Rhizophagus irregularis and Nitrogen Fixing Azotobacter with a Reduced Rate of Chemical Fertilizer Application Enhances Pepper Growth along with Fruits Biochemical and Mineral Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Treatments
2.3. Soil and Plant Sampling
2.4. Data Analysis
3. Result
3.1. Influence of Chemical Fertilizers and Bio-Fertilizer on Growth Attributes of Bell Pepper
3.2. Influence of Chemical Fertilizers and Bio-Fertilizer on Chemical Attributes of Bell Pepper
3.3. Influence of Chemical Fertilizers and Bio-Fertilizer on Mineral Content (mg/100 g Dry Weight), Root Mycorrhization (%) and Number of Spores
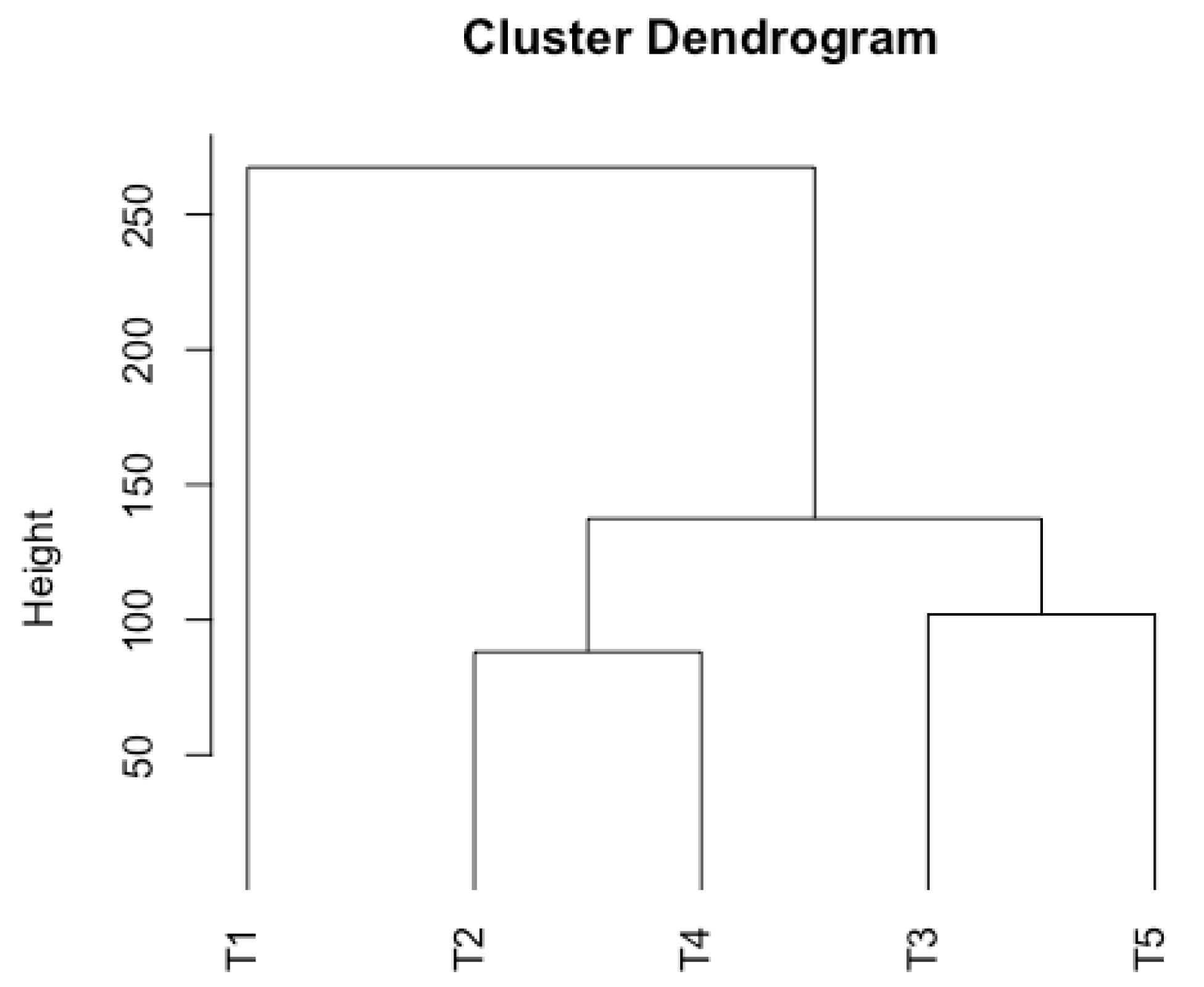
3.4. UPGMA Clustering and PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Wang, Z.; Jia, J.; Bai, L.; Liu, H.; Shen, S.; Yan, H. Newly designed molecularly imprinted 3-aminophenol-glyoxal-urea resin as hydrophilic solid-phase extraction sorbent for specific simultaneous determination of three plant growth regulators in green bell peppers. Food Chem. 2020, 311, 125999. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Estrada, C.E.; Gallardo-Velázquez, T.; Osorio-Revilla, G.; Castañeda-Pérez, E.; Meza-Márquez, O.G.; del Socorro López-Cortez, M.; Hernández-Martínez, D.M. Prediction of total phenolics, ascorbic acid, antioxidant capacities, and total soluble solids of Capsicum annuum L. (bell pepper) juice by FT-MIR and multivariate analysis. LWT Food Sci. Technol. 2020, 126, 109285. [Google Scholar] [CrossRef]
- Sharma, N.; Shukla, Y.R.; Singh, K.; Mehta, D.K. Soil fertility, nutrient uptake and yield of bell pepper as influenced by conjoint application of organic and inorganic fertilizers. Soil Sci. Plant Anal. 2020, 51, 1626–1640. [Google Scholar] [CrossRef]
- Raturi, H.C.; Uppal, G.S.; Singh, S.K.; Kachwaya, D.S. Effect of organic and inorganic nutrient sources on growth, yield and quality of bell pepper (Capsicum annuum L.) grown under polyhouse condition. J. Pharma. Phytochem. 2019, 8, 1788–1792. [Google Scholar]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-de-la-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of three nanoparticles (Se, Si and Cu) on the bioactive compounds of bell pepper fruits under saline stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Imadi, S.R.; Shahzadi, K.; Gul, A. Comparative Study of Three Different Fertilizers on Yield and Quality of Capsicum. In Crop Production Technologies for Sustainable Use and Conservation; Ozturk, M., Hakeem, K.R., Ashraf, M., Ahmad, M.S.A., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 155–173. [Google Scholar]
