Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design and Treatments
2.3. Soil Samples and Analysis
2.4. Statistical Analysis
3. Results
3.1. Changes in Soil Organic Carbon Content under Different Fertilization Treatments
3.2. Changes in Soil Particulate Organic Carbon Content under Different Fertilization Treatments
3.3. Changes in the Soil Light Fraction Organic Carbon Content under Different Fertilization Treatments
3.4. The Responses of SOC, POC and LFOC Stocks to Different Amounts of C Input
4. Discussion
4.1. Effect of Long-Term Fertilization on Soil Organic Carbon
4.2. Effects of Long-Term Fertilization on Selected Soil Organic Carbon Fractions
4.3. How to Maintain or Enhance Soil Organic Carbon in Agricultural Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, K.l.; Wang, J.Z.; Shao, X.F.; Xu, M.G.; Li, J.W.; Wang, X.J.; Murphy, D.V. Relative contribution of maize and external manure amendment to soil carbon sequestration in a long-term intensive maize cropping system. Sci. Rep. 2015, 5, 10791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.G.; Liang, G.Q.; Zhang, F.D. The Evolution of Soil Fertility in China; Agricultural Science and Technology Press: Beijing, China, 2006. [Google Scholar]
- Yang, Z.C.; Zhao, N.; Huang, F.; Lv, Y.Z. Long-term effects of different organic and inorganic fertilizer treatments on soil organic carbon sequestration and crop yields on the North China Plain. Soil Till. Res. 2015, 146, 47–52. [Google Scholar] [CrossRef]
- Young, L.M. Carbon sequestration in agriculture: The U.S. policy in context. Am. J. Agric. Econ. 2003, 85, 1164–1170. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Philos. T. R. Soc. B. 2008, 363, 789–813. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, R.; Kundu, S.; Srivastva, A.K.; Gupta, H.S.; Prakash, V.; Bhatt, J.G. Long term fertilization effects on soil organic carbon pools in a sandy loam soil of Indian sub-Himalayas. Plant Soil 2011, 341, 109–124. [Google Scholar] [CrossRef]
- Dou, X.; He, P.; Zhu, P.; Zhou, W. Soil organic carbon dynamics under long-term fertilization in a black soil of China: Evidence from stable C isotopes. Sci. Rep. 2016, 6, 21488. [Google Scholar] [CrossRef] [Green Version]
- Purakayastha, T.J.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-tern impact of fertilizers on soil organic carbon pools and sequestration rates in maize-wheat-cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Urkurkar, I.S.; Tiwari, A.; Chitale, S.; Bajpai, R.K. Influence of long-term use of inorganic and organic manures on soil fertility and sustainable productivity of rice (Oryza sativa) and wheat (Triticum aestivum) in Inceptisols. Indian J. Agric. Sci. 2010, 80, 208–212. [Google Scholar]
- Dong, W.Y.; Zhang, X.Y.; Wang, H.M.; Dai, X.Q.; Sun, X.M.; Qiu, W.W.; Yang, F.T. Effect of different fertilizer application on the soil fertility of paddy soils in red soil region of Southern China. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; An, S.S. Responses of soil nitrogen, phosphorous and organic matter to vegetation succession on the Loess Plateau of China. J. Arid Land 2015, 7, 216–223. [Google Scholar] [CrossRef]
- Li, J.; Wen, Y.C.; Li, X.H.; Li, Y.T.; Yang, X.D.; Lin, Z.; Song, Z.Z.; Cooper, J.M.; Zhao, B.Q. Soil labile organic carbon fraction and soil organic carbon stocks as affected by long-term organic and mineral fertilization regimes in the North China. Soil Till. Res. 2018, 175, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Conrad, K.A.; Dalal, R.C.; Allen, D.C.; Fujinuma, R.; Menzies, N.W. A free light fraction carbon and nitrogen, a physically uncomplexed soil organic matter distribution within subtropical grass. Soil Res. 2018, 56, 820–828. [Google Scholar] [CrossRef]
- Sequeira, C.H.; Alley, M.M.; Jones, B.P. Evaluation of potentially labile soil organic carbon and nitrogen fractionation procedures. Soil Biol. Biochem. 2011, 43, 438–444. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Global Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Gosling, P.; Parsons, N.; Bending, G.D. What are the primary factors controlling the light fraction and particulate soil organic matter content of agricultural soils? Biol. Fert. Soils 2013, 49, 1001–1014. [Google Scholar] [CrossRef]
- Yang, X.Y.; Ren, W.D.; Sun, B.H.; Zhang, S.L. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177, 49–56. [Google Scholar] [CrossRef]
- Soil Survey Staff. Key to Soil Taxonomy, 11th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2010.
