Degradation of Organics and Change Concentration in Per-Fluorinated Compounds (PFCs) during Ozonation and UV/H2O2 Advanced Treatment of Tertiary-Treated Sewage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Condition
2.2. Characteristics of Wastewater
2.3. Reagents and Chemicals
2.4. Analytical Procedure
3. Results
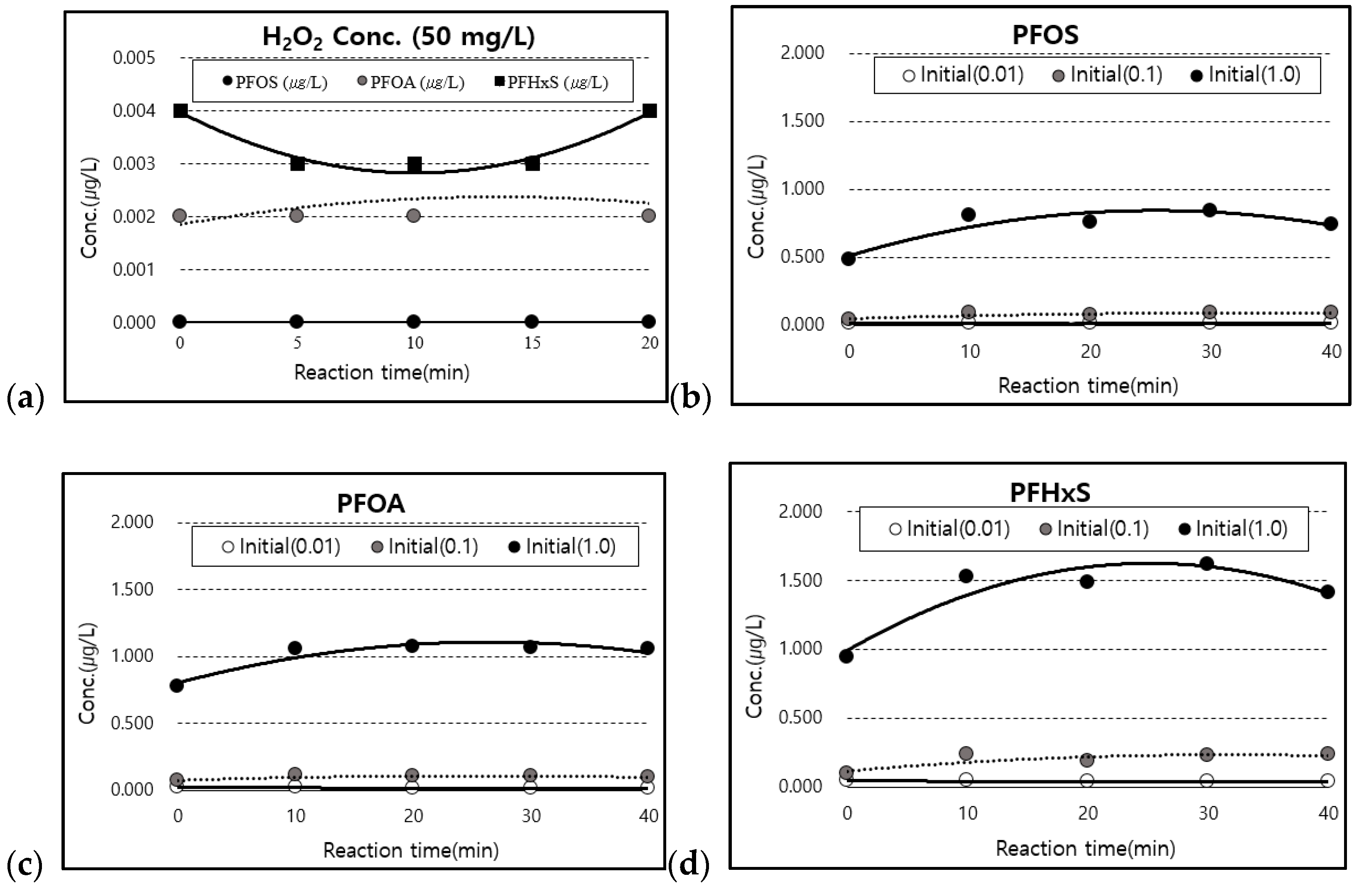
3.1. H2O2 Degradation
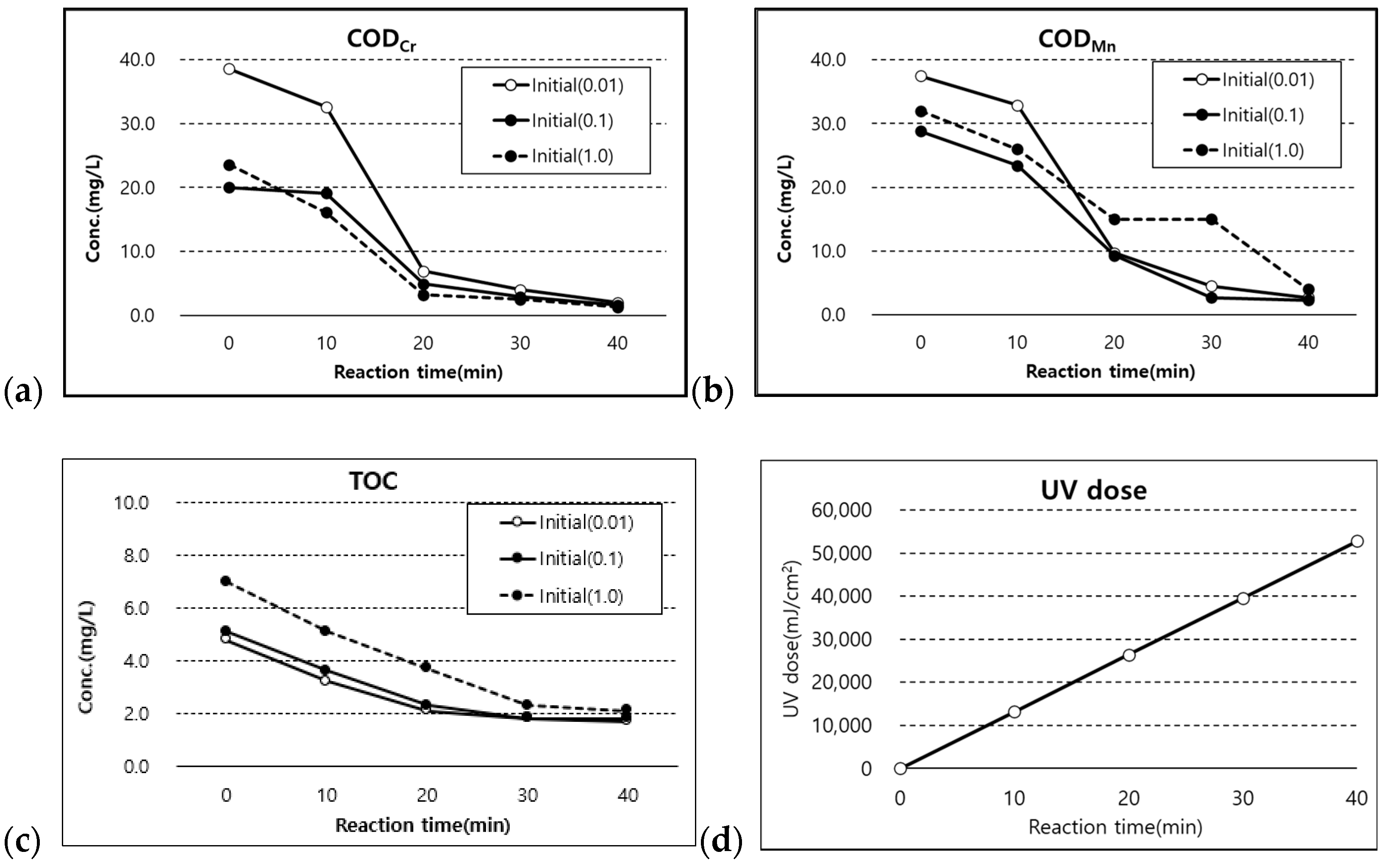
3.2. Degradation of CODMn, CODCr, and TOC with Spiking PFCs
3.3. Change in PFCs by UV/H2O2 with and without Spiking PFCs
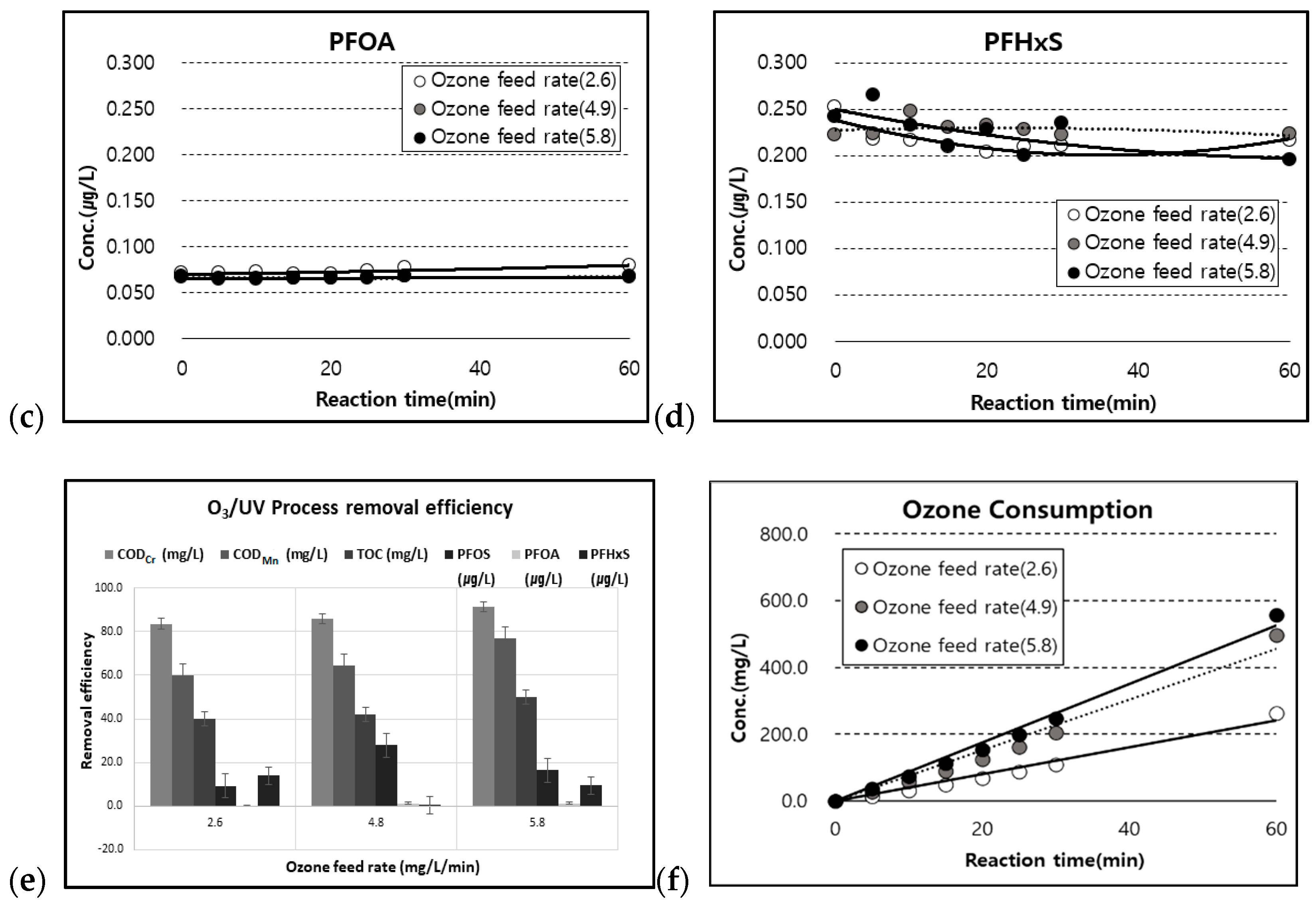
3.4. Effect of O3 and O3/UV on the Change in CODMn and CODCr
3.5. Effect of O3 on the TOC and PFCs Concentration
3.6. Effect of O3/UV on the TOC and PFC Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CODCr | chemical oxygen demand by dichromate |
| CODMn | chemical oxygen demand by permanganate |
| TOC | total organic carbon |
| AOP | advanced oxidation process |
| UV | ultraviolet |
| STP | sewage treatment plant |
| PFC | perfluorinated compound |
| PFHxS | perfluorohexanesulphonic acid |
| PFOS | perfluorooctanesulfonic acid |
| PFOA | perfluorooctanoic |
References
- Escher, B.I.; Fenner, K. Recent advances in environmental risk Assessment of transformation products. Environ. Sci. Technol. 2011, 45, 3835–3847. [Google Scholar] [CrossRef] [PubMed]
- Brucker-Davis, F.; Thayer, K.; Colborn, T. Significant effects of mild endogenous hormonal changes in humans: Considerations for low-dose testing. Environ. Health Perspect. 2001, 109, 21. [Google Scholar] [PubMed] [Green Version]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Barbash, J.E.; Gilliom, R.J. Occurrence of pesticides in shallow groundwater of the United States: Initial results from the National Water-Quality Assessment Program. Environ. Sci. Technol. 1998, 32, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Li, Q.; Feng, W. Application Progress of O3/UV Advanced Oxidation Technology in the Treatment of Organic Pollutants in Water. Sustainability 2022, 14, 1556. