Impact of River-Reservoir Hybrid System on Zooplankton Community and River Connectivity
Abstract
:1. Introduction
2. Materials and Methods
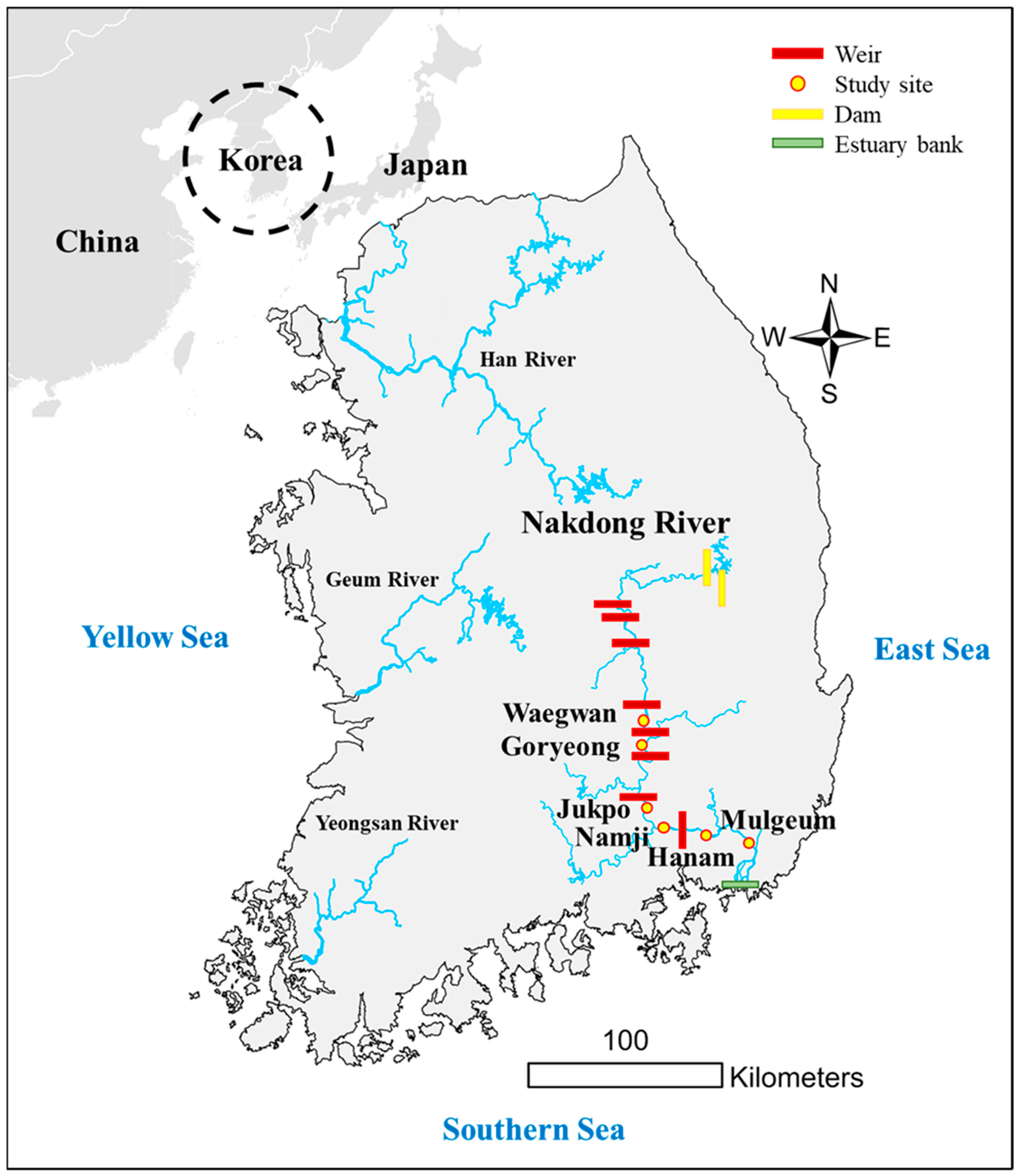
2.1. Study Sites
2.2. Field Survey
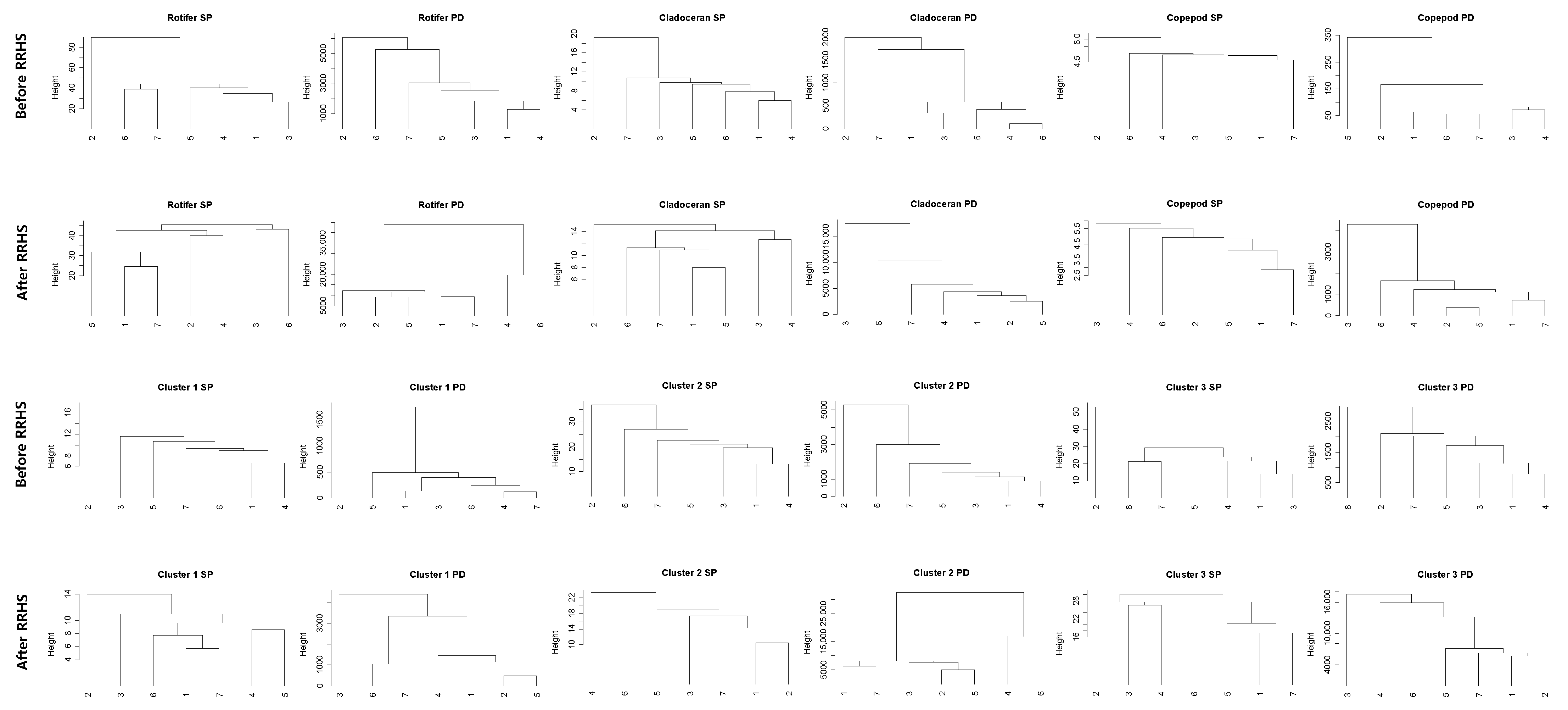
2.3. Data Analysis
3. Results
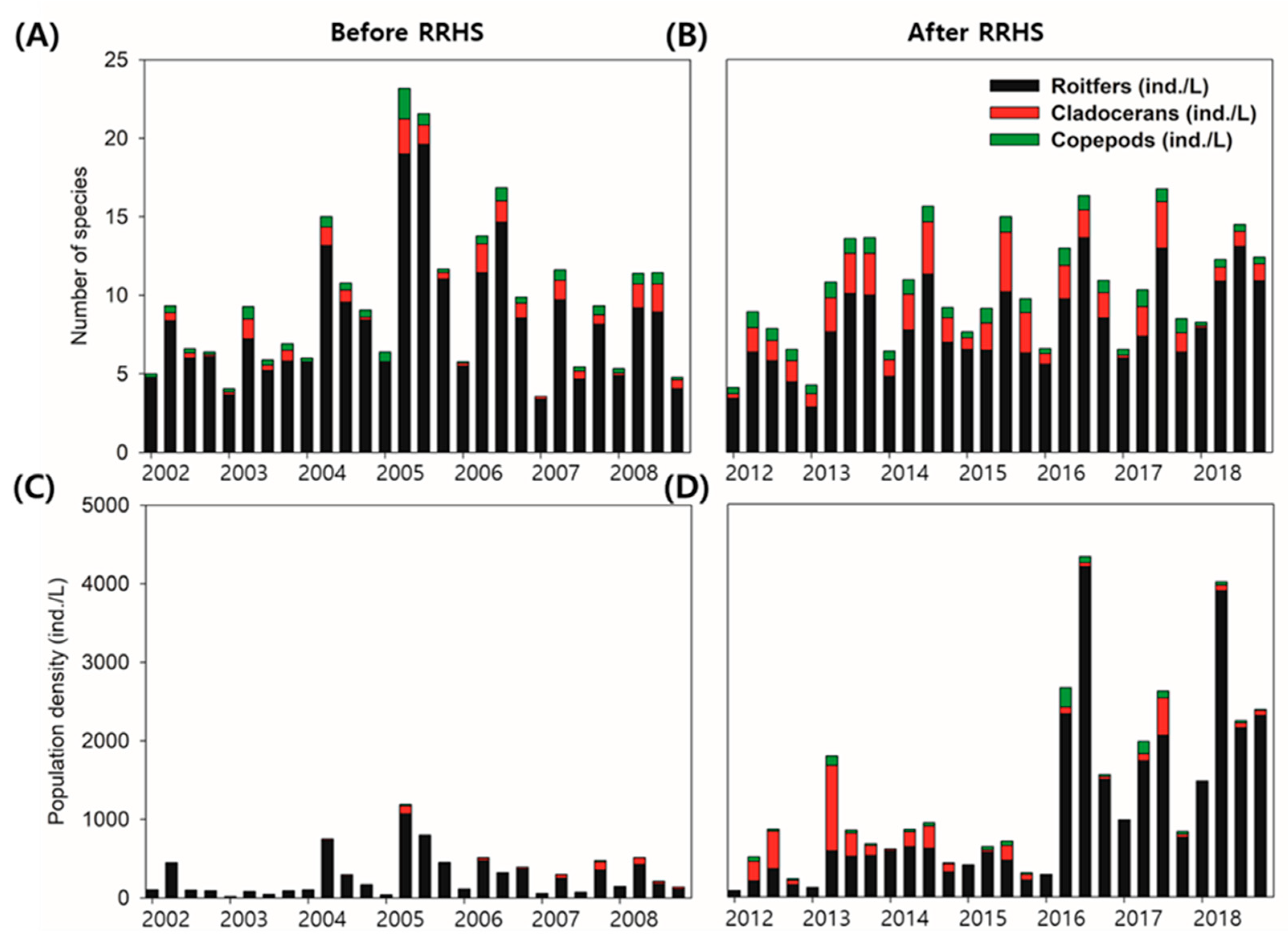
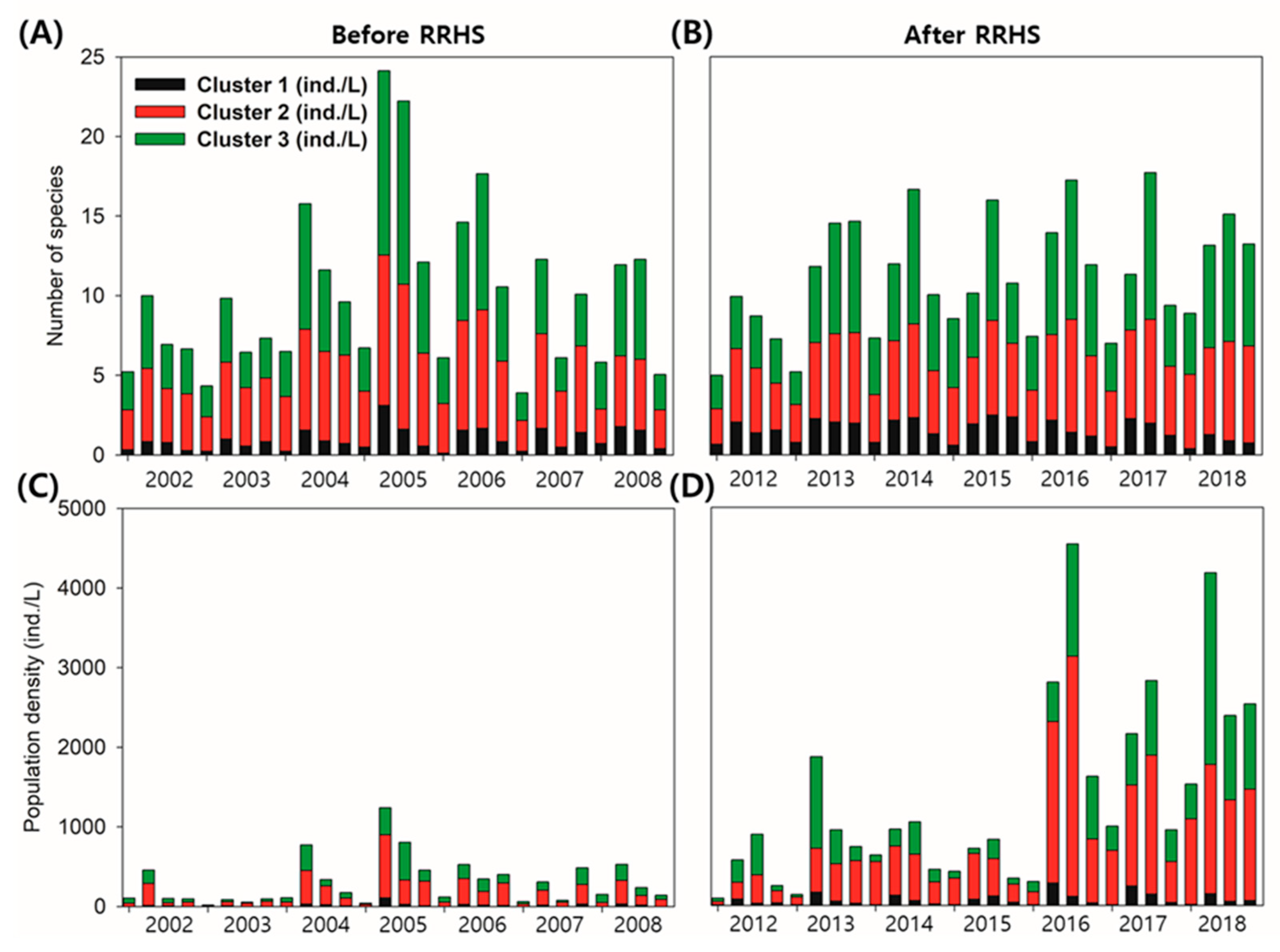
3.1. Time-Series Fluctuations of Zooplankton
3.2. Time-Series Trends and Longitudinal Patterns
4. Discussion
4.1. Importance of Long-Term Zooplankton Data
4.2. River–Reservoir Hybrid System with Zooplankton
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, K.H.; Jeong, K.S.; Joo, G.J.; Kim, H.W. The spring metazooplankton dynamics in the river-reservoir hybrid system (Nakdong River, Korea): Its role in controlling the phytoplankton biomass. Korean J. Ecol. Environ. 2003, 36, 420–426. [Google Scholar]
- Chen, W.; Olden, J.D. Designing flows to resolve human and environmental water needs in a dam-regulated river. Nat. Commun. 2017, 8, 2158. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poff, N.L.R.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, C.; Zhao, J.; Xia, J.; Yu, Q.; Eamus, D. Zooplankton in highly regulated rivers: Changing with water environment. Ecol. Eng. 2013, 58, 323–334. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakamura, F. Impacts of dam-regulated flows on channel morphology and riparian vegetation: A longitudinal analysis of Satsunai River. Japan. Landsc. Ecol. Eng. 2011, 7, 65–77. [Google Scholar] [CrossRef]
- Kim, D.K.; Jeong, K.S.; Chang, K.H.; La, G.H.; Joo, G.J.; Kim, H.W. Patterning zooplankton communities in accordance with annual climatic conditions in a regulated river system. Nakdong River, South Korea. Int. Rev. Hydrobiol. 2012, 97, 55–72. [Google Scholar] [CrossRef]