- Veihmeyer, F.J.; Hendrickson, A.H. Soil density and root penetration. Soil Sci. 1948, 65, 487–493. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2000; pp. 176–185. [Google Scholar]
- Walkley, A.J.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Qlsen, S.R.; Cole, C.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954.
- Cambardella, A.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P.; Townley–Smith, L. Light-fraction organic matter in soils from long-term crop rotation. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Frolking, S.; Harriss, R. Modeling carbon biogeochemistry in agricultural soils. Glob. Biogeochem. Cycles 1994, 8, 237–254. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, M.; He, X.; Zhang, W.; Huang, S.; Yang, X.; Liu, H.; Peng, C.; Shirato, Y.; Iizumi, T.; et al. Soil organic carbon sequestration in upland soils of northern China under variable fertilizer management and climate change scenarios. Glob. Biogeochem. Cycles 2014, 28, 319–333. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Beare, M.H.; McKim, U.F.; Skjemstad, J.O. Chemical and biological characteristics of physically uncomplexed organic matter. Soil Sci. Soc. Am. J. 2006, 70, 975–985. [Google Scholar] [CrossRef]
- Wander, M.M.; Yun, W.; Goldstein, W.A.; Aref, S.; Khan, S.A. Organic N and particulate organic matter fractions in organic and conventional farming systems with a history of manure application. Plant Soil 2007, 291, 311–321. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Li, P.Z.; Lin, X.X. Characteristics of organic materials decomposition in infertile red soils. Acta Ecol. Sin. 2002, 22, 1224–1230. [Google Scholar]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Malthi, S.S.; Lemke, R.; Wang, Z.H.; Chhabra, B.S. Tillage, nitrogen and crop residue effects on crop yield, 0nutrient uptake, soil quality, and greenhouse gas emissions. Soil Till. Res. 2006, 90, 171–183. [Google Scholar] [CrossRef]
