Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methodology
3. Results and Discussion
3.1. Characterization of BM As-Received
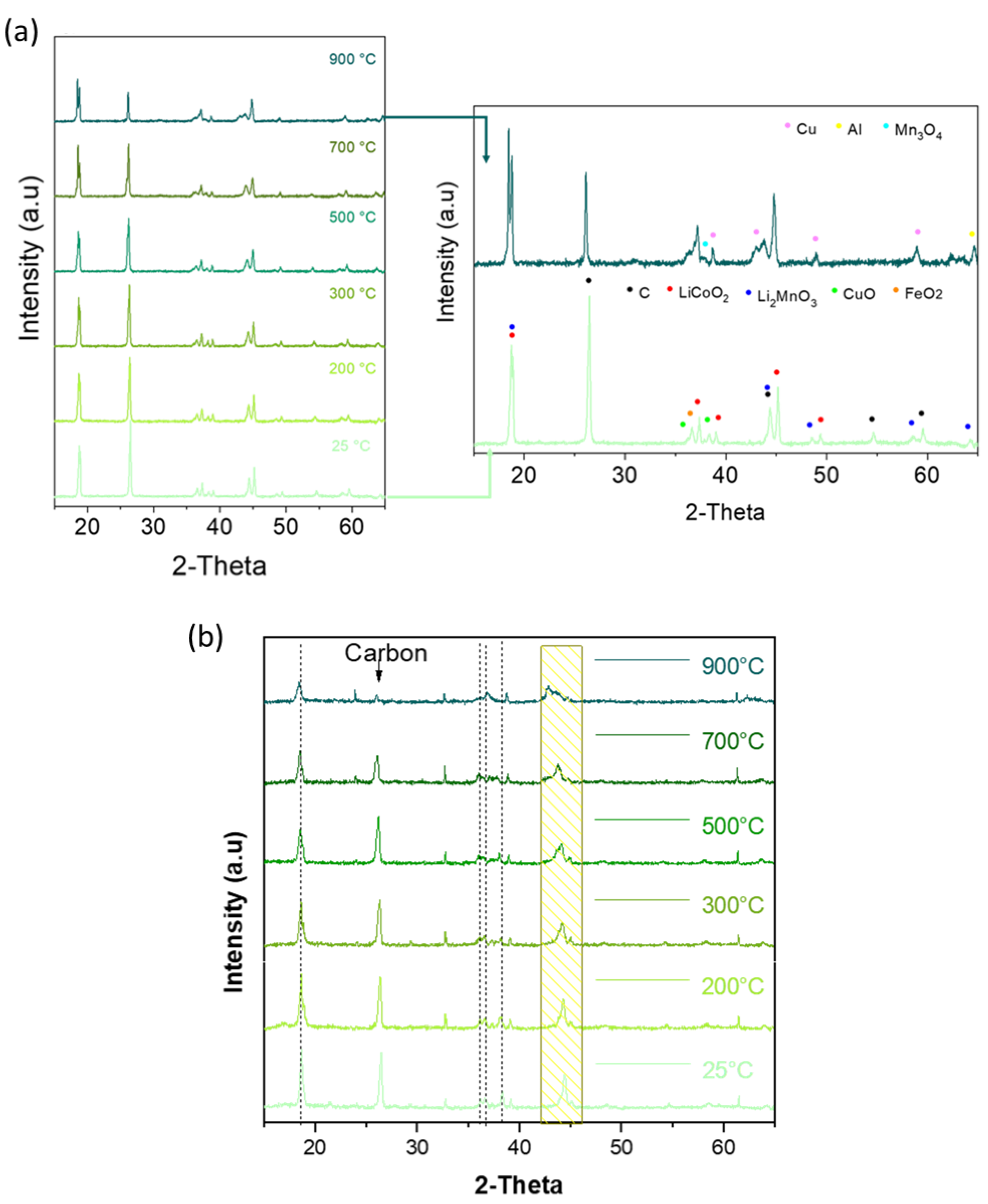
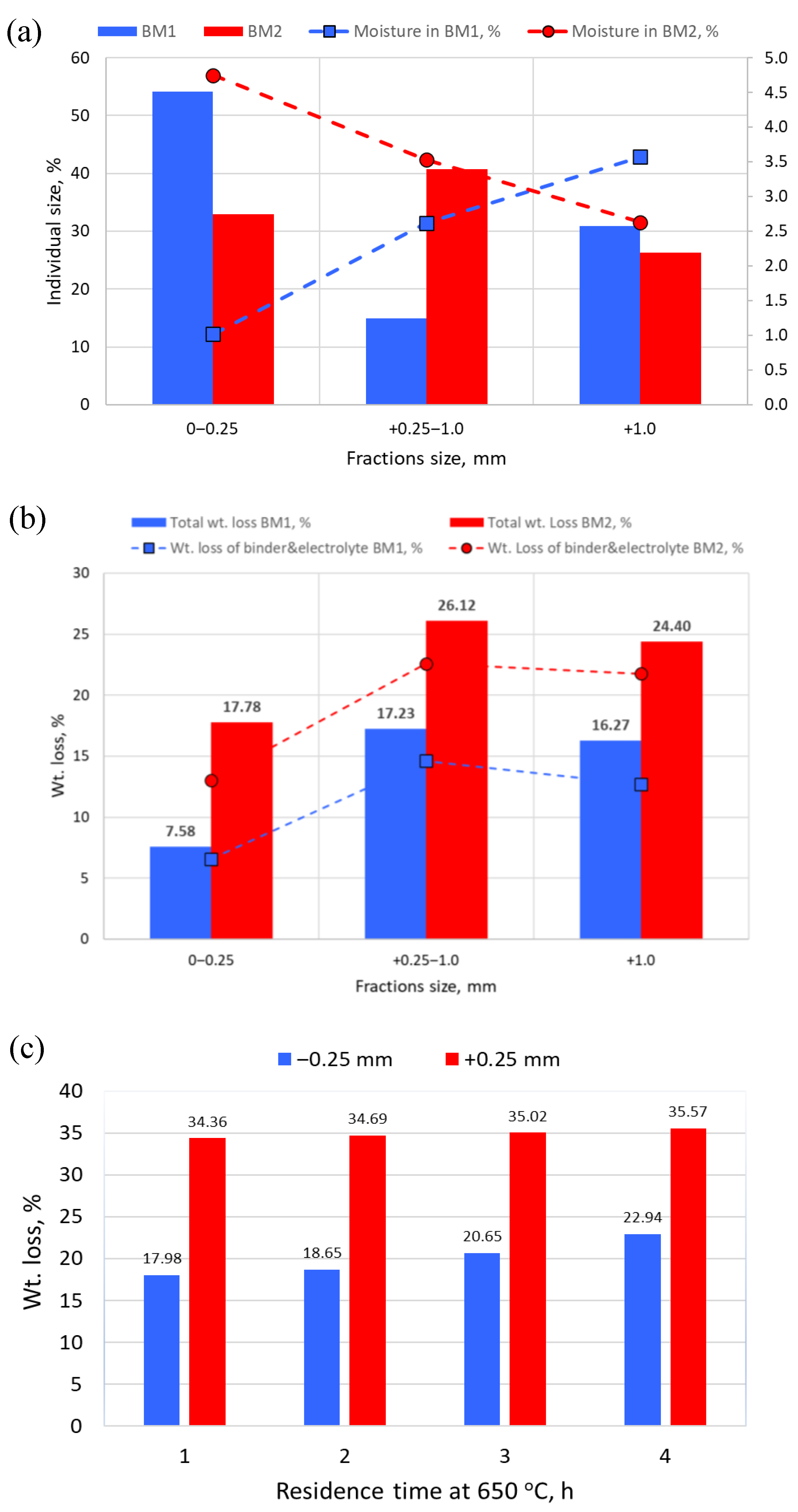
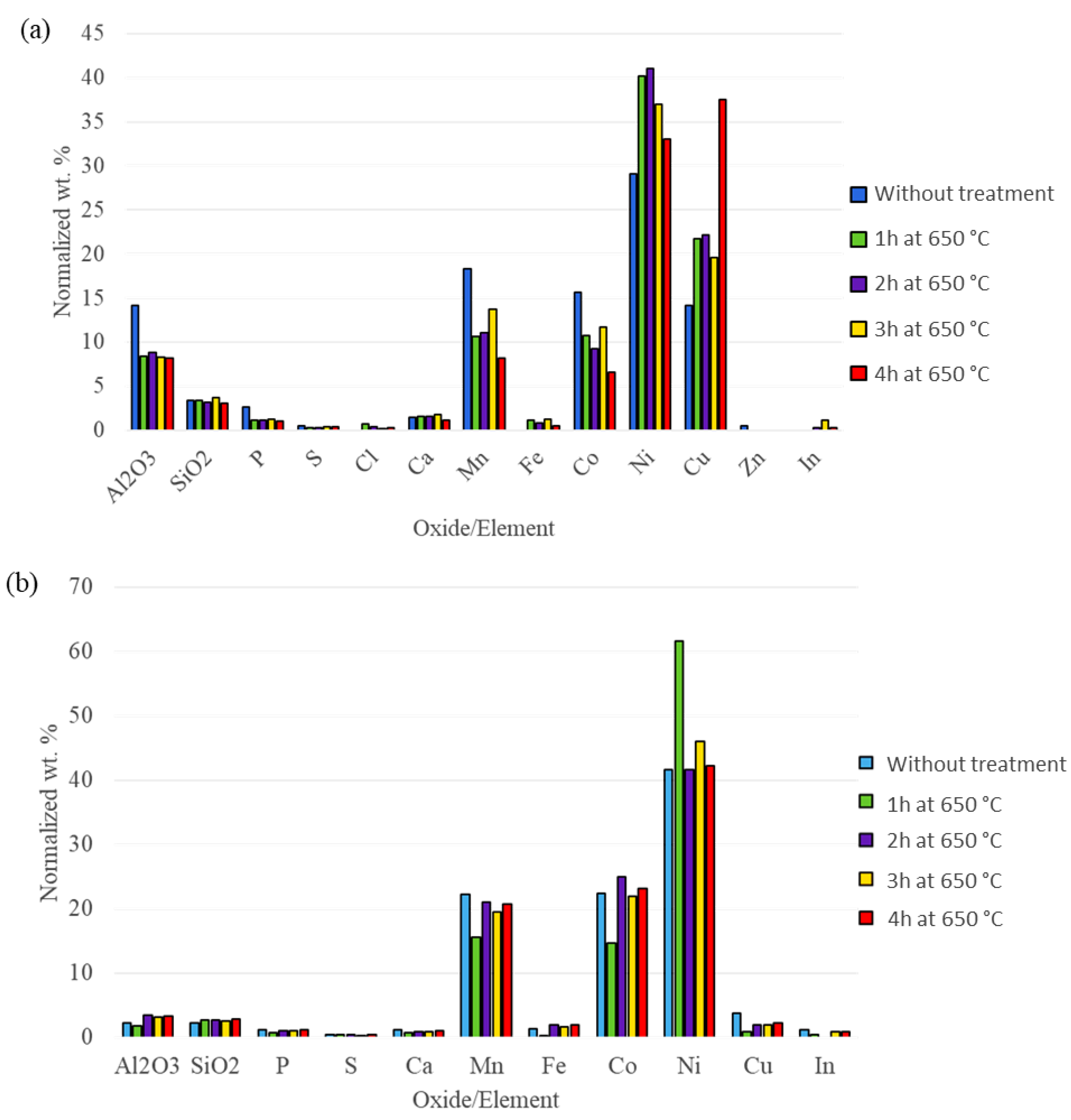