- Falke, J.A.; Gido, K.B. Spatial effects of reservoirs on stream fish assemblages in the Great Plains, USA. River Res. Appl. 2006, 22, 55–68. [Google Scholar] [CrossRef]
- Branco, P.; Segurado, P.; Santos, J.M.; Pinheiro, P.; Ferreira, M.T. Does longitudinal connectivity loss affect the distribution of freshwater fish? Ecol. Eng. 2012, 48, 70–78. [Google Scholar] [CrossRef]
- Zhao, K.; Song, K.; Pan, Y.; Wang, L.; Da, L.; Wang, Q. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecol. Indic. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Jo, H.; Jeppesen, E.; Ventura, M.; Buchaca, T.; Gim, J.S.; Yoon, J.D.; Kim, D.W.; Joo, G.J. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total Environ. 2019, 657, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Rourke, M.L.; Robinson, W.; Baumgartner, L.J.; Doyle, J.; Growns, I.; Thiem, J.D. Sequential fishways reconnect a coastal river reflecting restored migratory pathways for an entire fish community. Restor. Ecol. 2019, 27, 399–407. [Google Scholar] [CrossRef]
- Im, R.Y.; Ji, Y.K.; Nishihiro, J.; Joo, G.J. Large weir construction causes the loss of seasonal habitat in riverine wetlands: A case study of the four large river projects in South Korea. Ecol. Eng. 2020, 152, 105839. [Google Scholar] [CrossRef]
- Liu, J.; Soininen, J.; Han, B.; Declerck, S.A.J. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J. Biogeogr. 2013, 40, 2238–2248. [Google Scholar] [CrossRef]