- Rudrappa, L.; Purakayasha, T.J.; Singh, D.; Bhadraray, S. Long-term manuring and fertilization effects on soil organic carbon pools in a Typic Haplustepts of semi-arid subtropical India. Soil Till. Res. 2006, 88, 180–192. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Till. Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 3, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, D.; Yang, L. Long-term effect of chemical fertilizer, straw, and manure on labile organic matter fractions in a paddy soil. Biol. Fert. Soils 2007, 44, 93–101. [Google Scholar] [CrossRef]
- Whalen, J.K.; Hu, Q.; Liu, A. Compost applications increase water-stable aggregates in conventional and no-tillage systems. Soil Sci. Soc. Am. J. 2003, 67, 1842–1847. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Dan, C.O.; Fang, X.W.; He, Z.; Schmidt-Rohr, K. Influence of animal manure application on the chemical structures of soil organic matter as investigated by advanced solid-state NMR and FT-IR spectroscopy. Geoderma 2008, 146, 353–362. [Google Scholar] [CrossRef]
- Duan, Y.H.; Yang, H.B.; Shi, T.H.; Zhang, W.J.; Xu, M.G.; Gao, S.D. Long-term manure application to improve soil macroaggregates and plant-available nitrogen in a Mollisol. Soil Till. Res. 2021, 211, 105035. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Tuti, M.D.; Bisht, J.K.; Bhatt, J.C.; Gupta, H.S. Conservation tillage and fertilization impacts on soil aggregation and carbon pools in the Indian Himalayas under an irrigated rice-wheat rotation. Soil Sci. 2012, 177, 218–228. [Google Scholar] [CrossRef]
- Puget, P.; Drinkwater, L.E. Short-term dynamics of root- and shoot-derived carbon from a leguminous green manure. Soil Sci. Soc. Am. J. 2001, 65, 771–779. [Google Scholar] [CrossRef]
- Tuo, D.F.; Xu, M.X.; Li, Q.; Liu, S.H. Soil aggregate stability and associated structure affected by long-term fertilization for affected by long-term fertilization for a loessia soil on the Loess Plateau of China. Pol. J. Environ. Stud. 2017, 26, 827–835. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Ellert, B.H.; Carter, M.R.; Monreal, C.M. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.M.; Yan, J.; Han, X.Z.; Zou, W.X.; Chen, X.; Lu, X.C.; Feng, Y.T. Labile organic carbon fractions drive soil microbial communities after long-term fertilization. Glob. Ecol. Conserv. 2021, 32, e01867. [Google Scholar] [CrossRef]