3.2. Effect of Temperature on Mass and Chemical Composition
3.3. Heat Treatment of BM
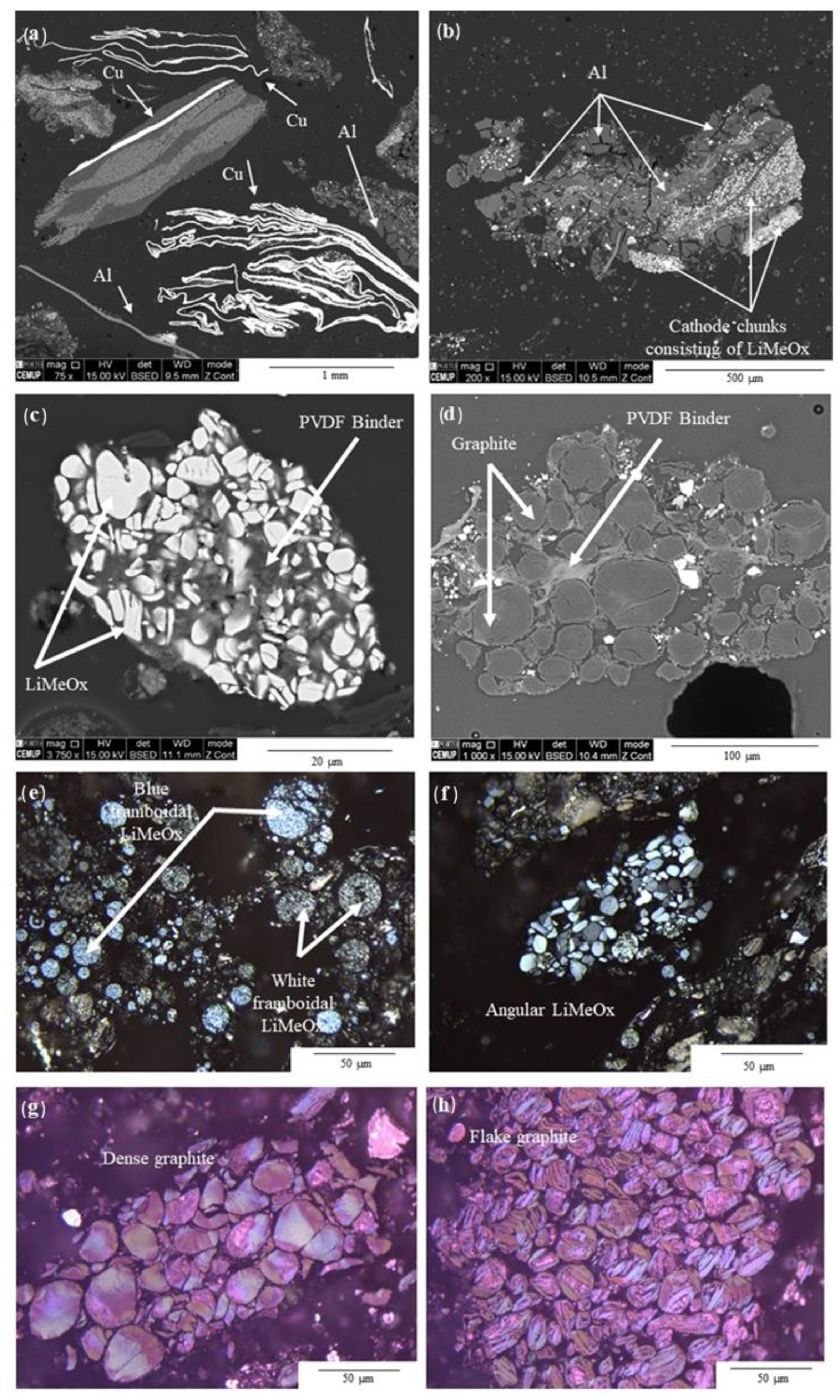
3.4. Morphological and Chemical Characterization of the BM after Pyrolysis
3.4.1. Binder and LiMeOx Morphological and Chemical Transformation
3.4.2. Graphite Optical and Physical Transformations
3.5. Gas Release from the BM under Pyrolytic Conditions
4. Conclusions
- LiMeOx occurs in the smaller-sized fractions as blue and white framboidal morphologies (LiMn, LiNiCo, and LiCoMnNi oxides) and as angular morphologies (LiCo oxide). BM1 was rich in LiCoO2, while BM2 was rich in LiNiCoMn oxides; however, the major component in both grades of BM was graphite. Thermal treatment led to a decrease in the compaction of the LiMeOx cathodes due to the removal of the PVDF binder and the decomposition of the LiMeOx into their respective metals.