- Lin, W.; Lin, M.; Zhou, H.; Wu, H.; Li, Z.; Lin, W. The effects of chemical and organic fertilizer usage on rhizosphere soil in tea orchards. PLoS ONE 2019, 14, e0217018. [Google Scholar] [CrossRef]
- Kumar, S.; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: A review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial inoculants for soil quality and plant health. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; Volume 22, pp. 281–307. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, S.Y.; Baek, D.Y.; Park, S.Y.; Lee, S.G.; Ryu, T.H.; Lee, S.K.; Kang, H.J.; Kwon, O.H.; Kil, M.; et al. A comparison of the nutrient composition and statistical profile in red pepper fruits (Capsicums annuum L.) based on genetic and environmental factors. Appl. Biol. Chem. 2019, 62, 48. [Google Scholar] [CrossRef]
- Yadav, R.; Ror, P.; Beniwal, R.; Kumar, S.; Ramakrishna, W. Bacillus sp. and arbuscular mycorrhizal fungi consortia enhance wheat nutrient and yield in the second-year field trial: Superior performance in comparison with chemical fertilizers. J. Appl. Microbiol. 2021, 132, 2203–2219. [Google Scholar] [CrossRef]
- Sumbul, A.; Ansari, R.A.; Rizvi, R.; Mahmood, I. Azotobacter: A potential bio-fertilizer for soil and plant health management. Saudi J. Biol. Sci. 2020, 27, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Wang, J.; Khan, A.; Ahmad, S.; Yang, L.; Ali, I.; Zeeshan, M.; Ullah, S.; Fahad, S.; Ali, S.; et al. Impact of the mixture verses solo residue management and climatic conditions on soil microbial biomass carbon to nitrogen ratio: A systematic review. Environ. Sci. Pollut. Res. 2021, 28, 64241–64252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Saini, I.; Kaushik, P.; Aldawsari, M.M.; Al Balawi, T.; Alam, P. Mycorrhizal fungi and Pseudomonas fluorescens application reduces root-knot nematode (Meloidogyne javanica) infestation in eggplant. Saudi J. Biol. Sci. 2021, 28, 3685–3691. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Khan, F.; Khan, A.; Wang, J. Soil fertility in response to urea and farmyard manure incorporation under different tillage systems in Peshawar, Pakistan. Int. J. Agric. Biol. 2018, 20, 1539–1547. [Google Scholar]
- Sofyan, E.T.; Sara, D.S.; Machfud, Y. The effect of organic and inorganic fertilizer applications on N, P-uptake, K-uptake and yield of sweet corn (Zea mays saccharata Sturt). IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012021. [Google Scholar] [CrossRef]
- Package and Practices for Cultivation of Vegetables. Available online: https://www.pau.edu/content/ccil/pf/pp_veg.pdf (accessed on 20 July 2019).
