PTFE-Modified Mn-Co-Based Catalytic Ceramic Filters with H2O Resistance for Low-Temperature NH3-SCR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalytic Ceramic Filters
2.2. Catalytic Activity Measurement
2.3. Characterization
3. Results and Discussions
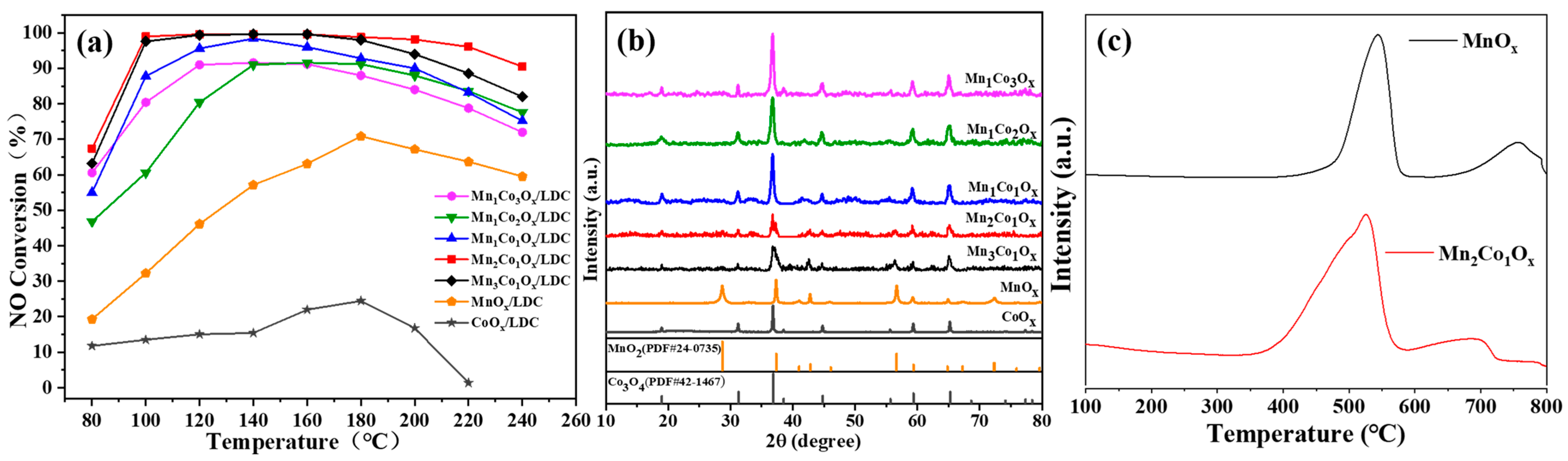
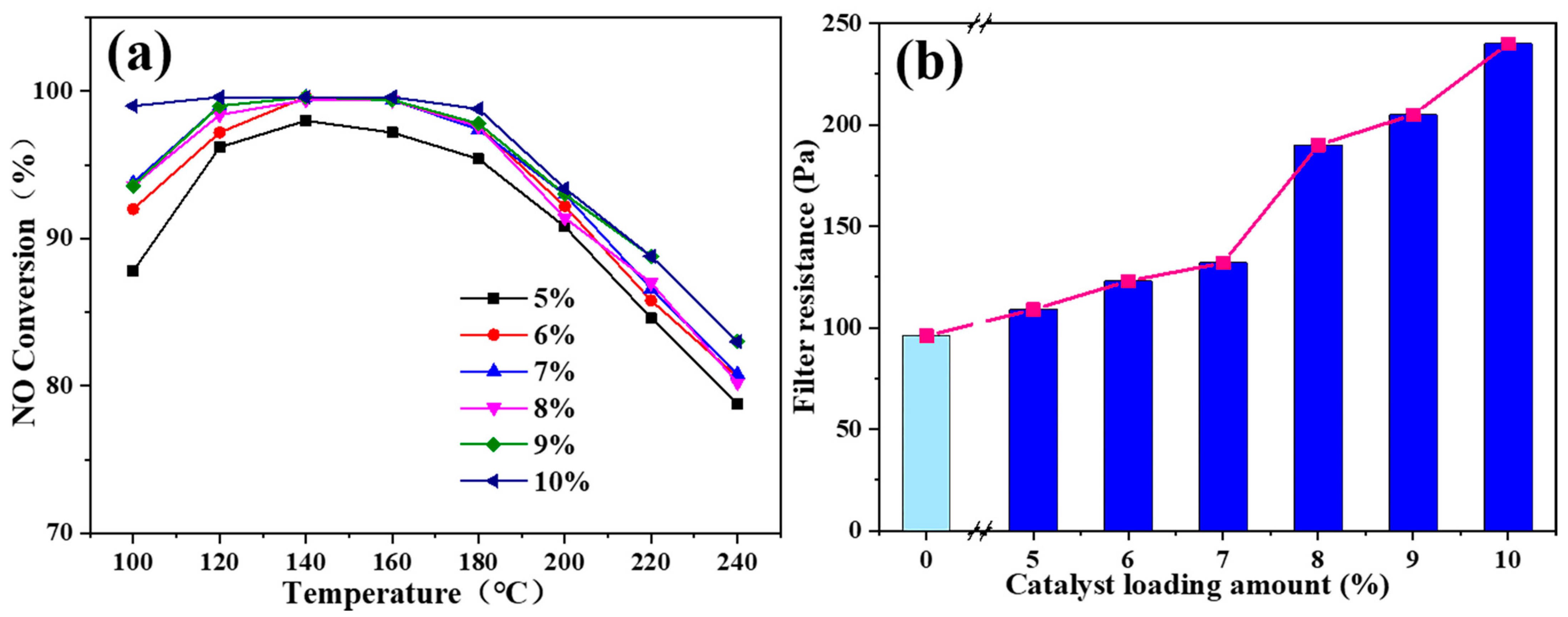
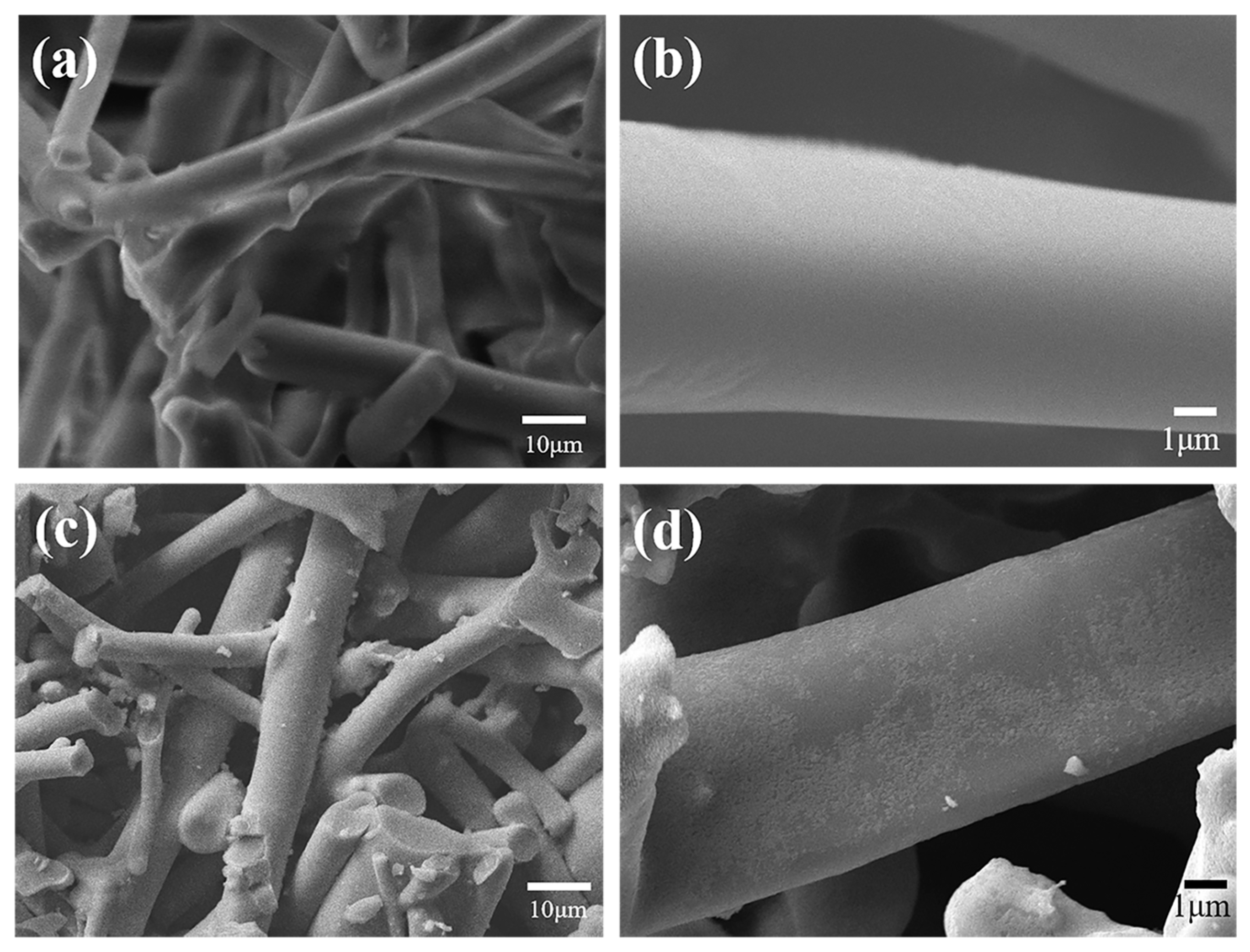
3.1. Denitrification and Filtration Performance of Mn-Co/LDC
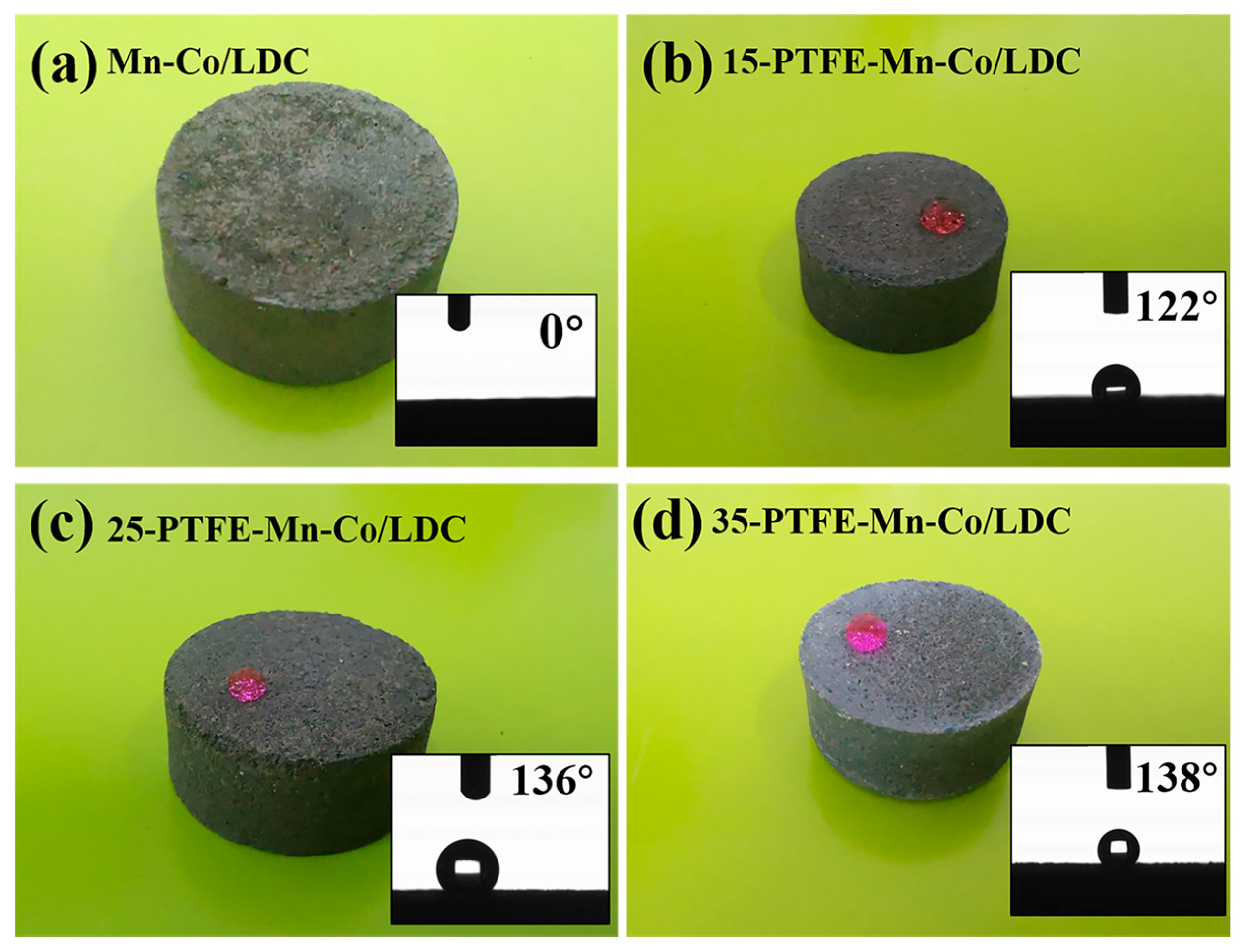
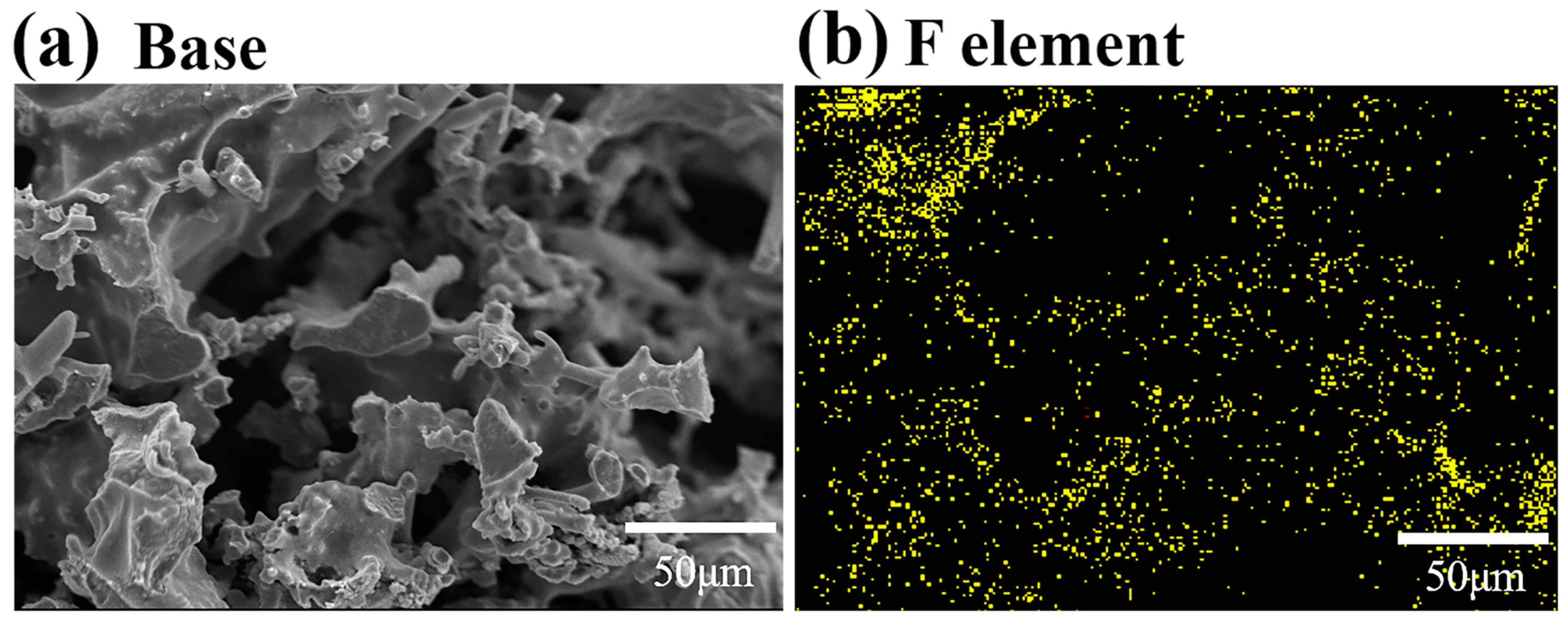
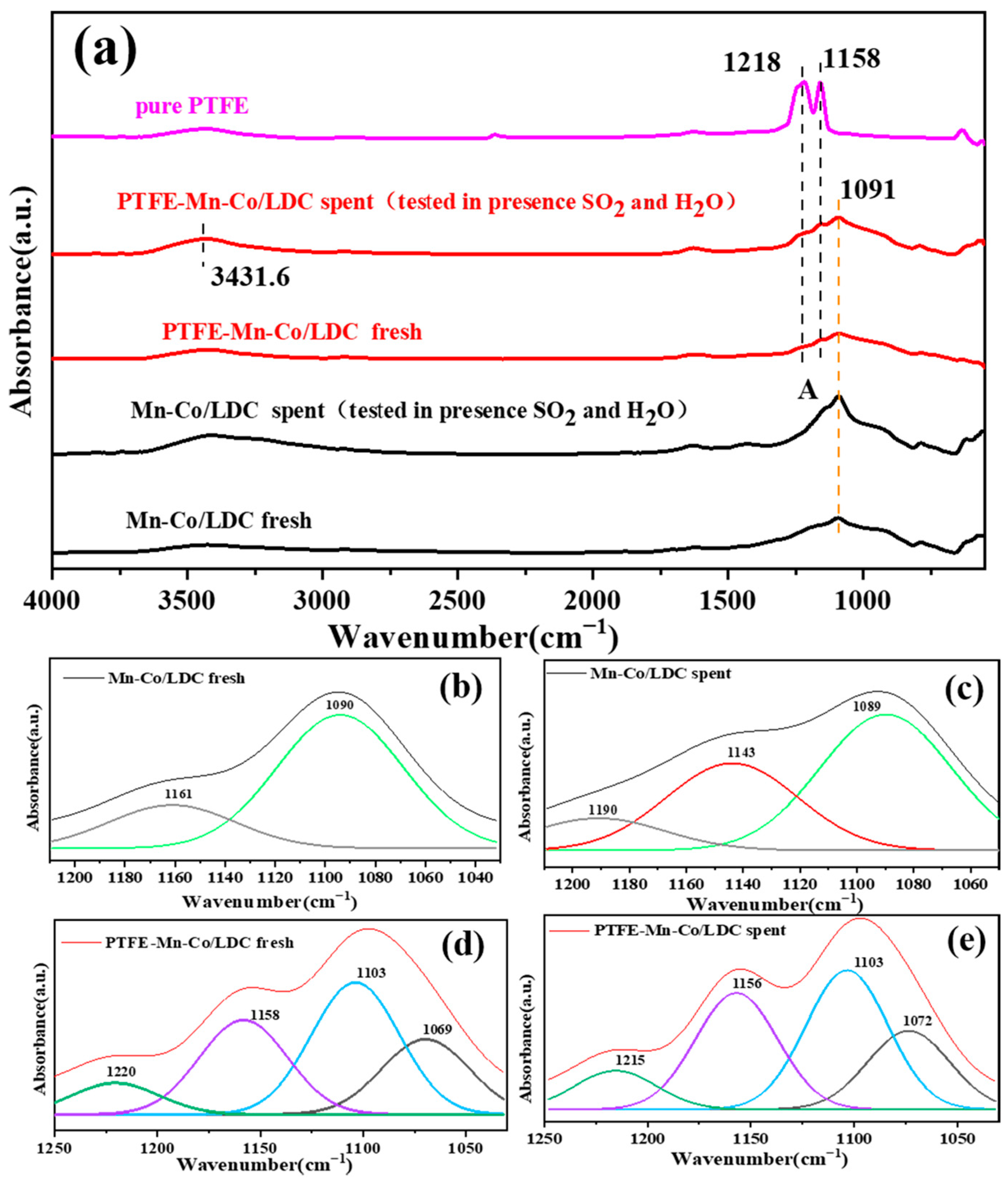
3.2. Improvement of H2O Resistance for Mn-Co/LDC
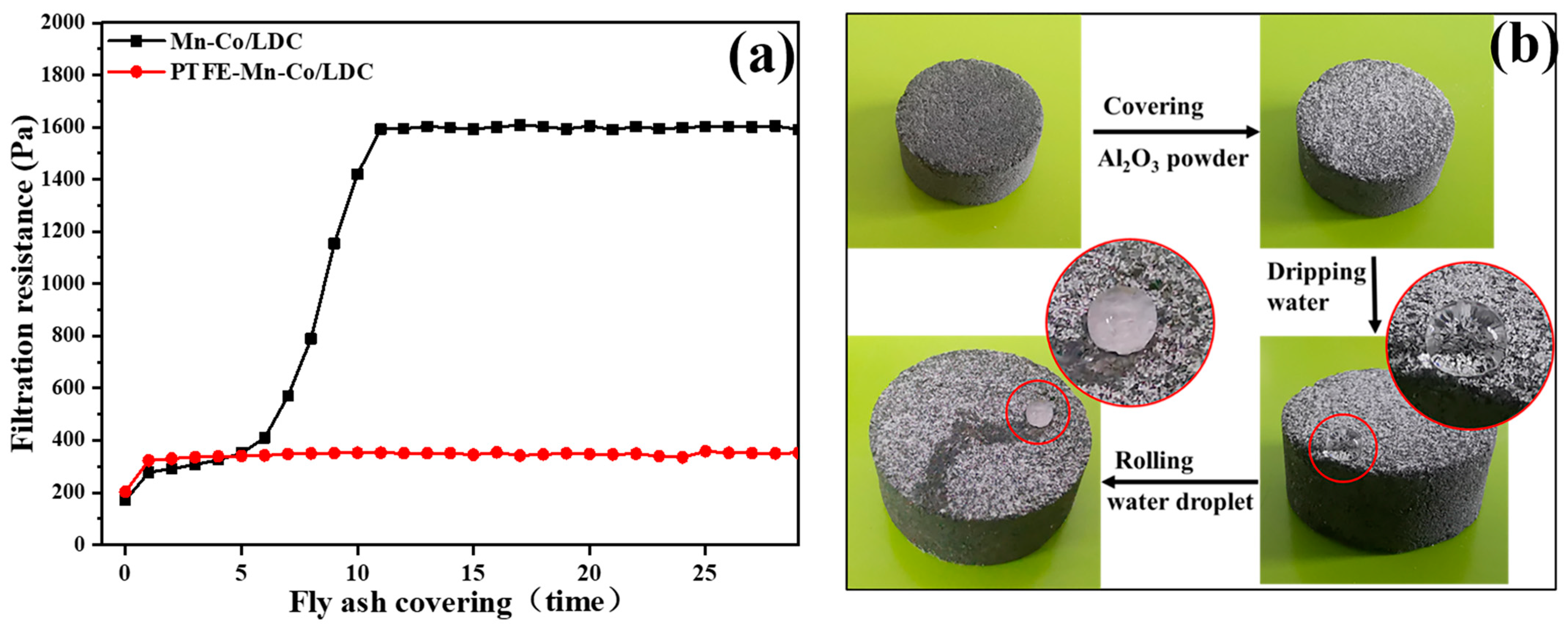
3.3. The Self-Cleaning Performance of PTFE-Mn-Co/LDC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, A.J.; Cole, J.M. Removal or storage of environmental pollutants and alternative fuel sources with inorganic adsorbents via host-guest encapsulation. J. Mater. Chem. A 2017, 5, 10746–10771. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.S.; Hsiau, S.S.; Smid, J.; Wu, J.F.; Ma, S.M. Removal of dust particles from fuel gas using a moving granular bed filter. Fuel 2016, 182, 174–187. [Google Scholar] [CrossRef]
- Hu, J.; Zhong, Z.X.; Zhang, F.; Xing, W.H.; Low, Z.X.; Fan, Y.Q. Coating of ZnO nanoparticles onto the inner pore channel surface of SiC foam to fabricate a novel antibacterial air filter material. Ceram. Int. 2015, 41, 7080–7090. [Google Scholar] [CrossRef]
