Modeling the Carbon Sequestration Potential of Multifunctional Agroforestry-Based Phytoremediation (MAP) Systems in Chinandega, Nicaragua
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Sites
2.1.1. The Picacho Airfield
2.1.2. El Ensayo
2.2. Exploration of Appropriate Phytoremediation Species for Chinandega
The Selection of the Species
- experimental data on soil phytoremediation capacity/pollution tolerance to heavy metals and other elementary pollutants,
- experimental data on soil phytoremediation capacity/pollution tolerance to POPs and other organic pollutants,
- data supporting that the species can be used in agroforestry systems, and
- data supporting that the species can be used for profit (e.g., food, firewood, timber, medical purposes, or animal feed).
2.3. The Four Scenarios
2.3.1. Scenario 1: Shading System with Teak (Tectona grandis) and Patchouli (Pogostemon cablin)
2.3.2. Scenario 2: Alley Cropping System Erythrina poeppigiana and Ricinus communis
2.3.3. Scenario 3: Silvopasture System of Cordia alliodora and Brachiaria ruziziensis
2.3.4. Scenario 4: Alley Cropping System of Gliricidia sepium and Amaranth (Amaranthus sp.)
2.4. Modeling of Carbon Sequestration Using CO2FIX
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Land Use. Our World Data. 2019. Available online: https://ourworldindata.org/land-use (accessed on 6 December 2021).
- Dubois, O. The State of the World's Land and Water Resources for Food and Agriculture: Managing Systems at Risk; Earthscan: London, UK, 2011. [Google Scholar]
- Willett, W.; Rockstrom, J.; Loken, B.; Springmann, M.; Lang, T.S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Baude, M.; Meyer, B.C.; Schindewolf, M. Land use change in an agricultural landscape causing degradation of soil based ecosystem services. Sci. Total Environ. 2019, 659, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V. An assessment of the global impact of 21st century land use change on soil erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef] [Green Version]
- FAO. Proceedings of the Global Symposium on Soil Pollution 2018; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Wiggering, H.; Dalchow, C.; Glemnitz, M.; Helming, K.; Müller, K.; Schultz, A.; Stachow, U.; Zander, P. Indicators for multifunctional land use—Linking socio-economic requirements with landscape potentials. Ecol. Indic. 2006, 6, 238–249. [Google Scholar] [CrossRef]
- Simelton, E.; Ostwald, M.; Osiru, M. Multifunctional land-use systems—A solution for food security in Africa? In Multifunctional Land Uses in Africa; Routledge: Oxfordshire, UK, 2019; pp. 1–21. [Google Scholar]
- Haller, H.; Jonsson, A. Growing food in polluted soils: A review of risks and opportunities associated with combined phytoremediation and food production (CPFP). Chemosphere 2020, 254, 126826. [Google Scholar] [CrossRef]
- Mander, Ü.; Helming, K.; Wiggering, H. Multifunctional land use: Meeting future demands for landscape goods and services. In Multifunctional Land Use; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–13. [Google Scholar]
- Kumar, A.; Cabral-Pinto, M.; Kumar, A.; Kumar, M.; Dinis, P.A. Estimation of Risk to the Eco-Environment and Human Health of Using Heavy Metals in the Uttarakhand Himalaya, India. Appl. Sci. 2020, 10, 7078. [Google Scholar] [CrossRef]
- Haller, H.; Jonsson, A.; Lacayo Romero, M.; Jarquín Pascua, M. Bioaccumulation and translocation of field-weathered toxaphene and other persistent organic pollutants in three cultivars of amaranth (A. cruentus ‘R127 México’, A. cruentus ‘Don León’ y A. caudatus ‘CAC 48 Perú’)—A field study from former cotton fields in Chinandega, Nicaragua. Ecol. Eng. 2018, 121, 65–71. [Google Scholar]
