Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira tamariscifolia
Abstract
:1. Introduction
2. Results and Discussion
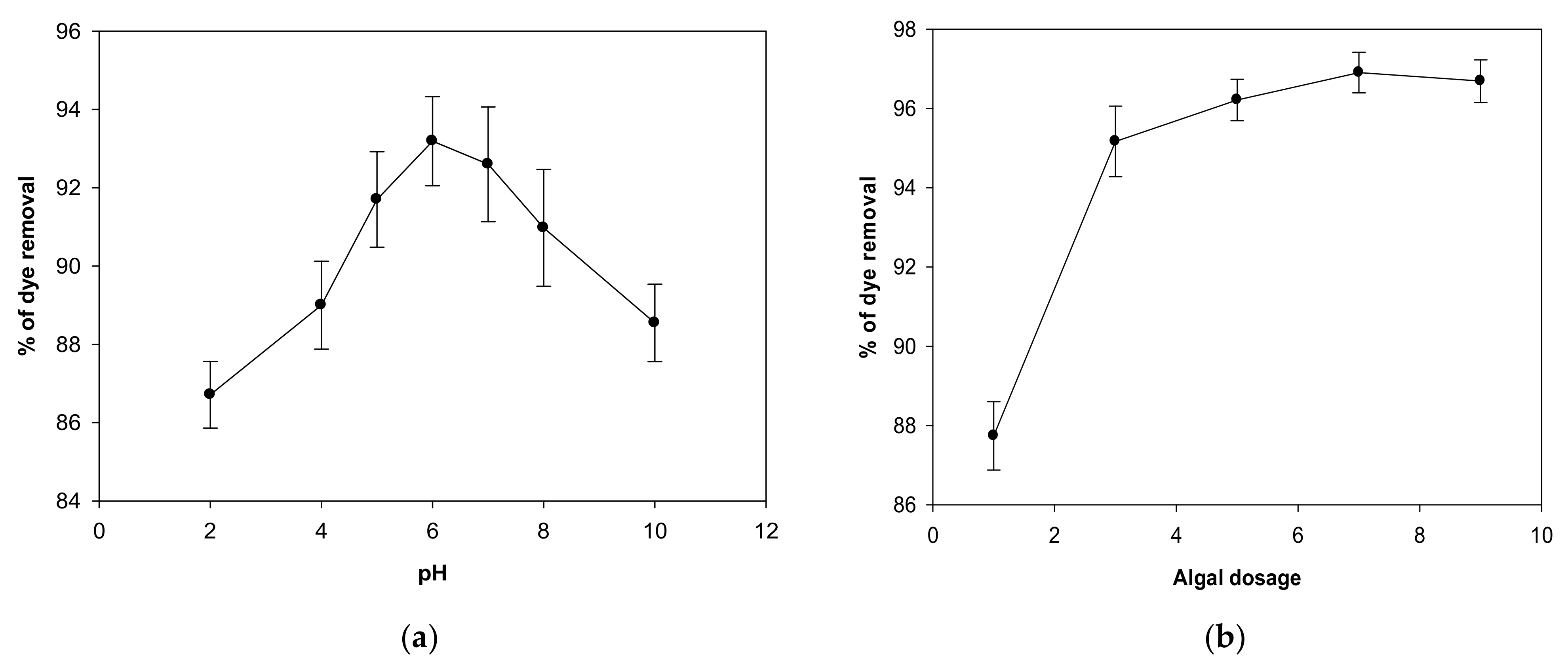
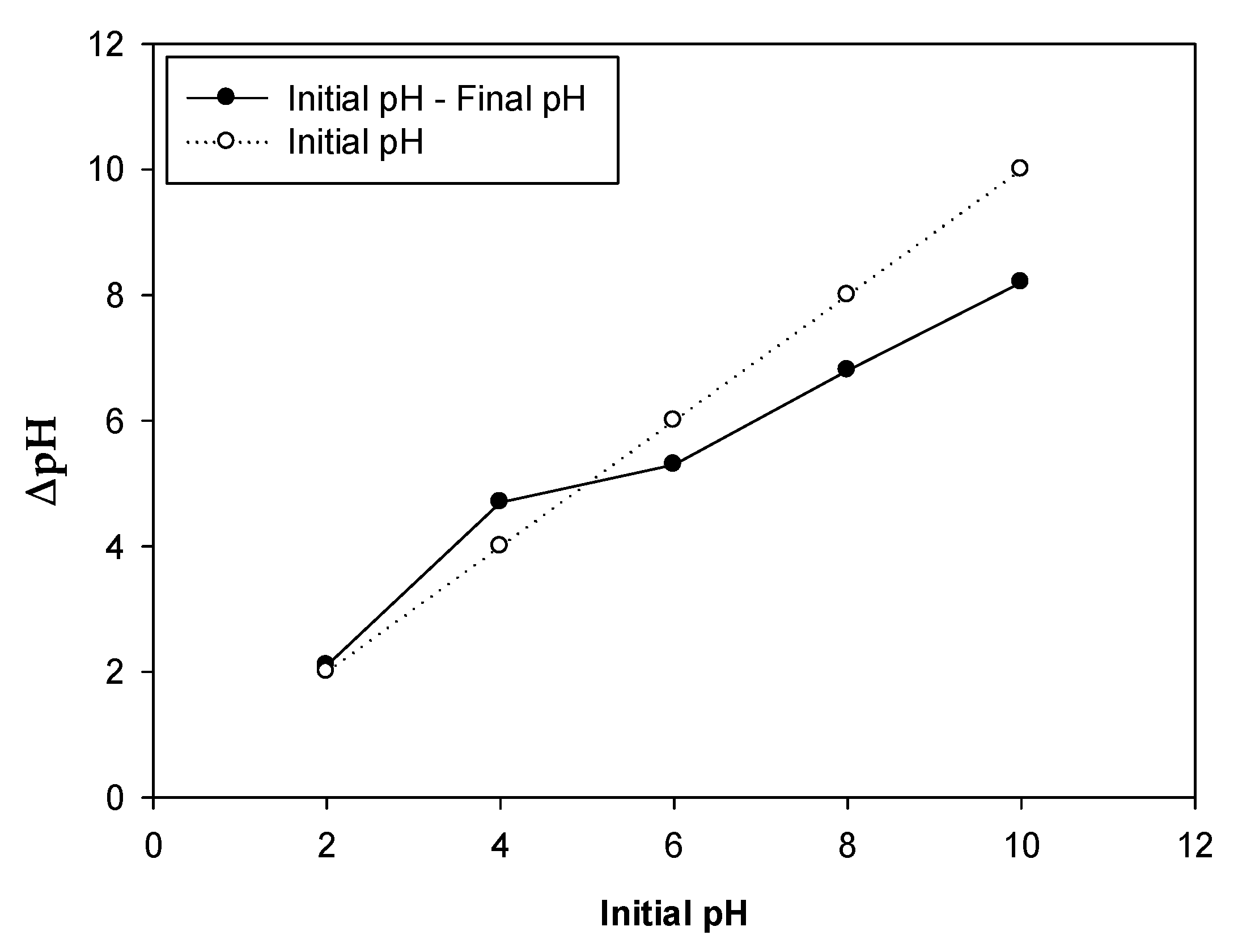
2.1. Impact of pH
2.2. Impact of Biomass Concentration
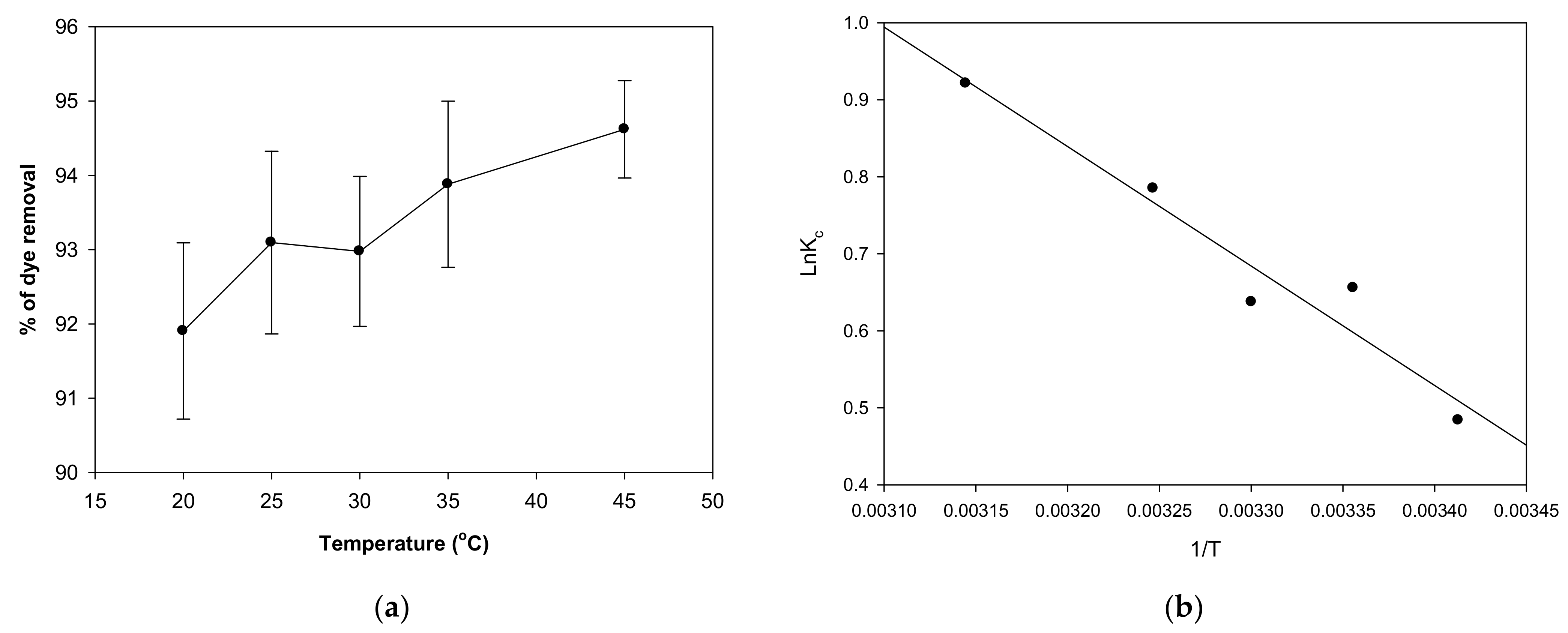
2.3. Impact of Temperature on MV 2B Biosorption and Thermodynamic Studies
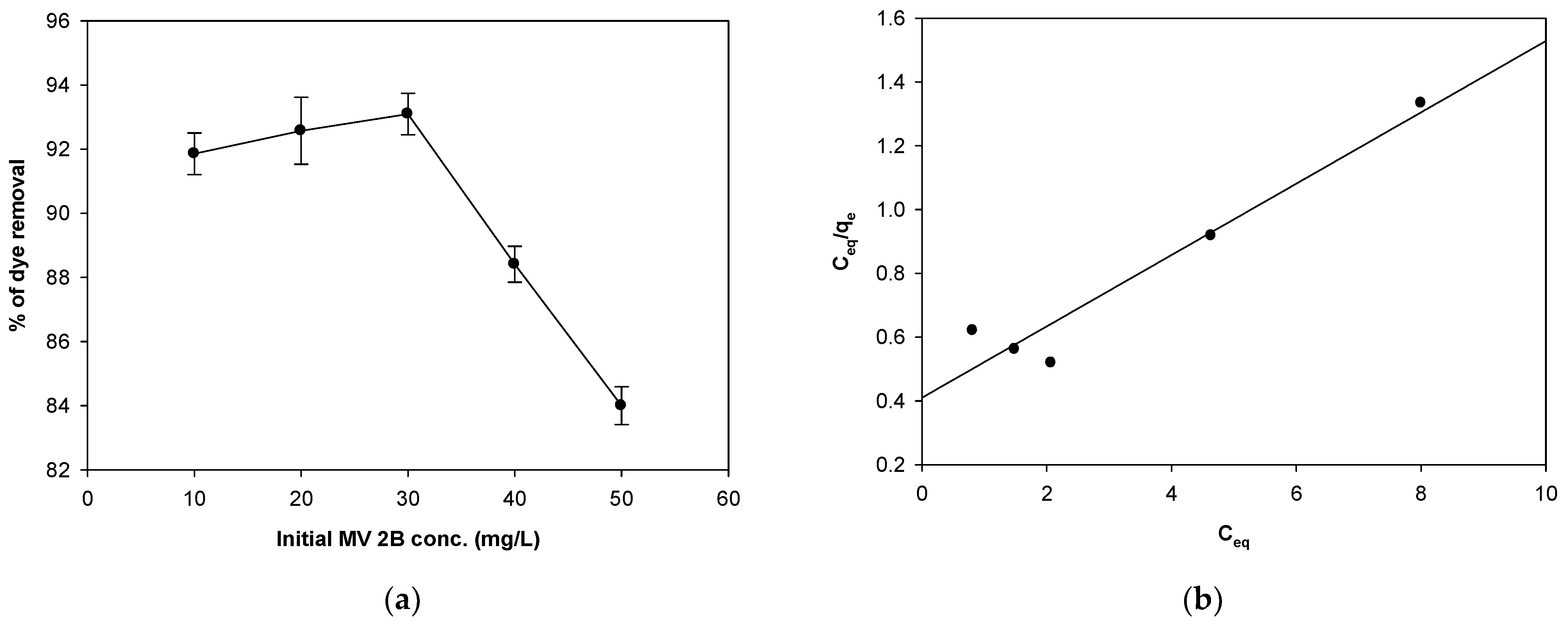
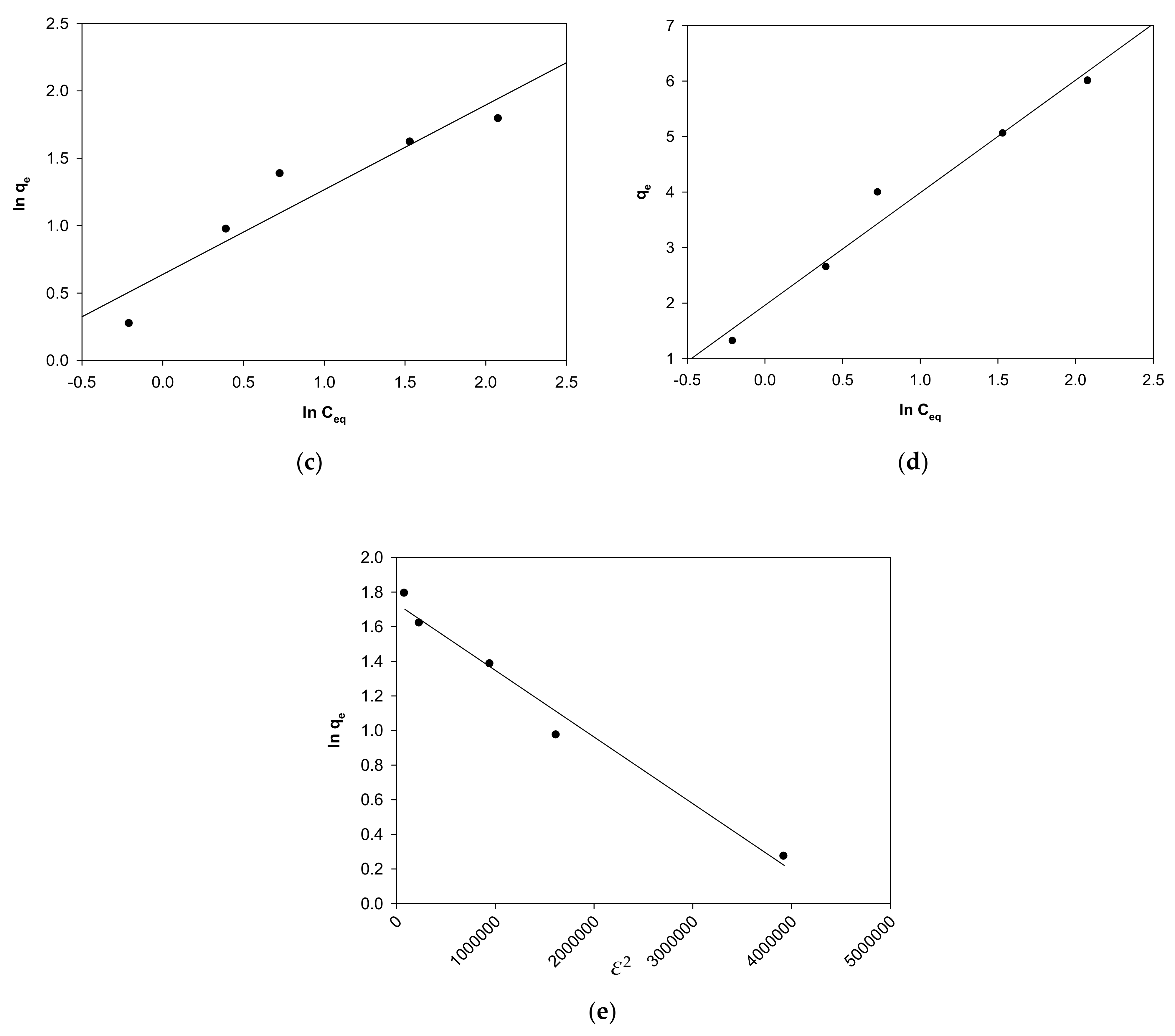
2.4. Impact of Initial MV 2B Concentration and Isothermal Studies
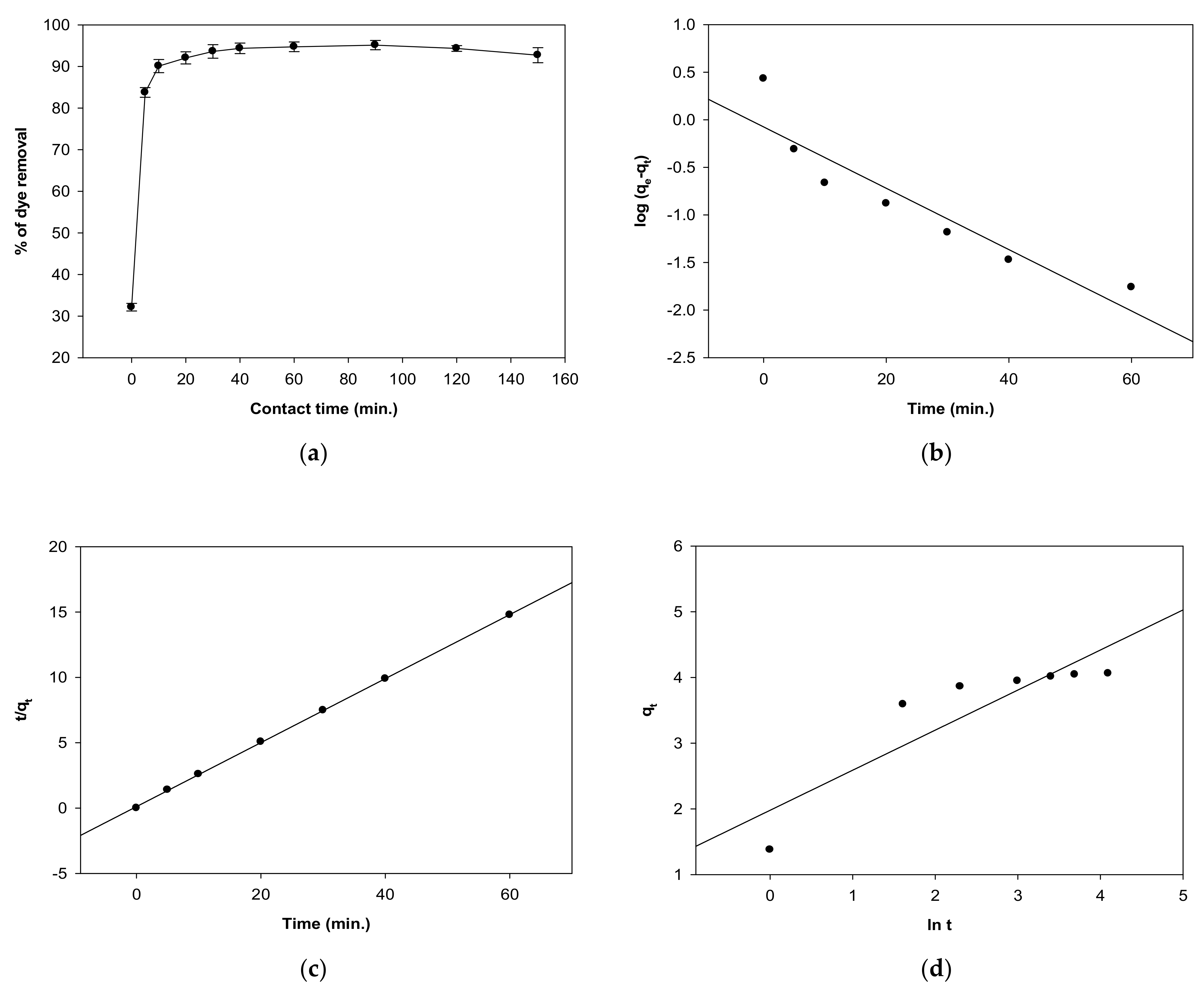
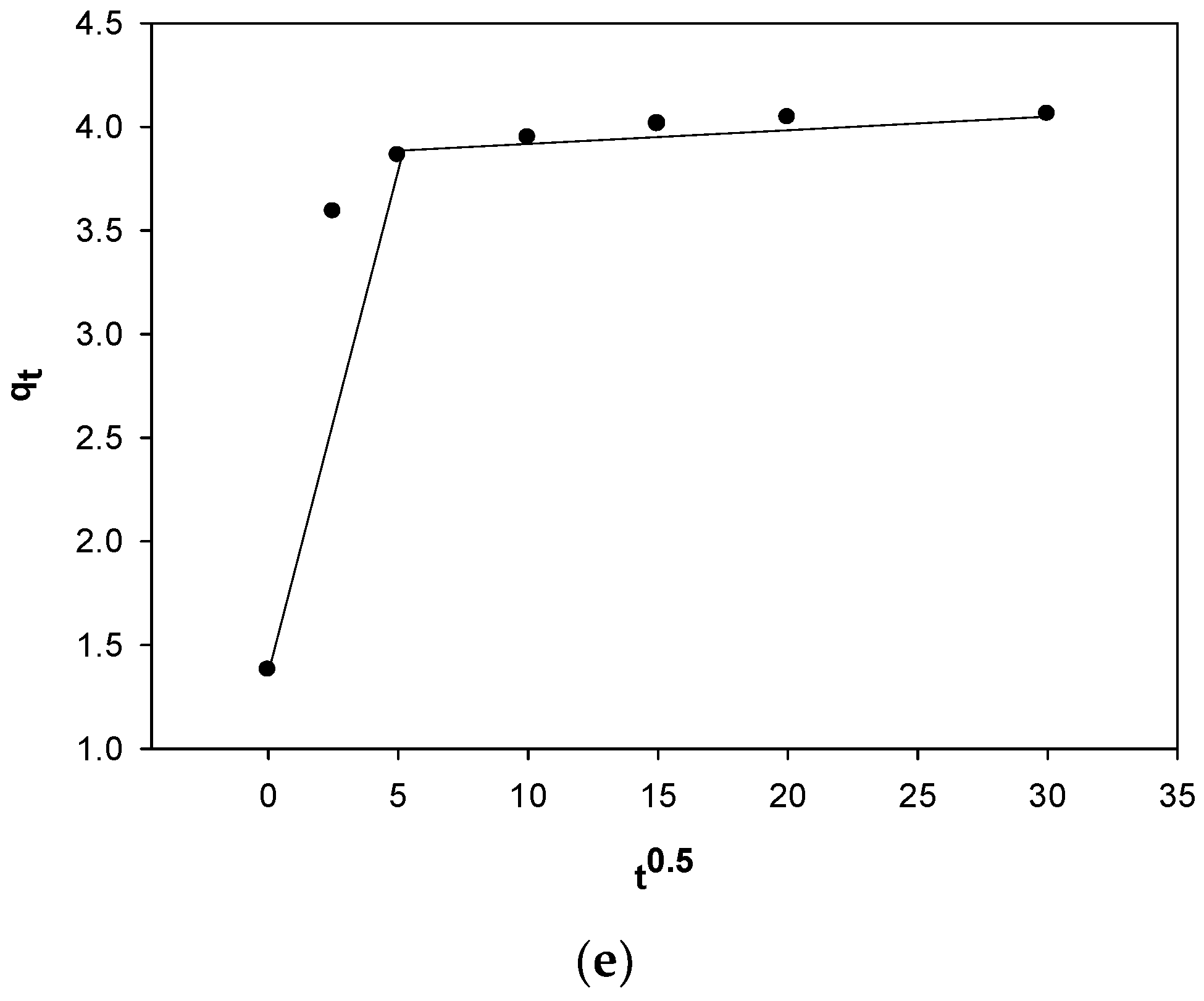
2.5. Impact of Contact Time on MV 2B Biosorption and Kinetic Studies
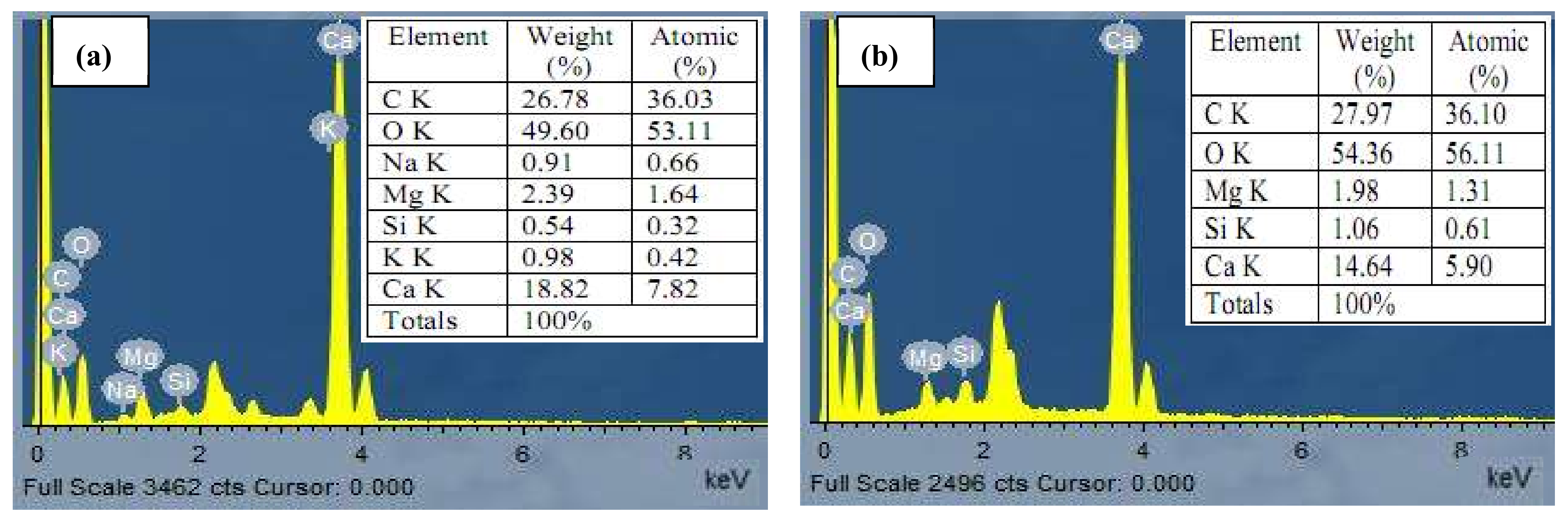
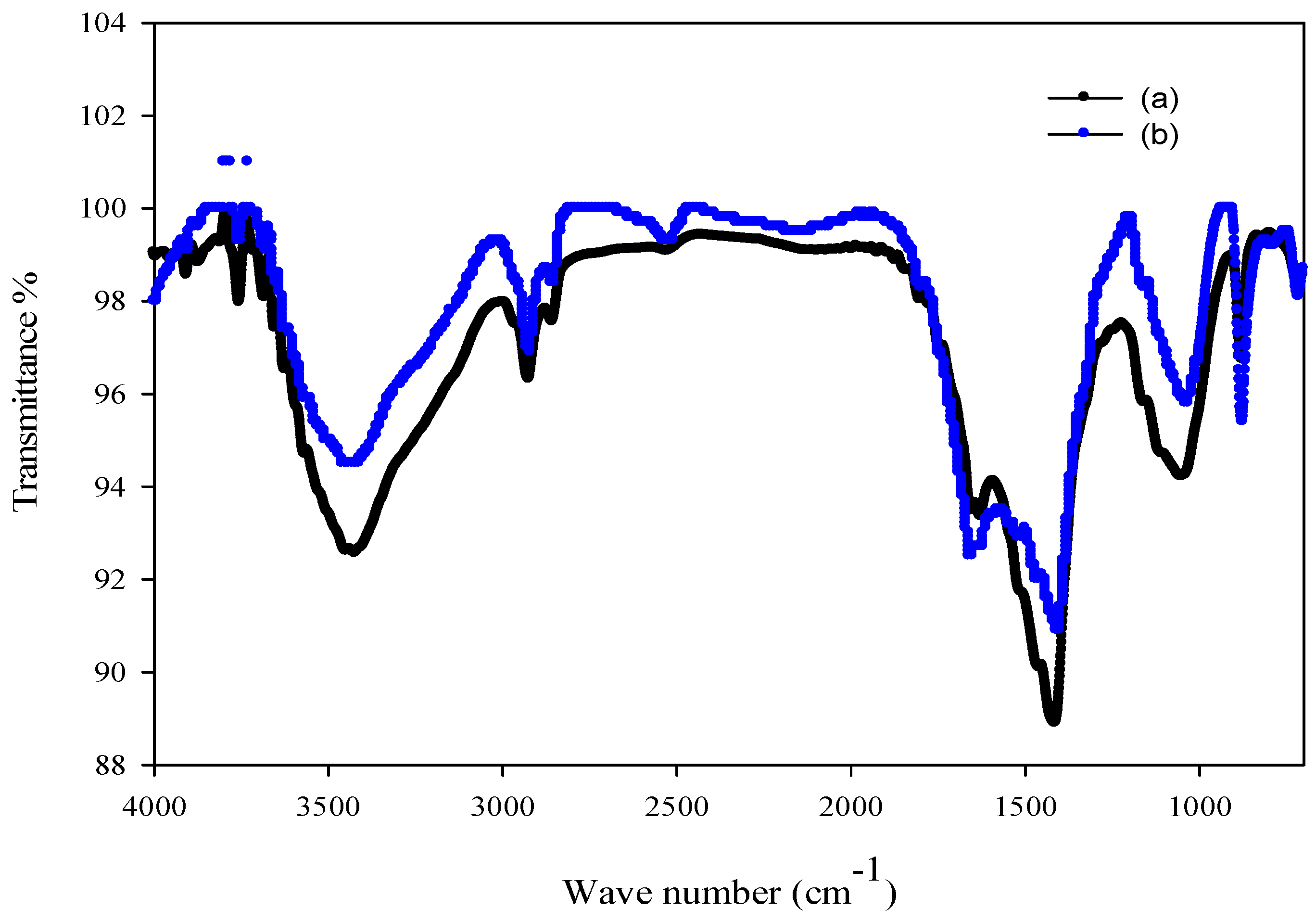
2.6. Characterization of Algal Biomass
2.6.1. SEM/EDX Analyses
2.6.2. FT-IR Study
2.6.3. Determination of pHpzc
2.7. Possible Mechanism of Dye Biosorption
3. Materials and Methods
3.1. Collection of Cystoseira tamariscifolia Seaweed
3.2. Preparation of Stock Solution
3.3. Biosorption Experiment
3.4. Thermodynamic Analysis
3.5. Modeling of Biosorption Isotherms
3.6. Modeling of Biosorption Kinetics
3.7. Characterization of Biosorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, M.; Thakore, S.; Jadeja, R. Removal of organic dyes using Fucus vesiculosus seaweed bioadsorbent an ecofriendly approach: Equilibrium, kinetics and thermodynamic studies. Environ. Chem. Ecotoxicol. 2022, 4, 67–77. [Google Scholar] [CrossRef]
- Chandhru, M.; Rani, S.K.; Vasimalai, N. Reductive degradation of toxic six dyes in industrial wastewater using diaminobenzoic acid capped silver nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104225. [Google Scholar] [CrossRef]
- Ayele, A.; Getachew, D.; Kamaraj, M.; Suresh, A. Phycoremediation of synthetic dyes: An effective and eco-friendly algal technology for the dye abatement. J. Chem. 2021, 2021, 9923643. [Google Scholar] [CrossRef]
- Seow, T.W.; Lim, C.K.; Nor, M.H.M.; Mubarak, M.F.M.; Lam, C.Y.; Yahya, A.; Ibrahim, Z. Review on wastewater treatment technologies. Int. J. Appl. Environ. Sci. 2016, 11, 111–126. [Google Scholar]