- Hashim, S.M.; Mohamed, A.R.; Bhatia, S. Catalytic inorganic membrane reactors: Present research and future prospects. Rev. Chem. Eng. 2011, 27, 157–178. [Google Scholar] [CrossRef]
- Kim, Y.G.; Choi, J.H.; Yu, L.; Bak, Y.C. Modification of V2O5-WO3/TiO2 Catalysts Supported on SiC Filter for NO Reduction at Low Temperature. Solid State Phenom. 2007, 124–126, 1713–1716. [Google Scholar] [CrossRef]
- Cho, E.H.; Suh, K.S.; Shin, M.C.; Shin, H.G.; Lee, H.S.; Niihara, K. Thermal and chemical degradation behavior of a catalytic ceramic filter for dust/NOx removal. J. Ceram. Process. Res. 2009, 10, 73–76. [Google Scholar] [CrossRef]
- Heidenreich, S.; Nacken, M.; Hackel, M.; Schaub, G. Catalytic filter elements for combined particle separation and nitrogen oxides removal from gas streams. Powder Technol. 2008, 180, 86–90. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.H.; Phule, A.D. Development of high performance catalytic filter of V2O5-WO3TiO2 supported-SiC for NOx reduction. Powder Technol. 2018, 327, 282–290. [Google Scholar] [CrossRef]
- Nacken, M.; Heidenreich, S.; Hackel, M.; Schaub, G. Catalytic activation of ceramic filter elements for combined particle separation, NOx removal and VOC total oxidation. Appl. Catal. B 2007, 70, 370–376. [Google Scholar] [CrossRef]
