Abstract
Global food production faces many challenges, including climate change, a water crisis, land degradation, and desertification. These challenges require research into non-traditional sources of human foods. Edible mushrooms are considered an important next-generation healthy food source. Edible mushrooms are rich in proteins, dietary fiber, vitamins, minerals, and other bioactive components (alkaloids, lactones, polysaccharides, polyphenolic compounds, sesquiterpenes, sterols, and terpenoids). Several bioactive ingredients can be extracted from edible mushrooms and incorporated into health-promoting supplements. It has been suggested that several human diseases can be treated with extracts from edible mushrooms, as these extracts have biological effects including anticancer, antidiabetic, antiviral, antioxidant, hepatoprotective, immune-potentiating, and hypo-cholesterolemic influences. The current study focuses on sustainable approaches for handling edible mushrooms and their secondary metabolites, including biofortification. Comparisons between edible and poisonous mushrooms, as well as the common species of edible mushrooms and their different bioactive ingredients, are crucial. Nutritional values and the health benefits of edible mushrooms, as well as different biomedical applications, have been also emphasized. Further research is needed to explore the economic sustainability of different medicinal mushroom bioactive compound extracts and their potential applications against emerging diseases such as COVID-19. New approaches such as nano-biofortification are also needed to supply edible mushrooms with essential nutrients and/or to increase their bioactive ingredients.
1. Introduction
More than 14,000 mushroom species have been identified, about 10% of which are macro-fungi. Edible mushrooms are macro-fungi that can be seen with the naked eye, i.e., fleshy fruit bodies of many species of mushrooms, and they have already been widely used in food and medicine due to their delicious taste and diverse physiological activities [1]. The fruiting bodies of edible mushrooms, which include about 700 species, can be safely consumed and are considered beneficial to human health [2]. Edible mushrooms are seen as an important source of food because of their high content of polysaccharides, dietary fiber, proteo-glucans, and vitamins such as riboflavin and thiamine [3,4,5]. Edible mushrooms also have many bioactive ingredients that are beneficial for human health such as antioxidants [6], terpenoids [7], lectins [8], phenolics/polyphenolics [9], polysaccharides [10], and ergosterols [11,12]. Not much is known regarding the capability of mushrooms to treat and/or prevent chronic diseases. However, several chronic diseases have been treated by consuming edible mushrooms (e.g., Coriolus versicolor, Ganoderma lucidum, Grifola frondose, Phellinus linteus, Pleurotus eryngii, and Poria cocos) such as cancer [13], diabetes mellitus [14], obesity [15,16], hyperlipidemia [10], cardiovascular and neurodegenerative diseases [17], and hypercholesterolemia [2].
Whether edible mushrooms are considered a sustainable healthy human food source mainly depends on the mushroom species and their chemical composition as well as the growing media. The safety and health of a mushroom is the main factor that controls its edibility. Many studies have confirmed that mushrooms grown in polluted soils threaten human health [13,18,19,20,21,22,23]. These studies focused on pollutants (e.g., heavy metals, organic toxicants, radioactive pollutants, or radionuclides) in mushrooms grown in polluted soils as reported in China [20,23], Iran [18,21], Poland [22,24,25], Spain [26], and Turkey [27,28]. However, further investigations on the consumption of edible mushrooms cultivated in polluted soils are needed before the association between pollutants and edible mushrooms can be more clearly understood [19].
Therefore, this review focuses on edible mushrooms and the differences between them and poisonous mushrooms. Common species of edible mushrooms will be discussed, including their bioactive ingredients content, nutritional values, and health benefits. The biomedical applications of edible mushrooms will also be reported.
2. Methodology of the Review
This review is an attempt to focus on the edible mushrooms and the difference between them and poisonous mushrooms. The methodology that was followed in this study depended on the collected materials from the common scientific websites or engines such as the databases of ScienceDirect, SpringerLink, PubMed, and MDPI. This is work based on combinations of key terms to identify useful documents, including edible mushrooms, poisonous mushrooms, nutritional value, and medicinal attributes. The collected information included original articles, review articles, and chapters in the English language, and more than 200 articles finally were selected. The collected information was analyzed and listed in tables and drawn in figures. Tables summarize much of the information by surveying the nutritional and medicinal attributes in collected articles on edible mushrooms, whereas figures include photos of edible and poisonous mushrooms along with comparisons between them. The studies were collected from the main places for mushroom production such as China, Iran, Poland, India, Brazil, Ireland, Taiwan, and Malaysia.
3. Sustainable Production of Edible Mushrooms
More than 100 countries cultivate edible mushrooms commercially using different systems and on different scales. The global production of mushrooms increases annually at growth rate of about 7%. The current global consumption of mushrooms is around 12.74 million tonnes, and it is predicted that the global production of mushrooms will scale to 20.84 million tonnes by 2026 [29]. Mushrooms can be classified into some categories including (1) cultivated mushrooms (which can be grown commercially by farmers using various strategies to produce for sale at supermarkets; these include enoki and oyster mushrooms), (2) wild mushrooms (which can grow on the root systems of trees in forests and are harvested by mushroom hunters—lots of wild mushrooms are poisonous), (3) medicinal mushrooms (several mushrooms have medicinal benefits but might not always be pleasant to consume), and (4) poisonous mushrooms, which have toxic substances or toxins [30]. Based on the growing method of mushrooms, they can be classified into saprotrophic mushrooms (sapro = rot; troph = eating), which grow on dead matter; mycorrhizal mushrooms (mykes = fungus; rhiza = root), which have a symbiotic relationship (sym = together; biosis = way of living) with crops or trees; and parasitic mushrooms, which infect and depend on its host plant and then kill it [30].
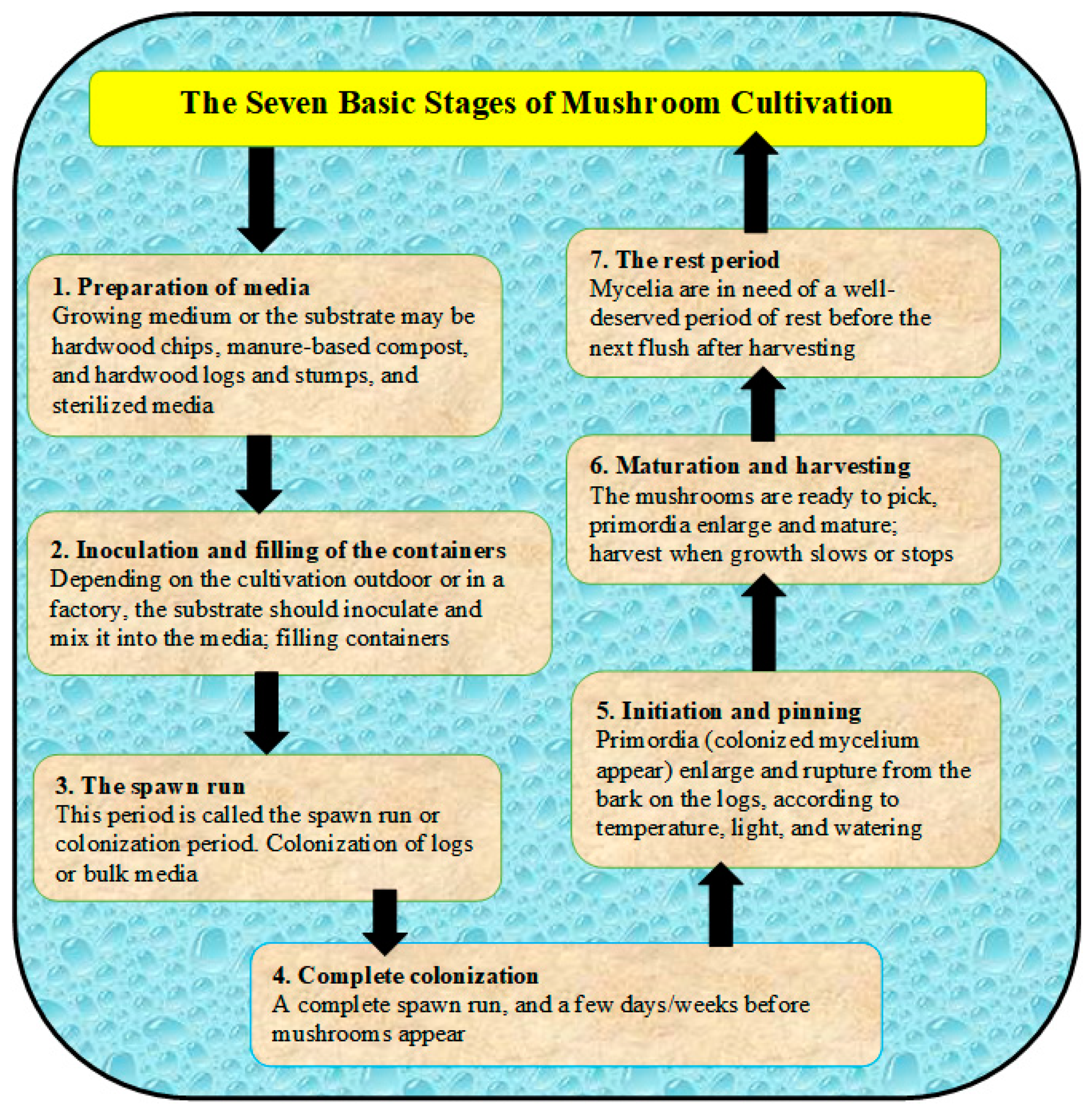
Most cultivated mushrooms have a basic life cycle, which includes the following stages (1) sporulation (i.e., production spores not seeds), (2) spore germination and the mating of cells, (3) colonization to complete fruiting initiated, (4) the formation of primordia, and (5) after fruiting or mature of mushrooms, spores release again and the cycle repeats (Figure 1) [31]. The cultivation steps can be summarized in terms of seven stages, as presented in Figure 2 and described in [31]. The sustainable production of mushrooms is important because mushrooms are considered edible foods and are high in protein content. There are many poisonous mushrooms, which must be clear to consumers in order to avoid causing serious ecological and health problems [32]. The huge amount of wastes that result from mushroom cultivation needs to be sustainably recycled for in order to protect the environment and to produce the bioenergy as well. Therefore, edible mushrooms can be promoted as an important agri-business activity to address many environmental issues, especially the ecological degradation [29,33]. The cultivation of mushrooms can be used to manage farm wastes by recycling them, as well as to dispose of spent mushroom substrate “SMS” (i.e., wastes remaining after harvesting mushrooms). On the other hand, the cultivation of mushrooms should protect from many competitor microorganisms and promote hygiene, which are controlled through the environmental conditions as presented in Table 1 [34].

Figure 1.
The industry of mushroom cultivation is considered an important industry, which needs certain steps to produce the edible mushrooms as presented in these photos. The first group of photos (1) includes mushroom fungi (Pleurotus ostreatus) culture, which should first be ready on the surface of agar plate beside the cultivation substrates, as well as tools for propagation of mushroom and poured media (heat treated at 95 °C, for 1 day). The inoculant and the millet spawn in a jar should be also prepared. The second and third group of photos (2,3) include the industrial preparing and filling of spawn in the factory, whereas the group of photos in (4) represent oyster mushroom production. These steps have been photographed from the factory of “Magyar Gomba Kertész Kft”, and all photos were taken by Gréta Törős, Debrecen University, Hungary.
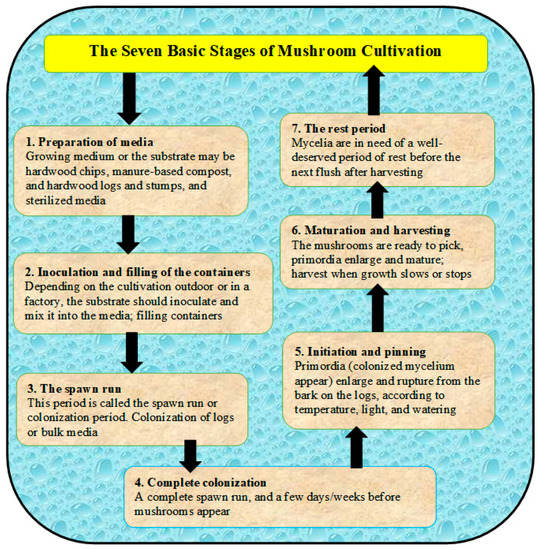
Figure 2.
Different stages of mushroom cultivation starting from preparation of media till harvesting.

Table 1.
The environmental conditions controlling the most commonly cultivated species as ideal values, but certain strains may exceed them.