- Carvalho, F.; Montenegro-Guillén, S.; Villeneuve, J.; Cattini, C.; Tolosa, I.; Bartocci, J.; Lacayo-Romero, M.; Cruz-Granja, A. Toxaphene residues from cotton fields in soils and in the coastal environment of Nicaragua. Chemosphere 2003, 53, 627–636. [Google Scholar] [CrossRef]
- Castilho, J.; Fenzl, N.; Guillen, S.M.; Nascimento, F. Organochlorine and organophosphorus pesticide residues in the Atoya river basin, Chinandega, Nicaragua. Environ. Pollut. 2000, 110, 523–533. [Google Scholar] [CrossRef]
- Moncrieff, J.E.; Bentley, L.R.; Palma, H.C. Investigating pesticide transport in the León-Chinandega aquifer, Nicaragua. Hydrogeol. J. 2008, 16, 183–197. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.R. Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Springer Science & Business Media: Berlin, Germany, 2011; Volume 8. [Google Scholar]
- Muschler, R.G.; Bonnemann, A. Potentials and limitations of agroforestry for changing land-use in the tropics: Experiences from Central America. For. Ecol. Manag. 1997, 91, 61–73. [Google Scholar] [CrossRef]
- Atangana, A.; Khasa, D.; Chang, S.; Degrande, A. Phytoremediation in tropical agroforestry. In Tropical Agroforestry; Springer: Dordrecht, The Netherlands, 2014; pp. 343–351. [Google Scholar]
- Dollinger, J.; Jose, S. Agroforestry for soil health. Agrofor. Syst. 2018, 92, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Feliciano, D.; Ledo, A.; Hillier, J.; Nayak, D.R. Which agroforestry options give the greatest soil and above ground carbon benefits in different world regions? Agric. Ecosyst. Environ. 2018, 254, 117–129. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Blanchart, E.; Skiba, U.; Hénault, C.; Harmand, J.-M. Changes in carbon stock and greenhouse gas balance in a coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agriculture. Ecosyst. Environ. 2012, 148, 102–110. [Google Scholar] [CrossRef]
- Liu, X.; Trogisch, S.; He, J.-S.; Niklaus, P.A.; Bruelheide, H.; Tang, Z.; Erfmeier, A.; Scherer-Lorenzen, M.; Pietsch, K.A.; Yang, B.; et al. Tree species richness increases ecosystem carbon storage in subtropical forests. Proc. R. Soc. B 2018, 285, 20181240. [Google Scholar] [CrossRef] [Green Version]
- Nath, T.K.; Jashimuddin, M.; Hasan, K.; Shahjahan, M.; Pretty, J. The sustainable intensification of agroforestry in shifting cultivation areas of Bangladesh. Agrofor. Syst. 2016, 90, 405–416. [Google Scholar] [CrossRef]
- Budowski, G. The scope and potential of agroforestry in Central America. Agrofor. Syst. 1993, 23, 121–131. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Azzarello, E.; Pandolfi, C.; Pollastri, S.; Masi, E.; Mugnai, S.; Mancuso, S. The use of trees in phytoremediation. CAB Reviews: Perspectives in Agriculture. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–15. [Google Scholar]
- Rockwood, D.; Naidu, C.V.; Carter, D.R.; Rahmani, M.; Spriggs, T.A.; Lin, C.; Alker, G.R.; Isebrands, J.G.; Segrest, S.A. Short-rotation woody crops and phytoremediation: Opportunities for agroforestry. Agrofor. Syst. 2004, 61, 51–63. [Google Scholar]
- Gómez, L.; Contreras, A.; Bolonio, D.; Quintana, J.; Oñate-Sánchez, L.; Merino, I. Phytoremediation with trees. Adv. Bot. Res. 2019, 89, 281–321. [Google Scholar]
- Haller, H.; Jonsson, A.; Fröling, M. Application of ecological engineering within the framework for strategic sustainable development for design of appropriate soil bioremediation technologies in marginalized regions. J. Clean. Prod. 2018, 172, 2415–2424. [Google Scholar] [CrossRef]
- Huang, Y.; Xi, Y.; Gan, L.; Johnson, D.; Wu, Y.; Ren, D.; Liu, H. Effects of lead and cadmium on photosynthesis in Amaranthus spinosus and assessment of phytoremediation potential. Int. J. Phytoremediat. 2019, 21, 1041–1049. [Google Scholar] [CrossRef]