- Graphite occurs in smaller size fractions as dense or flake morphologies. When compared to natural graphite, the graphite in the BM samples is disordered (based on petrographic and Raman structural analyses), and this indicates that the graphite used in batteries is not necessarily graphitic. Upon thermal treatment, the structure of the graphite becomes more ordered (based on petrographic and Raman structural analyses). The intensity of graphite XRD peaks decreased when heating the BM either in air or in N2 and can be attributed to the reduction of embedded cathodic LiMeOx with graphite.
- PVDF binder occurs on the surfaces of BM particles and hinders the recycling of BM. When heated, the PVDF partially decomposes into char, but new F cuboids and suspected carbonate crystals are also formed.
- Al- and Cu foils occur in larger size fractions and deteriorate during thermal treatment of BM.
- It was possible to detect several gas species during the pyrolytic treatment of the BM including DMC, DEC, oxygenated hydrocarbon (such as CH2O), hydrocarbons (such as CH4 and C6H6), and other miscellaneous gases (such as HBr, C2N2, HCN, and POF3). By increasing the pyrolysis temperature of the BM, higher mass loss and higher gas concentration were monitored.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. Global EV Outlook 2021—Accelerating Ambitions Despite the Pandemic; IEA: Paris, France, 2021; p. 101. [Google Scholar]
- Graham, J.D.; Rupp, J.A.; Brungard, E. Lithium in the green energy transition: The quest for both sustainability and security. Sustainability 2021, 13, 11274. [Google Scholar] [CrossRef]
- Dadé, M.; Wallmach, T.; Laugier, O. Detailed Microparticle Analyses Providing Process Relevant Chemical and Microtextural Insights into the Black Mass. Minerals 2022, 12, 119. [Google Scholar] [CrossRef]
- Gaines, L. Profitable Recycling of Low-Cobalt Lithium-Ion Batteries Will Depend on New Process Developments. One Earth 2019, 1, 413–415. [Google Scholar] [CrossRef]
- Fahimi, A.; Ducoli, S.; Federici, S.; Ye, G.; Mousa, E.; Frontera, P.; Bontempi, E. Evaluation of the sustainability of technologies to recycle spent lithium-ion batteries, based on embodied energy and carbon footprint. J. Clean. Prod. 2022, 338, 130493. [Google Scholar] [CrossRef]
- Halleux, V. New EU Regulatory Framework for Batteries-Setting Sustainability Requirements. © European Union 2022. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2021/689337/EPRS_BRI(2021)689337_EN.pdf (accessed on 1 December 2022).