- Fontana, I.B.; Peterson, M.; Cechinel, M.A.P. Application of brewing waste as biosorbent for the removal of metallic ions present in groundwater and surface waters from coal regions. J. Environ. Chem. Eng. 2018, 6, 660–670. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Biological Dyes and Stains: Synthesis and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B. Removal of methyl violet 2B from aqueous solution using Casuarina equisetifolia needle. Int. Sch. Res. Not. 2013, 2013, 619819. [Google Scholar] [CrossRef] [Green Version]
- Mehr, H.V.; Saffari, J.; Mohammadi, S.Z.; Shojaei, S. The removal of methyl violet 2B dye using palm kernel activated carbon: Thermodynamic and kinetics model. Int. J. Environ. Sci. Technol. 2020, 17, 1773–1782. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B. Removal of the methyl violet 2B dye from aqueous solution using sustainable adsorbent Artocarpus odoratissimus stem axis. Appl. Water Sci. 2017, 7, 3573–3581. [Google Scholar] [CrossRef] [Green Version]
- Loulidi, I.; Boukhlifi, F.; Ouchabi, M.; Amar, A.; Jabri, M.; Kali, A.; Chraibi, S.; Hadey, C.; Aziz, F. Adsorption of crystal violet onto an agricultural waste residue: Kinetics, isotherm, thermodynamics, and mechanism of adsorption. Sci. World J. 2020. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kumar, R.V.; Dhinakaran, G. Dye removal from aqueous solution using low cost adsorbent. Int. J. Environ. Sci. 2011, 1, 1492. [Google Scholar]
- Parmar, N.D.; Shukla, S.R. Decolourization of dye wastewater by microbial methods—A review. Indian J. Chem. Technol. (IJCT) 2018, 25, 315–323. [Google Scholar]
- Fawzy, M.A.; Issa, A.A. Bioremoval of heavy metals and nutrients from sewage plant by Anabaena oryzae and Cyanosarcina fontana. Int. J. Phytoremediat. 2016, 18, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Al-Zaban, M.I.; Albarakaty, F.M.; Abdelwahab, S.F.; Hassan, S.H.; Fawzy, M.A. Kinetic, Isotherm and Thermodynamic Aspects of Zn2+ Biosorption by Spirulina platensis: Optimization of Process Variables by Response Surface Methodology. Life 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Romauld, S.I.; Venkataraghavan, R.; Yuvaraj, D.; Devi, V.I.; Hashika, S. Mycoremediation of Hydrocarbon and its products using Fusarium oxysporum. Res. J. Pharm. Technol. 2019, 12, 4216–4224. [Google Scholar] [CrossRef]