- Govindaraju, M.; Fowmitha Banu, J.; Goel, M. CO2 Sequestration Through Phytoremediation Techniques with Special Emphasis on Urban Forestry to Mitigate Climate Change Impact. In Climate Change and Green Chemistry of CO2 Sequestration; Springer: Singapore, 2021; pp. 263–271. [Google Scholar]
- Eze, C.N.; Odoh, C.K.; Eze, E.A.; Orjiakor, P.I.; Enemuor, S.C.; Okobo, U.J. Chromium (III) and its effects on soil microbial activities and phytoremediation potentials of Arachis hypogea and Vigna unguiculata. Afr. J. Biotechnol. 2018, 17, 1207–1214. [Google Scholar]
- Yasmeen, T.; Li, A.; Iqbal, S.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Ali, S. Biotechnological tools in the remediation of cadmium toxicity. In Cadmium Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 497–520. [Google Scholar]
- Jaiswal, N.; Sachdev, S.; Tallapragada, S.; Singh, R.P. Phytoextraction Potential of Neem (Azadirachtaindica) for Cddetoxification from the Contaminated Soil. Clim. Chang. Environ. Sustain. 2018, 6, 154–159. [Google Scholar] [CrossRef]
- Pino, N.J.; Múnera, L.M.; Peñuela, G.A. Phytoremediation of soil contaminated with PCBs using different plants and their associated microbial communities. Int. J. Phytoremediat. 2019, 21, 316–324. [Google Scholar] [CrossRef]
- Martins, W.B.R.; Schwartz, G.; Ribeiro, S.S.; Ferreira, G.C.; de Souza Barbosa, R.; de Paula, M.T.; Barbosa, V.M.; de Assis Oliveira, F. Ecosystem restoration after bauxite mining: Favorable indicators for Technosols construction and soil management using liming and subsoiling. New For. 2021, 52, 971–994. [Google Scholar] [CrossRef]
- Giliba, R.A.; Boon, E.K.; Kayombo, C.J.; Chirenje, L.I.; Musamba, E.B.; Kashindye, A.M.; Mushi, J.R. Assessment of heavy metals in some edible and fodder plants from Mazimbu Village, Morogoro, Tanzania. J. Life Sci. 2011, 3, 93–96. [Google Scholar] [CrossRef]
- Zemunik, G.; Winter, K.; Turner, B.L. Toxic effects of soil manganese on tropical trees. Plant Soil 2020, 453, 343–354. [Google Scholar] [CrossRef]
- Gautam, M.; Pandey, D.; Agrawal, M. Phytoremediation of metals using lemongrass (Cymbopogon citratus (DC) Stapf.) grown under different levels of red mud in soil amended with biowastes. Int. J. Phytoremediat. 2017, 19, 555–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, L.A.; Piotto, F.A.; Nogueirol, R.C.; Azevedo, R.A. Use of non-hyperaccumulator plant species for the phytoextraction of heavy metals using chelating agents. Sci. Agric. 2013, 70, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Marrugo-Madrid, S.; Turull, M.; Montes, G.E.; Pico, M.V.; Marrugo-Negrete, J.L.; Díez, S. Phytoremediation of mercury in soils impacted by gold mining: A case-study of Colombia. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–160. [Google Scholar]
- Amin, H.; Ahmed Arain, B.; Abbasi, M.S.; Amin, F.; Jahangir, T.M.; Soomro, N.-U.-A. Evaluation of chromium phyto-toxicity, phyto-tolerance, and phyto-accumulation using biofuel plants for effective phytoremediation. Int. J. Phytoremediat. 2019, 21, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.J.; Nair, A.; Sajeshkumar, N.; Jose, P. Analysis of the phylloremediation capability of Mangifera indica in hydrocarbon polluted area: An outlook study. J. Med. Plants 2022, 10, 125–135. [Google Scholar]
- Andriya, N.N.; Hamim, H.; Sulistijorini, S.; Triadiati, T. The phytoremediation potential of non-edible oil-producing plants for gold mine tailings. Biodiversitas J. Biol. Divers. 2019, 20, 2949–2957. [Google Scholar] [CrossRef]
- Osmana, N.A.; Roslana, A.M.; Ibrahima, M.F.; Hassana, M.A. Potential use of Pennisetum purpureum for phytoremediation and bioenergy production: A mini review. Asia-Pac. J. Mol. Biol. Biotechnol. 2020, 28, 14–26. [Google Scholar] [CrossRef]
