Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wet Market Wastewater Sampling
2.2. Microalgae Scenedesmus sp. Preparation
2.3. Phycoremediation Experiments
2.4. Statistical Analysis
2.5. Microalgae Harvesting by Centrifugation
2.6. Flocculation Procedure Optimization
2.7. Microalgae Biomass as Fish Feeds Potential
2.7.1. Heavy Metals Content in Dry Biomass
2.7.2. Total Carbohydrate, Lipid and Protein
2.7.3. FTIR and GC–MS Analysis of Microalgae Scenedesmus sp.
3. Results and Discussion
3.1. Heavy Metals Removal by Phycoremediation
3.2. Microalgae Harvesting Efficiency
3.3. Analyses of Microalgae Biomass Protein–Lipid Content
3.4. Analyses of Heavy Metal Content in Dry Microalgae Biomass
3.5. Fatty Acid Analyses of Microalgae Biomass
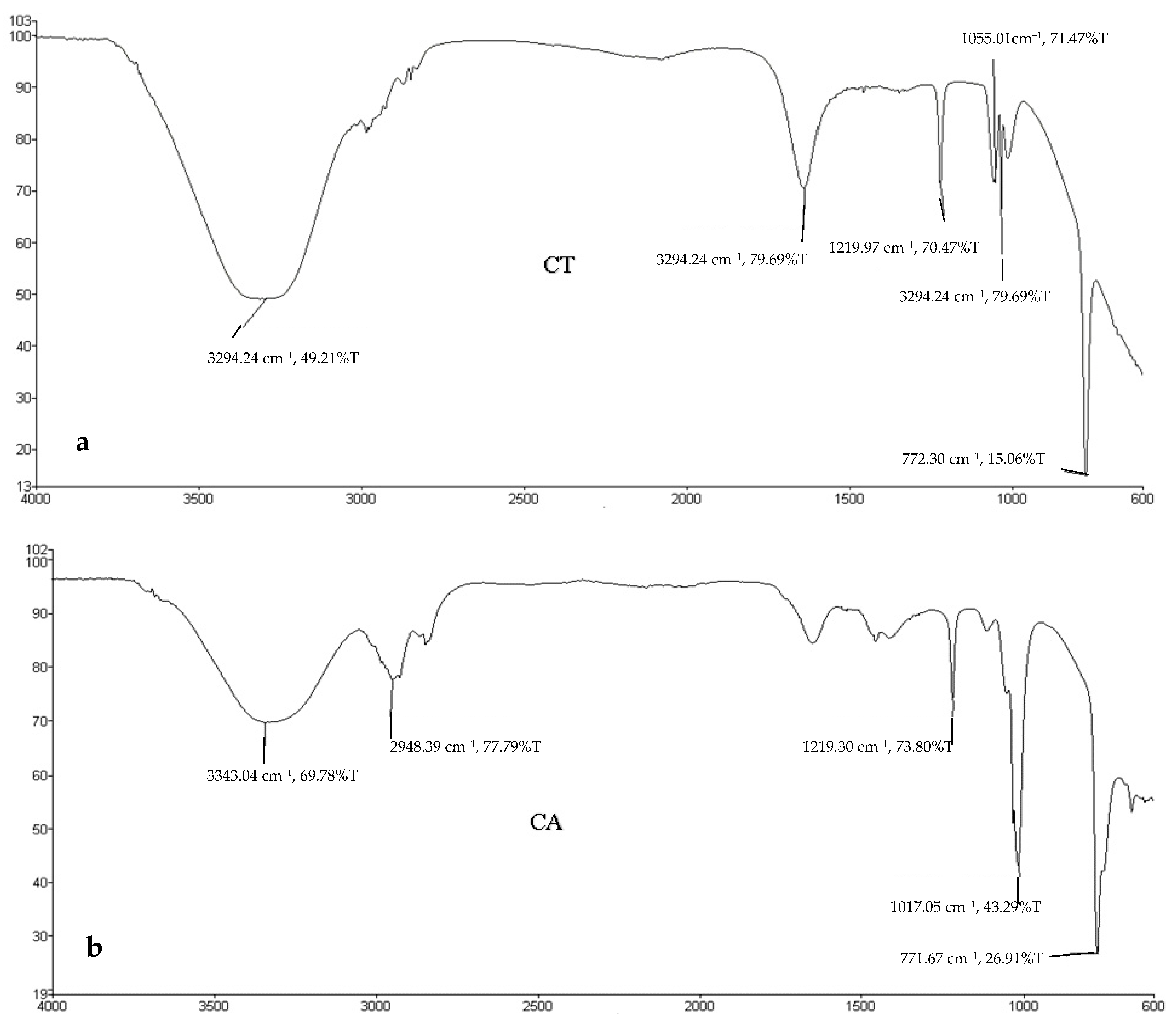
3.6. FTIR Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Badwy, T.M.; Ibrahim, E.M.; Zeinhom, M.M. Partial Replacement of Fish Meal with Dried Microalga (Chlorella spp. and Scenedesmus spp.) in Nile Tilapia (Oreochromis niloticus) Diets. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 801–811. [Google Scholar]
- Radhakrishnan, S.; Seenivasan, C.; Muralisankar, T. Effect of Dietary Replacement of Fishmeal with Chlorella Vulgaris on Growth Performance, Energy Utilisation and Digestive Enzymes in Macrobrachium Rosenbergii postlarvae. Int. J. Fish. Aquac. 2015, 7, 62–70. [Google Scholar]
- Tibaldi, E.; Chini Zittelli, G.; Parisi, G.; Bruno, M.; Giorgi, G.; Tulli, F.; Venturini, S.; Tredici, M.R.; Poli, B.M. Growth Performance and Quality Traits of European Sea Bass (D. labrax) Fed Diets Including Increasing Levels of Freeze-Dried Isochrysis sp. (T-ISO) Biomass as a Source of Protein and n-3 Long Chain PUFA in Partial Substitution of Fish Derivatives. Aquaculture 2015, 440, 60–68. [Google Scholar] [CrossRef]
- Tongsiri, S.; Mang-Amphan, K.; Peerapornpisal, Y. Effect of Replacing Fishmeal with Spirulina on Growth, Carcass Composition and Pigment of the Mekong Giant Catfish. Asian J. Agric. Sci. 2010, 2, 106–110. [Google Scholar]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Iconomou, D.; Muylaert, K. Applying Raw Poultry Litter Leachate for the Cultivation of Arthrospira platensis and Chlorella vulgaris. Algal Res. 2016, 13, 79–84. [Google Scholar] [CrossRef]