- Imran-Shaukat, M.; Wahi, R.; Ngaini, Z. The application of agricultural wastes for heavy metals adsorption: A meta-analysis of recent studies. Bioresour. Technol. Rep. 2022, 17, 100902. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Dahri, M.K.; Lim, L.B. Removal of methyl violet 2B dye from aqueous solution using Nepenthes rafflesiana pitcher and leaves. Appl. Water Sci. 2017, 7, 3859–3868. [Google Scholar] [CrossRef] [Green Version]
- Bonetto, L.R.; Ferrarini, F.; De Marco, C.; Crespo, J.S.; Guégan, R.; Giovanela, M. Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J. Water Process. Eng. 2015, 6, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Mahini, R.; Esmaeili, H.; Foroutan, R. Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis. Turk. J. Biochem. 2018, 43, 623–631. [Google Scholar] [CrossRef]
- Thomas, A.D.; Bohumil, V.; Alfonso, M. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar]
- Abbas, S.H.; Ismail, I.M.; Mostafa, T.M.; Sulaymon, A.H. Biosorption of heavy metals: A review. J. Chem. Sci. Technol. 2014, 3, 74–102. [Google Scholar]
- Doan, V.D.; Tran, T.K.N.; Nguyen, A.T.; Tran, V.A.; Nguyen, T.D.; Le, V.T. Comparative study on adsorption of cationic and anionic dyes by nanomagnetite supported on biochar derived from Eichhornia crassipes and Phragmites australis stems. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100569. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Ben Hamissa, A.M.; Fathallah, A.; Kortas, M.H.; Baklouti, T.; Mahjoub, B.; Seffen, M. Biosorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. J. Hazard. Mater. 2009, 170, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A review on biosorptive removal of dyes and heavy metals from wastewater using watermelon rind as biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; Rabei, N.H.; El-Malkey, S.E. Eco-friendly approach for biosorption of Pb2+ and carcinogenic Congo red dye from binary solution onto sustainable Ulva lactuca biomass. Sci. Rep. 2020, 10, 16021. