coffee Conversion Landscape in Sumber-Jaya (Lampung, Indonesia): From Allometric Equations to Land Use Change Analysis; Science in China Series C-Life Sciences; Suppl. Science in China Press: Beijing, China, 2002; Volume 45, pp. 75–86. [Google Scholar]
- Dixon, R.K. Agroforestry systems: Sources of sinks of greenhouse gases? Agrofor. Syst. 1995, 31, 99–116. [Google Scholar] [CrossRef]
- Nair, P.K. and Nair, V.D. Carbon storage in North American agroforestry systems. In The Potential of U.S. Forest Soils to Sequester Carbon and Mitigate the Greenhouse Effect; Kimble, J., Heath, L.S., Birdsey, R.A., Lal, R., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 333–346. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Birhane, E.; Ahmed, S.; Hailemariam, M.; Negash, M.; Rannestad, M.M.; Norgrove, L. Carbon stock and woody species diversity in homegarden agroforestry along an elevation gradient in southern Ethiopia. Agrofor. Syst. 2020, 94, 1099–1110. [Google Scholar] [CrossRef]
- Negash, M.; Starr, M. Biomass and soil carbon stocks of indigenous agroforestry systems on the south-eastern Rift Valley escarpment, Ethiopia. Plant Soil 2015, 393, 95–107. [Google Scholar] [CrossRef]
- Seta, T.; Demissew, S. Diversity and standing carbon stocks of native agroforestry trees in Wenago district, Ethiopia. J. Emerg. Trends Eng. Appl. Sci. (JETEAS) 2014, 5, 125–132. [Google Scholar]
- Jose, S. Agroforestry for ecosystem services and environmental benefits: An overview. Agrofor. Syst. 2009, 76, 1–10. [Google Scholar] [CrossRef]
- Mutuo, P.K.; Cadisch, G.; Albrecht, A.; Palm, C.A.; Verchot, L. Potential of agroforestry for carbon sequestration and mitigation of greenhouse gas emissions from soils in the tropics. Nutr. Cycl. Agroecosystems 2005, 71, 43–54. [Google Scholar] [CrossRef]
- Sauer, T.J.; Cambardella, C.A.; Brandle, J.R. Soil carbon and tree litter dynamics in a red cedar–scotch pine shelterbelt. Agrofor. Syst. 2007, 71, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Pandey, D.N. Carbon sequestration in agroforestry systems. Clim. Policy 2002, 2, 367–377. [Google Scholar] [CrossRef]
- Schulp, C.J.; Nabuurs, G.-J.; Verburg, P.H.; de Waal, R.W. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. The Storage and Production of Organic Matter in Tropical Forests and Their Role in the Global Carbon Cycle. Biotropica 1982, 14, 161. [Google Scholar] [CrossRef]
- Franks, P.; Hou-Jones, X.; Fikreyesus, D.; Sintayehu, M.; Mamuye, S.; Danso, E.Y.; Meshack, C.K.; McNicol, I.; Van Soesbergen, A. Reconciling forest Conservation with Food Production in Sub-Saharan Africa: Case Studies from Ethiopia, Ghana and Tanzania; International Institute for Environment and Development: London, UK, 2017; p. 111. [Google Scholar]
- Kanshie, T.K. Five thousand years of sustainability?: A case study on Gedeo land use (Southern Ethiopia). Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2002; p. 295. [Google Scholar]
- Abebe, T. Determinants of crop diversity and composition in Enset-Coffee agroforestry homegardens of Southern Ethiopia. J. Agric. Rural. Dev. Trop. Subtrop. 2013, 114, 29–38. [Google Scholar]
- Asfaw, Z.; Ågren, G.I. Farmers’ local knowledge and topsoil properties of agroforestry practices in Sidama, Southern Ethiopia. Agrofor. Syst. 2007, 71, 35–48. [Google Scholar] [CrossRef]
- Negash, M. Trees Management and Livelihoods in Gedeo’s Agroforests, Ethiopia. For. Trees Livelihoods 2007, 17, 157–168. [Google Scholar] [CrossRef]
- Negash, M.; Abdulkadir, A.; Hagberg, S. Farmers’ planting practices of Eucalyptus in Enset-Coffee based agroforestry system of Sidama, Ethiopia. Ethiop. J. Nat. Resour. 2005, 7, 239–251. [Google Scholar]