- Abdullah, M.; Fasola, M.; Muhammad, A.; Malik, S.A.; Bostan, N.; Bokhari, H.; Kamran, M.A.; Shafqat, M.N.; Alamdar, A.; Khan, M.; et al. Avian Feathers as a Non-Destructive Bio-Monitoring Tool of Trace Metals Signatures: A Case Study from Severely Contaminated Areas. Chemosphere 2015, 119, 553–561. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. Chem. Bio. Eng. Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Suresh Kumar, K.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A Promising Tool for Heavy Metal Remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Atari, L.; Esmaeili, S.; Zahedi, A.; Mohammadi, M.J.; Zahedi, A.; Babaei, A.A. Removal of Heavy Metals by Conventional Water Treatment Plants Using Poly Aluminum Chloride. Toxin Rev. 2019, 38, 127–134. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Jais, N.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Hashim, M.K.A. The dual roles of phycoremediation of wet market wastewater for nutrients and heavy metals removal and microalgae biomass production. Clean Technol. Environ. Policy 2016, 19, 37–52. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; Van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina Biomass Produced from Treatment of Aquaculture Wastewater as Agricultural Fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Leite, G.B.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Algal Biofuels: Challenges and Opportunities. Bioresour. Technol. 2013, 145, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Apandi, N.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Gani, P.; Ibrahim, A.; Kassim, A.H.M. Scenedesmus Biomass Productivity and Nutrient Removal from Wet Market Wastewater, A Bio-Kinetic Study. Waste Biomass Valorization 2019, 10, 2783–2800. [Google Scholar] [CrossRef]
- Gani, P.; Mohamed Sunar, N.; Matias-Peralta, H.; Abdul Latiff, A.A.; Mohamad Fuzi, S.F.Z. Growth of Microalgae Botryococcus sp. in Domestic Wastewater and Application of Statistical Analysis for the Optimization of Flocculation Using Alum and Chitosan. Prep. Biochem. Biotechnol. 2017, 47, 333–341. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Tiey, A.; Kassim, A.H.M. Optimising of Scenedesmus sp. Biomass 664 Production in Chicken Slaughterhouse Wastewater Using Response Surface Methodology and Potential Utilisation as Fish 665 Feeds. Environ. Sci. Pollut. Res. Int. 2019, 26, 12089–12108. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, A.L. A Simple Method for Isolation and Purification of Total Lipids from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rosmawanie, M.; Mohamed, R.; Al-Gheethi, A.; Pahazri, F.; Amir-Hashim, M.K.; Nur-Shaylinda, M.Z. Sequestering of pollutants from public market wastewater using Moringa oleifera and Cicer arietinum flocculants. J. Environ. Chem. Eng. 2018, 6, 2417–2428. [Google Scholar]