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Gernjak, W. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total Environ. 2019, 701, 135023. [Google Scholar] [CrossRef]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; Gunten, U.V. Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: Use of kinetic and water specific information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef]
- Cerreta, G.; Roccamante, M.A.; Patricia, P.B.; Oller, I.; Aguera, A.; Malato, S.; Rizzo, L. Advanced treatment of urban wastewater by UV-C/free chlorine process: Micro-pollutants removal and effect of UV-C radiation on trihalomethanes formation. Water Res. 2019, 169, 115220. [Google Scholar] [CrossRef]
- Boulanger, B.; Vargo, J.D.; Schnoor, J.L.; Hornbuckle, K.C. Evaluation of perfluorooctane surfactants in a wastewater treatment system and in a commercial surface protection product. Environ. Sci. Technol. 2005, 39, 5524–5530. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Higgins, C.P.; Huset, C.A.; Luthy, R.G.; Barofsky, D.F.; Field, J.A. Fluorochemical mass flows in a municipal wastewater treatment facility. Environ. Sci. Technol. 2006, 40, 7350–7357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.; Kim, H.; Han, G.; Kim, H.; Hwang, Y.; Kim, Y. Behavior of perfluorinated compounds in advanced water treatment plant. J. Korean Soc. Water Wastewater 2020, 34, 323–334. [Google Scholar] [CrossRef]
- Higgins, C.P.; Field, J.A.; Criddle, C.S.; Luthy, R.G. Quantitative determination of perfluorochemicals in sediments and domestic sludge. Environ. Sci. Technol. 2005, 39, 3946–3956. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Berger, U.; Järnberg, U.; Kallenborn, R. Perfluorinated alkylated substances (PFAS) in the European Nordic environment. Organohalogen Compd. 2004, 66, 4046–4052. [Google Scholar]