- Negash, M. The indigenous agroforestry systems of the south-eastern Rift Valley escarpment, Ethiopia: Their biodiversity, carbon stocks, and litterfall. Viikki Tropical Resources Institute (VITRI). Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 2013; p. 62. [Google Scholar]
- National Meteorology Agency. Climatic Data of South Nations and Nationalities Peoples Regional State; National Meteorology Agency: Hawassa, Ethiopia, 2019. [Google Scholar]
- Mebrate, B.T. Agroforestry Practices in Gedeo Zone, Ethiopia: A Geographical Analysis. Ph.D. Dissertation, Panjab University, Chandigarh, India, 2007; p. 188. [Google Scholar]
- Abebe, T. Diversity in Homegarden Agroforestry Systems of Southern Ethiopia. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands , 2005; p. 143. [Google Scholar]
- Asfaw, Z. Tree Species Diversity, Topsoil Conditions and Arbuscular Mycorrhizal Association in the Sidama Traditional Agroforestry Land Use, Southern Ethiopia. Ph.D. Dissertation, Swedish University of Agricultural Sciences, Department of Forest Management and Products, SLU, Uppsala, Sweden, 2003; p. 263. [Google Scholar]
- Bekele-Tesemma, A. Profitable Agroforestry Innovations for Eastern Africa: Experience from 10 Agroclimatic Zones of Ethiopia, India, Kenya, Tanzania, and Uganda; Regional Land Management Unit: Nairobi, Kenya, 2008; p. 374. [Google Scholar]
- Brandt, S.; Spring, A.; Hiebsch, C.; McCabe, J.T.; Tabogie, E.; Diro, M.; Wolde-michael, G.; Yntiso, G.; Shigeta, M.; Tesfaye, S. The “Tree against Hunger”. In Enset-Based Agricultural Systems in Ethiopia; American Association for the Advancement of Science: New York, NY, USA, 1997; p. 55. [Google Scholar]
- Zewdie, S.; Fetene, M.; Olsson, M. Fine root vertical distribution and temporal dynamics in mature stands of two enset (Enset ventricosum Welw Cheesman) clones. Plant Soil 2008, 305, 227–236. [Google Scholar] [CrossRef]
- Bizuayehu, T. On Sidama folk identification, naming, and classification of cultivated enset (Ensete ventricosum) varieties. Genet. Resour. Crop Evol. 2008, 55, 1359–1370. [Google Scholar] [CrossRef]
- Abebe, T.; Bongers, F. Land-use dynamics in enset-based agroforestry homegardens in Ethiopia. In Forest-People Interfaces; Arts, B., van Bommel, S., Ros-Tonen, M., Verschoor, G., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 69–85. [Google Scholar]
- Labouisse, J.-P.; Bellachew, B.; Kotecha, S.; Bertrand, B. Current status of coffee (Coffea arabica L.) genetic resources in Ethiopia: Implications for conservation. Genet. Resour. Crop. Evol. 2008, 55, 1079–1093. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Nemomissa, S.; Granhall, U. Composition of coffee shade tree species and density of indigenous arbuscular mycorrhizal fungi (AMF) spores in Bonga natural coffee forest, southwestern Ethiopia. For. Ecol. Manag. 2007, 241, 145–154. [Google Scholar] [CrossRef]
- Asfaw, Z.; Mulata, Y.; Assefa, B.; Abebe, T.; Duna, S.; Mulugeta, G.; Mebrahten, H.; Kassa, H. Enhancing the Role of Forestry in Building Climate Resilient Green Economy in Ethiopia: Strategy for Scalling Up Effective Forest Management Practices in Southern Nations, Nationalities and Peoples Regional State with Particular an Emphasis on Agroforestry; Center for International Forestry Research (CIFOR): Bogor, Indonesia, 2015; p. 66. [Google Scholar]
- Kuyah, S.; Dietz, J.; Muthuri, C.; Jamnadass, R.; Mwangi, P.; Coe, R.; Neufeldt, H. Allometric equations for estimating biomass in agricultural landscapes: I. Aboveground biomass. Agric. Ecosyst. Environ. 2012, 158, 216–224. [Google Scholar] [CrossRef]