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef] [Green Version]
- European Union. Directive 2008/68/EC of the European Parliament and of the Council of 24 September 2008 on the inland transport of dangerous goods. Off. J. Eur. Union L 2008, 230, 13–59. [Google Scholar]
- Petranikova, M.; Naharro, P.; Vieceli, N.; Lombardo, G.; Ebin, B. Recovery of critical metals from EV batteries via thermal treatment and leaching with sulfuric acid at ambient temperature. Waste Manag. 2022, 140, 164–172. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, C.; He, W.; Li, G.; Huang, J. A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them. Waste Manag. Res. 2018, 36, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Huang, Z.; Makuza, B.; Guo, X.; Tian, Q. Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review. Miner. Eng. 2021, 173, 107218. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Neumann, J.; Petronikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Hu, X.; Mousa, E.; Ånnhagen, L.; Musavi, Z.; Alemrajabi, M.; Hall, B.; Ye, G. Complex gas formation during combined mechanical and thermal treatments of spent lithium-ion-battery cells. J. Hazard. Mater. 2022, 431, 128541. [Google Scholar] [CrossRef] [PubMed]
- ISO 7404-2; Methods for the Petrographic Analysis of Coals. Part 2: Methods of Preparing Coal Samples. ISO (International Organisation for Standardisation): Geneva, Switzerland, 2009.
- ISO 7404-5; Methods for the Petrographic Analysis of Coals. Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. ISO (International Organisation for Standardisation): Geneva, Switzerland, 2009.
- Li, X.; Hayashi, J.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Rebelo, S.L.; Guedes, A.; Szefczyk, M.E.; Pereira, A.M.; Araújo, J.P.; Freire, C. Progress in Raman spectra analysis of covalently functionalized multiwalled carbon nanotubes: Unraveling disorder in graphitic materials. Phys. Chem. Chem. Phys. 2016, 18, 12784–12796. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.W.; Dallmeyer, I.; Johnson, T.J.; Brauer, C.S.; McEwen, J.-S.; Espinal, J.F.; Garcia-Perez, M. Structural analysis of char by Raman spectroscopy: Improving band assignments through computational calculations from first principles. Carbon 2016, 100, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Badenhorst, C. Char Extracted from Coal Ash as a Replacement for Natural Graphite. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2019. [Google Scholar]