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN—Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Omokpariola, D.O. Experimental Modelling Studies on the removal of crystal violet, methylene blue and malachite green dyes using Theobroma cacao (Cocoa Pod Powder). J. Chem. Lett. 2021, 2, 9–24. [Google Scholar]
- Sharma, K.; Sharma, S.; Sharma, V.; Mishra, P.K.; Ekielski, A.; Sharma, V.; Kumar, V. Methylene Blue Dye Adsorption from Wastewater Using Hydroxyapatite/Gold Nanocomposite: Kinetic and Thermodynamics Studies. Nanomaterials 2021, 11, 1403. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Low-cost biosorption of Methylene Blue and Congo Red from single and binary systems using Sargassum latifolium biorefinery waste/wastepaper xerogel: An optimization and modeling study. J. Appl. Phycol. 2021, 33, 675–691. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Use of algal biorefinery waste and waste office paper in the development of xerogels: A low cost and eco-friendly biosorbent for the effective removal of Congo red and Fe (II) from aqueous solutions. J. Environ. Manag. 2020, 262, 110380. [Google Scholar] [CrossRef]
- Kankılıç, G.B.; Metin, A.Ü.; Aluç, Y. Investigation on phenol degradation capability of Scenedesmus regularis: Influence of process parameters. Environ. Technol. 2018, 41, 1065–1073. [Google Scholar] [CrossRef]
- Shojaei, S.; Khammarnia, S.; Shojaei, S.; Sasani, M. Removal of reactive red 198 by nanoparticle zero valent iron in the presence of hydrogen peroxide. J. Water Environ. Nanotechnol. 2017, 2, 129–135. [Google Scholar]
- Ruscasso, F.; Bezus, B.; Garmendia, G.; Vero, S.; Curutchet, G.; Cavello, I.; Cavalitto, S. Debaryomyces hansenii F39A as biosorbent for textile dye removal. Rev. Argent. Microbiol. 2021, 53, 257–265. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Alharthi, S. Cellular responses and phenol bioremoval by green alga Scenedesmus abundans: Equilibrium, kinetic and thermodynamic studies. Environ. Technol. Innov. 2021, 22, 101463. [Google Scholar] [CrossRef]