- Tong, X.G.; Xu, M.G.; Wang, X.J.; Zhang, W.J.; Cong, R.H. Long-term fertilization effects on organic carbon fractions in a red soil of China. Catena 2010, 113, 251–259. [Google Scholar] [CrossRef]
- Blair, N.; Faulkner, R.D.; Till, A.R.; Korschens, M.; Schulz, E. Long-term management impacts on soil C, N and physical fertility: Part Ⅱ: Bad Lauchstadt static and extreme FYM experiments. Soil Till. Res. 2006, 91, 39–47. [Google Scholar] [CrossRef]
- Cai, A.D.; Xu, M.G.; Zhang, W.J.; Wang, B.R.; Cai, Z.J. Establishing and verification of the relationship between soil organic carbon storage and exogenous carbon input. J. Plant Nutr. Fertil. 2020, 26, 934–941. [Google Scholar]


| Treatments | Chemical Fertilizer | Manure (kg ha−1) | |
|---|---|---|---|
| Urea (kg N ha−1) | Calcium Superphosphate (kg P2O5 ha−1) | ||
| Control | 0 | 0 | 0 |
| N1P1 | 60 | 37.5 | 0 |
| N2P2 | 120 | 75 | 0 |
| N3P3 | 180 | 112.5 | 0 |
| N4P4 | 240 | 150 | 0 |
| N2P1M1 | 120 | 37.5 | 22,500 |
| N3P2M3 | 180 | 75 | 67,500 |
| N4P2M2 | 240 | 75 | 45,000 |
| M6 | 0 | 0 | 135,000 |
| Time | Treatments | pH | Bulk Density (g cm−3) | Porosity (%) | Clay (%) | Soil Organic Carbon (g kg−1) | Total Nitrogen (g kg−1) | Total Phoshorus (g kg−1) | Available Nitrogen (mg kg−1) | Available Phosphorus (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1992 | Initial | 8.40 ± 0.03 b | 1.21 ± 0.03 b | 54.40 ± 0.06 | 19.25 ± 0.45 a | 13.80 ± 0.03 c | 1.05 ± 0.02 c | 0.76 ± 0.02 e | 117.69 ± 0.41 c | 4.84 ± 0.29 g |
| 2016 | Control | 8.65 ± 0.03 a | 1.33 ± 0.01 a | nd | 17.21 ± 0.12 b | 9.82 ± 0.04 e | 0.83 ± 0.01 d | 0.70 ± 0.01 e | 62.76 ± 0.02f | 4.56 ± 0.05 g |
| N1P1 | 8.49 ± 0.04 b | 1.27 ± 0.02 a | nd | 16.24 ± 0.09 b | 9.80 ± 0.07 e | 1.02 ± 0.12 c | 0.61 ± 0.01f | 62.48 ± 2.03f | 10.06 ± 0.04 f | |
| N2P2 | 8.42 ± 0.01 b | 1.26 ± 0.06 a | nd | 20.63 ± 0.37 a | 10.52 ± 0.10 d | 1.28 ± 0.01 c | 0.80 ± 0.02 d | 70.90 ± 1.08 e | 22.29 ± 0.02 e | |
| N3P3 | 8.47 ± 0.05 b | 1.34 ± 0.02 a | nd | 14.80 ± 0.25 c | 10.26 ± 0.02 d | 1.29 ± 0.03 c | 0.83 ± 0.01 d | 84.81 ± 0.03 d | 33.60 ± 0.08 e | |
| N4P4 | 8.49 ± 0.02 b | 1.32 ± 0.04 a | nd | 18.52 ± 0.06 b | 9.65 ± 0.05 e | 1.36 ± 0.02 c | 1.25 ± 0.02 b | 100.95 ± 8.06 c | 43.99 ± 0.03 d | |