- Liu, S.; Xie, G.; Wang, L.; Cottenie, K.; Lid, D.; Wang, B. Different roles of environmental variables and spatial factors in structuring stream benthic diatom and macroinvertebrate in Yangtze River Delta, China. Ecol. Indic. 2016, 61, 602–611. [Google Scholar] [CrossRef]
- Souza, C.A.D.; Vieira, L.C.G.; Legendre, P.; Carvalho, P.D.; Velho, L.F.M.; Beisner, B.E. Damming interacts with the flood pulse to alter zooplankton communities in an Amazonian river. Freshw. Biol. 2019, 64, 1040–1053. [Google Scholar] [CrossRef]
- Kim, H.W.; Joo, G.J. The longitudinal distribution and community dynamics of zooplankton in a regulated large river: A case study of the Nakdong River (Korea). Hydrobiologia 2000, 438, 171–184. [Google Scholar] [CrossRef]
- Kamboj, V.; Kamboj, N. Spatial and temporal variation of zooplankton assemblage in the mining-impacted stretch of Ganga River, Uttarakhand, India. Environ. Sci. Pollut. Res. 2020, 27, 27135–27146. [Google Scholar] [CrossRef]
- Folt, C.L.; Burns, C.W. Biological drivers of zooplankton patchiness. Trends Ecol. Evol. 1999, 14, 300–305. [Google Scholar] [CrossRef]
- Ko, E.J.; Kim, D.K.; Jung, E.S.; Heo, Y.J.; Joo, G.J.; Kim, H.W. Comparison of Zooplankton Community Patterns in Relation to Sediment Disturbances by Dredging in the Guemho River, Korea. Water 2020, 12, 3434. [Google Scholar] [CrossRef]
- Pachepsky, E.; Lutscher, F.; Nisbet, R.M.; Lewis, M.A. Persistence, spread and the drift paradox. Theor. Popul. Biol. 2005, 67, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malazarte, J.M.; Lee, H.; Kim, H.W.; Sin, Y. Spatial and temporal dynamics of potentially toxic cyanobacteria in the riverine region of a temperate estuarine system altered by weirs. Water 2017, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Romo, S.; Soria, J.; Fernandez, F.; Ouahid, Y.; Barón-solá, Á. Water residence time and the dynamics of toxic cyanobacteria. Freshw. Biol. 2013, 58, 513–522. [Google Scholar] [CrossRef]