- Tang, L.; Huang, S.; Wang, Y.; Liang, D.; Li, Y.; Li, J.; Wang, Y.; Xie, Y.; Wang, W. Highly efficient, stable, and recyclable hydrogen manganese oxide/cellulose film for the extraction of lithium from seawater. ACS Appl. Mater. Interfaces 2020, 12, 9775–9781. [Google Scholar] [CrossRef]
- Fu, J.; Xin, Q.; Wu, X.; Chen, Z.; Yan, Y.; Liu, S.; Wang, M.; Xu, Q. Selective adsorption and separation of organic dyes from aqueous solution on polydopamine microspheres. J. Colloid Interface Sci. 2016, 461, 292–304. [Google Scholar] [CrossRef]
- Jafari, S.; Azizian, S.; Jaleh, B. Enhancement of methyl violet removal by modification of TiO2 nanoparticles with AgI. J. Ind. Eng. Chem. 2012, 18, 2124–2128. [Google Scholar] [CrossRef]
- Wu, J.S.; Liu, C.H.; Chu, K.H.; Suen, S.Y. Removal of cationic dye methyl violet 2B from water by cation exchange membranes. J. Membr. Sci. 2008, 309, 239–245. [Google Scholar] [CrossRef]
- Ofomaja, A.E.; Ho, Y.S. Effect of temperatures and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresour. Technol. 2008, 99, 5411–5417. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Anebi, P.O.; Ikudayisi, V.A. Biosorption of an anionic dye, eosin yellow onto pineapple peels: Isotherm and thermodynamic study. Int. Ann. Sci. 2018, 4, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Nnaji, C.C.; Agim, A.E.; Mama, C.N.; Emenike, P.C.; Ogarekpe, N.M. Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernel chaff. Sci. Rep. 2021, 11, 7808. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Abdus-Salam, N.; Ikudayisi-Ugbe, A.V.; Ugbe, F.A. Adsorptive removal of methylene blue from synthetic wastewater using date palm seeds, goethite and their composite. Acta Sci. Malays. (ASM) 2021, 5, 27–35. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Ahmad, N.; Bello, O.S. Adsorption kinetic studies for the removal of synthetic dye using durian seed activated carbon. J. Dispers. Sci. Technol. 2015, 36, 670–684. [Google Scholar] [CrossRef]