- Gao, F.Y.; Tang, X.L.; Yi, H.H.; Li, J.Y.; Zhao, S.Z.; Wang, J.G.; Chu, C.; Li, C.L. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Wu, R.; Li, L.; Zhang, N.; He, J.; Song, L.; Zhang, G.; Zhang, Z.; He, H. Enhancement of low-temperature NH3-SCR catalytic activity and H2O & SO2 resistance over commercial V2O5-MoO3/TiO2 catalyst by high shear-induced doping of expanded graphite. Catal. Today 2021, 376, 302–310. [Google Scholar] [CrossRef]
- Yang, S.J.; Liu, C.X.; Chang, H.Z.; Ma, L.; Qu, Z.; Yan, N.Q.; Wang, C.Z.; Li, J.H. Improvement of the Activity of gamma-Fe2O3 for the Selective Catalytic Reduction of NO with NH3 at High Temperatures: NO Reduction versus NH3 Oxidization. Ind. Eng. Chem. Res. 2013, 52, 5601–5610. [Google Scholar] [CrossRef]
- Zhang, N.N.; Xin, Y.; Wang, X.; Shao, M.F.; Li, Q.; Ma, X.C.; Qi, Y.X.; Zheng, L.R.; Zhang, Z.L. Iron-niobium composite oxides for selective catalytic reduction of NO with NH3. Catal. Commun. 2017, 97, 111–115. [Google Scholar] [CrossRef]
- Chitsazi, H.; Zhang, N.; Li, L.; Liu, X.; Wu, R.; He, J.; Song, L.; He, H. In-situ DRIFT assessment on strengthening effect of cerium over FeOx/TiO2 catalyst for selective catalytic reduction of NOx with NH3. J. Rare Earths 2021, 39, 526–531. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. New Mn-TiO2 aerogel catalysts for the low-temperature selective catalytic reduction of NOx. J. Sol.-Gel. Sci. Technol. 2021, 97, 302–310. [Google Scholar] [CrossRef]