- Kim, H.G.; Hong, S.; Kim, D.K.; Joo, G.J. Drivers shaping episodic and gradual changes in phytoplankton community succession: Taxonomic versus functional groups. Sci. Total Environ. 2020, 734, 138940. [Google Scholar] [CrossRef]
- Shen, J.; Qin, G.; Yu, R.; Zhao, Y.; Yang, J.; An, S.; Liu, R.; Leng, X.; Wan, Y. Urbanization has changed the distribution pattern of zooplankton species diversity and the structure of functional groups. Ecol. Indic. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Blondel, J. Guilds or functional groups: Does it matter? Oikos 2003, 100, 223–231. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.H.; Hwang, G.; Jun, M.S.; Choi, K. Eutrophication of reservoirs in South Korea. Limnology 2001, 2, 223–229. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Q.; Xu, D.; Lin, J.; Cheng, S.; Wu, Z. Effects of sediment dredging on water quality and zooplankton community structure in a shallow of eutrophic lake. J. Environ. Sci. 2010, 22, 218–224. [Google Scholar] [CrossRef]
- Braun, L.M.; Brucet, S.; Mehner, T. Top-down and bottom-up effects on zooplankton size distribution in a deep stratified lake. Aquat. Ecol. 2021, 55, 527–543. [Google Scholar] [CrossRef]
- Werner, E.E.; Hall, D.J. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 1974, 55, 1042–1052. [Google Scholar] [CrossRef]
- Manatunge, J.; Asaeda, T.; Priyadarshana, T. The influence of structural complexity on fish-zooplankton interactions: A study using artificial submerged macrophytes. Environ. Biol. Fishes 2000, 58, 425–438. [Google Scholar] [CrossRef]