- Adamczyk, Z.; Komorek, J.; Białecka, B.; Całus-Moszko, J.; Klupa, A. Possibilities of graphitization of unburned carbon from coal fly ash. Minerals 2021, 11, 1027. [Google Scholar] [CrossRef]
- Li, K.; Rimmer, S.M.; Liu, Q. Geochemical and petrographic analysis of graphitized coals from Central Hunan, China. Int. J. Coal Geol. 2018, 195, 267–279. [Google Scholar] [CrossRef]
- Kwiecińska, B.; Petersen, H.I. Graphite, semi-graphite, natural coke, and natural char classification—ICCP system. Int. J. Coal Geol. 2004, 57, 99–116. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. Environmentally friendly, and low-temperature approach for decomposition of polyvinylidene fluoride from the cathode electrode of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 12799–12806. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
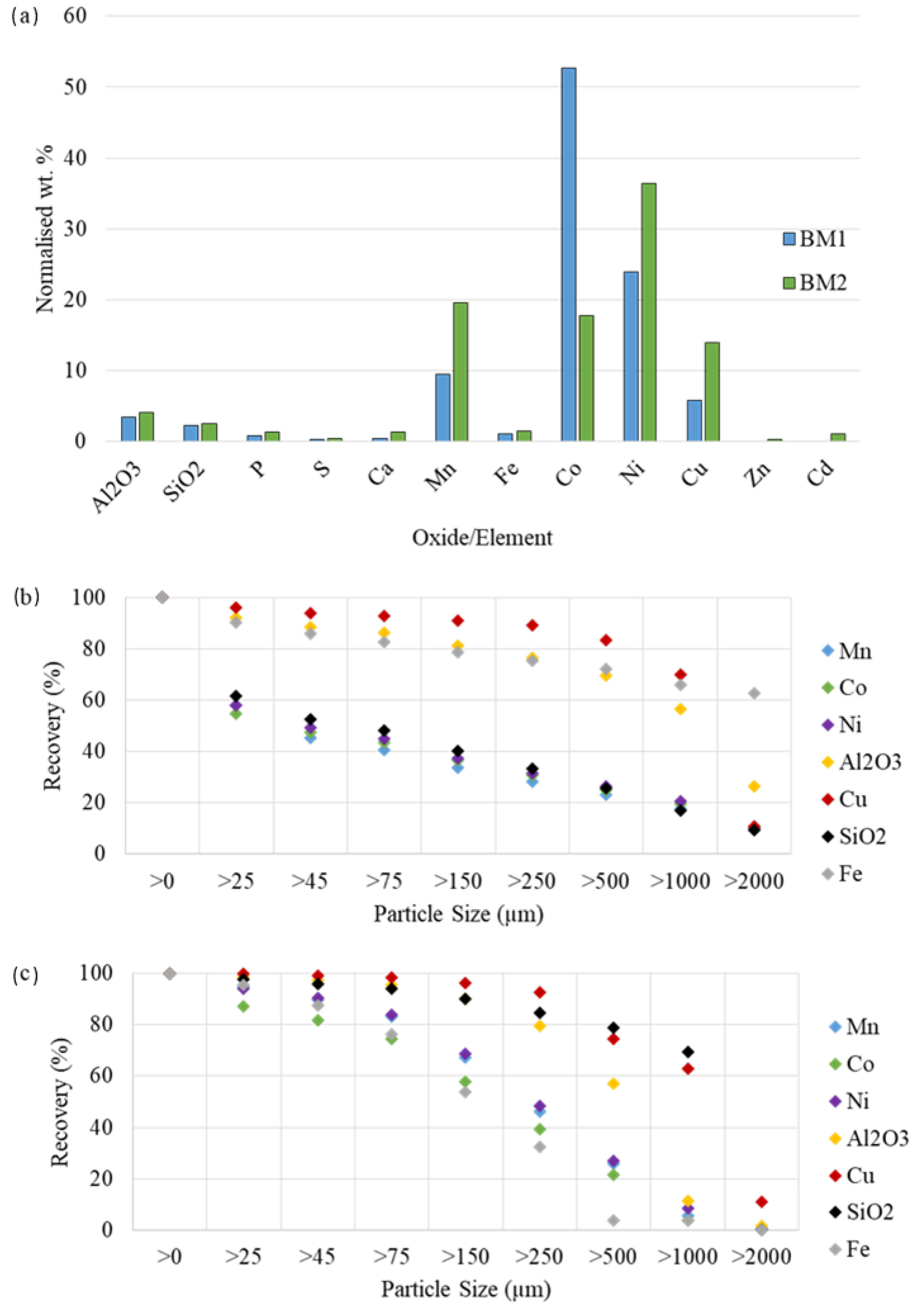






| Element | Co | Ni | Mn | Li | Cu | Al | Fe | C | Others |
|---|---|---|---|---|---|---|---|---|---|
| wt.%, Dry Basis | |||||||||
| BM1 | 17.5 | 5.1 | 3.0 | 3.9 | 3.9 | 1.6 | 1.6 | 36.0 | 27.4 |
| BM2 | 5.4 | 12.5 | 10.9 | 2.6 | 3.1 | 4.8 | 2.3 | 42.1 | 16.3 |
| Petrographic Reflectance Results | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Random Reflectance | Maximum Dense Graphite | Maximum Flake Graphite | ||||||||||
| Rr % | s | Rm % | S | Rm % | s | |||||||
| BM1 | 5.628 | 0.571 | 9.984 | 0.796 | 9.170 | 0.892 | ||||||
| BM2 | 4.764 | 0.645 | 9.868 | 1.526 | 7.994 | 1.385 | ||||||
| Raman microspectroscopy results | ||||||||||||
| G−D1 distance (cm−1) | D1/G integrated intensity ratio | D1/G FWHM ratio | ||||||||||
| Dense | Flake | Ordered | Average | Dense | Flake | Ordered | Average | Dense | Flake | Ordered | Average | |
| BM1 | 231 | 231 | - | 231 | 1.8 | 1.2 | - | 1.5 | 2.4 | 2.1 | - | 2.3 |
| BM2 | 215 | 232 | 230 | 225 | 1.5 | 1.6 | 1.1 | 1.3 | 1.1 | 0.9 | 2.3 | 1.3 |