- Chima, U.; Fredrick, C.; Alex, A.; Ikwumadi, E. Heavy metal contents of five major medicinal tree species in two communities reflecting high and low pollution gradients in Greater Port Harcourt city, Rivers state, Nigeria. Nat. Sci. 2021, 19, 16–26. [Google Scholar]
- Elshamy, M.M.; Heikal, Y.M.; Bonanomi, G. Phytoremediation efficiency of Portulaca oleracea L. naturally growing in some industrial sites, Dakahlia District, Egypt. Chemosphere 2019, 225, 678–687. [Google Scholar] [CrossRef]
- Ratna, S.; Rastogi, S.; Kumar, R. Phytoremediation: A synergistic interaction between plants and microbes for removal of unwanted chemicals/contaminants. In Microbes and Signaling Biomolecules Against Plant Stress; Springer: Singapore, 2021; pp. 199–222. [Google Scholar]
- Zhou, J.; Chen, L.; Peng, L.; Luo, S.; Zeng, Q. Phytoremediation of heavy metals under an oil crop rotation and treatment of biochar from contaminated biomass for safe use. Chemosphere 2020, 247, 125856. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Liu, S.; Zhao, Y.; Long, Y.; Pan, Y. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Use of sorghum crops for in situ phytoremediation of polluted soils. J. Agric. Sci. Technol. A 2011, 1, 693–702. [Google Scholar]
- Chan-Quijano, J.G.; Cach-Pérez, M.J.; Rodríguez-Robles, U. Phytoremediation of soils contaminated by hydrocarbon. In Phytoremediation; Springer: Cham, Switzerland, 2020; pp. 83–101. [Google Scholar]
- Mary Agbogidi, O.; Dickens Dolor, E.; Mercy Okechukwu, E. Evaluation of Tectona grandis (Linn.) and Gmelina arborea (Roxb.) for phytoremediation in crude oil contaminated soils. Agric. Conspec. Sci. 2007, 72, 149–152. [Google Scholar]
- Ruley, J.A.; Tumuhairwe, J.B.; Amoding, A.; Opolot, E.; Oryem-Origa, H.; Basamba, T. Assessment of plants for phytoremediation of hydrocarbon-contaminated soils in the Sudd Wetland of South Sudan. Plant Soil Environ. 2019, 65, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Baoune, H.; Aparicio, J.D.; Acuña, A.; El Hadj-khelil, A.O.; Sanchez, L.; Polti, M.A.; Alvarez, A. Effectiveness of the Zea mays-Streptomyces association for the phytoremediation of petroleum hydrocarbons impacted soils. Ecotoxicol. Environ. Saf. 2019, 184, 109591. [Google Scholar] [CrossRef]
- Nagarajaiah, C.; Kittur, B.H.; Mukthamath, U.; Venkatesh, L. Evaluation of medicinal and aromatic crops under teak based agroforestry system. Environ. Ecol. 2012, 30, 221–225. [Google Scholar]
- Sharanabasappa, P.A.; Madiwalar, S.; Channabasa, K.; Kumar, P. Performance of medicinal and aromatic plants as intercrops with teak. Karnataka J. Agric. Sci. 2007, 20, 179–180. [Google Scholar]
- Pérez, D.; Kanninen, M. Provisional equations for estimating total and merchantable volume of Tectona grandis trees in Costa Rica. For. Trees Livelihoods 2003, 13, 345–359. [Google Scholar]
- Kumar, D.; Bijalwan, A.; Kalra, A.; Dobriyal, M.J. Effect of shade and organic manure on growth and yield of patchouli [Pogostemon cablin (blanco) benth.] under teak (Tectona grandis lf) based agroforestry system. Indian 2016, 142, 1121–1129. [Google Scholar]
- Ribeiro, A.S.; Ribeiro, M.S.; Bertolucci, S.K.; Bittencourt, W.J.; Carvalho, A.A.; Tostes, W.N.; Alves, E.; Pinto, J.E. Colored shade nets induced changes in growth, anatomy and essential oil of Pogostemon cablin. An. Acad. Bras. Ciências 2018, 90, 1823–1835. [Google Scholar] [CrossRef]