- Saito, N.; Harada, K.; Inoue, K.; Sasaki, K.; Yoshinaga, T.; Koizumi, A. Perfluorooctanoate and perfluorooctane sulfonate concentrations in surface water in Japan. J. Occup. Health 2004, 46, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, E.; Taniyasu, S.; Yamashita, N.; Kannan, K. Perfluoroctanoic acid and perfluorooctane sulfonate in Michigan and New York waters. Organohalogen Compd. 2004, 66, 4069–4073. [Google Scholar]
- Hansen, K.J.; Johnson, H.O.; Eldridge, J.S.; Butenhoff, J.L.; Dick, L.A. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 2002, 36, 1681–1685. [Google Scholar] [CrossRef]
- Taniyasu, S.; Kannan, K.; So, M.K.; Gulkowska, A.; Sinclair, E.; Okazawa, T.; Yamashita, N. Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J. Chromatogr. A 2005, 1093, 89–97. [Google Scholar] [CrossRef]
- Lien, N.P.H.; Fujii, S.; Tanaka, S.; Nozoe, M.; Tanaka, H. Contamination of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in surface water of the Yodo River basin (Japan). Desalination 2008, 226, 338–347. [Google Scholar] [CrossRef]
- Yuan, F.; Hu, C.; Hu, X.; Qu, J.; Yang, M. Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2. Water Res. 2009, 43, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.R.; Saez, M.; Dolfing, J.; de Voogt, P. Biodegradation of perfluorinated compounds. Rev. Environ. Contam. Toxicol. 2008, 196, 53–71. [Google Scholar] [PubMed]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38–52. [Google Scholar] [CrossRef] [Green Version]
- Bliss, J. Removal of PFOA from Water Using UV Treatment, Chemical Oxidation and Adsorption by Activated Carbon and Zeolites; Major Qualify Project, Worcester Polytechnic Institute: Worceste, MA, USA, 2012. [Google Scholar]
- Hori, H.; Hayakawa, E.; Einaga, H. Decomposition of environmentally persistent perfluorooctanoic acid in water by photochemical approaches. Environ. Sci. Technol. 2004, 38, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, A.; Hayakawa, E. Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef]