- Hrbácek, J.; Dvorakova, M.; Korinek, V.; Prochazkova, L. Demonstration of the effect of the fish stock on the species composition of zooplankton and the intensity of metabolism of the whole plankton association. Int. Assoc. Theor. Appl. Limnol. Negot. 1961, 75, 1–65. [Google Scholar]
- Brooks, J.L.; Dodson, S.I. Predation, body size, and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Iglesias, C.; Mazzeo, N.; Goyenola, G.; Fosalba, C.; Teixeira De Mello, F.; García, S.; Jeppesen, E. Field and experimental evidence of the effect of Jenynsia multidentata, a small omnivorous-planktivorous fish, on the size distribution of zooplankton in subtropical lakes. Freshw. Biol. 2008, 53, 1797–1807. [Google Scholar] [CrossRef]
- Choi, J.Y.; La, G.H.; Kim, S.K.; Jeong, K.S.; Joo, G.J. Zooplankton community distribution in aquatic plants zone: Influence of epiphytic rotifers and cladocerans in accordance with aquatic plants cover and types. Korean J. Ecol. Environ. 2013, 6, 86–93. [Google Scholar] [CrossRef]
- Kim, H.W.; Hwang, S.J.; Chang, K.H.; Jang, M.H.; Joo, G.J.; Walz, N. longitudinal difference in zooplankton grazing on phyto-and bacterioplankton in the Nakdong River (Korea). Int. Rev. Hydrobiol. 2002, 87, 281–293. [Google Scholar] [CrossRef]
- Haney, J.F.; Hall, D.J. Sugar-coated Daphnia: A preservation technique for Cladocera. Limnol. Oceanogr. 1973, 18, 331–333. [Google Scholar] [CrossRef]
- Mizuno, T.; Takahashi, E. An Illustrated Guide to Freshwater Zooplankton in Japan; Tokai University Press: Tokyo, Japan, 1999. [Google Scholar]