- Masera, O.R.; Garza-Caligaris, J.; Kanninen, M.; Karjalainen, T.; Liski, J.; Nabuurs, G.; Pussinen, A.; de Jong, B.H.; Mohren, G. Modeling carbon sequestration in afforestation, agroforestry and forest management projects: The CO2FIX V. 2 approach. Ecol. Model. 2003, 164, 177–199. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Fernandes, J.R.; Gerenutti, M.; Gomes, H.M.; Ribeiro, R.; De Almeida, M.V. Evaluation of Ricinus communis L. for the phytoremediation of polluted soil with organochlorine pesticides. BioMed Res. Int. 2015, 2015, 549863. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulou, E.; Papatheohari, Y.; Zanetti, F.; Tsiotas, K.; Papamichael, I.; Christou, M.; Namatov, I.; Monti, A. Comparative studies on several castor (Ricinus communis L.) hybrids: Growth, yields, seed oil and biomass characterization. Ind. Crops Prod. 2015, 75, 8–13. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. A review on phytoremediation of crude oil spills. Water Air Soil Pollut. 2015, 226, 279. [Google Scholar] [CrossRef]
- Cook, B.G.; Pengelly, B.C.; Brown, S.; Donnelly, J.R.; Eagles, D.; Franco, A.; Hanson, J.; Mullen, B.; Partridge, I.; Peters, M.; et al. Brachiaria ruziziensis. In Tropical Forages: An Interactive Selection Tool; CSIRO Sustainable Ecosystems, Queensland Department of Primary Industries and Fisheries, Centro Internacional de Agricultura Tropical (CIAT), International Livestock Research Institute (ILRI): Brisbane, Australia, 2005. [Google Scholar]
- Eggleston, S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies: Hayama, Japan, 2006; Volume 5. [Google Scholar]
- Elevitch, C. Species profiles for Pacific Island agroforestry. In Permanent Agriculture Resources Series; Western Region Sustainable Agriculture Research and Education: Holualoa, HI, USA, 2006. [Google Scholar]
- Gunathilake, H.; Joseph, P.; Wickremasinghe, H.; Peiris, T. Sustainable Biomass Production in Sri Lanka and Possibilities for Agroforestry Intervention. In Proceedings of the International Conference on the Issues for Sustainable Use of Biomass Resources for Energy, Colombo, Sri Lanka, 15–17 August 2005; pp. 15–18. [Google Scholar]
- Smiley, G.; Kroschel, J. Temporal change in carbon stocks of cocoa–gliricidia agroforests in Central Sulawesi, Indonesia. Agrofor. Syst. 2008, 73, 219–231. [Google Scholar] [CrossRef]
- Law-Ogbomo, K.E.; Ajayi, S.O. Growth and yield performance of Amaranthus cruentus influenced by planting density and poultry manure application. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 195–199. [Google Scholar]
- Chunilall, V.; Kindness, A.; Jonnalagadda, S. Heavy metal uptake by two edible Amaranthus herbs grown on soils contaminated with lead, mercury, cadmium, and nickel. J. Environ. Sci. Health 2005, 40, 375–384. [Google Scholar] [CrossRef]
- Rahman, M.M.; Azirun, S.M.; Boyce, A.N. Enhanced accumulation of copper and lead in amaranth (Amaranthus paniculatus), Indian mustard (Brassica juncea) and sunflower (Helianthus annuus). PLoS ONE 2013, 8, e62941. [Google Scholar] [CrossRef] [Green Version]
- Shevyakova, N.; Cheremisina, A.; Kuznetsov, V.V. Phytoremediation potential of Amaranthus hybrids: Antagonism between nickel and iron and chelating role of polyamines. Russ. J. Plant Physiol. 2011, 58, 634–642. [Google Scholar] [CrossRef]
- Yuan, M.; He, H.; Xiao, L.; Zhong, T.; Liu, H.; Li, S.; Deng, P.; Ye, Z.; Jing, Y. Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sJN27. Chemosphere 2014, 103, 99–104. [Google Scholar] [CrossRef]
- Kim, D.-G.; Kirschbaum, M.U.; Beedy, T.L. Carbon sequestration and net emissions of CH4 and N2O under agroforestry: Synthesizing available data and suggestions for future studies. Agric. Ecosyst. Environ. 2016, 226, 65–78. [Google Scholar] [CrossRef]
- FAO. Global Soil Organic Carbon Map GLOSIS-GSOCmap (v1.5.0) Food and Agriculture Organization of the United Nations 2020. Available online: http://54.229.242.119/GSOCmap/ (accessed on 21 October 2021).