- Chen, J.; Zang, P.; Liu, J. Photodegration of perfluorooctanoic acid 185nm vacuum ultraviolet light. J. Environ. Sci. 2007, 19, 387–390. [Google Scholar] [CrossRef]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Oktan, A.B.S.K.N. Decomposition of Hydrogen Peroxide-Kinetics and Review of Chosen catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)–A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Aziz, K.H.H. Application of different advanced oxidation processes for the removal of chloroacetic acids using a planar falling film reactor. Chemosphere 2019, 228, 377–383. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Miessner, H.; Mueller, S.; Mahyar, A.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Comparative study on 2, 4-dichlorophenoxyacetic acid and 2, 4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J. Hazard. Mater 2018, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropo lutants by advanced oxidation technologies. J. Hazard. Mater. 2019, 363, 155–173. [Google Scholar]
- Aziz, K.H.H.; Miessner, H.; Mahyar, A.; Mueller, S.; Kalass, D.; Moeller, D.; Omer, K.M. Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge. Sep. Purif. Technol. 2019, 216, 51–57. [Google Scholar] [CrossRef]
- Liu, C.; Higgins, C.; Wang, F.; Shih, K. Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Sep. Purif. Technol. 2012, 91, 46–51. [Google Scholar] [CrossRef]
- Campbell, T.Y.; Vecitis, C.D.; Mader, B.T.; Hoffmann, M.R. Perfluorinated surfactant chain-length effects on sono-chemical kinetics. J. Phys. Chem. A 2009, 113, 9834–9842. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. J. Environ. Sci. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Kuo, J.; Wu, C.-H. Decomposition of perfluorooctanoic acid by ultraviolet light irradiation with Pb-modified titanium dioxide. J. Hazard. Mater. 2016, 303, 111–118. [Google Scholar] [CrossRef]