- Kuyah, S.; Dietz, J.; Muthuri, C.; Jamnadass, R.; Mwangi, P.; Coe, R.; Neufeldt, H. Allometric equations for estimating biomass in agricultural landscapes: II. Belowground biomass. Agric. Ecosyst. Environ. 2012, 158, 225–234. [Google Scholar] [CrossRef]
- Negash, M.; Starr, M.; Kanninen, M. Allometric equations for biomass estimation of Enset (Ensete ventricosum) grown in indigenous agroforestry systems in the Rift Valley escarpment of southern-eastern Ethiopia. Agrofor. Syst. 2012, 87, 571–581. [Google Scholar] [CrossRef]
- Negash, M.; Starr, M.; Kanninen, M.; Berhe, L. Allometric equations for estimating aboveground biomass of Coffea arabica L. grown in the Rift Valley escarpment of Ethiopia. Agrofor. Syst. 2013, 87, 953–966. [Google Scholar] [CrossRef]
- Roy, C. Options techniques et socio-economiques de reduction des emissions de CO2 et d’augmentation des stocks de carbone. Technical and socio-economical options for decreasing CO2 emissions and developing carbon fixation. Comptes Rendus-Acad. D Agric. Fr. 1999, 85, 311–320. [Google Scholar]
- Duguma, B.; Gockowski, J.; Bakala, J. Smallholder Cacao (Theobroma cacao Linn.) cultivation in agroforestry systems of West and Central Africa: Challenges and opportunities. Agrofor. Syst. 2001, 51, 177–188. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Global Forest Resource Assessment, Main Report no. 163.FAO (Food and Agriculture Organization of the United Nations); Food and Agriculture Organization (FAO): Rome, Italy, 2010. [Google Scholar]
- Silva, L.C.R.; Hoffmann, W.A.; Rossatto, D.R.; Haridasan, M.; Franco, A.C.; Horwath, W.R. Can savannas become forests? A coupled analysis of nutrient stocks and fire thresholds in central Brazil. Plant Soil 2013, 373, 829–842. [Google Scholar] [CrossRef]
- Charles, R.; Munishi, P.; Nzunda, E. Agroforestry as adaptation strategy under climate change in Mwanga District, Kilimanjaro, Tanzania. Int. J. Env. Prot. 2013, 3, 29–38. [Google Scholar]
- Tadesse, A.B.M.; Bajigo, M.T.A. Estimation of Carbon Stored in Agroforestry Practices in Gununo Watershed, Wolayitta Zone, Ethiopia. J. Ecosyst. Ecography 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Kumar, B.M. Carbon sequestration potential of tropical homegardens. In Tropical Homegardens; Kumar, B.M., Nair, P.K.R., Eds.; Advances in Agroforestry: New York, NY, USA, 2006; Volume 3, pp. 185–204. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Srivastava, P.; Kumar, A.; Behera, S.K.; Sharma, Y.K.; Singh, N. Soil carbon sequestration: An innovative strategy for reducing atmospheric carbon dioxide concentration. Biodivers. Conserv. 2012, 21, 1343–1358. [Google Scholar] [CrossRef]
- Soto-Pinto, L.; Anzueto, M.; Mendoza, J.; Ferrer, G.J.; De Jong, B. Carbon sequestration through agroforestry in indigenous communities of Chiapas, Mexico. Agrofor. Syst. 2009, 78, 39–51. [Google Scholar] [CrossRef]
- Lemenih, M.; Itanna, F. Soil carbon stocks and turnovers in various vegetation types and arable lands along an elevation gradient in southern Ethiopia. Geoderma 2004, 123, 177–188. [Google Scholar] [CrossRef]
- Swamy, S.; Puri, S. Biomass production and C-sequestration of Gmelina arborea in plantation and agroforestry system in India. Agrofor. Syst. 2005, 64, 181–195. [Google Scholar] [CrossRef]
- Brown, S.; Grais, A.; Ambagis, S. Baseline GHG Emissions from the Agricultural Sector and Mitigation Potential in Countries of East and West Africa; CCAFS Working Paper No. 13; CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS): Copenhagen, Denmark., 2012; Available online: www.ccafs.cgiar.org (accessed on 10 September 2020).