- Schelhaas, M.; Van Esch, P.; Groen, T.; De Jong, B.; Kanninen, M.; Liski, J.; Masera, O.; Mohren, G.; Nabuurs, G.; Palosuo, T. CO2FIX V 3.1 A Modelling Framework for Quantifying Carbon Sequestration in Forest Ecosystems; Alterra: Wageningen, The Netherlands, 2004; Alterra-Rapport 1068. [Google Scholar]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Gemmill-Herren, B.; D’Annolfo, R.; Graeub, B.E.; Cunningham, S.A.; Breeze, T.D. Farming Approaches for Greater Biodiversity, Livelihoods, and Food Security. Trends Ecol. Evol. 2017, 32, 68–80. [Google Scholar] [CrossRef]
- Van der Werf, H.M.G.; Knudsen, M.T.; Cederberg, C. Towards better representation of organic agriculture in life cycle assessment. Nat. Sustain. 2020, 3, 419–425. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C. Greenhouse gas mitigation in agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 789–813. [Google Scholar] [CrossRef] [Green Version]
- Ramón, F.; Lull, C. Legal measures to prevent and manage soil contamination and to increase food safety for consumer health: The case of Spain. Environ. Pollut. 2019, 250, 883–891. [Google Scholar] [CrossRef]
- Razikordmahaleh, L. Policy Research on Soil contamination to Achieve Food Safety. In Proceedings of the Global Symposium on Soil Pollution 2018; Food and Agriculture Organization (FAO): Rome, Italy, 2018. [Google Scholar]

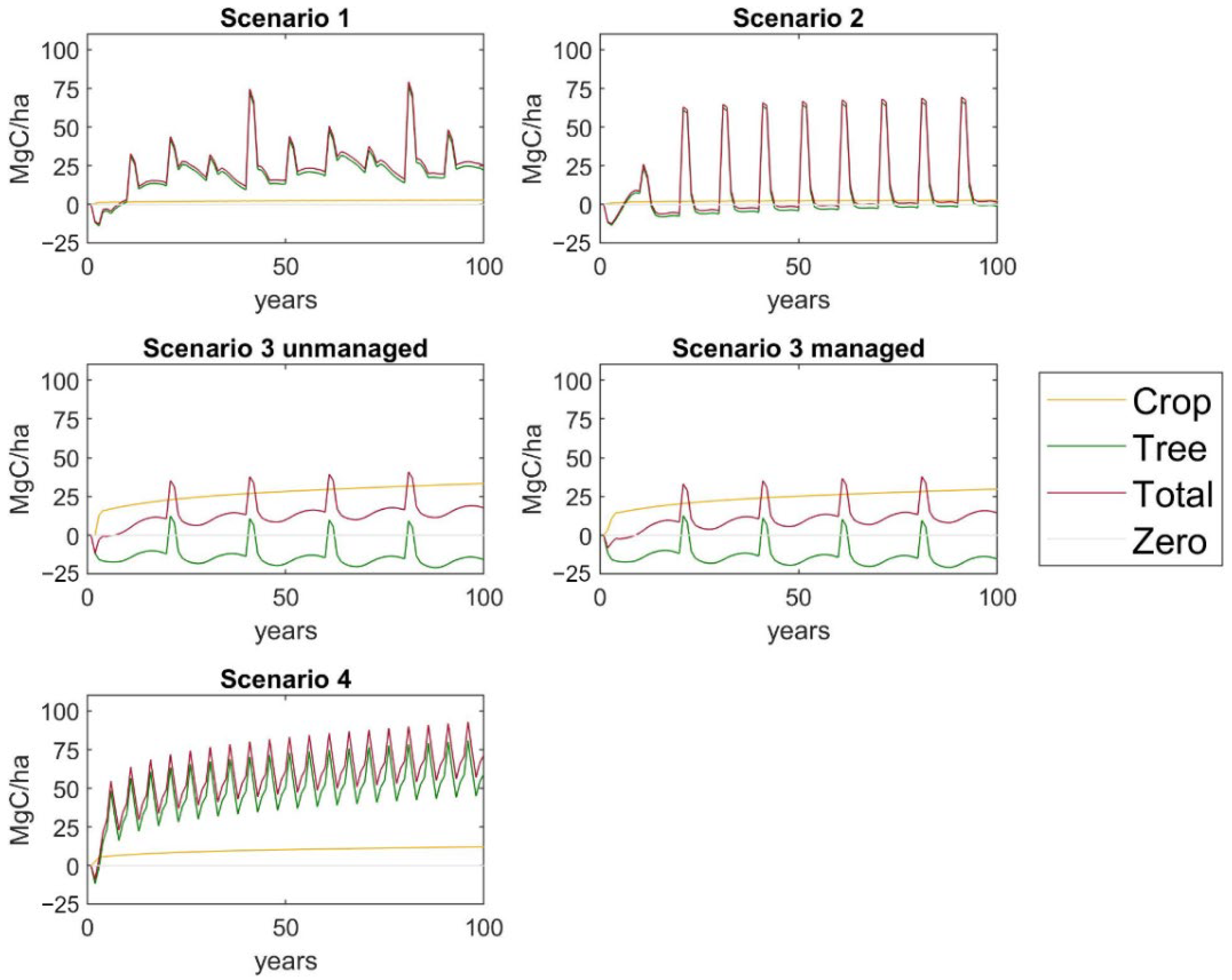

| Scenario | Density of Trees per Hectare before Thinning | Thinning Interval of Trees (Year) | Rotation Period of Trees/Crops (Year) | Harvests per Year of Crop | Tree Products Included |
|---|---|---|---|---|---|
| 1: T. grandis and P. cablin | 1111 | 3, 10, 20, 30 | 40/3 | 2 | Yes |
| 2: E. poeppigiana and R. communis | 837 | Annual pruning of leaves and branches | 10/5 | 1 | No |
| 3: C. alliodora and B. ruziziensis | 100 | None | 20/1 1 | Several 1 | Yes |