- Ajayan, K.V.; Selvaraju, M.; Unnikannan, P.; Sruthi, P. Phycoremediation of Tannery Wastewater Using Microalgae Scenedesmus Species. Int. J. Phytoremediat. 2015, 17, 907–916. [Google Scholar] [CrossRef]
- Boadi, N.; Twumasi, S. Heavy Metal Contamination in Canned Fish Marketed in Ghana. Am. J. Sci. Ind. Res. 2011, 2, 877–882. [Google Scholar] [CrossRef]
- Poon, W.C.; Herath, G.; Sarker, A.; Masuda, T.; Kada, R. River and Fish Pollution in Malaysia: A Green Ergonomics Perspective. Appl. Ergon. 2016, 57, 80–93. [Google Scholar] [CrossRef]
- Gani, P.; Mohamed Sunar, N.; Matias-Peralta, H.; Abdul Latiff, A.A.; Parjo, U.K.; Oyekanmi, A.A. Green Approach in the Bio-Removal of Heavy Metals from Wastewaters. In Proceedings of the International Symposium on Civil and Environmental Engineering 2016 (ISCEE 2016), Beijing, China, 29–30 October 2016; Volume 103, p. 06007. [Google Scholar]
- Sengar, R.M.S.; Singh, K.K.; Singh, S. Application of Phycoremediation Technology in the Treatment of Sewage Water to Reduce Pollution Load. Indian J. Sci. Res. 2011, 2, 33–39. [Google Scholar]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual Role of Microalgae: Phycoremediation of Domestic Wastewater and Biomass Production for Sustainable Biofuels Production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Solomon, S.G.; Okomoda, V.T.; Oda, S.O. Nutritional Value of Toasted Pigeon Pea, Cajanus cajan Seed and Its Utilization in the Diet of Clarias gariepinus (Burchell, 1822) Fingerlings. Aquac. Rep. 2017, 7, 34–39. [Google Scholar] [CrossRef]
- Mohd Apandi, N.; Muhamad, M.S.; Radin Mohamed, R.M.S.; Mohamed Sunar, N.; Al-Gheethi, A.; Gani, P.; Rahman, A. Optimizing of Microalgae Scenedesmus sp. Biomass Production in Wet Market Wastewater Using Response Surface Meth-709 odology. Sustainability 2021, 13, 2216. [Google Scholar] [CrossRef]
- Alipourzadeh, A.; Mehrnia, M.R.; Hallaj Sani, A.; Babaei, A. Application of Response Surface Methodology for Investigation of Membrane Fouling Behaviours in Microalgal Membrane Bioreactor: The Effect of Aeration Rate and Biomass Concentration. RSC Adv. 2016, 6, 111182–111189. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2015/186. Off. J. Eur. Union L. 2015. 31/11. Available online: https://www.legislation.gov.uk/eur/2015/186/adopted (accessed on 22 January 2022).