- Shu, Z.; Singh, A.; Klamerth, N.; McPhedran, K.; Bolton, J.B.; Belosevic, M.; El-Din, M.G. Pilot-scale UV/H2O2 advanced oxidation process for municipal reuse water: Assessing micropollutant degradation and estrogenic impacts on goldfish (Carassius auratus L.). Water Res. 2016, 101, 157–166. [Google Scholar] [CrossRef]
- Ulliman, S.; Miklos, D.; Hubner, U.; Drewes, J.E.; Linden, K.G. Improving UV/H2O2 performance following tertiary treatment of municipal wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1321–1330. [Google Scholar] [CrossRef]
- Kunacheva, C.; Tanak, S.; Fujii, S.; Boontanon, S.K.; Musirat, C.; Wongwattana, T.; Shivakoti, B.R. Mass flows of perfluorinated compounds (PFCs) in central wastewater treatment plants of industrial zones in Thailand. Chemosphere 2011, 83, 737–744. [Google Scholar] [CrossRef]
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Zhang Jackie, X. Treatment train approaches for the remediation of per-and polyfluoroalkyl substances (PFAS): A critical review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Derco, J.; Gotvajn, A.Z.; Cizmarova, O.; Dudas, J.; Sumegova, L. Removal of Micropollutants by Ozone-Based Process. Process 2021, 9, 1013. [Google Scholar] [CrossRef]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224–230. [Google Scholar] [CrossRef]
- Gao, P.; Cui, J.; Deng, Y. Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption–regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA). Chem. Eng. J. 2021, 405, 126698. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Smith, S.M.; Wantala, K.; Kajitvichyanukul, P. Photocatalytic remediation of persistent organic pollutants (POPs): A review. Arab. J. Chem. 2020, 13, 8309–8337. [Google Scholar] [CrossRef]






| Parameter | Concentration |
|---|---|
| pH | 6.80 |
| Temp | 20–25 °C |
| CODCr (mg/L) | 23–27 |
| CODMn (mg/L) | 21–32 |
| TOC (mg/L) | 5.5–6.0 |
| EC (μS/cm) | 350–355 |