- Isik, B.; Ugraskan, V.; Cankurtaran, O. Effective biosorption of methylene blue dye from aqueous solution using wild macrofungus (Lactarius piperatus). Sep. Sci. Technol. 2021, 57, 854–871. [Google Scholar] [CrossRef]
- Hasani, N.; Selimi, T.; Mele, A.; Thaçi, V.; Halili, J.; Berisha, A.; Sadiku, M. Theoretical, Equilibrium, Kinetics and Thermodynamic Investigations of Methylene Blue Adsorption onto Lignite Coal. Molecules 2022, 27, 1856. [Google Scholar] [CrossRef]
- Fawzy, M.A. Biosorption of copper ions from aqueous solution by Codium vermilara: Optimization, kinetic, isotherm and thermodynamic studies. Adv. Powder Technol. 2020, 31, 3724–3735. [Google Scholar] [CrossRef]
- Qian, H.; Huang, S.; Ba, Z.; Wang, W.; Yu, F.; Liang, D.; Xie, Y.; Wang, Y.; Wang, Y. HTO/Cellulose Aerogel for Rapid and Highly Selective Li+ Recovery from Seawater. Molecules 2021, 26, 4054. [Google Scholar] [CrossRef]
- Abou Oualid, H.; Abdellaoui, Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Abou Oualid, J. Eco-efficient green seaweed Codium decorticatum biosorbent for textile dyes: Characterization, mechanism, recyclability, and RSM optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef]
- Boudechiche, N.; Mokaddem, H.; Sadaoui, Z.; Trari, M. Biosorption of cationic dye from aqueous solutions onto lignocellulosic biomass (Luffa cylindrica): Characterization, equilibrium, kinetic and thermodynamic studies. Int. J. Ind. Chem. 2016, 7, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Karnakanti, S. Adsorption Kinetics of Malachite Green Dye Removal from Aqueous Solution by using Banana Stem. Int. J. Eng. Adv. Technol. 2021, 10, 215–220. [Google Scholar]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K. Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ash—Kinetic study and equilibrium isotherm analyses. Dyes Pigm. 2006, 69, 210–223. [Google Scholar] [CrossRef]
- Shakoor, S.; Nasar, A. Removal of methylene blue dye from artificially contaminated water using citrus limetta peel waste as a very low cost adsorbent. J. Taiwan Inst. Chem. Eng. 2016, 66, 154–163. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [Green Version]