4. Edible vs. Poisonous Mushrooms
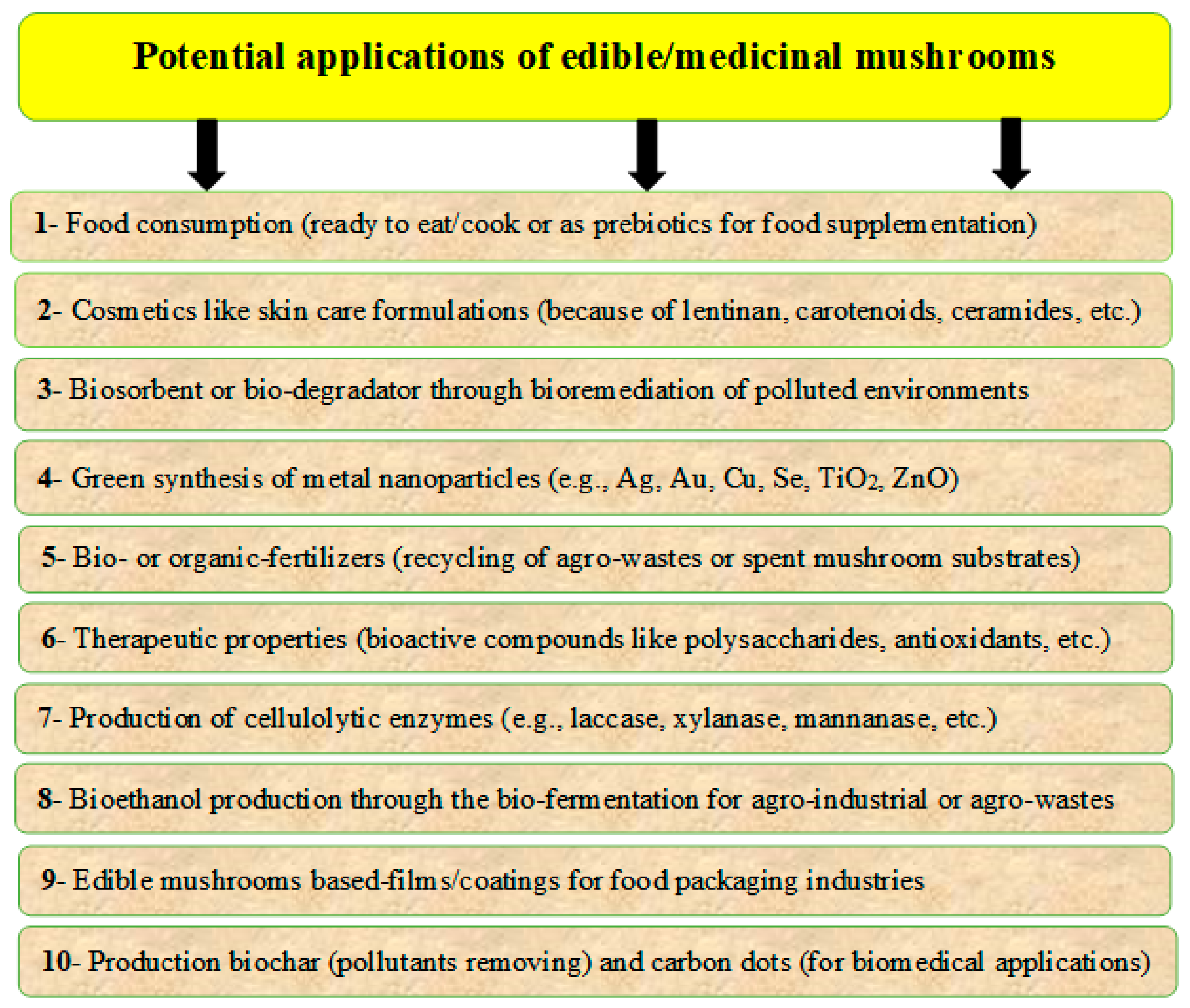
There are many categories of mushrooms, such as edible, non-edible, and poisonous mushrooms; wild and cultivated mushrooms; or medicinal and industrial mushrooms. Mushroom by-products, which may be generated during production and processing, represent a disposal problem. However, these by-products may be promising sources for several applications because of their nutritional and functional properties [35,36]. There may be many sustainable applications for these by-products, including the production of bioactive compounds for pharmaceutical and nutraceutical formulations, as well as several other applications such as animal feeds, fertilizers, energy production, bioremediation, bio-based materials, cosmetics, and cosmeceuticals. Many applications of edible mushrooms are presented in Figure 3, according to Kumar et al. [37], Zhang et al. [38], and Lopez-Hortas et al. [39].
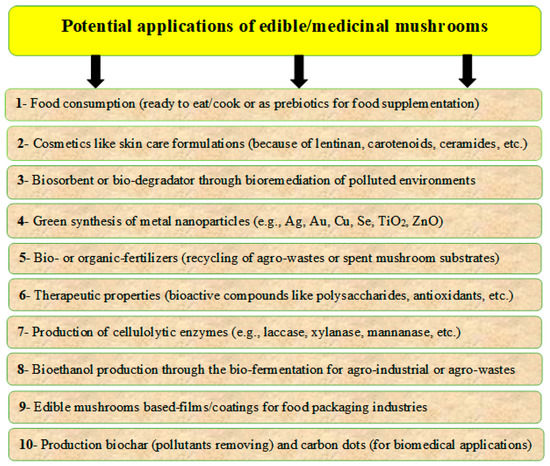
Figure 3.
Potential applications of different mushrooms and their by-products, which include using bioactive compounds in developing the pharmaceutical and nutraceutical formulations, in addition to applications such as animal feeds, bioremediation, fertilizers, energy production, bio-based materials, cosmetics, and cosmeceuticals.
Some poisonous species of mushrooms have toxins that are not destroyed under the high temperatures reached during cooking [40]. Several studies have been conducted on the identification of mushrooms, including their taxonomy, and naming indices can be found on many websites such as Index Fungorum (http://www.indexfungorum.org/, accessed on 15 January 2022), Myco-Bank Database (http://www.mycobank.org/, accessed on 15 January 2022), the British Mycological Society (https://www.britmycolsoc.org.uk/library/english-names, accessed on 15 January 2022), International Mycological Association, (https://www.ima-mycology.org/, accessed on 15 January 2022), Fungal databases (https://nt.ars-grin.gov/fungaldatabases/, accessed on 15 January 2022), NCBI or National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/taxonomy, accessed on 15 January 2022), and the Global Biodiversity Information Facility (https://www.gbif.org/species, accessed on 16 December 2021).
FAO has stated that reports on the identification of edible or poisonous mushroom species are based on named sources, and there is no test or characteristic to distinguish edible from poisonous mushrooms [41]. The edibility of mushrooms was simplified into the following categories based on the available information: (1) confirmed edible mushrooms (E1), (2) confirmed edible mushrooms with conditions (E2), (3) unconfirmed edible mushrooms (E3), and poisonous mushrooms (P) [41]. It is worth noting that some edible mushrooms are very similar in their appearance to the poisonous types and may grow in the same habitat; an example is Entoloma sarcopum (an edible mushroom) and Entoloma rhodopolium (a poisonous mushroom) [42] (Table 2; Figure 4). The edibility of mushrooms must be accurately identified based on different advanced methods such as sensory identification methods (e.g., morphological and odor methods), instrumental analysis methods (e.g., mass spectrometry, chromatographic, and spectral technology), and molecular biology methods (e.g., isothermal amplification technology, molecular marker technology, endogenous reference gene method, and sequencing technology). A review of this topic was provided by Wei et al. [43]. Edible and non-edible or poisonous mushrooms have recently been discussed in reviews such as Wu et al. [40], Li et al. [44], Ramírez-Terrazo et al. [45], Zhang et al. [46], and Wei et al. [43].

Table 2.
A list of some selected non-edible or poisonous mushroom species including the common English name and their family.

Figure 4.
List of some poisonous mushrooms including the scientific names for each one. Sources: by user Strobilomyces, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=1091222; by Onderwijsgek, CC BY-SA 3.0 nl, https://commons.wikimedia.org/w/index.php?curid=1428517; by Gerhard Koller (Gerhard), CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=18730680; by Alan Rockefeller, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=95423433; by Alan Rockefeller, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=61680344; by Renier van Rensburg, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=71032684; by Strobilomyces, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=438081; by MichalPL, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=63737437, accessed on 16 January 2022.
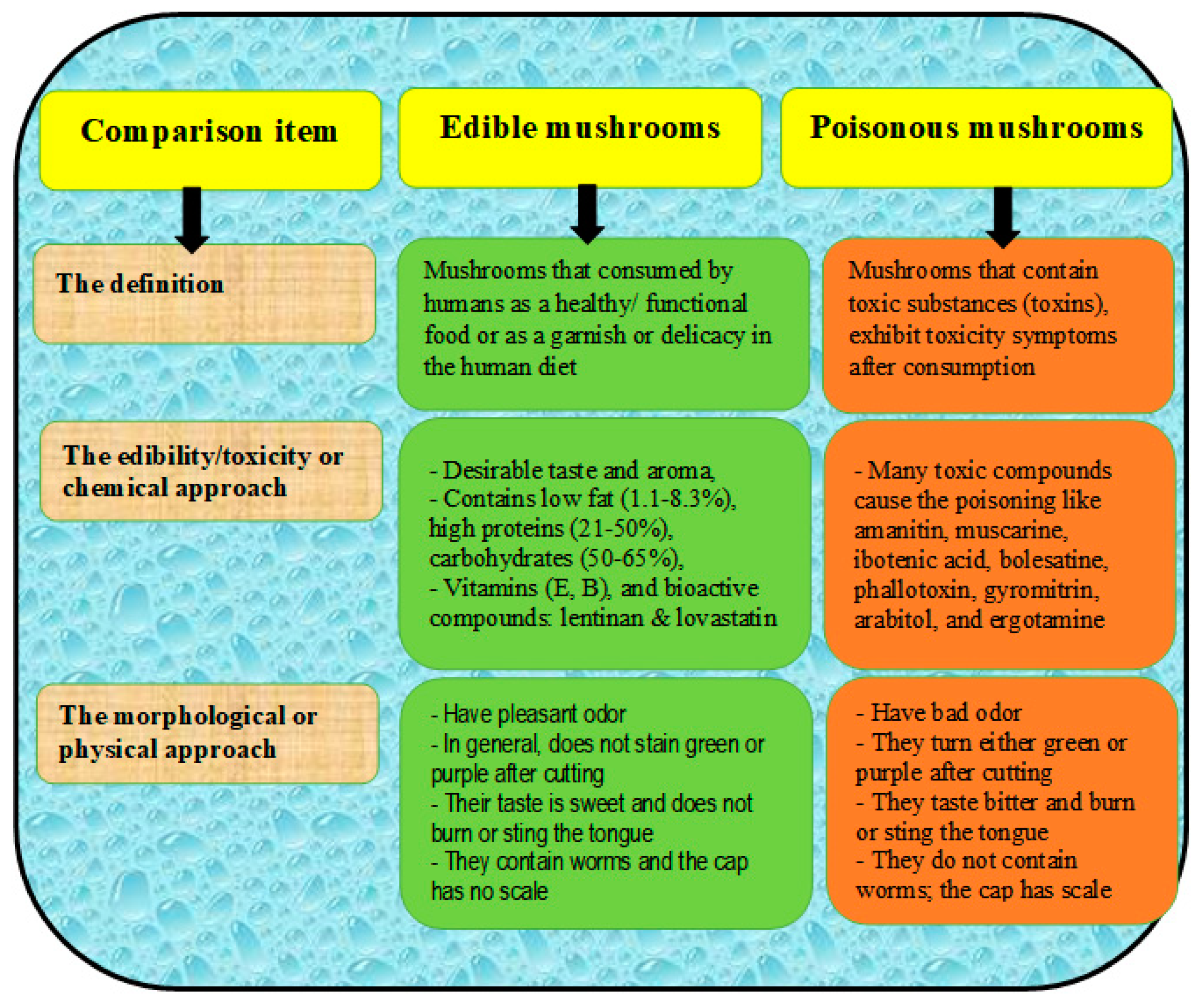
Several taxa of poisonous mushrooms have been reported particularly among the most common wild mushrooms, such as Amanita, Chlorophyllum, Cantharocybe, Inocybe, Entoloma, Leccinellum, Russula, and Xerocomus [49,50,51]. Poisonous mushrooms have toxic compounds that usually cause problems due to unintentional ingestion, with these problems including endocrine toxicity, neurotoxicity, cytotoxicity, myotoxicity, and gastrointestinal disturbances (Table 3) [52]. Many poisonous species produce neurotoxic secondary metabolites such as muscarine or psilocybin [51], alpha-amanitin and amanitin [53], coprine [50], gyromitrin [52], ibotenic acid [54], orellanine [55], phallotoxin [56], and russuphelins [57]. Differences between edible and poisonous mushrooms are of important concern for human health, which mainly depends on the following criteria: the edible mushrooms should have a pleasant odor and sweet taste and not be stained green or purple under cutting; the should not burn or sting the tongue when a piece is tasted; and there should be no scales on the cap [58]. A comparison between edible and poisonous mushrooms can be found in Figure 5 [58].

Table 3.
A list of main toxins, clinical features, and their relationships to poisonous mushrooms based on the classification of poisonous mushrooms.
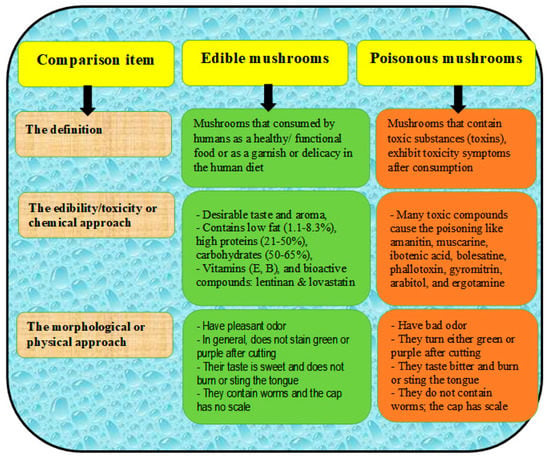
Figure 5.
A comparison between edible and poisonous mushrooms, including morphological and chemical approaches.
5. Common Species of Edible Mushrooms
Edible mushrooms are the fleshy fruit bodies of many species of macro-fungi that can be used in the human diet (Table 4). The edibility criteria of mushrooms may depend on the absence of poisonous substances or toxins that are detrimental to human health. Edible mushrooms are mainly consumed for their nutritional value, medicinal features, and sweet taste. Several species of edible mushrooms are found the wild and raised for harvest, but some species are difficult to cultivate. Several articles or reviews have reported about edible mushrooms from different points of view:

Table 4.
List of selected edible mushroom species as reported in the literature.
- (1)
- Studies on edible mushrooms harvested from polluted areas with a focus on Africa [13], China [20,23,61,62], Greece [63], Iran [18,21], Poland [22,24,25,64,65,66], Turkey [27,28,67,68,69,70,71,72], and Spain [26] or on Europe as a whole, e.g., Świsłowski et al. [73], or on the global level, e.g., Dowlati et al. [18].
- (2)
- A study focusing on locals’ perspective concerning the changing of the abundance of wild edible mushrooms, which has decreased due to direct exploitation by 31% and land use change by 38% of all taxa [74].