- Gonçalves-de-Albuquerque, C.F.; Medeiros-de-Moraes, I.M.; de Jesus Oliveira, F.M.; Burth, P.; Bozza, P.T.; Castro Faria, M.V.; Silva, A.R.; de Castro-Faria-Neto, H.C. Omega-9 Oleic Acid Induces Fatty Acid Oxidation and Decreases Organ Dysfunction and Mortality in Experimental Sepsis. PLoS ONE 2016, 11, e0153607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Long, L.; Sun, X.; Wu, H.; Li, T.; Xiang, W. Optimization of Medium Using Response Surface Methodology for Lipid Production by Scenedesmus sp. Mar. Drugs 2014, 12, 1245–1257. [Google Scholar] [CrossRef] [Green Version]
- Gour, R.S.; Chawla, A.; Singh, H.; Chauhan, R.S.; Kant, A. Characterization and Screening of Native Scenedesmus sp. Isolates Suitable for Biofuel Feedstock. PLoS ONE 2016, 11, e0155321. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. A Study on Effective Lipid Extraction Methods from Certain Fresh Water Microalgae. Elixir Int. J. 2011, 39, 4589–4591. [Google Scholar]
- Mehta, S.K.; Gaur, J.P. Use of Algae for Removing Heavy Metal Ions from Wastewater: Progress and Prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.M.; Latiff, A.A.A.; Parjo, U.K.; Embong, Z.; Khalid, A.; Tajudin, S.A.A. The Potential of Biodiesel Production from Botryococcus sp. Biomass after Phycoremediation of Domestic and Industrial Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2016, 160, 012048. [Google Scholar] [CrossRef]
- Sulaymon, A. Column Biosorption of Lead, Cadmium, Copper, and Arsenic Ions onto Algae. J. Bioprocess. Biotech. 2013, 3, 100128. [Google Scholar] [CrossRef]











| Parameter | Unit | Value |
|---|---|---|
| Turbidity | NTU | 112 |
| pH | - | 6.8 |
| Chemical oxygen demand | (mg/L) | 3506 |
| Biochemical oxygen demand | (mg/L) | 1784 |
| Dissolved oxygen | (mg/L) | 3.72 |
| Total suspended solid | (mg/L) | 225 |
| Ferum, Fe | (ppb) | 5050 |
| Cadmium, Cd | (ppb) | 17 |
| Chromium, Cr | (ppb) | 194 |
| Variables | Range and Level | ||
|---|---|---|---|
| −1 (Min) | 0 (Medium) | +1 (Max) | |
| pH | 5 | 8.5 | 12 |
| Coagulant dosage (mg/L) | 30 | 105 | 180 |
| Run | Actual Factor Values | Response Values (Harvesting Efficiency) | ||||
|---|---|---|---|---|---|---|
| Cajanus cajan (CC) | Cicer arietinum (CA) | |||||
| pH | Coagulant Dosage (mg/L) | Actual (Experiment) | Predicted (RSM Model) | Actual (Experiment) | Predicted (RSM Model) | |
| 1 | 5 | 30 | 44.56 | 45.22 | 34.78 | 34.52 |
| 2 | 12 | 30 | 70.21 | 69.54 | 45 | 44.72 |
| 3 | 5 | 180 | 72.33 | 73.99 | 56.9 | 58.19 |
| 4 | 12 | 180 | 88.9 | 89.23 | 71.23 | 72.5 |
| 5 | 5 | 105 | 65.21 | 62.89 | 62.33 | 61.3 |
| 6 | 12 | 105 | 82.33 | 82.67 | 74.54 | 73.55 |
| 7 | 8.5 | 30 | 62.11 | 62.12 | 56.83 | 57.37 |
| 8 | 8.5 | 180 | 88.34 | 86.35 | 85.65 | 83.09 |