- Escudero, L.B.; Smichowski, P.N.; Dotto, G.L. Macroalgae of Iridaea cordata as an efficient biosorbent to remove hazardous cationic dyes from aqueous solutions. Water Sci. Technol. 2017, 76, 3379–3391. [Google Scholar] [CrossRef]
- Gokulan, R.; Avinash, A.; Prabhu, G.G.; Jegan, J. Remediation of remazol dyes by biochar derived from Caulerpa scalpelliformis—An eco-friendly approach. J. Environ. Chem. Eng. 2019, 7, 103297. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Hifney, A.F.; Adam, M.S.; Al-Badaani, A.A. Biosorption of cobalt and its effect on growth and metabolites of Synechocystis pevalekii and Scenedesmus bernardii: Isothermal analysis. Environ. Technol. Innov. 2020, 19, 100953. [Google Scholar] [CrossRef]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of potential contaminants in processed biomass using fourier transform infrared spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Teodosio Melo, K.R.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly (ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Marycz, K. A comprehensive analysis of biosorption of metal ions by macroalgae using ICP-OES, SEM-EDX and FTIR techniques. PLoS ONE 2018, 13, e0205590. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ponce, L.; Granado-Castro, M.D.; Casanueva-Marenco, M.J.; Galindo-Riaño, M.D.; Díaz-de-Alba, M. Sherry wine industry by-product as potential biosorbent for the removal of Cr (VI) from aqueous medium. Biomass Convers. Biorefinery 2021, 1–19. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.A. Phycoremediation and adsorption isotherms of cadmium and copper ions by Merismopedia tenuissima and their effect on growth and metabolism. Environ. Toxicol. Pharmacol. 2016, 46, 116–121. [Google Scholar] [CrossRef]
- Jiang, R.; Tian, J.; Zheng, H.; Qi, J.; Sun, S.; Li, X. A novel magnetic adsorbent based on waste litchi peels for removing Pb (II) from aqueous solution. J. Environ. Manag. 2015, 155, 24–30. [Google Scholar] [CrossRef]

| ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (kJ/mol) | R2 | ||||
|---|---|---|---|---|---|---|---|
| 293 K | 298 K | 303 K | 308 K | 318 K | |||
| −1.18 | −1.62 | −1.60 | −2.01 | −2.43 | 12.87 | 0.048 | 0.940 |