- (3)
- Studies on the bioactive properties of different edible mushrooms, including total phenolic content, phenolic acid, antioxidant capacities, and antimicrobial activity in Pleurotus sajor-caju wild edible mushroom [75], in Agaricus bisporus, Cantharellus cibarius, Boletus edulis, Lactarius deliciosus, Lentinus edodes, Ganoderma lucidum, Hericium erinaceus, Morchella spp., and Pleurotus ostreatus [76], and in Pleurotus ostreatus [77]; polysaccharide in many edible mushrooms [78]; and bioactive components in several species of edible mushrooms [79].
- (4)
- Studies on determining the amount of potentially bioavailable compounds such as phenolic compounds in mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes [80].
- (5)
- Studies on integrated methods (morphological and molecular approaches) for the identification of bioactive compounds [81], or using only molecular strategies for identification [82].
- (6)
- Studies on the mineral and nutritional composition of wild edible mushrooms [82,83,84,85] and the nutritional value and biological properties of some edible mushrooms [86].
- (7)
- Studies on using mushroom-derived bioactive compounds against SARS-CoV-2 infection [87,88].
6. Bioactive Ingredients of Edible Mushrooms
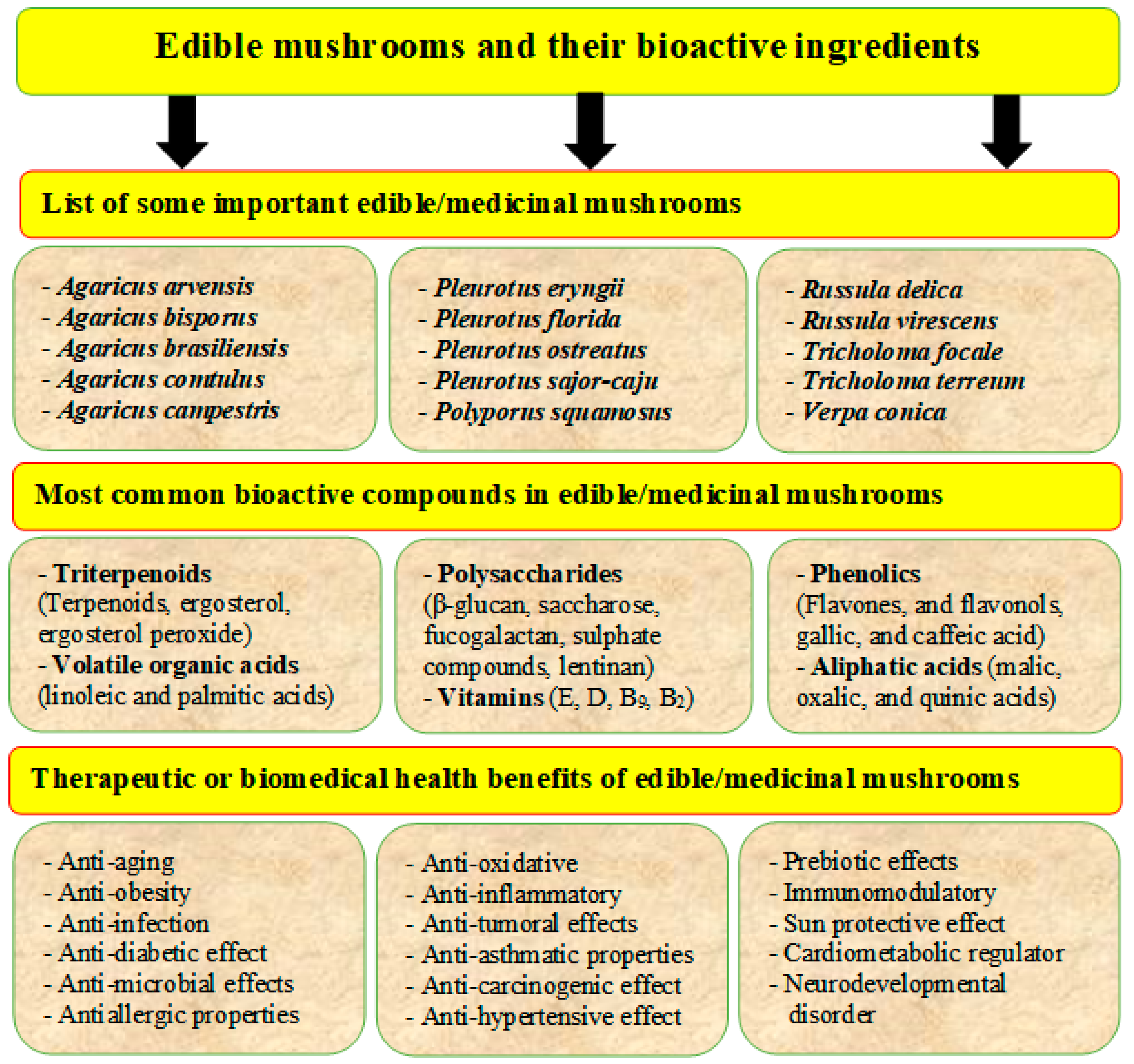
Edible mushrooms contain various bioactive ingredients such as proteins, polysaccharides, polyunsaturated fatty acids (PUFA), dietary fibers, amino acids, vitamins, and minerals (Table 5). They have essential health effects, such as antioxidant, antimicrobial, immune-stimulatory, and anticancer, cholesterol-lowering properties (Figure 6) [12,37,38,39]. Several significant components and secondary metabolites dominate their biological activity. Lectins are carbohydrate-binding proteins that can be found in many types of edible mushrooms such as Phaseolus vulgaris, Agaricus campestris, Agaricus bisporus, Grifola frondosa, Boletus satanus, Flammulina velutipes, Tricholoma mongolicum, Ganoderma lucidum, and Volvariella volvacea. Lectins have been shown to increase insulin secretion, activate the immune system, and have anticancer effects [8]. Lectins can also play essential roles in physiological processes such as dormancy, growth, morphogenesis, morphological changes, and molecular recognition in the early stages of mycorrhization. Lectins isolated from the edible mushroom Clitocybe nebularis exhibit immunostimulatory effects on the most potent antigen-presenting cells, dendritic cells [94].

Table 5.
A list of some phenolic compounds, polysaccharides, proteins, and triterpenoids found in selected edible mushrooms species.
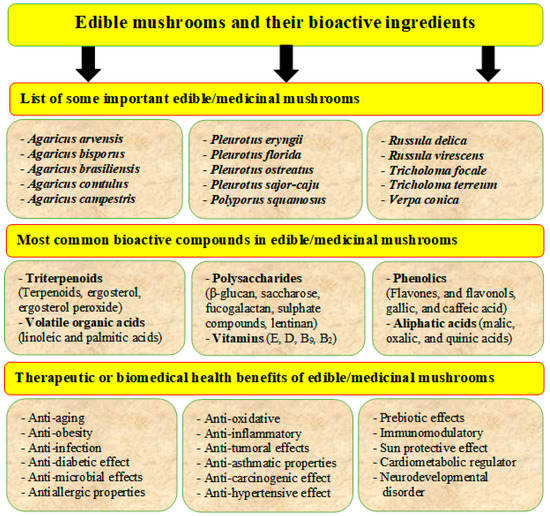
Figure 6.
Edible mushrooms are an important source of human food and have several bioactive ingredients (phenols, lectins, terpenoids, flavonoids, etc.) and bio-medicinal activities such as anticancer, antidiabetic, antiaging, antiviral, antioxidant, and anti-inflammatory.
Glucans are one of the unique ingredients in mushrooms that have immune-stimulatory, anticancer, anti-inflammatory, and antioxidant effects. They can be found in different types of edible mushrooms such as Jelly ears (Auricularia auricular), Reishi (Ganoderma lucidum), Shiitake (Lentinula edodes), and Oyster (Pleurotus ostreatus) [95]. For example, beta-glucan, isolated from Pleurotus pulmonarius, has potent anti-inflammatory and antinociceptive properties that inhibit pro-inflammatory cytokines [96]. Glucans isolated from Pleurotus pulmonarius also suppressed colon carcinogenesis associated with colitis by regulating cell proliferation, inducing apoptosis, and suppressing inflammation [97]. Beta-glucan is a high-molecular-weight polysaccharide of glucose bound by glycosidic bonds.
Phenolic compounds are secondary metabolites of edible mushrooms. Polyphenols have been extensively studied and shown to be effective against a variety of health complications. Phenolic acids such as p-hydroxybenzoic, cinnamic, gallic, salicylic, p-coumaric acids, syringic acids, caffeic, ferulic, chlorogenic, and flavonoid can be found in mushrooms [130]. Gallic, caffeic, and p-coumaric acids are the main phenolic groups and play an essential role in the biological activity of mushrooms [131]. Phenolic compounds have high antioxidant activity. Polyphenols from edible mushrooms such as Meripilus giganteus, Agaricus hydnum, and Rufescens silvaticus have high antioxidant capacity [80]. Phenolic compounds have also shown anticancer activity against kidney cancer cell lines and human ovarian cancer cell lines [6]. Flavonoid compounds, including myricetin, rutin, naringenin, quercetin, morin, and hesperetin, are included in the polyphenol content, and they exhibit antiproliferative effect [132].
Terpenoids such as monoterpenoids, sesquiterpenoids, diterpenoids, and triterpenoids, are essential components in mushrooms. They have been shown to have antimicrobial, anticancer, anticholinesterase, anti-inflammatory, antimalarial, and antioxidant properties [106]. Currently, about 285 types of terpenoids have been discovered in mushrooms and have medicinal properties. For example, ganoderic acids are a lanastanoid type triterpenoid and have been isolated from Ganoderma amboinense, Ganoderma lucidum, etc. Ergosterol, the principal sterol in most edible mushrooms, is a valuable dietary precursor of vitamin D2 and a natural antioxidant [133]. Phytosterols, such as ergosterols and ergosterol peroxide, have been shown to be more potent than the nonsteroidal anti-inflammatory drug indomethacin, as shown by their 50% inhibitory effect. High levels of ergosterols can be found in Agaricus bisporus, Lentinus edodes, Grifola frondose, Pleurotus ostreatus, Agaricus bisporus, and Agaricus bisporus [12].
7. Nutritional Values and Health Benefits of Edible Mushrooms
As a crucial source of food for humans for thousands of years, the medicinal and organoleptic properties provided by the chemical composition and nutritional value of edible mushrooms have been reported by several researchers (Figure 7). The consumption of edible and medicinal mushrooms in Eastern and Western countries has gradually increased in recent decades [134]. Agaricus bisporus, Lentinus edodes, and Pleurotus spp. are presently the most common cultivated edible mushrooms, with China as the largest producer of these mushrooms in the world [86]. Edible mushrooms are known for their high contents of carbohydrates, protein, and crude fibers, as well as different bioactive compounds, which provide both nutritional and health benefits for humans [86,135]. The relative content of these nutritional components differs by species and between countries, as reported in Table 6.

Figure 7.
Photos of some common edible mushrooms and their scientific names. Sources: by frankenstoen from Portland, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=7304024; CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=191739; by Dan Molter (shroomydan), CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=9822537; by TOMMES-WIKI, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=27753655; by Strobilomyces, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=179148; by Archenzo, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=24474; by voir ci-dessous, CC BY 3.0, https://commons.wikimedia.org/w/index.php?curid=3330811; by Mыць Дeниc, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1334049, accessed on 16 January 2020.

Table 6.
General nutritional values (based on dry weight) of some common edible mushrooms from different sources.
The main nutritional compounds that edible mushrooms are rich in include proteins, carbohydrates, crude fiber, total phenolic compounds, and vitamins and many minerals. Several studies have discussed the nutritional value of edible mushrooms from different locations such as Argentina [136], Bangladesh [85], China [136,137], Chile [86], India [83,138], and Turkey [9,76]. Many reports have also focused on one or more edible mushrooms such as Agaricus bisporus [24,139]; Lactarius deliciosus [140]; Pleurotus citrinopileatus [91]; Helvella leucopus and Morchella pulchella [9]; Pleurotus sajor-caju and Calocybe indica [141]; Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes [80]; some studies have focused on several species, e.g., Altaf et al. [83], Das et al. [135], and Taskın et al. [6]. Several myco-chemical structures and compositions can be found in edible mushrooms, including phenolic compounds, fatty acids, terpenoids, lipids, polysaccharides, and proteins, which are important compounds for human health. Saini et al. [11] reported the biologically active nutraceutical compounds present in edible mushrooms, including ergosterol, proteins, and fatty acids content (388 mg/100 g DW, 4.5–37.4%, and 1.75–15.5%, respectively).
The biofortification approach can be defined as a method by which pollutants and/or biodegradation from the environment can be removed or blocked from microorganisms such as bacteria, fungi, or algae. It is considered a sustainable agricultural strategy and a cost-effective tool to increase the bioavailability or content of essential nutrients in the edible parts of cultivated plants and reduce malnutrition [150]. Some edible mushrooms have been biofortified with nutrients, particularly selenium [151], which is applied to produce Se enrichment and increase bioactive metabolites of Pleurotus ostreatus [152], Cordyceps militaris [153], Hericium erinaceus [154], and Ganoderma lucidum [155]. Nano-biofortification is also an important approach that recently has been confirmed for using nano-selenium as anti-COVID-19 nanoparticles [156,157].
8. Biomedical Applications of Edible Mushrooms
Medicinal mushrooms can be defined as mushrooms that are used to modulate human immune responses such as antimicrobial, anticancer, and antioxidant (Table 7). These mushrooms have important compounds such as alkaloids, polyphenols, polysaccharides, sesquiterpenes, terpenoids, and metal chelating agents for treatment different human diseases [17]. Medicinal mushrooms are gaining more recognition. Consequently, this is creating new paths for researchers to understand how these types of mushrooms can play a role against human diseases such as neurodegenerative diseases (i.e., Alzheimer’s disease, Huntington’s disease, multiple sclerosis, Parkinson’s disease, motor neuron disease, and prion disease) through mechanisms such as regulating neurotrophins synthesis, reducing oxidative stress, neuroinflammation, protecting neurons, and modulation of acetylcholinesterase activity [17,158]. These strategies may include roles of mushrooms such as (1) antibacterial antibiotics, (2) antimycotics, (3) biofilm inhibitors, (4) anticancer agents, (5) antidiabetes, (6) improving nerve functioning, (7) cardiovascular disease control, (8) antiviral agents, (9) prebiotic, and (10) immunosuppressive and immunomodulatory agents [158,159].