| N2P1M1 | 8.38 ± 0.03 b | 1.20 ± 0.01 b | nd | 17.55 ± 0.13 b | 14.57 ± 0.09 c | 1.34 ± 0.01 c | 0.83 ± 0.01 d | 85.05 ± 0.05 d | 24.74 ± 0.04 e | |
| N3P2M3 | 8.16 ± 0.05 c | 1.22 ± 0.01 b | nd | 16.51 ± 0.21 b | 17.88 ± 0.27 b | 2.17 ± 0.04 a | 1.24 ± 0.02 b | 126.23 ± 0.12 b | 170.51 ± 0.09 b | |
| N4P2M2 | 8.30 ± 0.08 b | 1.16 ± 0.04 b | nd | 16.83 ± 0.17 b | 14.65 ± 0.84 c | 1.93 ± 0.03 b | 0.95 ± 0.02 c | 101.66 ± 0.09 c | 86.97 ± 0.10 c | |
| M6 | 7.82 ± 0.02 d | 1.16 ± 0.03 b | nd | 12.66 ± 0.04 d | 25.11 ± 0.34 a | 2.69 ± 0.05 a | 1.48 ± 0.03 a | 150.80 ± 0.13 a | 211.24 ± 0.12 a |
| Year | Soil Particulate Organic Carbon (g kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | 1992 | 1996 | 2001 | 2006 | 2011 | 2016 | |
| Control | 2.91 ± 0.11 a | 2.38 ± 0.05 Bb | 1.80 ± 0.10 Dd | 2.05 ± 0.06 Cc | 2.00 ± 0.05 Cc | 1.87 ± 0.01 Ed | |
| N1P1 | 2.91 ± 0.11 a | 2.39 ± 0.01 Bb | 1.80 ± 0.08 Dc | 2.37 ± 0.08 Bb | 2.06 ± 0.05 Cc | 2.31 ± 0.03 Db | |
| N2P2 | 2.91 ± 0.11 a | 2.64 ± 0.07 ABab | 1.83 ± 0.03 Dc | 2.26 ± 0.07 Bb | 2.25 ± 0.12 Cb | 2.15 ± 0.02 Db | |
| N3P3 | 2.91 ± 0.11 a | 2.07 ± 0.12 Cb | 1.89 ± 0.05 Dc | 2.16 ± 0.08 Bb | 2.13 ± 0.04 Cb | 1.75 ± 0.01 Fc | |
| N4P4 | 2.91 ± 0.11 a | 2.52 ± 0.12 ABab | 2.16 ± 0.10 Cb | 2.03 ± 0.02 Cbc | 1.87 ± 0.05 Cc | 1.56 ± 0.01 Fd | |
| N2P1M1 | 2.91 ± 0.11 a | 2.59 ± 0.01 ABb | 2.20 ± 0.04 Cc | 2.75 ± 0.14 Bab | 2.94 ± 0.10 Ba | 3.08 ± 0.28 Ca | |
| N3P2M3 | 2.91 ± 0.11 a | 2.90 ± 0.16 Aa | 2.73 ± 0.07 Ba | 3.06 ± 0.08 ABa | 3.14 ± 0.03 ABa | 3.30 ± 0.01 Ba | |
| N4P2M2 | 2.91 ± 0.11 a | 2.03 ± 0.05 Cc | 2.66 ± 0.01 Bb | 3.03 ± 0.10 ABa | 3.02 ± 0.11 ABa | 2.98 ± 0.01 Ca | |
| M6 | 2.91 ± 0.11 a | 3.18 ± 0.10 Aa | 3.09 ± 0.04 Aa | 3.36 ± 0.08 Aa | 3.52 ± 0.19 Aa | 3.65 ± 0.01 Aa | |
| Year | Light Fraction Organic Carbon (g kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | 1992 | 1996 | 2001 | 2006 | 2011 | 2016 | |
| Control | 2.14 ± 0.04 a | 2.01 ± 0.02 Ba | 2.20 ± 0.05 Ba | 1.13 ± 0.01 Db | 0.92 ± 0.01 Db | 1.06 ± 0.10 Eb | |
| N1P1 | 2.14 ± 0.04 a | 1.75 ± 0.02 Cb | 2.05 ± 0.04 Ba | 1.43 ± 0.02 Cb | 0.74 ± 0.01 Dc | 1.52 ± 0.05 Db | |