- Ng, D.; Acharya, D.; Wang, X.D.; Easton, C.D.; Wang, J.X.; Xie, Z.L. Low temperature SCR of NOx over Mn/Fe mixed oxides catalyst: Comparison of synthesis methods. J. Chem. Technol. Biotechnol. 2021, 96, 2681–2695. [Google Scholar] [CrossRef]
- Zabihi, V.; Eikani, M.H.; Ardjmand, M.; Latifi, S.M.; Salehirad, A. Selective catalytic reduction of NO by Fe-Mn nanocatalysts: Effect of structure type. Environ. Sci. Pollut. Res. 2021, 28, 39159–39167. [Google Scholar] [CrossRef]
- Yang, B.; Zheng, D.H.; Shen, Y.S.; Qiu, Y.S.; Li, B.; Zeng, Y.W.; Shen, S.B.; Zhu, S.M. Influencing factors on low-temperature deNO(x) performance of Mn-La-Ce-Ni-O-x/PPS catalytic filters applied for cement kiln. J. Ind. Eng. Chem. 2015, 24, 148–152. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Guo, Y.; He, J.; Wu, R.; Song, L.; Zhang, G.; Zhao, J.; Wang, D.; He, H. A MnO2-based catalyst with H2O resistance for NH3-SCR: Study of catalytic activity and reactants-H2O competitive adsorption. Appl. Catal. B 2020, 270, 118860. [Google Scholar] [CrossRef]
- Urbanas, D.; Baltrenaite-Gediene, E. Selective Catalytic Reduction of NO by NH3 over Mn-Cu Oxide Catalysts Supported by Highly Porous Silica Gel Powder: Comparative Investigation of Six Different Preparation Methods. Catalysts 2021, 11, 702. [Google Scholar] [CrossRef]
