Design of UVA Ultraviolet Disinfection System for Nutrient Solution Residual Liquid and Development of Microbial Online Monitoring System
Abstract
:1. Introduction
2. Materials and Methods
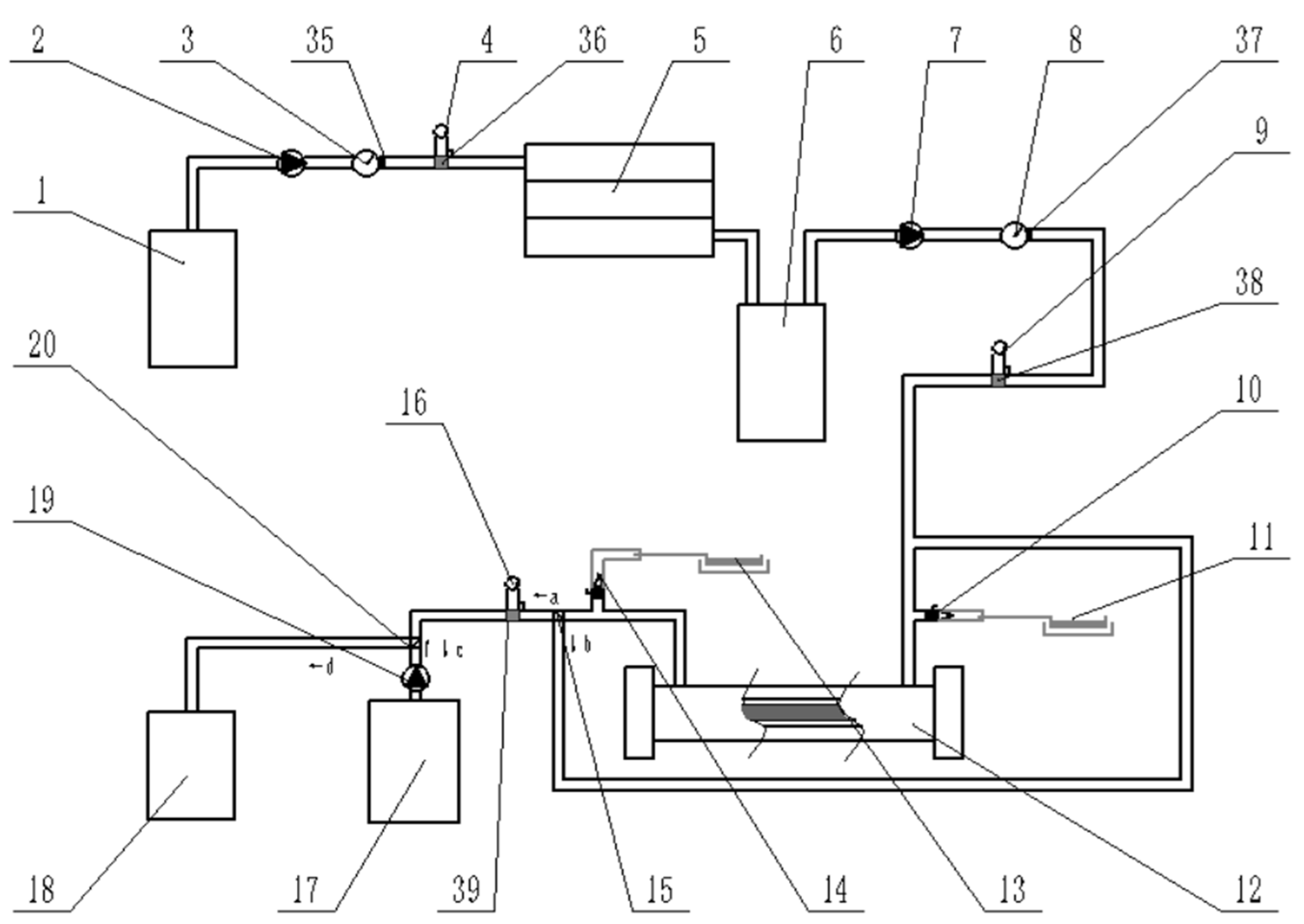
2.1. Design of Piping System for Residual Liquid Treatment
Residual Liquid Treatment Piping System
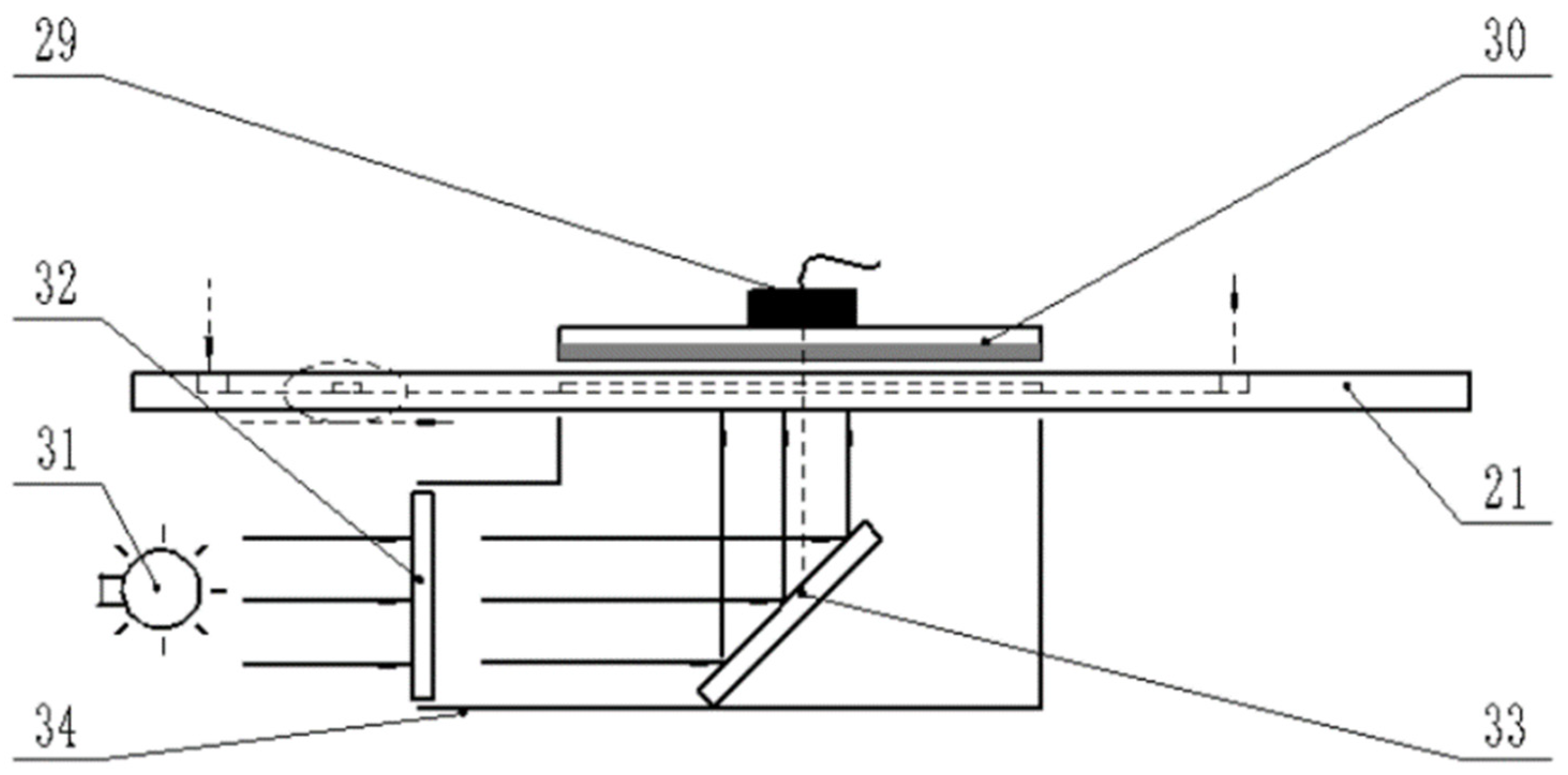