| 4: G. sepium and Amaranthus sp. | 4200 (pollarded) | Biannual pruning | 5/1 | 2 | No |
| Scenario | Soil | Total Including Products | Total Excluding Products | ||
|---|---|---|---|---|---|
| Mg C ha−1 yr−1 | Mg C ha−1 yr−1 | Mg CO2eq ha−1 yr−1 | Mg C ha−1 yr−1 | Mg CO2eq ha−1 yr−1 | |
| Scenario 1 | 0.5 | 2.2 | 8.0 | 1.2 | 4.4 |
| Scenario 2 | 0.7 | no products | no products | 0.7 | 2.7 |
| Scenario 3 unmanaged | 0.4 | 0.7 | 2.7 | 0.5 | 1.7 |
| Scenario 3 managed | 0.4 | 0.7 | 2.5 | 0.4 | 1.5 |
| Scenario 4 | 0.9 | no products | no products | 1.0 | 3.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kåresdotter, E.; Bergqvist, L.; Flores-Carmenate, G.; Haller, H.; Jonsson, A. Modeling the Carbon Sequestration Potential of Multifunctional Agroforestry-Based Phytoremediation (MAP) Systems in Chinandega, Nicaragua. Sustainability 2022, 14, 4932. https://doi.org/10.3390/su14094932
Kåresdotter E, Bergqvist L, Flores-Carmenate G, Haller H, Jonsson A. Modeling the Carbon Sequestration Potential of Multifunctional Agroforestry-Based Phytoremediation (MAP) Systems in Chinandega, Nicaragua. Sustainability. 2022; 14(9):4932. https://doi.org/10.3390/su14094932
Chicago/Turabian StyleKåresdotter, Elisie, Lisa Bergqvist, Ginnette Flores-Carmenate, Henrik Haller, and Anders Jonsson. 2022. "Modeling the Carbon Sequestration Potential of Multifunctional Agroforestry-Based Phytoremediation (MAP) Systems in Chinandega, Nicaragua" Sustainability 14, no. 9: 4932. https://doi.org/10.3390/su14094932
APA StyleKåresdotter, E., Bergqvist, L., Flores-Carmenate, G., Haller, H., & Jonsson, A. (2022). Modeling the Carbon Sequestration Potential of Multifunctional Agroforestry-Based Phytoremediation (MAP) Systems in Chinandega, Nicaragua. Sustainability, 14(9), 4932. https://doi.org/10.3390/su14094932