- Gao, E.H.; Pan, H.; Zhang, W.; Li, Y.N.; Cao, G.H.; Bernards, M.T.; He, Y.; Shi, Y. Insights on the mechanism of enhanced selective catalytic reduction of NO with NH3 over Zr-doped MnCr2O4: A combination of in situ DRIFTS and DFT. Chem. Eng. J. 2020, 386, 123956. [Google Scholar] [CrossRef]
- Li, W.M.; Liu, H.D.; Chen, Y.F. Fabrication of MnOx-CeO2-Based Catalytic Filters and Their Application in Low-Temperature Selective Catalytic Reduction of NO with NH3. Ind. Eng. Chem. Res. 2020, 59, 12657–12665. [Google Scholar] [CrossRef]
- Fang, Q.; Zhu, B.; Sun, Y.; Song, W.; Xu, M. Effects of alkali metal poisoning and cobalt modification on the NH3 adsorption behavior on the MnxOy/Ni (1 1 1) surface: A DFT-D study. Appl. Surf. Sci. 2020, 509, 144901. [Google Scholar] [CrossRef]
- Zhang, N.; He, H.; Wang, D.; Li, Y. Challenges and opportunities for manganese oxides in low-temperature selective catalytic reduction of NOx with NH3: H2O resistance ability. J. Solid State Chem. 2020, 289, 121464. [Google Scholar] [CrossRef]
- Pan, S.W.; Luo, H.C.; Li, L.; Wei, Z.L.; Huang, B.C. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Chitsazi, H.; Wu, R.; Zhang, N.; He, J.; Zhang, G.; He, H. Enhancement of low-temperature NH3-SCR catalytic activity and H2O resistance ability over MnOx/TiO2 catalyst by expanded graphite. Catal. Lett. 2020, 150, 2688–2694. [Google Scholar] [CrossRef]