| PFOS (μg/L) | 0.000 |
| PFOA (μg/L) | 0.001–0.002 |
| Pump | SPE Pump | Analytical Pump | ||||
|---|---|---|---|---|---|---|
| Column | BDS Hypersil C8 (I.D. 3.0 mm, × L 50 mm, 3.0 μm) | Accucore RP-MS (I.D. 2.1 mm, × L 100 mm, 2.6 μm) | ||||
| Mobile phase | A: Ammonium acetate B: Methanol | A: Ammonium acetate B: Methanol | ||||
| Time (min) | A % | B % | Time (min) | A % | B % | |
| 0–1.2 | 98 | 2 | 0–1.2 | 98 | 2 | |
| 1.2–2.0 | 40 | 60 | 1.2–1.3 | 98 | 2 | |
| 2.0–12.0 | 5 | 95 | 1.3–15.0 | 98 | 2 | |
| 12.0–15.0 | 5 | 95 | 15.0–15.1 | 98 | 2 | |
| 15.0–15.1 | 98 | 2 | ||||
| Injection volume | 1 mL | |||||
| Item | Condition |
|---|---|
| Source | Electrospray ionization (ESI) |
| Polarity | Negative |
| Spray voltage (V) | 3500 |
| Sheath gas (Arb) | 40 |
| Vaporizer temperature (°C) | 325 |
| Aux gas (Arb) | 10 |
| Ion sweep gas (Arb) | 1 |
| Collision energy (V) | 16.79, 10.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, J.; Lee, J.-y.; Ha, H.-J.; Yi, I.G.; Hong, D.-H.; Park, C.-M.; Lee, M.-Y.; Kim, I. Degradation of Organics and Change Concentration in Per-Fluorinated Compounds (PFCs) during Ozonation and UV/H2O2 Advanced Treatment of Tertiary-Treated Sewage. Sustainability 2022, 14, 5597. https://doi.org/10.3390/su14095597
Akter J, Lee J-y, Ha H-J, Yi IG, Hong D-H, Park C-M, Lee M-Y, Kim I. Degradation of Organics and Change Concentration in Per-Fluorinated Compounds (PFCs) during Ozonation and UV/H2O2 Advanced Treatment of Tertiary-Treated Sewage. Sustainability. 2022; 14(9):5597. https://doi.org/10.3390/su14095597
Chicago/Turabian StyleAkter, Jesmin, Jai-yeop Lee, Hyun-Ju Ha, In Geol Yi, Da-Hye Hong, Chang-Min Park, Mok-Young Lee, and Ilho Kim. 2022. "Degradation of Organics and Change Concentration in Per-Fluorinated Compounds (PFCs) during Ozonation and UV/H2O2 Advanced Treatment of Tertiary-Treated Sewage" Sustainability 14, no. 9: 5597. https://doi.org/10.3390/su14095597