- Oueslati, I.; Allamano, P.; Bonifacio, E.; Claps, P. Vegetation and Topographic Control on Spatial Variability of Soil Organic Carbon. Pedosphere 2013, 23, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Zhu, H.; Guo, S. Soil organic carbon as a function of land use and topography on the Loess Plateau of China. Ecol. Eng. 2015, 83, 249–257. [Google Scholar] [CrossRef]
- Kang, B.T.; Caveness, F.E.; Tian, G.; Kolawole, G.O. Longterm alley cropping with four hedgerow species on an Alfisol in southwestern Nigeria–effect on crop performance, soil chemical properties and nematode population. Nutr. Cycl. Agroecosystems 1999, 54, 145–155. [Google Scholar] [CrossRef]
- Alegre, J. Soil and water conservation by contour hedging in the humid tropics of Peru. Agric. Ecosyst. Environ. 1996, 57, 17–25. [Google Scholar] [CrossRef]
- Kirsten, M.; Kaaya, A.; Klinger, T.; Feger, K.-H. Stocks of soil organic carbon in forest ecosystems of the Eastern Usambara Mountains, Tanzania. CATENA 2016, 137, 651–659. [Google Scholar] [CrossRef]
- Kinoshita, R.; Roupsard, O.; Chevallier, T.; Albrecht, A.; Taugourdeau, S.; Ahmed, Z.; van Es, H.M. Large topsoil organic carbon variability is controlled by Andisol properties and effectively assessed by VNIR spectroscopy in a coffee agroforestry system of Costa Rica. Geoderma 2016, 262, 254–265. [Google Scholar] [CrossRef]
- Noponen, M.R.; Edwards-Jones, G.; Haggar, J.P.; Soto, G.; Attarzadeh, N.; Healey, J.R. Greenhouse gas emissions in coffee grown with differing input levels under conventional and organic management. Agric. Ecosyst. Environ. 2012, 151, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.B.; Méndez, V.E. Interactions between Carbon Sequestration and Shade Tree Diversity in a Smallholder Coffee Cooperative in El Salvador. Conserv. Biol. 2013, 28, 489–497. [Google Scholar] [CrossRef]