- National Institute of Environmental Research. Cladocera: A Practical Guide TO Common Freshwater Zooplankton; National Institute of Environmental Research: Gyeonggi-do, Korea, 2016. (In Korean) [Google Scholar]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014, 61, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Pohlert, T. Non-Parametric Trend Tests and Change-Point Detection. Thorsten Pohlert. 2016. Available online: https://cran.r-project.org/web/packages/trend/vignettes/trend.pdf (accessed on 9 February 2022).
- Gavrilov, M.B.; Tošić, I.; Marković, S.B.; Unkašević, M.; Petrović, P. Analysis of annual and seasonal temperature trends using the Mann-Kendall test in Vojvodina, Serbia. Idojaras 2016, 120, 183–198. [Google Scholar]
- Sakoe, H.; Chiba, S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans. Acoust. Speech Signal Process. 1978, 26, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Sardá-Espinosa, A. Comparing time-series clustering algorithms in R using the dtwclust Package. R Package Vignette 2017, 12, 41. [Google Scholar]
- Kim, D.K.; Jeong, K.S.; McKay, R.I.B.; Chon, T.S.; Joo, G.J. Machine learning for predictive management: Short and long term prediction of phytoplankton biomass using genetic algorithm based recurrent neural networks. Int. J. Environ. Res. 2012, 6, 95–108. [Google Scholar]
- Czerniawski, R.; Domagała, J. Small dams profoundly alter the spatial and temporal composition of zooplankton communities in running waters. Int. Rev. Hydrobiol. 2014, 99, 300–311. [Google Scholar] [CrossRef]