| N2P2 | 2.14 ± 0.04 a | 1.62 ± 0.04 Cb | 2.13 ± 0.01 Ba | 0.72 ± 0.01 Ec | 1.06 ± 0.06 Cbc | 1.49 ± 0.07 Db | |
| N3P3 | 2.14 ± 0.04 a | 1.93 ± 0.09 Ca | 2.18 ± 0.01 Ba | 0.67 ± 0.02 Ec | 0.79 ± 0.02 Dc | 1.50 ± 0.01 Db | |
| N4P4 | 2.14 ± 0.04 a | 2.21 ± 0.09 ABa | 2.34 ± 0.06 ABa | 1.16 ± 0.04 Dc | 1.46 ± 0.01 Bb | 2.29 ± 0.12 Ca | |
| N2P1M1 | 2.14 ± 0.04 b | 2.51 ± 0.02 Aa | 2.70 ± 0.04 Aa | 1.41 ± 0.08 Cd | 1.36 ± 0.04 Bd | 1.68 ± 0.04 Dc | |
| N3P2M3 | 2.14 ± 0.04 bc | 2.41 ± 0.13 Ab | 2.76 ± 0.02 Ab | 2.08 ± 0.02 ABc | 1.62 ± 0.03 ABd | 3.22 ± 0.11 Ba | |
| N4P2M2 | 2.14 ± 0.04 c | 2.06 ± 0.02 Bc | 2.40 ± 0.04 ABb | 1.86 ± 0.04 Bd | 1.49 ± 0.05 Be | 2.98 ± 0.12 BCa | |
| M6 | 2.14 ± 0.04 c | 2.77 ± 0.07 Ab | 2.62 ± 0.01 Ab | 2.78 ± 0.02 Ab | 2.80 ± 0.13 Ab | 3.78 ± 0.36 Aa | |
| Treatment | SOC | POC | LFOC | |||
|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | 0–20 cm | 20–40 cm | |
| Control | 9.82 ± 0.04 Ea | 8.90 ± 0.05 Db | 1.87 ± 0.01 Ea | 0.89 ± 0.01 Eb | 1.06 ± 0.10 Ea | 0.97 ± 0.01 Da |
| N1P1 | 9.80 ± 0.07 Ea | 6.57 ± 0.02 Eb | 2.31 ± 0.03 Da | 0.91 ± 0.03 Eb | 1.52 ± 0.05 Da | 1.27 ± 0.04 Cb |
| N2P2 | 10.52 ± 0.10 Da | 8.98 ± 0.05 Db | 2.15 ± 0.02 Da | 1.93 ± 0.05 Bb | 1.49 ± 0.07 Da | 1.01 ± 0.01 Db |
| N3P3 | 10.26 ± 0.02 Da | 9.37 ± 0.09 CDb | 1.75 ± 0.01 Fa | 1.17 ± 0.04 Db | 1.50 ± 0.01 Da | 0.98 ± 0.02 Db |
| N4P4 | 9.65 ± 0.05 Ea | 6.57 ± 0.02 Eb | 1.56 ± 0.01 Fa | 0.93 ± 0.02 Eb | 2.29 ± 0.12 Ca | 0.62 ± 0.02 Eb |
| N2P1M1 | 14.57 ± 0.09 Ca | 12.07 ± 0.17 Bb | 3.08 ± 0.08 Ca | 1.48 ± 0.03 Cb | 1.68 ± 0.04 Da | 1.29 ± 0.03 Cb |
| N3P2M3 | 17.88 ± 0.27 Ba | 12.74 ± 0.09 Bb | 3.30 ± 0.01 Ba | 2.06 ± 0.02 Bb | 3.22 ± 0.11 Ba | 1.79 ± 0.02 Bb |
| N4P2M2 | 14.65 ± 0.04 Ca | 10.03 ± 0.29 Cb | 2.98 ± 0.01 Ca | 1.64 ± 0.01 Cb | 2.98 ± 0.12 BCa | 1.34 ± 0.01 Cb |
| M6 | 25.11 ± 0.34 Aa | 13.62 ± 0.20 Ab | 3.65 ± 0.01 Aa | 2.37 ± 0.06 Ab | 3.78 ± 0.36 Aa | 2.87 ± 0.03 Ab |
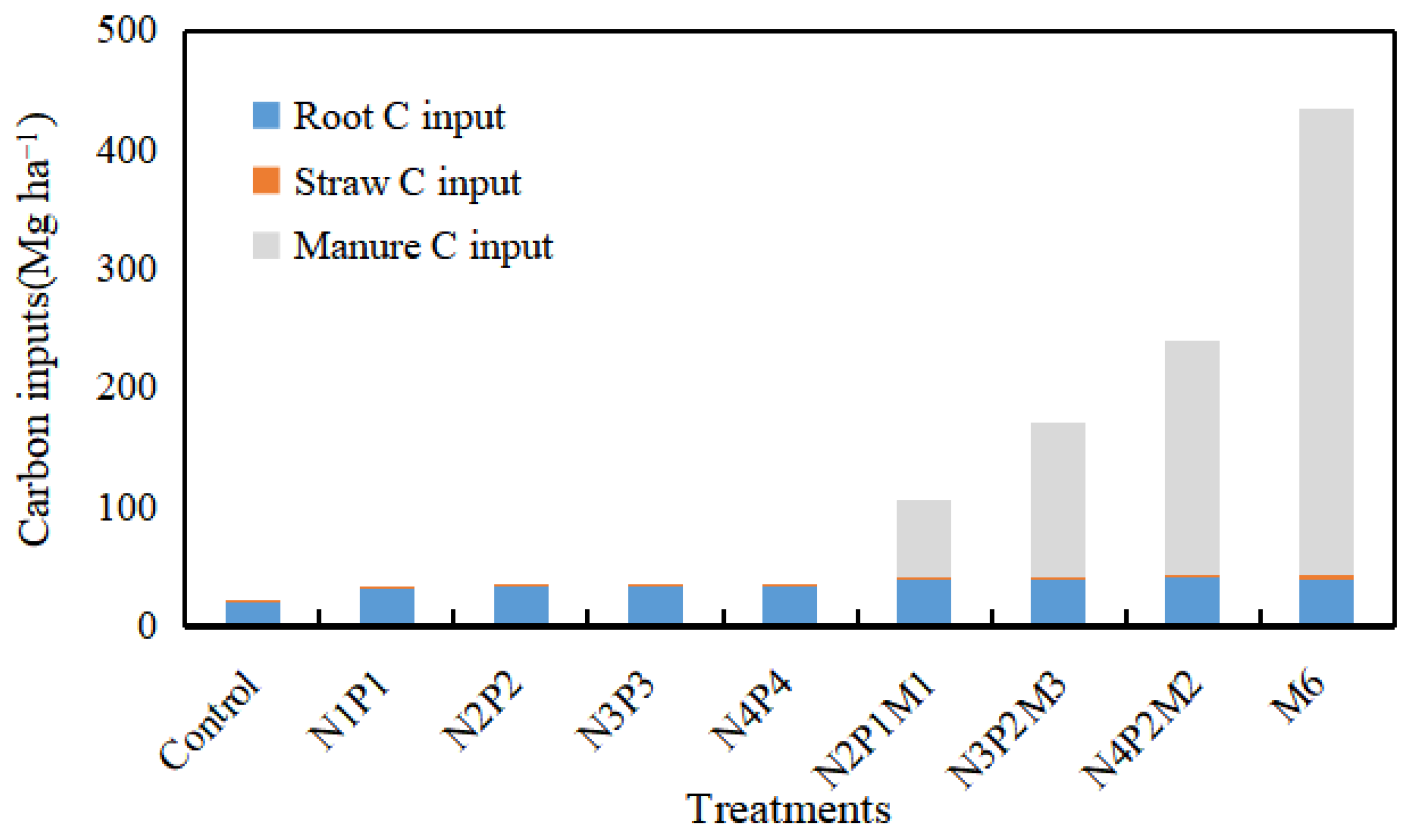
| Treatment | Total C Input (Mg ha−1) | ∆Cstocks (Mg ha−1) | CSR (Mg ha−1 y−1) | CSE (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SOC | POC | LFOC | SOC | POC | LFOC | SOC | POC | LFOC | ||
| Control | 20.54 ± 0.04 F | |||||||||