| Langmuir | Freundlich | Temkin | D–R | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax (mg/g) | b (L/mg) | RL | R2 | Kf (mg/g) | n | R2 | B | A (L/mg) | b (J/mol) | R2 | qo (mg/g) | Β × 10−7 (mol2/J2) | E (kJ/mol) | R2 |
| 10.0 | 0.24 | 0.07–0.23 | 0.94 | 1.89 | 1.59 | 0.88 | 2.03 | 2.64 | 1220 | 0.97 | 5.66 | 4.0 | 11.2 | 0.979 |
| Adsorbents | Adsorption Conditions | qmax (mg/g) | References | ||
|---|---|---|---|---|---|
| pH | Temperature (oC) | Adsorbent Dose (g/L) | |||
| HNT-Fe3O4 composite | 4.2 | 25 | 1.5 | 20.4 | [19] |
| Padina sanctae-crucis | 8 | 25 | 2 | 10.02 | [20] |
| TiO2P25 | - | 30 | 1 | 4.6 | [39] |
| Modified cation exchange membrane | - | 25 | - | 10.1 | [40] |
| Wood of Monsunya (tree) | 10 | 25 | 4 | 17.7 | [41] |
| Cystoseira tamariscifolia | 6 | 25 | 7 | 10 | Present study |
| Pseudo-First Order | Pseudo-Second Order | Elovich | Intra-Particle Diffusion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe (exp.) (mg/g) | qe (cal.) (mg/g) | K1 (min−1) | R2 | qe (cal.) (mg/g) | K2 (g/mg min) | R2 | α (mg/g min) | β (g/mg) | R2 | Ki1 (mg/g min1/2) | Ki2 (mg/g min1/2) | Ci (mg/g) | R21 | R22 |
| 4.07 | 1.19 | 0.014 | 0.86 | 4.08 | 0.53 | 0.999 | 756.5 | 1.64 | 0.787 | 0.496 | 4.0 | 6.05 | 0.83 | 0.803 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zaban, M.I.; Alharbi, N.K.; Albarakaty, F.M.; Alharthi, S.; Hassan, S.H.A.; Fawzy, M.A. Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira tamariscifolia. Sustainability 2022, 14, 5285. https://doi.org/10.3390/su14095285
Al-Zaban MI, Alharbi NK, Albarakaty FM, Alharthi S, Hassan SHA, Fawzy MA. Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira tamariscifolia. Sustainability. 2022; 14(9):5285. https://doi.org/10.3390/su14095285
Chicago/Turabian StyleAl-Zaban, Mayasar I., Nada K. Alharbi, Fawziah M. Albarakaty, Sarah Alharthi, Sedky H. A. Hassan, and Mustafa A. Fawzy. 2022. "Experimental Modeling Investigations on the Biosorption of Methyl Violet 2B Dye by the Brown Seaweed Cystoseira tamariscifolia" Sustainability 14, no. 9: 5285. https://doi.org/10.3390/su14095285