Table 7.
A survey of some pharmacological or medicinal activities in selected edible mushrooms species according to published studies, study model, and the suggested disease being treated.
Several studies have been published about different therapeutic applications of medicinal mushrooms [178]. These studies depend on the kind of bioactive compounds and mushroom species, which can be divided into the following groups:
- (1)
- Anticancer group
This group includes many kinds of cancers such as colorectal, oral, renal, and cervical cancers besides leukemia, as well as breast, liver, lung, and prostate cancer. The main bioactive mushroom extracts that can be used against cancers may include methanol, polysaccharides, ganoderic acid, and ethanol extracts. Medicinal mushrooms that have these bioactive compounds include Agaricus brasiliensis, Boletus edulis, Ganoderma lucidum, Pleurotus ostreatus, P. pulmonarius, Ophiocordyceps sobolifera, Sanghuangprous vaninii, and Tremella mesenterica [93,158,162,163,179,180].
- (2)
- Antidiabetic group
This includes many mushroom species such as Grifola frondose and Phellinus linteus, which have different polysaccharides as bioactive compounds [14,158,181,182].
- (3)
- Cardiovascular diseases group
This includes species such as Agaricus bisporus and Tricholoma matsutakei. The crude extract of peptides found in these mushrooms may be useful in treating these diseases [158,183].
- (4)
- Immune-function group
This includes many species such as Pleurotus ostreatus and Trametes pubescens. The lectine extracts from these mushrooms may boost immune function [15,158,159,163].
- (5)
- Rheumatoid arthritis group
This includes some mushroom species such as Grifola frondose and Psilocybin spp. The active metabolite psilocin or polysaccharides extracts may help with rheumatoid arthritis [15,184].
- (6)
- Antiviral group
Major human viral diseases include human immunodeficiency virus (HIV), herpes simplex virus (HSV), hepatitis B and C viruses (HBV, HCV), and influenza. There are many antiviral compounds that can be extracted from various mushroom species [10,158,159,185]. Medicinal mushrooms can be divided into following groups based on three items mushroom species, extract of bioactive compounds, and virus disease group [186].
- (i)
- Antiviral mushrooms against HIV
This group includes mushrooms that have bioactive compounds that are active against human immunodeficiency virus (HIV), which include Lentinus edodes (polycarboxylated water-solubilized lignin), Pleurotus ostreatus (ubiquitin-like protein), Ganoderma lucidum (several triterpenoids), and Ganoderma colossus (lanostane triterpenes).
- (ii)
- Antiviral mushrooms against HSV
This group includes mushrooms that have bioactive compounds that act against Herpes simplex virus (HSV), which includes Agaricus brasiliensis (sulfated polysaccharide HSV-1), and Ganoderma lucidum (GLPG or Ganoderma lucidum proteoglycan).
- (iii)
- Antiviral mushrooms against influenza
This group includes mushrooms that have bioactive compounds that act against influenza virus (H5N1), which includes Phellinus baumii (hispidin), Inonotus hispidus (phenolic extracts), and Phellinus igniarius (sesquiterpenoid).
- (iv)
- Antiviral mushrooms against hepatitis virus
This group includes mushrooms that have bioactive compounds that act against hepatitis virus B or C (HBV, HCV), which include Antrodia camphorate (polysaccharides for HBV), Ganoderma lucidum (ganoderic acid), Lentinula edodes (mycelia solid culture extract for HCV), and Pleurotus ostreatus (lectin HBV). Recently, some published studies have reported about the biological role of bioactive compounds from mushrooms against COVID-19 such as polysaccharides [87,187], colossolactones, and ergosterol [88].
- (v)
- Antiviral mushrooms against other viruses
This group includes mushrooms that have bioactive compounds that act against other viruses, which include Hypsizygus marmoreus (sterols for Epstein–Barr virus), Auricularia auricular (polysaccharides for Newcastle disease virus), and Agaricus blazei (Murill extract for foot-and-mouth disease virus).
- (7)
- Group of neurodegenerative diseases
Some medicinal mushrooms such as Grifola frondosa, Hericium erinaceus, Lignosus rhinocerotis, Paxillus panuoides, Pleurotus giganteus, and Sarcodon scabrosus can improve human cognitive functions. Many bioactive ingredients can be used from this group of mushrooms against many diseases such as hericenones, scabronines, ganoderic acids, and aqueous and ethanolic extracts [17,188].
- (8)
- DNA damage group
This group includes mushrooms such as Agaricus blazei and Ganoderma lucidum, which have extracts such as polysaccharides that may work against DNA damage [189].
- (9)
- Antiaging group
This includes Tricholoma lobayense and Ganoderma lucidum, which could supply polysaccharide TLH-3 Ergosterol ganodermasidase, which acts against aging [10,15].
- (10)
- Antiobesity group
Many bioactive compounds can be extracted from mushroom species and used against obesity, including Pleurotus eryngii, P. sajor-caju, and Ophiocordyceps sinensis. These compounds may include β-glucan water extract and Cordyceps sinensis polysaccharide [16,142,190,191].
9. Bioactive Compounds from Edible Mushrooms and Toxic Dose
In general, the link between medicinal edible mushrooms and the impacts of their bioactive compounds in preventing human diseases (mycotherapy) is still in need of more investigation. Thus, a growing concern in mycotherapy is required from the scientific community to expand clinical trials to explore the potential benefits of the safe consumption of these mushrooms [192]. The bioactive compounds found in mushrooms may have important effects on human health. Mushrooms are most often taken into the body through ingestion, but their mechanisms need to be studied in both in vivo and in vitro clinical studies in order for their actions to be fully understood. Research should also be undertaken to standardize all production stages of edible mushrooms from cultivation through the extraction process, commercial preparation, regulation, and precise monitoring for high quality level [192], as set by the European Food Safety Authority (EFSA). This issue has been discussed by many studies, as reported by [74,79].
The bioactive compounds and their efficiency depend on several factors, from cultivation to the handling or processing of these bioactives to protect them from any harsh environments and to improve their bio-availability and biological activities upon human digestion [193]. The characterization of these bioactive components also depends on the kind of methodology and instrumentation used during their measurement, such as biochemical and high-performance liquid chromatography (HPLC) [194] or UHPLC-Mass Spectrometry-(MS)/MS [12], as well as all processing and extraction tools such as the drying temperature during processing [195] and hot water during extraction [196]. Therefore, it is difficult to establish the standardized doses for each edible mushroom for each bioactive compound required to achieve its desired biological effect.
It is assumed that up to 3.8 million fungal species inhabit the Earth. Around 120,000 of those are currently known, and more than 27,000 are macromycetes. Only about 500 species of macromycetes are known to be poisonous for humans [197]. As mentioned above, several studies have confirmed the beneficial attributes of consuming edible mushrooms. On the other hand, several potential risks to human health may occur when edible mushrooms grow in polluted soils and are ingested [13,198]. These risks mainly depend on the kind of pollutants, their consumed concentration, and the mushroom species [64,199]. The levels of pollutants, especially toxic elements in mushrooms before marketing, should be continually evaluated to assess the human health risk [21]. Poisonous mushrooms are considered a significant cause of “emergency medicine” when they cause mycotoxicity [198]. Poisonous mushrooms contain a variety of various toxins such as satratoxins [200], muscarine, amanitin, gyromitrin, ibotenic acid, orellanine, and russuphelins, which can differ markedly in their toxicity (Table 2). It is not easy to demarcate between edible and poisonous mushroom species because there are several cultivated and edible wild species that have dual potential health benefits and toxic compounds [198]. Some edible species may cause cardiac toxicity, allergic reactions, rhabdomyolysis, and mutagenic effects, as observed in laboratory rodents or in assays on cell cultures [198].
The mechanisms of interactions of nutritional or toxic compounds with the human body is of crucial concern. These mechanisms may include their toxic or harmful effects on the human endocrine, metabolic, and nervous systems. The recent increase in global consumption of edible mushrooms, with fresh mushroom of up to 300 g per day, may represent a public health concern [201]. Many studies using tumor-bearing animals, cultured cancer cells, and clinical trials are required to investigate these mechanisms [202]. Several poisoning cases resulting from deadly toxic mushrooms such as Trichoderma cornu-damae have been reported (Table 3), but toxicological analyses of these poisonings are still rare [200]. Cytotoxic mushroom poisoning may result from toxins of α-amanitin, amanitin, and orellanine, and their mechanisms may include (1) liver damage 1–3 days after ingestion due to hepatotoxic and nephrotoxic effects (such as mushrooms of Amanita phalloides, A. virosa, A. verna, A. exitialis); (2) gastrointestinal disturbances and liver and renal failure (such as Amanita verna, Lepiota helveola, and Galerina marginata); (3) kidney failure or early primary nephrotoxicity (Amanita smithiana, and A. pseudoporphyria); and (4) delayed primary nephrotoxicity (Cortinarius orellanus, and C. rubellus). More mechanisms and the mushrooms involved are listed in Table 2. The most distinguished poisonous mushrooms in Table 1 and Table 2 may include some species of Agaricus, Amanita, Gyromitra, Tricholoma, and Russula. In this context, a comprehensive review of the activity of bioactivities in Pleurotus spp. and their mechanism of action was reported by Cateni et al. [10].
10. Antinutritional Properties of Mushrooms
Apart from the many advantages resulting from the consumption of plant foods, some plants (e.g., legumes) and mushrooms possess potential deleterious effects due to their content of toxins or antinutrients [203,204,205]. Antinutrients are defined as natural or synthetic compounds, which can interfere with the uptake/absorption of nutrients by humans, and limit the bioavailability of essential nutrients causing a serious harmful impact on human health [203,205]. Antinutrients may include chitin, glucosinolates, lectins, phytates, oxalates, saponins, or tannins, which plants need in order to protect themselves from the surrounding stressful conditions. On the other hand, these antinutrients have become well-known to possess many beneficial effects and therapeutic potential on many human diseases [206].
Although several benefits of edible mushroom have been reported in different fields, as discussed earlier, including nano-biotechnology [33,207] and the production of bioactive compounds [79], some reports discussed how mushroom products can be hard to digest due to their content of antinutrients such as chitin (e.g., [203,208]). Some other reports have confirmed that edible mushroom consumption may be associated with a lower risk of mortality [209]. In general, edible mushrooms contain about 1.87–7.25% chitin, and many published studies involve the chitin in mushrooms from different points of view such as the production of chitinase [210], wound treatment [211], isolation of chitin–glucan complex [212,213], production of chitin/cellulose nanofiber [214], and production of chitin nanopaper [215].
11. General Discussion
The main intent of this review was to summarize the information related to edible mushrooms with a focus on their nutritional and medicinal applications. Mushroom by-products, which may be generated during their production and processing, may represent a disposal problem. However, these by-products may be promising sources for several applications because of their nutritional and functional properties [35,36]. There may be many sustainable applications for these by-products, including the production of bioactive compounds for pharmaceutical and nutraceutical formulations, as well as several other applications such as animal feeds, fertilizers, energy production, bioremediation, bio-based materials, cosmetics, and cosmeceuticals [33,91,216]. The sustainable productivity of edible/medicinal mushrooms has been reported by many researchers such as Shirur et al. [29], which could be used in industrial applications (Kumar et al. [37]), for the production of healthy food products (Zhang et al. [38]), for healthy and more sustainable foods (Das et al. [135], and for promising strategies of sustainable and novel food production (Lopez-Hortas et al. [39]).
On the other hand, toxic or poisonous mushrooms are considered an important issue, which still needs more exploration in terms of their toxins. Despite the health problems associated with poisonous mushrooms, the use of their main bioactive substances in such mushrooms in clinical applications is possible, as reported by many researchers (e.g., Patocka et al. [217]). These toxins could be applied in many respects, including biotechnology, biological control, and anticancer medicine. There is a need also for the discovery of more toxic species of mushrooms, and their toxins, which are expected to be increasingly known based on toxicological analyses.
12. Conclusions
Mushrooms are seen as an important source of food and are thought to play a crucial role in a healthy diet. The nutritional values of edible mushrooms results from their low content of calories, fat, and sodium and their high content of dietary fiber, proteins, vitamins, and essential nutrients, such as Cu, Mn, Se, and Zn. Several secondary metabolites (i.e., bioactive compounds) can be extracted from many edible medicinal mushrooms such as β-glucan, calcaelin, ergosterol, ganoderic acid, flavonoids, hispolon, laccase, lectins, lentinan, phenolics, proteoglycan, nucleotides, nucleosides, and triterpenoids. These compounds could offer various health benefits such as those provided by antioxidant, antiaging, anticancer, antimicrobial, anti-inflammatory, antiobesity, and immunomodulatory agents. These biological activities can reduce heart disease and hypertension, regulate metabolic disorders and body functions, improve gut health, boost the immune system, and help in weight loss. Edible mushrooms have become more popular as health promoters, with great concern given to different research activities looking into bioactive ingredients in several mushroom species. These bioactive compounds are needed to fortify or supply various staple food products. Although these bioactive components of edible mushrooms are crucial for human health, there is also an urgent need for testing such compounds against recent diseases such as coronavirus disease (COVID-19). Further investigations are needed on the nano-biofortification of different mushrooms, their bioactive compounds and therapeutic properties, and the health benefits of edible mushrooms for a healthy lifestyle.