- Thompson, I.D.; Ferreira, J.; Gardner, T.; Guariguata, M.; Koh, L.P.; Okabe, K.; Barlow, K.V.; Kurz, W.A.; Spalding, M.; van Vliet, N. Forest Biodiversity, Carbon and Other Ecosystem Services: Relationships and Impacts of Deforestation and Forest Degradation. Understanding Relationships between Biodiversity, Carbon, Forests and People: The Key to Achieving REDD+ Objectives; A Global Assessment Report IUFRO World Series; IUFRO: Vienna, Austria, 2012; Volume 31, pp. 21–51. [Google Scholar]
- Henry, M.; Tittonell, P.; Manlay, R.; Bernoux, M.; Albrecht, A.; Vanlauwe, B. Biodiversity, carbon stocks and sequestration potential in aboveground biomass in smallholder farming systems of western Kenya. Agric. Ecosyst. Environ. 2009, 129, 238–252. [Google Scholar] [CrossRef]
- Mandal, R.A.; Jha, P.K.; Dutta, I.C.; Thapa, U.; Karmacharya, S.B. Carbon Sequestration in Tropical and Subtropical Plant Species in Collaborative and Community Forests of Nepal. Adv. Ecol. 2016, 2016, 1529703. [Google Scholar] [CrossRef] [Green Version]
- Takimoto, A.; Nair, V.D.; Nair, P.K.R. Contribution of trees to soil carbon sequestration under agroforestry systems in the West African Sahel. Agrofor. Syst. 2008, 76, 11–25. [Google Scholar] [CrossRef]
- Thangata, P.; Hildebrand, P. Carbon stock and sequestration potential of agroforestry systems in smallholder agroecosystems of sub-Saharan Africa: Mechanisms for ‘reducing emissions from deforestation and forest degradation’ (REDD+). Agric. Ecosyst. Environ. 2012, 158, 172–183. [Google Scholar] [CrossRef]
- Kaur, B.; Gupta, S.; Singh, G. Soil carbon, microbial activity and nitrogen availability in agroforestry systems on moderately alkaline soils in northern India. Appl. Soil Ecol. 2000, 15, 283–294. [Google Scholar] [CrossRef]