- Planque, B.; Taylor, A.H. Long-term changes in zooplankton and the climate of the North Atlantic. ICES J. Mar. Sci. 1998, 55, 644–654. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. Adaptive monitoring: A new paradigm for long-term research and monitoring. Trends Ecol. Evol. 2009, 24, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, D.B.; Likens, G.E.; Andersen, A.; Bowman, D.; Bull, C.M.; Burns, E.; Dickman, C.R.; Hoffmann, A.A.; Keith, D.A.; Liddell, M.J.; et al. Value of long-term ecological studies. Austral Ecol. 2012, 37, 745–757. [Google Scholar] [CrossRef]
- Lovett, G.M.; Burns, D.A.; Driscoll, C.T.; Jenkins, J.C.; Mitchell, M.J.; Rustad, L.; Haeuber, R. Who needs environmental monitoring? Front. Ecol. Environ. 2007, 5, 253–260. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Burgman, M. Practical Conservation Biology; CSIRO: Canberra, Australia, 2005. [Google Scholar]
- Gurav, M.N.; Pejaver, M.K. Survey of rotifers to evaluate the water quality of the river Gadhi and its reservoir. Ecol. Environ. Conserv. 2013, 19, 417–423. [Google Scholar]
- Gutkowska, A.; Paturej, E.; Kowalska, E. Rotifer trophic state indices as ecosystem indicators in brackish coastal waters. Oceanologia 2013, 55, 887–899. [Google Scholar] [CrossRef]
- Krzysztoń, W.; Kosiba, J. Variations in zooplankton functional groups density in freshwater ecosystems exposed to cyanobacterial blooms. Sci. Total Environ. 2020, 730, 139044. [Google Scholar] [CrossRef]


| Category | Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|---|
| Species | Rotifers | 3 | 63 | 53 |
| Cladocerans | 12 | 10 | 11 | |
| Copepods | 8 | 2 | 3 | |
| Total | 23 | 75 | 67 | |
| Swimming type | Planktonic | 19 | 0 | 67 |
| Mixing | 3 | 23 | 0 | |
| Epiphytic | 1 | 52 | 0 | |
| Total | 23 | 75 | 67 | |
| Length (µm) | Minimum | 733 | 50 | 70 |
| Maximum | 2000 | 810 | 700 | |
| Category | Before RRHS | After RRHS | |||
|---|---|---|---|---|---|