Author Contributions
J.P. and H.E.-R. developed the idea and outline of the review; K.B. wrote the section of bioactive ingredients; N.A. wrote the nutritional section; Y.E. wrote the biomedical applications of the manuscript; the section on common species was written by X.L., G.T. and P.H.; and the other sections were written by H.E.-R., K.B., X.L. and N.A.; and Y.E. thoroughly revised the manuscript and finalized it. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the support of the 2020-1.1.2-PIACI-KFI-2020-00100 Project “Development of innovative food raw materials based on Maillard reaction by functional transformation of traditional and exotic mushrooms for food and medicinal purposes”. The research was also supported by the Stipendium Hungaricum Scholarship Program (SH ID:140993). The work/publication is supported by the EFOP-3.6.3-VEKOP-16-2017-00008 project. The project is co-financed by the European Union and the European Social Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
All authors thank Eric C. Brevik (College of Agricultural, Life, and Physical Sciences, Southern Illinois University, Carbondale, IL, USA) for his revising and editing the MS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qing, Z.; Cheng, J.; Wang, X.; Tang, D.; Liu, X.; Zhu, M. The Effects of Four Edible Mushrooms (Volvariella volvacea, Hypsizygus marmoreus, Pleurotus ostreatus and Agaricus bisporus) on Physicochemical Properties of Beef Paste. LWT 2021, 135, 110063. [Google Scholar] [CrossRef]
- Li, M.; Yu, L.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q.; Tian, F. Role of Dietary Edible Mushrooms in the Modulation of Gut Microbiota. J. Funct. Foods 2021, 83, 104538. [Google Scholar] [CrossRef]
- Balan, V.; Munafo, J.P.; Pattathil, S.; Merritt, B.B.; Venketachalam, S.; Ng, W.-O. Protocols to Evaluate the Nutritional and Potential Health Benefits of Edible Mushrooms. CBIOT 2018, 7, 34–58. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.; James, A.; Black, L. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef] [PubMed]
- Taşkın, H.; Süfer, Ö.; Attar, Ş.H.; Bozok, F.; Baktemur, G.; Büyükalaca, S.; Kafkas, N.E. Total Phenolics, Antioxidant Activities and Fatty Acid Profiles of Six Morchella Species. J. Food Sci. Technol. 2021, 58, 692–700. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An Emerging Resource for Therapeutic Terpenoids. 3 Biotech 2019, 9, 369. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins Purified from Medicinal and Edible Mushrooms: Insights into Their Antiviral Activity against Pathogenic Viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Acar, İ.; Blando, F.; Gul, B.; Greco, A.; Mukemre, M.; Uzun, Y.; Dalar, A. The Phenolic Profile and Biological Activities of the Wild-Edible Mushrooms Helvella leucopus and Morchella pulchella. Food Meas. 2021, 15, 555–566. [Google Scholar] [CrossRef]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in Wild and Cultivated Mushrooms: Nutrition and Health. Phytochem. Rev. 2021, 1–45. [Google Scholar] [CrossRef]
- Saini, R.K.; Rauf, A.; Khalil, A.A.; Ko, E.-Y.; Keum, Y.-S.; Anwar, S.; Alamri, A.; Rengasamy, K.R.R. Edible Mushrooms Show Significant Differences in Sterols and Fatty Acid Compositions. S. Afr. J. Bot. 2021, 141, 344–356. [Google Scholar] [CrossRef]
- Nowak, R.; Nowacka-Jechalke, N.; Pietrzak, W.; Gawlik-Dziki, U. A New Look at Edible and Medicinal Mushrooms as a Source of Ergosterol and Ergosterol Peroxide-UHPLC-MS/MS Analysis. Food Chem. 2022, 369, 130927. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Tagwireyi, C.; Musiyiwa, K.; Chipurura, B.; Nyamangara, J.; Sanganyado, E.; Chaukura, N. Occurrence, Behavior, and Human Exposure and Health Risks of Potentially Toxic Elements in Edible Mushrooms with Focus on Africa. Environ. Monit. Assess. 2021, 193, 302. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of Edible Mushroom as a Potent Therapeutics for the Diabetes and Obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal Mushroom: Boon for Therapeutic Applications. 3 Biotech 2018, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic Applications of Mushrooms and Their Biomolecules along with a Glimpse of in Silico Approach in Neurodegenerative Diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Dowlati, M.; Sobhi, H.R.; Esrafili, A.; FarzadKia, M.; Yeganeh, M. Heavy Metals Content in Edible Mushrooms: A Systematic Review, Meta-Analysis and Health Risk Assessment. Trends Food Sci. Technol. 2021, 109, 527–535. [Google Scholar] [CrossRef]
- El-Ramady, H.; Llanaj, X.; Prokisch, J. Edible Mushroom Cultivated in Polluted Soils and Its Potential Risks on Human Health: A Short Communication. Egypt. J. Soil Sci. 2021, 61, 1–10. [Google Scholar] [CrossRef]
- Ernst, A.-L.; Reiter, G.; Piepenbring, M.; Bässler, C. Spatial Risk Assessment of Radiocesium Contamination of Edible Mushrooms–Lessons from a Highly Frequented Recreational Area. Sci. Total Environ. 2022, 807, 150861. [Google Scholar] [CrossRef]
- Karami, H.; Shariatifar, N.; Nazmara, S.; Moazzen, M.; Mahmoodi, B.; Mousavi Khaneghah, A. The Concentration and Probabilistic Health Risk of Potentially Toxic Elements (PTEs) in Edible Mushrooms (Wild and Cultivated) Samples Collected from Different Cities of Iran. Biol. Trace Elem. Res. 2021, 199, 389–400. [Google Scholar] [CrossRef]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of Radioisotopes and Heavy Metals in Selected Species of Mushrooms. Food Chem. 2022, 367, 130670. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Zhou, Q.; Li, Z.; Li, W.; Zhang, J.; Tuo, F. Radionuclide Content and Risk Analysis of Edible Mushrooms in Northeast China. Radiat. Med. Prot. 2021, 2, 165–170. [Google Scholar] [CrossRef]
- Siwulski, M.; Budka, A.; Rzymski, P.; Gąsecka, M.; Kalač, P.; Budzyńska, S.; Magdziak, Z.; Niedzielski, P.; Mleczek, P.; Mleczek, M. Worldwide Basket Survey of Multielemental Composition of White Button Mushroom Agaricus Bisporus. Chemosphere 2020, 239, 124718. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Budzyńska, S.; Proch, J.; Gąsecka, M.; Niedzielski, P.; Rzymski, P. A Comparison of Toxic and Essential Elements in Edible Wild and Cultivated Mushroom Species. Eur. Food Res. Technol. 2021, 247, 1249–1262. [Google Scholar] [CrossRef]
- Melgar, M.J.; García, M.Á. Natural Radioactivity and Total K Content in Wild-Growing or Cultivated Edible Mushrooms and Soils from Galicia (NW, Spain). Environ. Sci. Pollut. Res. 2021, 28, 52925–52935. [Google Scholar] [CrossRef] [PubMed]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Metal Concentrations of Wild Mushroom Species Collected from Belgrad Forest (Istanbul, Turkey) with Their Health Risk Assessments. Environ. Sci. Pollut. Res. 2021, 28, 36193–36204. [Google Scholar] [CrossRef] [PubMed]
- Keskin, F.; Sarikurkcu, C.; Akata, I.; Tepe, B. Element Concentration, Daily Intake of Elements, and Health Risk Indices of Wild Mushrooms Collected from Belgrad Forest and Ilgaz Mountain National Park (Turkey). Environ. Sci. Pollut. Res. 2021, 28, 51544–51555. [Google Scholar] [CrossRef] [PubMed]
- Shirur, M.; Barh, A.; Annepu, S.K. Sustainable Production of Edible and Medicinal Mushrooms: Implications on Mushroom Consumption. In Climate Change and Resilient Food Systems; Hebsale Mallappa, V.K., Shirur, M., Eds.; Springer: Singapore, 2021; pp. 315–346. ISBN 978-981-334-537-9. [Google Scholar]
- Korman, R. Growing Mushrooms: The Complete Grower’s Guide to Becoming a Mushroom Expert and Starting Cultivation at Home; Amazon Digital Services LLC: Seattle, WA, USA, 2020; ISBN 978-1-65911-727-1. [Google Scholar]
- Cotter, T. Organic Mushroom Farming and Mycoremediation: Simple to Advanced and Experimental Techniques for Indoor and Outdoor Cultivation; Chelsea Green Publishing: White River Junction, VT, USA, 2015. [Google Scholar]
- Shirur, M. Entrepreneurial Behaviour and Socio Economic Analysis of Mushroom Growers in Karnataka. Indian J. Agric. Sci. 2017, 6, 840–845. [Google Scholar]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Arevalo, W. DIY Mushroom Cultivation: Growing Mushrooms at Home for Food, Medicine, and Soil. New Society Publishers, Gabriola Island, BC V0R 1X0, Canada. Available online: https://www.amazon.com/DIY-Mushroom-Cultivation-Mushrooms-Homesteader/dp/0865718954 (accessed on 17 March 2022).
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.M.M.B.; Freitas, A.C.; Ferreira, I.C.F.R.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef]
- Wang, C.-Y. A Review on the Potential Reuse of Functional Polysaccharides Extracted from the By-Products of Mushroom Processing. Food Bioprocess Technol. 2020, 13, 217–228. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. JoF 2021, 7, 427. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy Function and High Valued Utilization of Edible Fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Domínguez, H. Update on Potential of Edible Mushrooms: High-value Compounds, Extraction Strategies and Bioactive Properties. Int. J. Food Sci. Technol. 2022, 57, 1378–1385. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.-W.; Yang, Z.-L.; Bau, T.; Li, T.-H.; Dai, Y.-C. Resource Diversity of Chinese Macrofungi: Edible, Medicinal and Poisonous Species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Boa, E. Wild Edible Fungi a Global Overview of Their Use and Importance to People. Available online: https://www.fao.org/3/y5489e/y5489e00.htm (accessed on 7 January 2022).
- Aoki, W.; Watanabe, M.; Watanabe, M.; Kobayashi, N.; Terajima, J.; Sugita-Konishi, Y.; Kondo, K.; Hara-kudo, Y. Discrimination between Edible and Poisonous Mushrooms among Japanese Entoloma sarcopum and Related Species Based on Phylogenetic Analysis and Insertion/Deletion Patterns of Nucleotide Sequences of the Cytochrome Oxidase 1 Gene. Genes Genet. Syst. 2020, 95, 133–139. [Google Scholar] [CrossRef]
- Wei, Y.; Li, L.; Liu, Y.; Xiang, S.; Zhang, H.; Yi, L.; Shang, Y.; Xu, W. Identification Techniques and Detection Methods of Edible Fungi Species. Food Chem. 2022, 374, 131803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S.C.; Perez-Moreno, J.; Rahman, M.M.; Rashid, M.H.; Phengsintham, P.; Rizal, L.; et al. Reviewing the World’s Edible Mushroom Species: A New Evidence-based Classification System. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1982–2014. [Google Scholar] [CrossRef]
- Ramírez-Terrazo, A.; Adriana Montoya, E.; Garibay-Orijel, R.; Caballero-Nieto, J.; Kong-Luz, A.; Méndez-Espinoza, C. Breaking the Paradigms of Residual Categories and Neglectable Importance of Non-Used Resources: The “Vital” Traditional Knowledge of Non-Edible Mushrooms and Their Substantive Cultural Significance. J. Ethnobiol. Ethnomed. 2021, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mo, M.; Yang, L.; Mi, F.; Cao, Y.; Liu, C.; Tang, X.; Wang, P.; Xu, J. Exploring the Species Diversity of Edible Mushrooms in Yunnan, Southwestern China, by DNA Barcoding. JoF 2021, 7, 310. [Google Scholar] [CrossRef] [PubMed]
- GBIF The Global Biodiversity Information Facility. SPECIES SYNONYM. Available online: https://www.gbif.org/search?q=SPECIES%20SYNONYM (accessed on 15 December 2021).
- BMS The British Mycological Society. Available online: https://www.britmycolsoc.org.uk (accessed on 7 January 2022).