- Lehmann, J.; Peter, I.; Steglich, C.; Gebauer, G.; Huwe, B.; Zech, W. Below-ground interactions in dryland agroforestry. For. Ecol. Manag. 1998, 111, 157–169. [Google Scholar] [CrossRef]
- Sarvade, S.; Singh, R.; Prasad, H.; Prasad, D. Agroforestry practices for improving soil nutrient status. Pop. Kheti 2014, 2, 60–64. [Google Scholar]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Gill, A.S.; Burman, D. Production management of field crops in agroforestry systems. In Recent Advances in Agronomy; Singh, G., Kolar, J.S.W., Sekhon, H.S., Eds.; Indian Society of Agronomy: New Delhi, India, 2002; pp. 523–542. [Google Scholar]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Komicha, N.D.; Nigatu, L.; Mohamed, M. Physico-chemical properties of soil under the canopies of Faidherbia albida (Delile) A. Chev and Acacia tortilis (Forssk.) Hayen in park land agroforestry system in Central Rift Valley, Ethiopia. J. Hortic. For. 2018, 10, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.K.; Nair, P.K.R.; Nair, V.D.; Kumar, B.M. Carbon storage in relation to soil size-fractions under tropical tree-based land-use systems. Plant Soil 2009, 328, 433–446. [Google Scholar] [CrossRef]
- Alfaia, S.S.; Ribeiro, G.A.; Nobre, A.D.; Luizão, R.C.; Luizão, F.J. Evaluation of soil fertility in smallholder agroforestry systems and pastures in western Amazonia. Agric. Ecosyst. Environ. 2004, 102, 409–414. [Google Scholar] [CrossRef]



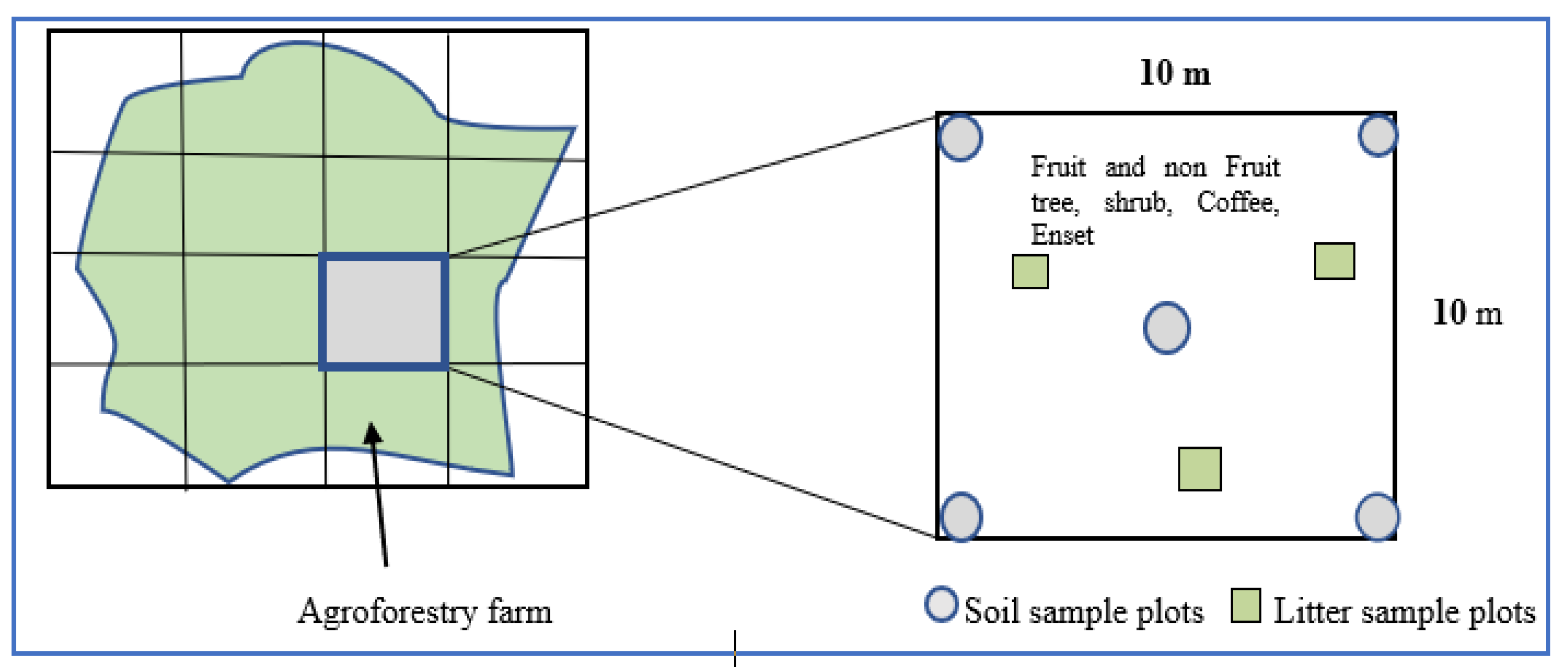


| Characteristics | Research Sites | ||
|---|---|---|---|
| Sisota | Golla | Chichu | |
| Location | Dilla Zuria district, SNNPRS | Dilla Zuria district, SNNPRs | Dilla Zuria district, SNNPRS |
| Altitude | 1760–1830 m asl | 1665–1732 m asl | 1544–1587 m asl |
| Topography | Steep slope land feature, azimuth: southwest facing | Slightly steep to medium, azimuth: southwest facing | Gentle slope, azimuth: southwest facing |