- Adesemoye, A.O.; Kloepper, J.W. Plant–microbe interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Kennedy, D.M.; Duncan, J.M.; Dugard, P.I.; Topham, P.H. Virulence and aggressiveness of single-zoospore isolates of Phytophthora fragariae. Plant Path. 1986, 35, 344–354. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Rodríguez, G.; Strecker, J.; Brewer, M.; Gonzalo, M.J.; Anderson, C.; Lang, L.; Sullivan, D.; Wagner, E.; Strecker, B.; Drushal, R.; et al. Tomato Analyzer Version 3 User Manual. 2010. Available online: https://vanderknaaplab.uga.edu/files/Tomato_Analyzer_3.0_Manual.pdf (accessed on 29 March 2022).
- Strecker, J.; Rodríguez, G.; Njanji, I.; Thomas, J.; Jack, A.; Darrigues, A.; Hall, J.; Dujmovic, N.; Gray, S.; van der Knaap, E.; et al. Tomato Analyzer Color Test Manual Version 3. 2010. Available online: https://vanderknaaplab.uga.edu/files/Color_Test_3.0_Manual.pdf (accessed on 29 March 2022).
- MFDS. Minerals. In MFDS Food Code; Notifcation No 2019-31, 2019.4.26; MFDS: Cheongju-si, Korea, 2019; Chapter 8. [Google Scholar]
- Klein, B.P.; Perry, A.K. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J. Food Sci. 1982, 47, 941–945. [Google Scholar] [CrossRef]
- Popelka, P.; Jevinová, P.; Šmejkal, K.; Roba, P. Determination of capsaicin content and pungency level of different fresh and dried chilli peppers. Folia Veter. 2017, 61, 11–16. [Google Scholar] [CrossRef]
- Sądej, W.; Żołnowski, A.C. Comparison of the Effect of Various Long-term Fertilization Systems on the Content and Fractional Composition of Humic Compounds in Lessive Soil. Plant Soil Environ. 2019, 21, 172–180. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; MeftahKadmiri, I. Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front. Microbiol. 2021, 12, 354. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Ordookhani, K.; Khavazi, K.; Moezzi, A.; Rejali, F. Influence of PGPR and AMF on antioxidant activity, lycopene and potassium contents in tomato. Afr. J. Agric. Res. 2010, 5, 1108–1116. [Google Scholar]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant: A review. Front. Plant Sci. 2021, 12, 1355. [Google Scholar] [CrossRef]
- Bisht, A.; Garg, N. AMF species improve yielding potential of Cd stressed pigeonpea plants by modulating sucrose-starch metabolism, nutrients acquisition and soil microbial enzymatic activities. Plant Growth Regul. 2022, 96, 409–430. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zahra, T.R. A comparative study of sweet pepper fruits nutritional composition produced under conventional and organic systems. Int. J. Agric. Sci. 2014, 10, 8–14. [Google Scholar]
- Gupta, S.; Kaushal, R.; Sood, G.; Bhardwaj, S.; Chauhan, A. Indigenous Plant Growth Promoting Rhizobacteria and Chemical Fertilizers: Impact on Soil Health and Productivity of Capsicum (Capsicum Annuum L.) in North Western Himalayan Region. Soil Sci. Plant Anal. 2021, 52, 948–963. [Google Scholar] [CrossRef]