- Tawatsin, A.; Parnmen, S.; Thavara, U.; Siriyasatien, P.; Kongtip, P. Mushroom Poisoning in Thailand: Incidence and Intoxication to Human Health. Med. Res. Arch. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Parnmen, S.; Nooron, N.; Leudang, S.; Sikaphan, S.; Polputpisatkul, D.; Rangsiruji, A. Phylogenetic Evidence Revealed Cantharocybe virosa (Agaricales, Hygrophoraceae) as a New Clinical Record for Gastrointestinal Mushroom Poisoning in Thailand. Toxicol. Res. 2020, 36, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Parnmen, S.; Nooron, N.; Leudang, S.; Sikaphan, S.; Polputpisatkul, D.; Pringsulaka, O.; Binchai, S.; Rangsiruji, A. Foodborne Illness Caused by Muscarine-Containing Mushrooms and Identification of Mushroom Remnants Using Phylogenetics and LC-MS/MS. Food Control 2021, 128, 108182. [Google Scholar] [CrossRef]
- White, J.; Weinstein, S.A.; De Haro, L.; Bédry, R.; Schaper, A.; Rumack, B.H.; Zilker, T. Mushroom Poisoning: A Proposed New Clinical Classification. Toxicon 2019, 157, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.A.; Tuncok, Y. Mushroom-Related Toxins, Alpha Amanitin, and Usage of Antioxidants: Directions toward Antioxidant Capacity. In Toxicology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 447–456. ISBN 978-0-12-819092-0. [Google Scholar]
- Beuhler, M.C. Overview of Mushroom Poisoning. In Critical Care Toxicology; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2103–2128. ISBN 978-3-319-17899-8. [Google Scholar]
- Hedman, H.; Holmdahl, J.; Mölne, J.; Ebefors, K.; Haraldsson, B.; Nyström, J. Long-Term Clinical Outcome for Patients Poisoned by the Fungal Nephrotoxin Orellanine. BMC Nephrol. 2017, 18, 121. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Chemical Reactivity and Bioactivity Properties of the Phallotoxin Family of Fungal Peptides Based on Conceptual Peptidology and DFT Study. Heliyon 2019, 5, e02335. [Google Scholar] [CrossRef] [PubMed]
- Govorushko, S.; Rezaee, R.; Dumanov, J.; Tsatsakis, A. Poisoning Associated with the Use of Mushrooms: A Review of the Global Pattern and Main Characteristics. Food Chem. Toxicol. 2019, 128, 267–279. [Google Scholar] [CrossRef]
- Ukwuru, M.; Muritala, A.; Lu, E. Edible and Non-Edible Wild Mushrooms: Nutrition, Toxicity and Strategies for Recognition. J. Clin. Nutr. Metab. 2018, 2, 9. [Google Scholar]
- Karimi, G.; Razavi, B.M. Poisonous Mushrooms. In Clinical Toxinology; Gopalakrishnakone, P., Faiz, S.M.A., Gnanathasan, C.A., Habib, A.G., Fernando, R., Yang, C.-C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–18. ISBN 978-94-007-6288-6. [Google Scholar]
- Kumar, S.; Jain, T.; Banerjee, D. Fungal Diseases and Their Treatment: A Holistic Approach. In Pathogenicity and Drug Resistance of Human Pathogens; Hameed, S., Fatima, Z., Eds.; Springer: Singapore, 2019; pp. 111–134. ISBN 978-981-329-448-6. [Google Scholar]
- Falandysz, J.; Zhang, J.; Saniewski, M. 137Cs, 40K, and K in Raw and Stir-Fried Mushrooms from the Boletaceae Family from the Midu Region in Yunnan, Southwest China. Environ. Sci. Pollut. Res. 2020, 27, 32509–32517. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, G.; Wang, L. Assessment of Potential Human Health Risk of Trace Element in Wild Edible Mushroom Species Collected from Yunnan Province, China. Environ. Sci. Pollut. Res. 2020, 27, 29218–29227. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of Heavy Metals by Wild Edible Mushrooms with Respect to Soil Substrates in the Athens Metropolitan Area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. The Level of Selected Organochlorine Compounds Residues in Popular Edible Mushrooms from North-Eastern Poland. Food Chem. 2021, 353, 129441. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Budka, A.; Siwulski, M.; Budzyńska, S.; Kalač, P.; Karolewski, Z.; Lisiak-Zielińska, M.; Kuczyńska-Kippen, N.; Niedzielski, P. Anthropogenic Contamination Leads to Changes in Mineral Composition of Soil- and Tree-Growing Mushroom Species: A Case Study of Urban vs. Rural Environments and Dietary Implications. Sci. Total Environ. 2021, 809, 151162. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzyńska, S.; Szostek, M.; Kuczyńska-Kippen, N.; Kalač, P.; Niedzielski, P.; Gąsecka, M.; et al. Toxicological Risks and Nutritional Value of Wild Edible Mushroom Species—A Half-Century Monitoring Study. Chemosphere 2021, 263, 128095. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Akata, I.; Guven, G.; Tepe, B. Metal Concentration and Health Risk Assessment of Wild Mushrooms Collected from the Black Sea Region of Turkey. Environ. Sci. Pollut. Res. 2020, 27, 26419–26441. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Akata, I.; Tepe, B. Metal Concentration and Health Risk Assessment of Eight Russula Mushrooms Collected from Kizilcahamam-Ankara, Turkey. Environ. Sci. Pollut. Res. 2021, 28, 15743–15754. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Popović-Djordjević, J.; Solak, M.H. Wild Edible Mushrooms from Mediterranean Region: Metal Concentrations and Health Risk Assessment. Ecotoxicol. Environ. Saf. 2020, 190, 110058. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sarikurkcu, R.T.; Akata, I.; Tepe, B. Metal Concentration and Health Risk Assessment of Fifteen Wild Mushrooms Collected from the Ankara University Campus (Turkey). Environ. Sci. Pollut. Res. 2020, 27, 32474–32480. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Yildiz, D.; Akata, I.; Tepe, B. Evaluation of the Metal Concentrations of Wild Mushroom Species with Their Health Risk Assessments. Environ. Sci. Pollut. Res. 2021, 28, 21437–21454. [Google Scholar] [CrossRef] [PubMed]
- Türkmen, M.; Budur, D. Heavy Metal Contaminations in Edible Wild Mushroom Species from Turkey’s Black Sea Region. Food Chem. 2018, 254, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Świsłowski, P.; Dołhańczuk-Śródka, A.; Rajfur, M. Bibliometric Analysis of European Publications between 2001 and 2016 on Concentrations of Selected Elements in Mushrooms. Environ. Sci. Pollut. Res. 2020, 27, 22235–22250. [Google Scholar] [CrossRef] [PubMed]
- Schunko, C.; Li, X.; Klappoth, B.; Lesi, F.; Porcher, V.; Porcuna-Ferrer, A.; Reyes-García, V. Local Communities’ Perceptions of Wild Edible Plant and Mushroom Change: A Systematic Review. Glob. Food Secur. 2022, 32, 100601. [Google Scholar] [CrossRef]
- Kandasamy, S.; Chinnappan, S.; Thangaswamy, S.; Balakrishnan, S.; Khalifa, A.Y.Z. Assessment of Antioxidant, Antibacterial Activities and Bioactive Compounds of the Wild Edible Mushroom Pleurotus Sajor-Caju. Int. J. Pept. Res. Ther. 2020, 26, 1575–1581. [Google Scholar] [CrossRef]
- Alkin, M.; Söğüt, E.; Seydim, A.C. Determination of Bioactive Properties of Different Edible Mushrooms from Turkey. Food Meas. 2021, 15, 3608–3617. [Google Scholar] [CrossRef]
- Goswami, B.; Majumdar, S.; Das, A.; Barui, A.; Bhowal, J. Evaluation of Bioactive Properties of Pleurotus Ostreatus Mushroom Protein Hydrolysate of Different Degree of Hydrolysis. LWT 2021, 149, 111768. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically Active Polysaccharide from Edible Mushrooms: A Review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive Components of Mushrooms: Processing Effects and Health Benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Kała, K.; Krakowska, A.; Szewczyk, A.; Ostachowicz, B.; Szczurek, K.; Fijałkowska, A.; Muszyńska, B. Determining the Amount of Potentially Bioavailable Phenolic Compounds and Bioelements in Edible Mushroom Mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes. Food Chem. 2021, 352, 129456. [Google Scholar] [CrossRef] [PubMed]
- Mešić, A.; Šamec, D.; Jadan, M.; Bahun, V.; Tkalčec, Z. Integrated Morphological with Molecular Identification and Bioactive Compounds of 23 Croatian Wild Mushrooms Samples. Food Biosci. 2020, 37, 100720. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R.; Rao, S.R. Molecular Strategies for Identification and Characterization of Some Wild Edible Mushrooms of Nagaland, India. Mol. Biol. Rep. 2020, 47, 621–630. [Google Scholar] [CrossRef]
- Altaf, U.; Lalotra, P.; Sharma, Y.P. Nutritional and Mineral Composition of Four Wild Edible Mushrooms from Jammu and Kashmir, India. Indian Phytopathol. 2020, 73, 313–320. [Google Scholar] [CrossRef]
- Atri, N.S.; Sharma, Y.P.; Kumar, S. Mridu Wild Edible Mushrooms of North West Himalaya: Their Nutritional, Nutraceutical, and Sociobiological Aspects. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications; Satyanarayana, T., Das, S.K., Johri, B.N., Eds.; Springer: Singapore, 2019; pp. 533–563. ISBN 9789811384868. [Google Scholar]
- Sifat, N.; Lovely, F.; Zihad, S.M.N.K.; Hossain, M.G.; Shilpi, J.A.; Grice, I.D.; Mubarak, M.S.; Uddin, S.J. Investigation of the Nutritional Value and Antioxidant Activities of Common Bangladeshi Edible Mushrooms. Clin. Phytosci. 2020, 6, 88. [Google Scholar] [CrossRef]
- Jacinto-Azevedo, B.; Valderrama, N.; Henríquez, K.; Aranda, M.; Aqueveque, P. Nutritional Value and Biological Properties of Chilean Wild and Commercial Edible Mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides Obtained from Natural Edible Sources and Their Role in Modulating the Immune System: Biologically Active Potential That Can Be Exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Rangsinth, P.; Sillapachaiyaporn, C.; Nilkhet, S.; Tencomnao, T.; Ung, A.T.; Chuchawankul, S. Mushroom-Derived Bioactive Compounds Potentially Serve as the Inhibitors of SARS-CoV-2 Main Protease: An in Silico Approach. J. Tradit. Complementary Med. 2021, 11, 158–172. [Google Scholar] [CrossRef]
- Gurbuz, I.B. Nongreen Revolution: A Case Study of Wild-Grown Edible Mushroom. Environ. Sci. Pollut. Res. 2019, 26, 7954–7959. [Google Scholar] [CrossRef] [PubMed]
- Isik, H. Fatty Acid Contents of Three Wild Edible Mushroom Species. Chem. Nat. Compd. 2020, 56, 1114–1116. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the Nutritional and Functional Properties of Pleurotus citrinopileatus Mushrooms through the Exploitation of Winery and Olive Mill Wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, P.; Cheng, J.; Yang, H.; Zou, J.; Liu, X. The Role of Endogenous Enzyme from Straw Mushroom (Volvariella volvacea) in Improving Taste and Volatile Flavor Characteristics of Cantonese Sausage. LWT 2022, 154, 112627. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Ng, T.B. Interrelationship among Paraptosis, Apoptosis and Autophagy in Lung Cancer A549 Cells Induced by BEAP, an Antitumor Protein Isolated from the Edible Porcini Mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 188, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pohleven, J.; Brzin, J.; Vrabec, L.; Leonardi, A.; Čokl, A.; Štrukelj, B.; Kos, J.; Sabotič, J. Basidiomycete Clitocybe nebularis Is Rich in Lectins with Insecticidal Activities. Appl. Microbiol. Biotechnol. 2011, 91, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Pardhi, P.; Bhadoriya, S.S.; Jain, N.; Rai, G.; Jain, A.P. Antioxidant Potential of White Oyster Culinary-Medicinal Mushroom, Pleurotus florida (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.J.; Rezoagli, E.; Pogue, R.; Simonassi-Paiva, B.; Abidin, I.I.Z.; Fehrenbach, G.W.; O’Neil, E.; Major, I.; Laffey, J.G.; Rowan, N. Immunomodulatory Activity of β-Glucan Polysaccharides Isolated from Different Species of Mushroom—A Potential Treatment for Inflammatory Lung Conditions. Sci. Total Environ. 2022, 809, 152177. [Google Scholar] [CrossRef]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the Edible Mushroom Pleurotus pulmonarius Inhibit Colitis-Associated Colon Carcinogenesis in Mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Socaci, S.; Dulf, F.; Diaconeasa, Z.; Fărcaș, A.; Tofană, M.; Semeniuc, C. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef]
- Alshammaa, D.A.S. Phytochemical Investigation and Quantitative Comparison of Ergosterol Between Agaricus bisporus and Pleurotus ostreatus by HPLC and GC-MS Methods. Int. J. Pharm. Sci. Rev. Res. 2017, 44, 215–220. [Google Scholar]
- Verma, N.K.; Singh, A.P.; Singh, V.K. Agaricus bisporus (Fungi) Chemical Constituents and Pharmacological Activities—A Review. Asian J. Phytomedicine Clin. Res. 2019, 7, 82–87. [Google Scholar]
- Liu, G.; Ye, J.; Li, W.; Zhang, J.; Wang, Q.; Zhu, X.; Miao, J.; Huang, Y.; Chen, Y.; Cao, Y. Extraction, Structural Characterization, and Immunobiological Activity of ABP Ia Polysaccharide from Agaricus bisporus. Int. J. Biol. Macromol. 2020, 162, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Li, Z.-H.; Dong, Z.-J.; Su, J.; Li, Y.; Liu, J.-K. Non-Isoprenoid Botryane Sesquiterpenoids from Basidiomycete Boletus edulis and Their Cytotoxic Activity. Nat. Prod. Bioprospect. 2011, 1, 29–32. [Google Scholar] [CrossRef]
- Žurga, S.; Nanut, M.P.; Kos, J.; Sabotič, J. Fungal Lectin MpL Enables Entry of Protein Drugs into Cancer Cells and Their Subcellular Targeting. Oncotarget 2017, 8, 26896–26910. [Google Scholar] [CrossRef][Green Version]
- Luo, A.; Luo, A.; Huang, J.; Fan, Y. Purification, Characterization and Antioxidant Activities in Vitro and in Vivo of the Polysaccharides from Boletus edulis Bull. Molecules 2012, 17, 8079–8090. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P.; Kozak, L. Phenolic and Flavonoid Content in Hericium erinaceus, Ganoderma lucidum, and Agrocybe aegerita under Selenium Addition. Acta Aliment. 2016, 45, 300–308. [Google Scholar] [CrossRef]
- Surup, F.; Hennicke, F.; Sella, N.; Stroot, M.; Bernecker, S.; Pfütze, S.; Stadler, M.; Rühl, M. New Terpenoids from the Fermentation Broth of the Edible Mushroom Cyclocybe aegerita. Beilstein J. Org. Chem. 2019, 15, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Maro, A.D.; Iglesias, R.; Citores, L.; Ferreras, J.M.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Motoshima, R.A.; Rosa, T.D.F.; Mendes, L.D.C.; da Silva, E.V.; Viana, S.R.; do Amaral, B.S.; de Souza, D.H.; Lião, L.M.; da Silva, M.D.L.C.; de Sousa, L.R.; et al. Inhibition of Leishmania Amazonensis Arginase by Fucogalactan Isolated from Agrocybe aegerita Mushroom. Carbohydr. Polym. 2018, 201, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, P.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Borawska, M.H.; Socha, K. The Two Faces of Coprinus comatus—Functional Properties and Potential Hazards. Phytother. Res. 2020, 34, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Dulay, M.R.; Sanguesa, K.B.; Ablaza, J.; Joson, A.J.M.; Peria, J.N.T.; Quejada, C.S.; Basa, J.O.; Castro, M. Bioactive Myco-Nutrients of Aseptically Cultured Fruiting Bodies of coprinus comatus (o.f. Müll.) Pers. On Rice Bran-Enriched Ruminants’ Dung. Available online: https://www.semanticscholar.org/paper/BIOACTIVE-MYCO-NUTRIENTS-OF-ASEPTICALLY-CULTURED-OF-Dulay-Sanguesa/6ab75659b7e5c608804664d0bc0d3618a33798ad (accessed on 7 January 2022).