| Plant species coverage | Enset-dominated | Coffee and enset-dominated | Fruit tree-, coffee-, and enset- dominated |
| AF handling activity | Tree trimming, lopping, tightening, ripping of unwanted plants. Enset leaves and foliage of Millettia sp. used for manuring and floor covering | Trimming, lopping, pollarding, tightening, ripping of unwanted plants. Enset leaves, herbaceous plants and foliage of Millettia spp. used for manuring and floor covering | Tree trimming, pollarding. Farm house waste, ash and coffee peelings used as compost |
| Major food and cash crops, vegetables | Enset, taro, yam, kale | Coffee, enset, banana, taro, yam, sweet potatoes | Fruit, coffee, enset maize, haricot bean, sweet potatoes |
| Average distance from the next town (market) | 10 km | 8 km | 5 km |
| Biomass | C–Ft–E AF | Enset AF | C–E AF | F | p |
|---|---|---|---|---|---|
| Aboveground biomass a | 255.9 ± 294.0 | 81.1 ± 69.0 | 126.7 ± 145.1 | 4.4 | 0.017 |
| Belowground biomass b | 72.2 ± 69.9 | 26.9 ± 21.1 | 39.4 ± 39.4 | 4.8 | 0.012 |
| Agroforestry total biomass | 328.1 ± 364 | 108.0 ± 90.0 | 166.1 ± 184.4 | 4.5 | 0.016 |
| Agroforestry System | Woody | Enset | Coffee | Agroforestry Total Biomass | ||
|---|---|---|---|---|---|---|
| Fruit | Nonfruit | Total | ||||
| C–Ft–E based AF | 154.1 ± 158.8 a | 141.6 ± 283 a | 295.7 ± 372.0 a | 6.8 ± 3.7 a | 25.6 ± 41.5 a | 328.1 ± 364 a |
| Enset based AF | 29.7 ± 61.2 b | 45.3 ± 67.2 a | 75.0 ± 94.0 b | 29.0 ± 15.6 ab | - | 108.0 ± 90 b |
| C–E based AF | 31.7 ± 132.4 b | 97.7 ± 128.9 a | 129.4 ± 186.4 b | 19.1 ± 10.2 ac | 17.6 ± 6.5 a | 166.1 ± 184 b |
| C Stock | C–Ft–E AF | Enset AF | C–E AF | F | p |
|---|---|---|---|---|---|
| Aboveground C a | 83.8 ± 63.0 | 39.1 ± 32.0 | 37.8 ± 17.3 | 3.9 | 0.033 |
| Belowground C b | 24.4 ± 16.2 | 12.6 ± 9.7 | 12.7 ± 5.8 | 3.6 | 0.042 |