- Saia, S.; Aissa, E.; Luziatelli, F.; Ruzzi, M.; Colla, G.; Ficca, A.G.; Cardarelli, M.; Rouphael, Y. Growth-promoting bacteria and arbuscular mycorrhizal fungi differentially benefit tomato and corn depending upon the supplied form of phosphorus. Mycorrhiza 2020, 30, 133–147. [Google Scholar] [CrossRef]
- Hossain, A.; Ali, M.E.; Maitra, S.; Bhadra, P.; Rahman, M.M.E.; Ali, S.; Aftab, T. The role of soil microorganisms in plant adaptation to abiotic stresses: Current scenario and future perspectives. In Plant Perspectives to Global Climate Changes; Aftab, T., Roychoudhury, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 233–278. [Google Scholar]
- Shahein, M.M.; El-Sayed, S.F.; Taha, S.S. Impact of bio-and sources of organic fertilizers on sweet pepper vegetative growth, yield and quality under protected cultivation condition. Biosci. Res. 2018, 15, 453–465. [Google Scholar]
- Rueda-Puente, E.O.; Murillo-Amador, B.; Castellanos-Cervantes, T.; García-Hernández, J.L.; Tarazòn-Herrera, M.A.; Medina, S.M.; Barrera, L.E.G. Effects of plant growth promoting bacteria and mycorrhizal on Capsicum annuum L. var. aviculare ([Dierbach] D’Arcy and Eshbaugh) germination under stressing abiotic conditions. Plant Physiol. Biochem. 2010, 48, 724–730. [Google Scholar] [CrossRef]
- Fawzy, Z.F.; El-Bassiony, A.M.; Li, Y.; Ouyang, Z.; Ghonam, A.A. Effect of Mineral, Organic and Bio-N Fertilizers on Growth, Yield and Fruit Quality of Sweet Pepper. J. Appl. Sci. Res. 2012, 8, 3921–3933. [Google Scholar]
- Kashyap, A.S.; Thakur, A.K.; Thakur, N. Effect of organic manures and biofertilizers on the productivity of tomato and bell pepper under Mid-Hill conditions of Himachal Pradesh. Int. J. Econ. Plants 2014, 1, 9–12. [Google Scholar]
- Chetri, D.A.; Singh, A.K.; Singh, V.B. Effect of integrated nutrient management on yield, quality and nutrient uptake by capsicum (Capsicum annum) cv. California wonder. J. Soil Crop 2012, 22, 44–48. [Google Scholar]
- Gokul, D.; Poonkodi, P.; Angayarkanni, A. Effects of inorganic fertilizers, organic manures, biofertilizers and magnesium sulfate on yield attributes, yield and quality of chilli. Int. J. Anal. Exp. Modal Anal. 2021, 13, 779–783. [Google Scholar]
- Sani, M.N.H.; Yong, J.W.H. Harnessing Synergistic Biostimulatory Processes: A Plausible Approach for Enhanced Crop Growth and Resilience in Organic Farming. Biology 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Hariyono, D.; Ali, F.Y.; Nugroho, A. Increasing the Growth and Development of Chili-Pepper under Three Different Shading Condition in Response to Biofertilizers Application. Agrivita J. Agric. Sci. 2021, 43, 198–208. [Google Scholar] [CrossRef]
- Uresti-Porras, J.G.; Cabrera-De-La Fuente, M.; Benavides-Mendoza, A.; Olivares-Sáenz, E.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of Graft and Nano ZnO on Nutraceutical and Mineral Content in Bell Pepper. Plants 2021, 10, 2793. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bohra, J.S. Effect of NPKS and Zn application on growth, yield, economics and quality of baby corn. Arch. Agron. Soil Sci. 2014, 60, 1193–1206. [Google Scholar] [CrossRef]