- Zhao, H.; Zhang, J.; Liu, X.; Yang, Q.; Dong, Y.; Jia, L. The Antioxidant Activities of Alkalic-Extractable Polysaccharides from Coprinus comatus on Alcohol-Induced Liver Injury in Mice. Sci. Rep. 2019, 8, 11695. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, N.; Yanni, A.E.; Koutrotsios, G.; Aloupi, M. Bioactive Microconstituents and Antioxidant Properties of Wild Edible Mushrooms from the Island of Lesvos, Greece. Food Chem. Toxicol. 2013, 55, 378–385. [Google Scholar] [CrossRef]
- Feussi Tala, M.; Qin, J.; Ndongo, J.T.; Laatsch, H. New Azulene-Type Sesquiterpenoids from the Fruiting Bodies of Lactarius deliciosus. Nat. Prod. Bioprospect. 2017, 7, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Khaund, P.; Joshi, S.R. Enzymatic Profiling of Wild Edible Mushrooms Consumed by the Ethnic Tribes of India. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 263–271. [Google Scholar] [CrossRef]
- Su, S.; Ding, X.; Fu, L.; Hou, Y. Structural Characterization and Immune Regulation of a Novel Polysaccharide from Maerkang Lactarius deliciosus Gray. Int. J. Mol. Med. 2019, 44, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Saha, A.K.; Datta, B.K. Bioactive Compounds with Special References to Anticancer Property of Oyster Mushroom. J. Pharmacogn. Phytochem. 2018, 7, 2694–2698. [Google Scholar]
- Souilem, F.; Fernandes, Â.; Calhelha, R.C.; Barreira, J.C.M.; Barros, L.; Skhiri, F.; Martins, A.; Ferreira, I.C.F.R. Wild Mushrooms and Their Mycelia as Sources of Bioactive Compounds: Antioxidant, Anti-Inflammatory and Cytotoxic Properties. Food Chem. 2017, 230, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, Y.; Qiang, Z. A Potent Pharmacological Mushroom: Pleurotus eryngii. Fungal Genom. Biol. 2016, 6, 1–5. [Google Scholar] [CrossRef]
- Alam, N.; Yoon, K.; Shin, P.; Cheong, J.-C.; Yoo, Y.; Lee, T.-S. Antioxidant, Phenolic Compounds Concentration, Xanthine Oxidase and Tyrosinase Inhibitory Activities of Pleurotus cornucopiae. Aust. J. Basic Appl. Sci. 2011, 5, 229–239. [Google Scholar]
- Lee, S.R.; Lee, D.; Lee, H.-J.; Noh, H.J.; Jung, K.; Kang, K.S.; Kim, K.H. Renoprotective Chemical Constituents from an Edible Mushroom, Pleurotus cornucopiae in Cisplatin-Induced Nephrotoxicity. Bioorg. Chem. 2017, 71, 67–73. [Google Scholar] [CrossRef]
- Golak-Siwulska, I.; Kałużewicz, A.; Spiżewski, T.; Siwulski, M.; Sobieralski, K. Bioactive Compounds and Medicinal Properties of Oyster Mushrooms (Pleurotus sp.). Folia Hortic. 2018, 30, 191–201. [Google Scholar] [CrossRef]
- Minato, K.; Ohara, A.; Mizuno, M. A Proinflammatory Effect of the β -Glucan from Pleurotus cornucopiae Mushroom on Macrophage Action. Mediat. Inflamm. 2017, 2017, 8402405. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ye, L.; Kang, Z.; Jia, D.; Yang, L.; Zhang, B. LC-MS-Based Metabolomic Approach Revealed the Significantly Different Metabolic Profiles of Five Commercial Truffle Species. Front. Microbiol. 2019, 10, 2227. [Google Scholar] [CrossRef]
- Chen, H.-P.; Zhao, Z.-Z.; Li, Z.-H.; Huang, Y.; Zhang, S.-B.; Tang, Y.; Yao, J.-N.; Chen, L.; Isaka, M.; Feng, T.; et al. Anti-Proliferative and Anti-Inflammatory Lanostane Triterpenoids from the Polish Edible Mushroom Macrolepiota procera. J. Agric. Food Chem. 2018, 66, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Nowacka-Jechalke, N.; Juda, M.; Malm, A. The Preliminary Study of Prebiotic Potential of Polish Wild Mushroom Polysaccharides: The Stimulation Effect on Lactobacillus Strains Growth. Eur. J. Nutr. 2018, 57, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of Bioactivities and Phenolic Contents of Wild Edible Mushrooms from Northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Du, F.; Zhang, G.-Q.; Wang, H.-X.; Ng, T.-B. Purification a Laccase Exhibiting Dye Decolorizing Ability from an Edible Mushroom Russula virescens. Int. Biodeterior. Biodegrad. 2013, 82, 33–39. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated Modification, Characterization, and Potential Bioactivities of Polysaccharide from the Fruiting Bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Tejedor-Calvo, E.; Morales, D.; Marco, P.; Sánchez, S.; Garcia-Barreda, S.; Smiderle, F.R.; Iacomini, M.; Villalva, M.; Santoyo, S.; Soler-Rivas, C. Screening of Bioactive Compounds in Truffles and Evaluation of Pressurized Liquid Extractions (PLE) to Obtain Fractions with Biological Activities. Food Res. Int. 2020, 132, 109054. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Sarikurkcu, C.; Yalcin, O.U.; Cengiz, M.; Gungor, H. Metal Concentration, Phenolics Profiling, and Antioxidant Activity of Two Wild Edible Melanoleuca Mushrooms (M. cognata and M. stridula). Microchem. J. 2019, 150, 104172. [Google Scholar] [CrossRef]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The Contribution of Phenolic Acids to the Anti-Inflammatory Activity of Mushrooms: Screening in Phenolic Extracts, Individual Parent Molecules and Synthesized Glucuronated and Methylated Derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef]
- Saltarelli, R.; Palma, F.; Gioacchini, A.M.; Calcabrini, C.; Mancini, U.; De Bellis, R.; Stocchi, V.; Potenza, L. Phytochemical Composition, Antioxidant and Antiproliferative Activities and Effects on Nuclear DNA of Ethanolic Extract from an Italian Mycelial Isolate of Ganoderma lucidum. J. Ethnopharmacol. 2019, 231, 464–473. [Google Scholar] [CrossRef]
- Papoutsis, K.; Grasso, S.; Menon, A.; Brunton, N.P.; Lyng, J.G.; Jacquier, J.-C.; Bhuyan, D.J. Recovery of Ergosterol and Vitamin D2 from Mushroom Waste-Potential Valorization by Food and Pharmaceutical Industries. Trends Food Sci. Technol. 2020, 99, 351–366. [Google Scholar] [CrossRef]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A Review of Cultivation Strategies, Bioactivity, and Application of Mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Di Anibal, C.; Farenzena, S.; Rodríguez, M.S.; Albertengo, L. Chemical Composition and Nutritional Value of Argentine Commercial Edible Mushrooms. J. Verbr. Lebensm. 2015, 10, 155–164. [Google Scholar] [CrossRef]
- Yu, Q.; Guo, M.; Zhang, B.; Wu, H.; Zhang, Y.; Zhang, L. Analysis of Nutritional Composition in 23 Kinds of Edible Fungi. J. Food Qual. 2020, 2020, 8821315. [Google Scholar] [CrossRef]
- Ao, T.; Deb, C.R. Nutritional and Antioxidant Potential of Some Wild Edible Mushrooms of Nagaland, India. J. Food Sci. Technol. 2019, 56, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Xu, Z.; Fu, L.; Feng, S.; Yuan, M.; Huang, Y.; Liao, J.; Zhou, L.; Yang, H.; Ding, C. Chemical Composition, Antioxidant and Antihyperglycemic Activities of the Wild Lactarius deliciosus from China. Molecules 2019, 24, 1357. [Google Scholar] [CrossRef]
- Das, P.; Sikdar, S.R.; Samanta, A. Nutritional Analysis and Molecular Characterization of Hybrid Mushrooms Developed through Intergeneric Protoplast Fusion between Pleurotus Sajor-Caju and Calocybe indica with the Purpose to Achieve Improved Strains. World J. Microbiol. Biotechnol. 2021, 37, 69. [Google Scholar] [CrossRef]
- Wunjuntuk, K.; Ahmad, M.; Techakriengkrai, T.; Chunhom, R.; Jaraspermsuk, E.; Chaisri, A.; Kiwwongngam, R.; Wuttimongkolkul, S.; Charoenkiatkul, S. Proximate Composition, Dietary Fibre, Beta-Glucan Content, and Inhibition of Key Enzymes Linked to Diabetes and Obesity in Cultivated and Wild Mushrooms. J. Food Compos. Anal. 2022, 105, 104226. [Google Scholar] [CrossRef]
- Nayak, P.C.; Raju, C.V.; Lakshmisha, I.P.; Singh, R.R.; Sofi, F.R. Influence of Button Mushroom (Agaricus bisporus) on Quality and Refrigerated Storage Stability of Patties Prepared from Sutchi Catfish (Pangasius hypophthalmus). J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Jo, K.; Lee, J.; Jung, S. Quality Characteristics of Low-Salt Chicken Sausage Supplemented with a Winter Mushroom Powder. Korean J. Food Sci. Anim. Resour. 2018, 38, 768–779. [Google Scholar] [CrossRef]
- Mau, J.-L.; Lin, H.-C.; Ma, J.-T.; Song, S.-F. Non-Volatile Taste Components of Several Speciality Mushrooms. Food Chem. 2001, 73, 461–466. [Google Scholar] [CrossRef]
- Teklit, G.A. Chemical Composition and Nutritional Value of the Most Widely Used Mushrooms Cultivated in Mekelle Tigray Ethiopia. J. Nutr. Food Sci. 2015, 5, 1. [Google Scholar] [CrossRef]
- Michael, H.W.; Bultosa, G.; Pant, L.M. Nutritional Contents of Three Edible Oyster Mushrooms Grown on Two Substrates at Haramaya, Ethiopia, and Sensory Properties of Boiled Mushroom and Mushroom Sauce: Nutrient of Edible Oyster Mushrooms. Int. J. Food Sci. Technol. 2011, 46, 732–738. [Google Scholar] [CrossRef]
- Crisan, E.V.; Sands, A. Nutritional Value. In Biology and Cultivation of Edible Mushrooms; Chang, S.T., Hayer, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 138–168. [Google Scholar]
- Ghosh, K. A Review: Edible Mushrooms as Source of Dietary Fiber and Its Health Effects. J. Phys. Sci. 2016, 21, 129–137. [Google Scholar]
- Koç, E.; Karayiğit, B. Assessment of Biofortification Approaches Used to Improve Micronutrient-Dense Plants that Are a Sustainable Solution to Combat Hidden Hunger. J. Soil Sci. Plant Nutr. 2021, 4, 1–26. [Google Scholar] [CrossRef]
- Kora, A.J. Nutritional and Antioxidant Significance of Selenium-Enriched Mushrooms. Bull. Natl. Res. Cent. 2020, 44, 34. [Google Scholar] [CrossRef]
- Siwulski, M.; Budzyńska, S.; Rzymski, P.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Mleczek, M. The Effects of Germanium and Selenium on Growth, Metalloid Accumulation and Ergosterol Content in Mushrooms: Experimental Study in Pleurotus ostreatus and Ganoderma lucidum. Eur. Food Res. Technol. 2019, 245, 1799–1810. [Google Scholar] [CrossRef]
- Hu, T.; Liang, Y.; Zhao, G.; Wu, W.; Li, H.; Guo, Y. Selenium Biofortification and Antioxidant Activity in Cordyceps militaris Supplied with Selenate, Selenite, or Selenomethionine. Biol. Trace Elem. Res. 2019, 187, 553–561. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium Biofortification in Hericium erinaceus (Lion’s Mane Mushroom) and Its in Vitro Bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, S.; Wang, L.; Wei, Z.; Zhao, L.; Shi, G.; Ding, Z. Influence of Selenium Biofortification on the Growth and Bioactive Metabolites of Ganoderma lucidum. Foods 2021, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Elbasiouny, H.; Elbehiry, F.; Elsakhawy, T.; Omara, A.E.-D.; Amer, M.; Bayoumi, Y.; Shalaby, T.A.; Eid, Y.; et al. Nano-Biofortification of Different Crops to Immune against COVID-19: A Review. Ecotoxicol. Environ. Saf. 2021, 222, 112500. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B.; Yu, Y.-L.; Chen, C.; Li, Y.-F. Using Nano-Selenium to Combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discov. Today 2021, 27, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Bertollo, A.G.; Mingoti, M.E.D.; Plissari, M.E.; Betti, G.; Roman Junior, W.A.; Luzardo, A.R.; Ignácio, Z.M. Agaricus blazei Murrill Mushroom: A Review on the Prevention and Treatment of Cancer. Pharmacol. Res. Mod. Chin. Med. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H.; Cai, M.; Liang, X.; Wu, X.; Wang, A.; Chen, X.; Li, X.; Xiao, C.; Huang, L.; et al. Whole Genome Sequencing of an Edible and Medicinal Mushroom, Russula griseocarnosa, and Its Association with Mycorrhizal Characteristics. Gene 2022, 808, 145996. [Google Scholar] [CrossRef]
- He, P.-Y.; Hou, Y.-H.; Yang, Y.; Li, N. The Anticancer Effect of Extract of Medicinal Mushroom Sanghuangprous vaninii against Human Cervical Cancer Cell via Endoplasmic Reticulum Stress-Mitochondrial Apoptotic Pathway. J. Ethnopharmacol. 2021, 279, 114345. [Google Scholar] [CrossRef]