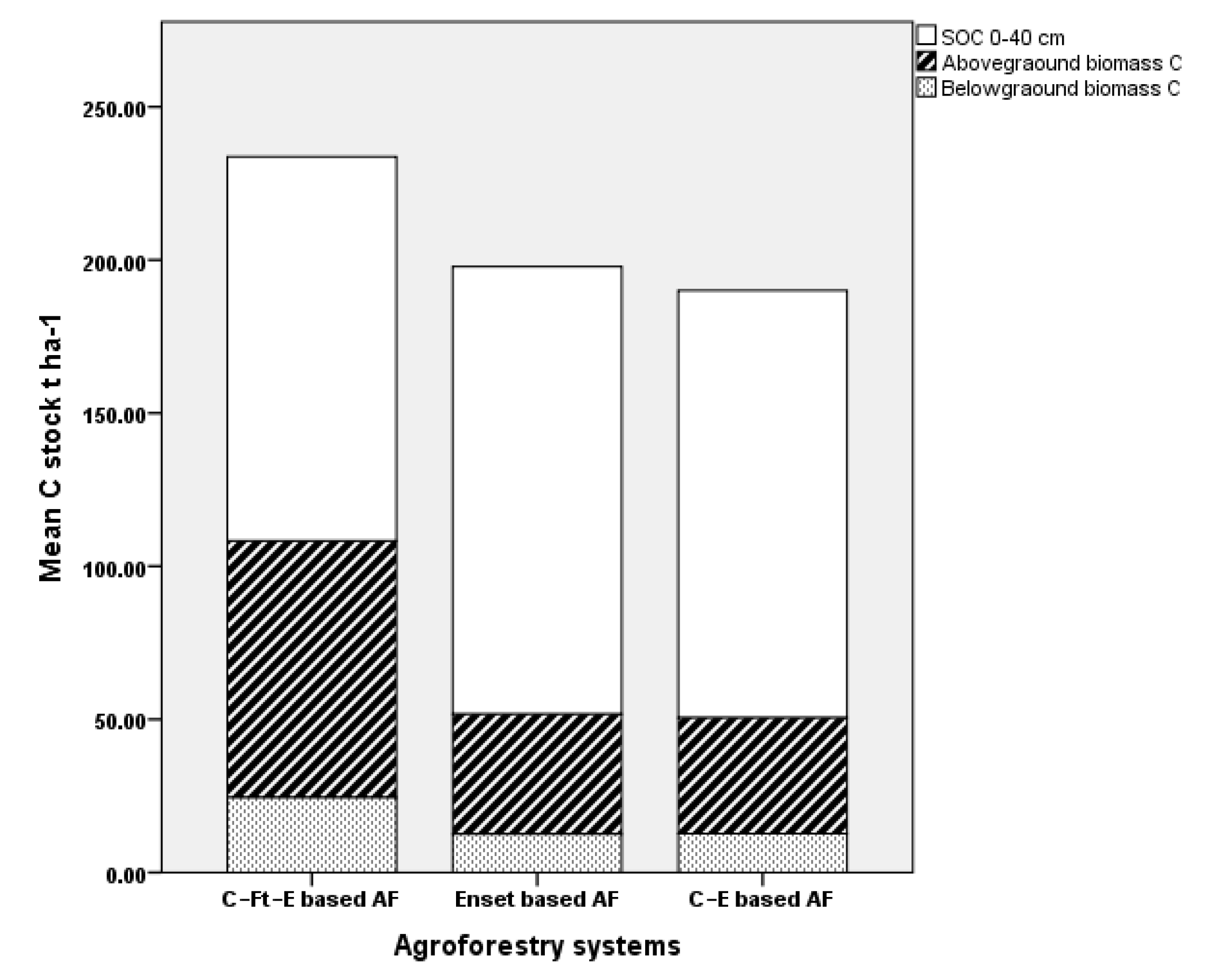
| Total biomass | 108.2 ± 79.2 a | 51.7 ± 41.7 b | 50.5 ± 23.1 b | 3.8 | 0.034 |
| SOC 0–20 | 75.7 ± 14.2 | 81.7 ± 14.4 | 76.9 ±18.3 | 0.4 | 0.665 |
| SOC 20–40 | 49.8 ± 7.5 | 64.4 ± 16.3 | 62.7 ± 21.2 | 8.9 | 0.103 |
| SOC 0–40 | 125.5 ± 17.3 a | 146.1 ± 26.5 a | 139.6 ± 25.4 a | 14.7 | 0.152 |
| Agroforestry total | 233.3 ± 81.0 a | 197.8 ± 58.7 a | 190.1 ± 29.8 a | 0.1 | 0.243 |
| Land Use Type | n | Mean ± SD | t | df | Sig. (2-Tailed) | |
|---|---|---|---|---|---|---|
| Pair 1 | SOC Coffee–Ft–Enset AF | 10 | 125.5 ± 17.3 | 5.0 | 9 | 0.001 ** |
| SOC monocrop plot | 10 | 90.5 ± 15.3 | ||||
| Pair 2 | SOC Enset based AF | 10 | 146.1 ± 26.5 | 0.4 | 9 | 0.688 NS |
| SOC monocrop plot | 10 | 141.8 ± 28.1 | ||||
| Pair 3 | SOC Coffee–Enset AF | 10 | 139.6 ± 25.4 | 5.4 | 9 | 0.000 ** |
| SOC monocrop plot | 10 | 95.3 ± 14.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfay, H.M.; Negash, M.; Godbold, D.L.; Hager, H. Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift-Valley Landscapes, Ethiopia. Sustainability 2022, 14, 4716. https://doi.org/10.3390/su14084716
Tesfay HM, Negash M, Godbold DL, Hager H. Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift-Valley Landscapes, Ethiopia. Sustainability. 2022; 14(8):4716. https://doi.org/10.3390/su14084716
Chicago/Turabian StyleTesfay, Hafte Mebrahten, Mesele Negash, Douglas L. Godbold, and Herbert Hager. 2022. "Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift-Valley Landscapes, Ethiopia" Sustainability 14, no. 8: 4716. https://doi.org/10.3390/su14084716
APA StyleTesfay, H. M., Negash, M., Godbold, D. L., & Hager, H. (2022). Assessing Carbon Pools of Three Indigenous Agroforestry Systems in the Southeastern Rift-Valley Landscapes, Ethiopia. Sustainability, 14(8), 4716. https://doi.org/10.3390/su14084716