| 9 | 8.5 | 105 | 76.77 | 77.52 | 83.43 | 85.18 |
| 10 | 8.5 | 105 | 78.2 | 77.52 | 80.23 | 85.18 |
| 11 | 8.5 | 105 | 75.43 | 77.52 | 82.21 | 85.18 |
| 12 | 8.5 | 105 | 77.21 | 77.52 | 85.69 | 85.18 |
| 13 | 8.5 | 105 | 78.2 | 77.52 | 92.33 | 85.18 |
| Type of Coagulant | Source | Sum of Squares | Df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|---|
| Cajanus cajan | Model | 1633.76 | 5 | 326.75 | 118.31 | <0.0001 |
| A—pH | 586.87 | 1 | 586.87 | 212.49 | <0.0001 | |
| B—Coagulant dosage | 880.64 | 1 | 880.64 | 318.86 | <0.0001 | |
| AB | 20.61 | 1 | 20.61 | 7.46 | 0.0293 | |
| A2 | 62 | 1 | 62 | 22.45 | 0.0021 | |
| B2 | 29.77 | 1 | 29.77 | 10.78 | 0.0134 | |
| Residual | 19.33 | 7 | 2.76 | |||
| Lack of fit | 14.01 | 3 | 4.67 | |||
| Pure error | 5.33 | 4 | 1.33 | 3.51 | 0.1285 | |
| Cor total | 1653.09 | 12 | ||||
| R2 = 0.9883, Adj. R2 = 0.9800, Pred, R2 = 0.9206, Adeq precision = 38.981 | ||||||
| Type of Coagulant | Source | Sum of Squares | Df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|---|
| Cicer arietinum | Model | 3615.16 | 5 | 723.03 | 50.6 | <0.0001 |
| C—pH | 225.22 | 1 | 225.22 | 15.76 | 0.0054 | |
| D—Coagulant dosage | 992.53 | 1 | 992.53 | 69.46 | <0.0001 | |
| CD | 4.22 | 1 | 4.22 | 0.3 | 0.6036 | |
| C2 | 870.41 | 1 | 870.41 | 60.91 | <0.0001 | |
| D2 | 617.08 | 1 | 617.08 | 43.18 | 0.0003 | |
| Residual | 100.03 | 7 | 14.29 | |||
| Lack of fit | 13.07 | 3 | 4.36 | |||
| Pure error | 86.96 | 4 | 21.74 | 0.2 | 0.8912 | |
| Cor total | 3715.19 | 12 | ||||
| R2 = 0.9731, Adj. R2 = 0.9538, Pred, R2 = 0.9353, Ad. precision = 19.726 | ||||||
| Type of Coagulant | Quadratic Equation (Coded Factors) | Quadratic Equation (Actual Factors) |
|---|---|---|
| Cajanus cajan | +77.52 + 9.89A + 12.11B − 2.27AB − 4.74A2 − 3.28B2 | −5.56 + 10.31A + 0.36B − 8.65AB − 0.39A2 − 5.84B2 |
| Cicer arietinum | +85.18 + 6.13C + 12.86D + 1.03CD − 17.75C2 − 14.95D2 | −78.211 + 25.98C + 0.70D + 3.91CD − 1.45C2 − 2.66D2 |
| Composition | CT | CA | CC | Malaysian Quality Fish Feed Standards | Yaakob et al. [23] | ||
|---|---|---|---|---|---|---|---|
| Grade A | Grade B | Grade C | |||||
| Protein (%) | 36.5 | 45.8 | 43.6 | 65 | 60 | 55 | 29.9 |
| Lipid (%) | 16 | 15 | 13 | 12 | 13 | 13 | 25.75 |
| Heavy Metals | Microalgae Biomass Concentration (ug/kg as in ppb) | Remarks | Max. Content in ug/kg (ppb) (EU) 2015/186 | ||
|---|---|---|---|---|---|
| CA | CC | CT | |||
| Ferum (Fe) | 252 ± 1.3 | 325 ± 0.3 | 220 ± 2.41 | Low | 50,000 |
| Chromium (Cr) | 0.221 ± 0.66 | 0.114 ± 0.45 | 0.35 ± 0.75 | Low | 20 |
| Cadmium (Cd) | 0.513 ± 3.9 | n.d. | 0.152 ± 2.3 | Low | 10,000 |
| Arsenic (As) | n.d. | n.d. | 0.33 ± 3.1 | Low | 2000 |
| Mercury (Hg) | 1.41 ± 0.11 | 1.03 ± 0.25 | 1.22 ± 2.3 | Low | 25,000 |