- Roesti, D.; Gaur, R.; Johri, B.N.; Imfeld, G.; Sharma, S.; Kawaljeet, K.; Aragno, M. Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol. Biochem. 2006, 38, 1111–1120. [Google Scholar] [CrossRef]
- Kim, K.; Yim, W.; Trivedi, P.; Madhaiyan, M.; Deka Boruah, H.P.; Islam, M.; Lee, G.; Sa, T. Synergistic effects of inoculating arbuscular mycorrhizal fungi and Methylobacteriumoryzae strains on growth and nutrient uptake of red pepper (Capsicum annuum L.). Plant Soil 2010, 327, 429–440. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Kumar, A.; Naqvi, S.D.Y.; Kaushik, P.; Khojah, E.; Amir, M.; Alam, P.; Samra, B.N. Rhizophagus irregularis and Nitrogen Fixing Azotobacter Enhances Greater Yam (Dioscorea alata) Biochemical Profile and Upholds Yield under Reduced Fertilization. Saudi J. Biol. Sci. 2022, 29, 3694–3703. [Google Scholar] [CrossRef]

| Variables | T1 (75%CF ± SD) | T2 (100%CF ± SD) | T3 (75%CF + RI ± SD) | T4 (75%CF + AC ± SD) | T5 (75%CF + RI + AC ± SD) |
|---|---|---|---|---|---|
| Perimeter | 25.85 ± 2.83 e * | 33.73 ± 0.63 d | 38.15 ± 1.05 c | 41.44 ± 0.73 b | 45.72 ± 1.10 a |
| Area (cm2) | 19.94 ± 3.80 e | 51.83 ± 1.07 d | 59.13 ± 0.95 c | 63.15 ± 0.44 b | 72.57 ± 0.18 a |
| Width Mid-height | 3.36 ± 0.79 d | 6.26 ± 0.50 c | 7.87 ± 0.11 b | 8.15 ± 0.05 b | 8.88 ± 0.10 a |
| Maximum Width | 5.21 ± 0.43 d | 7.23 ± 0.18 c | 8.22 ± 0.02 b | 8.33 ± 0.02 b | 9.19 ± 0.24 a |
| Height Mid width | 6.42 ± 0.63 e | 8.37 ± 0.01 d | 8.99 ± 0.14 c | 9.96 ± 0.21 b | 11.07 ± 0.73 a |
| Maximum Height | 6.61 ± 1.15 d | 9.37 ± 0.05 c | 10.14 ± 0.09 c | 11.09 ± 0.08 b | 12.48 ± 0.08 a |
| Curved Height | 7.84 ± 0.91 e | 10.97 ± 0.18 d | 12.60 ± 0.09 c | 13.49 ± 0.10 b | 14.35 ± 0.48 a |
| Shoulder Height | 0.01 ± 0.00 c | 0.24 ± 0.05 b | 0.48 ± 0.02 a | 0.50 ± 0.00 a | 0.51 ± 0.00 a |
| Pepper Pericarp Boundary | 18.12 ± 1.73 e | 26.08 ± 0.29 d | 29.22 ± 0.57 c | 32.40 ± 0.60 b | 35.87 ± 0.91 a |
| Pepper Pericarp Area | 25.82 ± 2.14 e | 41.12 ± 0.85 d | 52.37 ± 0.78 c | 58.67 ± 0.67 b | 63.33 ± 0.78 a |
| Pepper Pericarp Thickness | 0.69 ± 0.33 c | 1.38 ± 0.02 b | 1.54 ± 0.01 ab | 1.65 ± 0.04 a | 1.74 ± 0.00 a |
| Days to 50 flowering | 40.06 ± 2.64 a | 36.39 ± 0.53 a | 36.22 ± 5.29 a | 32.01 ± 1.76 b | 28.39 ± 0.95 b |
| Number of marketable fruits per plant | 11.41 ± 0.89 d | 22.34 ± 0.27 c | 24.60 ± 3.04 b | 26.14 ± 0.97 b | 29.40 ± 0.91 a |
| Fruit length (cm) | 4.82 ± 0.58 d | 6.57 ± 0.04 c | 6.85 ± 0.03 bc | 7.10 ± 0.10 ab | 7.34 ± 0.09 a |
| Fruit width (cm) | 4.76 ± 0.24 c | 6.94 ± 0.05 b | 6.77 ± 0.11 b | 7.01 ± 0.34 ab | 7.33 ± 0.05 a |
| Average fruit weight (g) | 62.57 ± 6.84 d | 79.24 ± 3.41 c | 83.59 ± 1.41 c | 109.18 ± 1.54 b | 133.73 ± 1.25 a |