| N1P1 | 33.08 ± 0.96 E | −5.02 ± 0.10 F | 1.21 ± 0.01 D | 1.75 ± 0.07 E | −0.21 ± 0.01 F | 0.05 ± 0.01 C | 0.07 ± 0.01 D | −40.0 ± 0.01 F | 9.63 ± 0.01 B | 13.95 ± 0.01 B |
| N2P2 | 36.22 ± 0.36 E | 4.66 ± 0.17 E | 3.76 ± 0.07 C | 1.45 ± 0.09 E | 0.19 ± 0.01 E | 0.16 ± 0.01 B | 0.06 ± 0.01 D | 29.73 ± 0.01 B | 24.00 ± 0.01 A | 9.22 ± 0.01 C |
| N3P3 | 36.02 ± 0.70 E | 5.27 ± 0.13 E | 0.86 ± 0.08 E | 1.43 ± 0.04 E | 0.22 ± 0.01 E | 0.04 ± 0.01 C | 0.06 ± 0.01 D | 34.05 ± 0.01 A | 5.56 ± 0.01 C | 9.21 ± 0.01 C |
| N4P4 | 35.32 ± 0.65 E | −4.71 ± 0.08 F | −0.42 ± 0.06 F | 2.24 ± 0.18 D | −0.20 ± 0.01 F | −0.02 ± 0.01 D | 0.09 ± 0.01 CD | −31.84 ± 0.01 F | −2.82 ± 0.01 F | 15.18 ± 0.02 A |
| N2P1M1 | 106.53 ± 1.53 D | 21.08 ± 0.32 C | 4.74 ± 0.03 B | 2.47 ± 0.09 D | 0.88 ± 0.02 C | 0.20 ± 0.01 A | 0.10 ± 0.01 C | 24.51 ± 0.01 C | 5.51 ± 0.01 C | 2.87 ± 0.01 E |
| N3P2M3 | 237.32 ± 1.67 B | 26.24 ± 0.39 B | 5.84 ± 0.03 A | 6.68 ± 0.13 B | 1.09 ± 0.02 B | 0.24 ± 0.01 A | 0.28 ± 0.01 A | 12.11 ± 0.01 D | 2.69 ± 0.01 D | 3.08 ± 0.01 D |
| N4P2M2 | 174.45 ± 2.70 C | 17.43 ± 0.36 D | 4.09 ± 0.02 B | 5.07 ± 0.13 C | 0.73 ± 0.02 D | 0.17 ± 0.01 B | 0.21 ± 0.01 B | 11.32 ± 0.01 D | 2.66 ± 0.01 D | 3.29 ± 0.01 D |
| M6 | 435.59 ± 3.36 A | 31.00 ± 0.53 A | 5.21 ± 0.06 A | 8.12 ± 0.39 A | 1.29 ± 0.03 A | 0.22 ± 0.01 A | 0.34 ± 0.02 A | 7.47 ± 0.01 E | 1.26 ± 0.01 E | 1.96 ± 0.01 F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Cheng, M.; Wen, Y.; Darboux, F. Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China. Sustainability 2022, 14, 5109. https://doi.org/10.3390/su14095109
Xiang Y, Cheng M, Wen Y, Darboux F. Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China. Sustainability. 2022; 14(9):5109. https://doi.org/10.3390/su14095109
Chicago/Turabian StyleXiang, Yun, Man Cheng, Yongli Wen, and Frédéric Darboux. 2022. "Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China" Sustainability 14, no. 9: 5109. https://doi.org/10.3390/su14095109
APA StyleXiang, Y., Cheng, M., Wen, Y., & Darboux, F. (2022). Soil Organic Carbon Sequestration under Long-Term Chemical and Manure Fertilization in a Cinnamon Soil, Northern China. Sustainability, 14(9), 5109. https://doi.org/10.3390/su14095109