| Compound | Molecular Formula | Molecular Weight | Algal Oil (%) | Compound Possible Applications (Dr. Duke’s Phytochemical and Ethnobotanical Database, 1994) |
|---|---|---|---|---|
| CT | ||||
| Oleic Acid | C18H34O2 | 282.5 | 50 | Antiandrogenic, preventive, antifungal, anti-inflammatory, cancer, polyunsaturated fatty acid, antiarthritic, antifungal |
| Tridecanoic acid | C13H26O2 | 214.3 | 25 | Enzyme inhibitors, antifungal agents, histamine antagonists |
| Compound | Molecular Formula | Molecular weight | Area (%) | Compound Possible applications (Dr. Duke’s Phytochemical and Ethnobotanical Database, 1994) |
| CC | ||||
| Oleic acid | C18H34O2 | 282.5 | 40 | Antiandrogenic, preventive, antifungal, anti-inflammatory, cancer, polyunsaturated fatty acid, antiarthritic, antifungal |
| Linolenic Acid | C18H30O2 | 278.4 | 30 | Antioxidant, anti-inflammatory and antinociceptive activities |
| 2-Trifluoroacetoxypentadecane | C17H31F3O2 | 324.428 | 17 | Antibacterial activity against Salmonella typhi, Pseudomonas eurogenosa, Bacillus subtilis, Streptococcus faecalis, and Staphylococcus aureus |
| CA | ||||
| Oleic Acid | C18H34O2 | 282.5 | 53 | Antiandrogenic, preventive, antifungal, anti-inflammatory, cancer, polyunsaturated fatty acid, antiarthritic, antifungal |
| 6-Octadecenoic acid, (Z)- | C18H34O2 | 282.5 | 15 | Antifungal, anti-inflammatory, anticancer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apandi, N.M.; Gani, P.; Sunar, N.M.; Mohamed, R.M.S.R.; AlGheethi, A.; Apandi, A.M.; Nagarajah, R.; Shaari, N.A.R.; Cheong, K.; Rahman, R.A. Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds. Sustainability 2022, 14, 5090. https://doi.org/10.3390/su14095090
Apandi NM, Gani P, Sunar NM, Mohamed RMSR, AlGheethi A, Apandi AM, Nagarajah R, Shaari NAR, Cheong K, Rahman RA. Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds. Sustainability. 2022; 14(9):5090. https://doi.org/10.3390/su14095090
Chicago/Turabian StyleApandi, Najeeha Mohd, Paran Gani, Norshuhaila Mohamed Sunar, Radin Maya Saphira Radin Mohamed, Adel AlGheethi, Affah Mohd Apandi, Ramathasan Nagarajah, Noor Afifee Raihan Shaari, Kelly Cheong, and Roshanida A. Rahman. 2022. "Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds" Sustainability 14, no. 9: 5090. https://doi.org/10.3390/su14095090
APA StyleApandi, N. M., Gani, P., Sunar, N. M., Mohamed, R. M. S. R., AlGheethi, A., Apandi, A. M., Nagarajah, R., Shaari, N. A. R., Cheong, K., & Rahman, R. A. (2022). Scenedesmus sp. Harvesting by Using Natural Coagulant after Phycoremediation of Heavy Metals in Different Concentrations of Wet Market Wastewater for Potential Fish Feeds. Sustainability, 14(9), 5090. https://doi.org/10.3390/su14095090