| Marketable yield per plant (kg) | 0.70 ± 0.10 e | 1.70 ± 0.10 d | 1.94 ± 0.05 b | 1.83 ± 0.06 c | 2.69 ± 0.01 a |
| Plant height cm. | 51.75 ± 0.50 c | 71.75 ± 0.50 b | 74.68 ± 0.90 b | 73.10 ± 0.29 b | 80.41 ± 5.35 a |
| TSS (%) | 2.82 ± 0.56 b | 4.12 ± 0.16 a | 4.33 ± 0.06 a | 4.29 ± 0.03 a | 4.56 ± 0.14 a |
| Ascorbic Acid | 128.50 ± 0.95 c | 131.33 ± 1.06 c | 149.00 ± 5.59 b | 153.50 ± 4.84 ab | 160.50 ± 5.64 a |
| Capsaicin content | 3.55 ± 0.09 d | 4.48 ± 0.20 c | 5.44 ± 0.16 b | 6.11 ± 0.10 a | 6.31 ± 0.19 a |
| Variables | T1 (75%CF ± SD) | T2 (100%CF ± SD) | T3 (75%CF + RI ± SD) | T4 (75%CF + AC ± SD) | T5 (75%CF + RI + AC ± SD) |
|---|---|---|---|---|---|
| Calcium (mg/100 g dry weight) | 42.21 ± 3.81 e * | 88.32 ± 12.10 b | 56.70 ± 9.12 c | 61.10 ± 3.57 d | 98.62 ± 10.45 a |
| Magnesium (mg/100 g dry weight) | 38.22 ± 12.15 d | 113 ± 20.23 b | 94.44 ± 13.65 c | 90.35 ± 9.12 c | 131.57 ± 4.56 a |
| Phosphorus (mg/100 g dry weight) | 102.22 ± 18.06 d | 250.62 ± 7.81 b | 280 ± 32.12 a | 220.29 ± 16.22 c | 290.68 ± 12.80 a |
| Sulfate (mg/100 g dry weight) | 66.15 ± 5.18 c | 260 ± 10.32 a | 210 ± 20.75 b | 200 ± 4.56 b | 264 ± 5.80 a |
| Iron (mg/100 g dry weight) | 2.88 ± 0.98 d | 7.12 ± 1.95 a | 5.58 ± 4.12 c | 6.88 ± 2.11 b | 7.00 ± 3.12 a |
| Sodium (mg/100 g dry weight) | 5.50 ± 1.50 c | 18.34 ± 1.75 b | 19.22 ± 4.12 b | 21.21 ± 2.15 a | 21.08 ± 1.88 a |
| Root Mycorrhization (%) | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 45.72 ± 8.62 b | 0.00 ± 0.00 c | 55.31 ± 12.17 a |
| Number of Spores | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 81.36 ± 5.88 b | 0.00 ± 0.00 c | 112.25 ± 9.22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.; Sharma, V.; Delta, A.K.; Kaushik, P. Rhizophagus irregularis and Nitrogen Fixing Azotobacter with a Reduced Rate of Chemical Fertilizer Application Enhances Pepper Growth along with Fruits Biochemical and Mineral Composition. Sustainability 2022, 14, 5653. https://doi.org/10.3390/su14095653
Sharma M, Sharma V, Delta AK, Kaushik P. Rhizophagus irregularis and Nitrogen Fixing Azotobacter with a Reduced Rate of Chemical Fertilizer Application Enhances Pepper Growth along with Fruits Biochemical and Mineral Composition. Sustainability. 2022; 14(9):5653. https://doi.org/10.3390/su14095653
Chicago/Turabian StyleSharma, Meenakshi, Vandana Sharma, Anil Kumar Delta, and Prashant Kaushik. 2022. "Rhizophagus irregularis and Nitrogen Fixing Azotobacter with a Reduced Rate of Chemical Fertilizer Application Enhances Pepper Growth along with Fruits Biochemical and Mineral Composition" Sustainability 14, no. 9: 5653. https://doi.org/10.3390/su14095653
APA StyleSharma, M., Sharma, V., Delta, A. K., & Kaushik, P. (2022). Rhizophagus irregularis and Nitrogen Fixing Azotobacter with a Reduced Rate of Chemical Fertilizer Application Enhances Pepper Growth along with Fruits Biochemical and Mineral Composition. Sustainability, 14(9), 5653. https://doi.org/10.3390/su14095653