- Zhang, G.D.; Huang, X.S.; Tang, Z.C. Enhancing Water Resistance of a Mn-Based Catalyst for Low Temperature Selective Catalytic Reduction Reaction by Modifying Super Hydrophobic Layers. ACS Appl. Mater. Interfaces 2019, 11, 36598–36606. [Google Scholar] [CrossRef]
- Hu, S.; Xiao, C.J.; Zhu, Z.L.; Luo, S.Z.; Wang, C. Catalytic Activity of Hydrophobic Pt/C/PTFE Catalysts of Different PTFE Content for Hydrogen-Water Liquid Exchange Reaction. At. Energy Sci. Technol. 2007, 41, 527–532. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Miller, C.; Perez, G.; Carlton, H.; Huitink, D.; Beckford, S.; Zou, M. Effect of Cu nanoparticles on the tribological performance of polydopamine plus polytetrafluoroethylene coatings in oil-lubricated condition. Appl. Surf. Sci. 2021, 565, 150525. [Google Scholar] [CrossRef]
- Yoon, S.; Sim, H.M.; Cho, S.; Ko, H.; Park, Y.; Kim, Y.; Kim, H.K. Highly Stretchable, Conductive Polymer Electrodes with a Mixed AgPdCu and PTFE Network Interlayer for Stretchable Electronics. Adv. Mater. Interfaces 2021, 8, 2001500. [Google Scholar] [CrossRef]
- Zhang, N.Q.; Li, L.C.; Zhang, B.B.; Sun, Y.M.; Song, L.Y.; Wu, R.; He, H. Polytetrafluoroethylene modifying: A low cost and easy way to improve the H2O resistance ability over MnOx for low-temperature NH3-SCR. J. Environ. Chem. Eng. 2019, 7, 103044. [Google Scholar] [CrossRef]