- Li, Y.-M.; Zhong, R.-F.; Chen, J.; Luo, Z.-G. Structural Characterization, Anticancer, Hypoglycemia and Immune Activities of Polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, J.; Li, Z.; Liu, H.; Wang, Y. Traditional Uses, Chemical Components and Pharmacological Activities of the Genus Ganoderma P. Karst.: A Review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory Activities of Polysaccharides from Ganoderma on Immune Effector Cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Chen, B.-S.; Dai, H.-Q.; Ren, J.-W.; Zhou, L.-W.; Wu, S.-H.; Liu, H.-W. Sesquiterpenes and Polyphenols with Glucose-Uptake Stimulatory and Antioxidant Activities from the Medicinal Mushroom Sanghuangporus sanghuang. Chin. J. Nat. Med. 2021, 19, 693–699. [Google Scholar] [CrossRef]
- Kurd-Anjaraki, S.; Ramezan, D.; Ramezani, S.; Samzadeh-Kermani, A.; Pirnia, M.; Shahi, B.Y. Potential of Waste Reduction of Agro-Biomasses through Reishi Medicinal Mushroom (Ganoderma lucidum) Production Using Different Substrates and Techniques. Acta Ecol. Sin. 2021, 42, 90–101. [Google Scholar] [CrossRef]
- Hyder, M.S.; Dutta, S.D. Mushroom-Derived Polysaccharides as Antitumor and Anticancer Agent: A Concise Review. Biocatal. Agric. Biotechnol. 2021, 35, 102085. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Ladányi, M.; Omran, M.; Papp, V.; Ronkainen, V.-P.; Pónya, Z.; Papp, I.; Némedi, E.; Kiss, A. Co-Encapsulation of Probiotic Lactobacillus acidophilus and Reishi Medicinal Mushroom (Ganoderma lingzhi) Extract in Moist Calcium Alginate Beads. Int. J. Biol. Macromol. 2021, 192, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected Edible Medicinal Mushrooms from Pleurotus Genus as an Answer for Human Civilization Diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of Neuroinflammatory Pathways by Medicinal Mushrooms, with Particular Relevance to Alzheimer’s Disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Gründemann, C.; Reinhardt, J.K.; Lindequist, U. European Medicinal Mushrooms: Do They Have Potential for Modern Medicine?—An Update. Phytomedicine 2020, 66, 153131. [Google Scholar] [CrossRef]
- Neergheen, V.S.; Hip Kam, A.; Pem, Y.; Ramsaha, S.; Bahorun, T. Regulation of Cancer Cell Signaling Pathways as Key Events for Therapeutic Relevance of Edible and Medicinal Mushrooms. Semin. Cancer Biol. 2020, 80, 145–156. [Google Scholar] [CrossRef]
- Karuppaiya, P.; Akbar, A.K. Mycotherapy of Antrodia Salmonea: A Taiwanese Medicinal Mushroom. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 409–419. ISBN 9789811363818. [Google Scholar]
- Fung, S.-Y.; Tan, C.-S. Tiger Milk Mushroom (The Lignosus trinity) in Malaysia: A Medicinal Treasure Trove. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 349–369. ISBN 9789811363818. [Google Scholar]
- Yongabi, K.A. African Medicinal Mushrooms: Source of Biopharmaceuticals for the Treatment of Noncommunicable Diseases—A Review. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 335–347. ISBN 9789811363818. [Google Scholar]
- Venturella, G.; Saporita, P.; Gargano, M.L. Current Research on Medicinal Mushrooms in Italy. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 317–333. ISBN 9789811363818. [Google Scholar]
- Chan, X.H.; Sabaratnam, V.; Abdullah, N.; Phan, C.-W. A 53-Year Bibliometric and Scientometric Analysis of Research in Culinary and Medicinal Mushrooms. Int. J. Med. Mushrooms 2020, 22, 521–534. [Google Scholar] [CrossRef]
- Buranrat, B.; Sangdee, K.; Thammawat, S.; Sangdee, A. Mechanisms of Crude Protein from Medicinal Mushroom Ophiocordyceps sobolifera against Human Breast MCF-7 Cancer Cells. Biologia 2020, 75, 1759–1768. [Google Scholar] [CrossRef]
- Rizzo, G.; Goggi, S.; Giampieri, F.; Baroni, L. A Review of Mushrooms in Human Nutrition and Health. Trends Food Sci. Technol. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic Potential of Mushrooms in Diabetes Mellitus: Role of Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Deveci, E.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. Inhibitory Activities of Medicinal Mushrooms on α-Amylase and α-Glucosidase-Enzymes Related to Type 2 Diabetes. S. Afr. J. Bot. 2021, 137, 19–23. [Google Scholar] [CrossRef]
- Krittanawong, C.; Isath, A.; Hahn, J.; Wang, Z.; Fogg, S.E.; Bandyopadhyay, D.; Jneid, H.; Virani, S.S.; Tang, W.H.W. Mushroom Consumption and Cardiovascular Health: A Systematic Review. Am. J. Med. 2021, 134, 637–642.e2. [Google Scholar] [CrossRef]
- Lin, D.S. Case Report: Resolution of Rheumatoid Arthritis in a Patient Consuming Psilocybin Mushrooms. J. Healthc. 2020, 3, 25–32. [Google Scholar] [CrossRef]
- Ellan, K.; Thayan, R.; Raman, J.; Hidari, K.I.P.J.; Ismail, N.; Sabaratnam, V. Anti-Viral Activity of Culinary and Medicinal Mushroom Extracts against Dengue Virus Serotype 2: An in-Vitro Study. BMC Complementary Altern. Med. 2019, 19, 260. [Google Scholar] [CrossRef]
- Pradeep, P.; Manju, V.; Ahsan, M.F. Antiviral Potency of Mushroom Constituents. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 275–297. ISBN 9789811363818. [Google Scholar]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The Antiviral, Anti-Inflammatory Effects of Natural Medicinal Herbs and Mushrooms and SARS-CoV-2 Infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Lee, W.; Fujihashi, A.; Govindarajulu, M.; Ramesh, S.; Deruiter, J.; Majrashi, M.; Almaghrabi, M.; Nadar, R.M.; Moore, T.; Agrawal, D.C.; et al. Role of Mushrooms in Neurodegenerative Diseases. In Medicinal Mushrooms; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 223–249. ISBN 9789811363818. [Google Scholar]
- Živković, L.; Bajić, V.; Bruić, M.; Borozan, S.; Popić, K.; Topalović, D.; Santibanez, J.; Spremo-Potparević, B. Antigenotoxic and Antioxidant Potential of Medicinal Mushrooms (Immune assist) against DNA Damage Induced by Free Radicals—An in Vitro Study. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2019, 845, 403078. [Google Scholar] [CrossRef]
- Khatun, M.A.; Sato, S.; Konishi, T. Obesity Preventive Function of Novel Edible Mushroom, Basidiomycetes-X (Echigoshirayukidake): Manipulations of Insulin Resistance and Lipid Metabolism. J. Tradit. Complementary Med. 2020, 10, 245–251. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. JoF 2021, 7, 397. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. IJMS 2021, 22, 634. [Google Scholar] [CrossRef]
- Shah, A.; ul Ashraf, Z.; Gani, A.; Masoodi, F.A.; Gani, A. β-Glucan from Mushrooms and Dates as a Wall Material for Targeted Delivery of Model Bioactive Compound: Nutraceutical Profiling and Bioavailability. Ultrason. Sonochem. 2022, 82, 105884. [Google Scholar] [CrossRef] [PubMed]
- Moumita, S.; Das, B. Assessment of the Prebiotic Potential and Bioactive Components of Common Edible Mushrooms in India and Formulation of Synbiotic Microcapsules. LWT 2022, 156, 113050. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Magdziak, Z.; Budzyńska, S.; Stuper-Szablewska, K.; Niedzielski, P.; Mleczek, M. The Effect of Drying Temperature on Bioactive Compounds and Antioxidant Activity of Leccinum scabrum (Bull.) Gray and Hericium erinaceus (Bull.) Pers. J. Food Sci. Technol. 2020, 57, 513–525. [Google Scholar] [CrossRef]
- Saetang, N.; Rattanapot, T.; Manmai, N.; Amornlerdpison, D.; Ramaraj, R.; Unpaprom, Y. Effect of Hot Water Extraction Process on Schizophyllan from Split Gill Mushroom. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Khovpachev, A.A.; Basharin, V.A.; Chepur, S.V.; Volobuev, S.V.; Yudin, M.A.; Gogolevsky, A.S.; Nikiforov, A.S.; Kalinina, L.B.; Tyunin, M.A. Actual Concepts of Higher Fungi’s Toxins: Simple Nitrogen-Containing Compounds. Biol. Bull. Rev. 2021, 11, 198–212. [Google Scholar] [CrossRef]
- Nieminen, P.; Mustonen, A.-M. Toxic Potential of Traditionally Consumed Mushroom Species—A Controversial Continuum with Many Unanswered Questions. Toxins 2020, 12, 639. [Google Scholar] [CrossRef]
- Golovko, O.; Kaczmarek, M.; Asp, H.; Bergstrand, K.-J.; Ahrens, L.; Hultberg, M. Uptake of Perfluoroalkyl Substances, Pharmaceuticals, and Parabens by Oyster Mushrooms (Pleurotus ostreatus) and Exposure Risk in Human Consumption. Chemosphere 2022, 291, 132898. [Google Scholar] [CrossRef]
- Ohta, H.; Watanabe, D.; Nomura, C.; Saito, D.; Inoue, K.; Miyaguchi, H.; Harada, S.; Aita, Y. Toxicological Analysis of Satratoxins, the Main Toxins in the Mushroom Trichoderma Cornu-Damae, in Human Serum and Mushroom Samples by Liquid Chromatography–Tandem Mass Spectrometry. Forensic Toxicol. 2021, 39, 101–113. [Google Scholar] [CrossRef]
- Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; Bouziane, H.; López-Castillo, J.G.; Palma, M.; Barbero, G.F. Toxic Elements and Trace Elements in Macrolepiota procera Mushrooms from Southern Spain and Northern Morocco. J. Food Compos. Anal. 2022, 108, 104419. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom Extracts and Compounds with Suppressive Action on Breast Cancer: Evidence from Studies Using Cultured Cancer Cells, Tumor-Bearing Animals, and Clinical Trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef] [PubMed]
- Oly-Alawuba, N.; Obiakor-Okeke, P.N. Antinutrient Profile of Three Mushroom Varieties Consumed in Amaifeke, Orlu, Imo State. Food Sci. Qual. Manag. 2014, 32, 1. [Google Scholar]
- Kim, O.-H.; Booth, C.J.; Choi, H.S.; Lee, J.; Kang, J.; Hur, J.; Jung, W.J.; Jung, Y.-S.; Choi, H.J.; Kim, H.; et al. High-Phytate/Low-Calcium Diet Is a Risk Factor for Crystal Nephropathies, Renal Phosphate Wasting, and Bone Loss. eLife 2020, 9, e52709. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Vetter, J. Chitin Content of Cultivated Mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Food Chem. 2007, 102, 6–9. [Google Scholar] [CrossRef]
- Ba, D.M.; Gao, X.; Al-Shaar, L.; Muscat, J.; Chinchilli, V.M.; Ssentongo, P.; Zhang, X.; Liu, G.; Beelman, R.B.; Richie, J.P. Prospective Study of Dietary Mushroom Intake and Risk of Mortality: Results from Continuous National Health and Nutrition Examination Survey (NHANES) 2003-2014 and a Meta-Analysis. Nutr. J. 2021, 20, 80. [Google Scholar] [CrossRef]
- Urbina-Salazar, A. del R.; Inca-Torres, A.R.; Falcón-García, G.; Carbonero-Aguilar, P.; Rodríguez-Morgado, B.; del Campo, J.A.; Parrado, J.; Bautista, J. Chitinase Production by Trichoderma Harzianum Grown on a Chitin-Rich Mushroom Byproduct Formulated Medium. Waste Biomass Valorization 2019, 10, 2915–2923. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, L.; Qu, H.; Zhou, H.; Yang, H.; Chen, H. Physicochemical Characterization, Adsorption Function and Prebiotic Effect of Chitin-Glucan Complex from Mushroom Coprinus comatus. Int. J. Biol. Macromol. 2022, 206, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yoshida, S.; Mitani, N.; Egusa, M.; Takagi, M.; Izawa, H.; Matsumoto, T.; Kaminaka, H.; Ifuku, S. Disease Resistance and Growth Promotion Activities of Chitin/Cellulose Nanofiber from Spent Mushroom Substrate to Plant. Carbohydr. Polym. 2022, 284, 119233. [Google Scholar] [CrossRef]
- Mat Zin, M.I.; Jimat, D.N.; Wan Nawawi, W.M.F. Physicochemical Properties of Fungal Chitin Nanopaper from Shiitake (L. edodes), Enoki (F. velutipes) and Oyster Mushrooms (P. ostreatus). Carbohydr. Polym. 2022, 281, 119038. [Google Scholar] [CrossRef]
- Khoo, S.C.; Ma, N.L.; Peng, W.X.; Ng, K.K.; Goh, M.S.; Chen, H.L.; Tan, S.H.; Lee, C.H.; Luang-In, V.; Sonne, C. Valorisation of Biomass and Diaper Waste into a Sustainable Production of the Medical Mushroom Lingzhi Ganoderma Lucidum. Chemosphere 2022, 286, 131477. [Google Scholar] [CrossRef]
- Patocka, J.; Wu, R.; Nepovimova, E.; Valis, M.; Wu, W.; Kuca, K. Chemistry and Toxicology of Major Bioactive Substances in Inocybe Mushrooms. IJMS 2021, 22, 2218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).