| p | Z Value | p | Z Value | ||
| Species | Total | 0.325 | 0.985 | 0.000 | 3.586 |
| Rotifers | 0.597 | 0.529 | 0.000 | 4.936 | |
| Cladocerans | 0.001 | 3.404 | 0.027 | −2.215 | |
| Copepods | 0.327 | 0.981 | 0.025 | −2.237 | |
| Cluster 1 | 0.017 | 2.393 | 0.060 | −1.878 | |
| Cluster 2 | 0.848 | 0.192 | 0.000 | 5.248 | |
| Cluster 3 | 0.262 | 1.121 | 0.006 | 2.726 | |
| Population density | Total | 0.075 | 1.778 | 0.000 | 5.738 |
| Rotifers | 0.197 | 1.289 | 0.000 | 7.075 | |
| Cladocerans | 0.000 | 4.007 | 0.004 | −2.900 | |
| Copepods | 0.271 | 1.102 | 0.589 | 0.540 | |
| Cluster 1 | 0.034 | 2.124 | 0.083 | 1.733 | |
| Cluster 2 | 0.056 | 1.914 | 0.000 | 6.278 | |
| Cluster 3 | 0.115 | 1.578 | 0.000 | 5.497 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.-J.; Jung, E.; Do, Y.; Joo, G.-J.; Kim, H.-W.; Jo, H. Impact of River-Reservoir Hybrid System on Zooplankton Community and River Connectivity. Sustainability 2022, 14, 5184. https://doi.org/10.3390/su14095184
Ko E-J, Jung E, Do Y, Joo G-J, Kim H-W, Jo H. Impact of River-Reservoir Hybrid System on Zooplankton Community and River Connectivity. Sustainability. 2022; 14(9):5184. https://doi.org/10.3390/su14095184
Chicago/Turabian StyleKo, Eui-Jeong, Eunsong Jung, Yuno Do, Gea-Jae Joo, Hyun-Woo Kim, and Hyunbin Jo. 2022. "Impact of River-Reservoir Hybrid System on Zooplankton Community and River Connectivity" Sustainability 14, no. 9: 5184. https://doi.org/10.3390/su14095184
APA StyleKo, E.-J., Jung, E., Do, Y., Joo, G.-J., Kim, H.-W., & Jo, H. (2022). Impact of River-Reservoir Hybrid System on Zooplankton Community and River Connectivity. Sustainability, 14(9), 5184. https://doi.org/10.3390/su14095184