- Zhao, L.; Li, K.; Wu, R.A.; Zhang, H.; Jin, J. Catalytic filter for the removal of dust and NOx at low temperature. Mater. Res. Express. 2020, 7, 125502. [Google Scholar] [CrossRef]
- Song, X.Y.; Jian, B.H.; Jin, J. Preparation of porous ceramic membrane for gas-solid separation. Ceram. Int. 2018, 44, 20361–20366. [Google Scholar] [CrossRef]
- Kim, T.I.; Tahk, D.; Lee, H.H. Wettability-controllable super water- and moderately oil-repellent surface fabricated by wet chemical etching. Langmuir 2009, 25, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.X.; Liu, T.; Zhao, N.; Yang, X.Y.; Deng, L.D. Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3. J. Environ. Sci. 2010, 22, 1447–1454. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. New insights into the various decomposition and reactivity behaviors of NH4HSO4 with NO on V2O5/TiO2 catalyst surfaces. Chem. Eng. J. 2016, 283, 846–854. [Google Scholar] [CrossRef]


| Catalysts | SBET (m2 g−1) a | Pore Volume (cm3 g−1) b | Pore Diameter (nm) c |
|---|---|---|---|
| Mn2Co1Ox | 32.64 | 0.07 | 6.67 |
| MnOx | 21.16 | 0.05 | 7.34 |
| Element | O K | Al K | Si K | Mn K | Co K | Total |
|---|---|---|---|---|---|---|
| Element wt.% | 39.03 | 11.88 | 28.21 | 13.19 | 7.69 | 100 |
| Atom % | 57.33 | 10.35 | 23.61 | 5.64 | 3.07 | 100 |