| Mean Random Reflectance | Maximum Dense Graphite | Maximum Flake Graphite | ||||
|---|---|---|---|---|---|---|
| Rr % | s | Rm % | s | Rm % | s | |
| BM1 +1 mm 550 °C 15 min | 4.404 | 0.503 | 8.797 | 0.922 | 8.064 | 0.768 |
| BM1 +0.25–1mm 550 °C 15 min | 4.555 | 0.348 | 9.360 | 0.745 | 8.862 | 0.767 |
| BM1 −0.25 mm 550 °C 15 min | 5.098 | 0.393 | 10.091 | 0.897 | 9.321 | 0.759 |
| BM2 +1 mm 550 °C 15 min | 4.599 | 0.558 | 9.904 | 0.874 | 9.186 | 0.943 |
| BM2 +0.25–1 mm 550 °C 15 min | 4.975 | 0.375 | 9.399 | 0.794 | 8.294 | 0.858 |
| BM2 −0.25 mm 550 °C 15 min | 4.935 | 0.324 | 9.373 | 0.583 | 8.467 | 0.782 |
| BM2 +0.25 mm 650 °C 1 h | 5.371 | 0.533 | 10.310 | 0.805 | 9.389 | 1.125 |
| BM2 +0.25 mm 650 °C 2 h | 5.298 | 0.517 | 10.452 | 1.207 | 9.887 | 1.320 |
| BM2 +0.25 mm 650 °C 3 h | 5.098 | 0.765 | 10.881 | 1.074 | 9.614 | 1.392 |
| BM2 +0.25 mm 650 °C 4 h | 5.333 | 0.589 | 10.099 | 0.989 | 9.457 | 1.186 |
| BM2 −0.25 mm 650 °C 1 h | 5.616 | 0.606 | 11.412 | 0.877 | 10.185 | 1.231 |
| BM2 −0.25 mm 650 °C 2 h | 5.520 | 0.582 | 10.732 | 1.357 | 10.230 | 1.061 |
| BM2 −0.25 mm 650 °C 3 h | 5.250 | 0.531 | 10.700 | 0.697 | 9.960 | 0.820 |
| BM2 −0.25 mm 650 °C 4 h | 5.614 | 0.593 | 11.108 | 1.224 | 9.899 | 1.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability 2023, 15, 15. https://doi.org/10.3390/su15010015
Mousa E, Hu X, Ånnhagen L, Ye G, Cornelio A, Fahimi A, Bontempi E, Frontera P, Badenhorst C, Santos AC, et al. Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability. 2023; 15(1):15. https://doi.org/10.3390/su15010015
Chicago/Turabian StyleMousa, Elsayed, Xianfeng Hu, Ludvig Ånnhagen, Guozhu Ye, Antonella Cornelio, Ario Fahimi, Elza Bontempi, Patrizia Frontera, Charlotte Badenhorst, Ana Cláudia Santos, and et al. 2023. "Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries" Sustainability 15, no. 1: 15. https://doi.org/10.3390/su15010015
APA StyleMousa, E., Hu, X., Ånnhagen, L., Ye, G., Cornelio, A., Fahimi, A., Bontempi, E., Frontera, P., Badenhorst, C., Santos, A. C., Moreira, K., Guedes, A., & Valentim, B. (2023). Characterization and Thermal Treatment of the Black Mass from Spent Lithium-Ion Batteries. Sustainability, 15(1), 15. https://doi.org/10.3390/su15010015