| Catalysts | Evaluation Conditions | Temperature/°C | NO Conversion | References |
|---|---|---|---|---|
| PTFE-Mn-Co/LDC | [NO] = [NH3] =500 ppm, [O2] =6%, [H2O] = 5% | 140 | 98% | This work |
| PTFE-Mn-Co/LDC | [NO] = [NH3] =500 ppm, [O2] =6%, [H2O] = 10% | 140 | 86% | This work |
| Mn-Ce/TiO2 | [NO] = [NH3] =600 ppm, [O2] =3%, [H2O] = 3% | 140 | 57% | [36] |
| Co1Mn4Ce5Ox | [NO] = [NH3] =500 ppm, [O2] =5%, [H2O] = 10% | 175 | 75% | [11] |
| MnO2-PTFE | [NO] = [NH3] =1000 ppm [O2] =6%, [H2O] =10%, [SO2] = 50 ppm | 180 | 87% | [32] |
| OMS-2 | [NO] = [NH3] =500 ppm, [O2] =5%, [H2O] =10% | 150 | 55% | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhou, T.; Xu, X.; Han, C.; Zhang, H.; Jin, J. PTFE-Modified Mn-Co-Based Catalytic Ceramic Filters with H2O Resistance for Low-Temperature NH3-SCR. Sustainability 2022, 14, 5353. https://doi.org/10.3390/su14095353
Li K, Zhou T, Xu X, Han C, Zhang H, Jin J. PTFE-Modified Mn-Co-Based Catalytic Ceramic Filters with H2O Resistance for Low-Temperature NH3-SCR. Sustainability. 2022; 14(9):5353. https://doi.org/10.3390/su14095353
Chicago/Turabian StyleLi, Kun, Tao Zhou, Xinqiang Xu, Changye Han, Hua Zhang, and Jiang Jin. 2022. "PTFE-Modified Mn-Co-Based Catalytic Ceramic Filters with H2O Resistance for Low-Temperature NH3-SCR" Sustainability 14, no. 9: 5353. https://doi.org/10.3390/su14095353