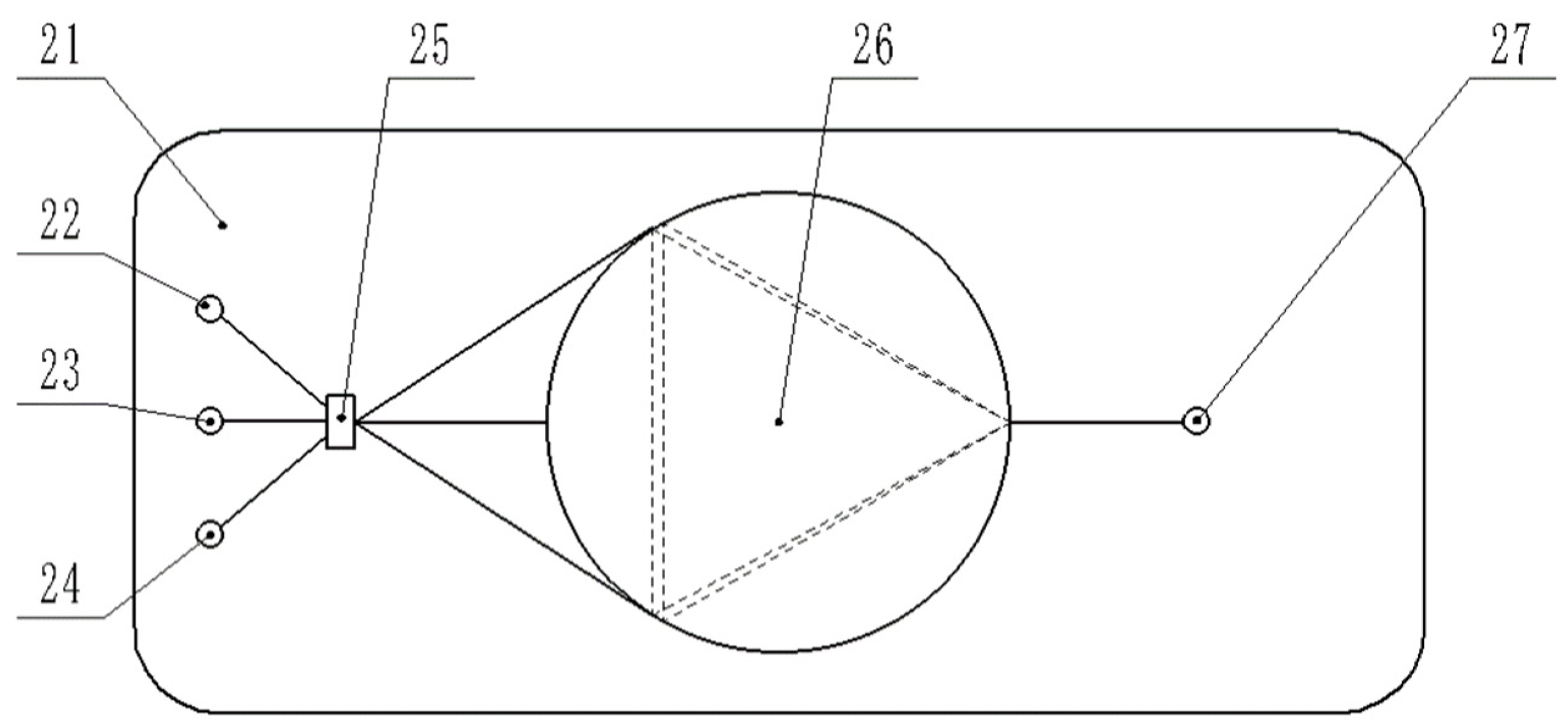
2.2. Design of Online Monitoring System for Residual Liquid Microorganisms Based on Microfluidic Chip
2.2.1. Microbiological Monitoring System Model
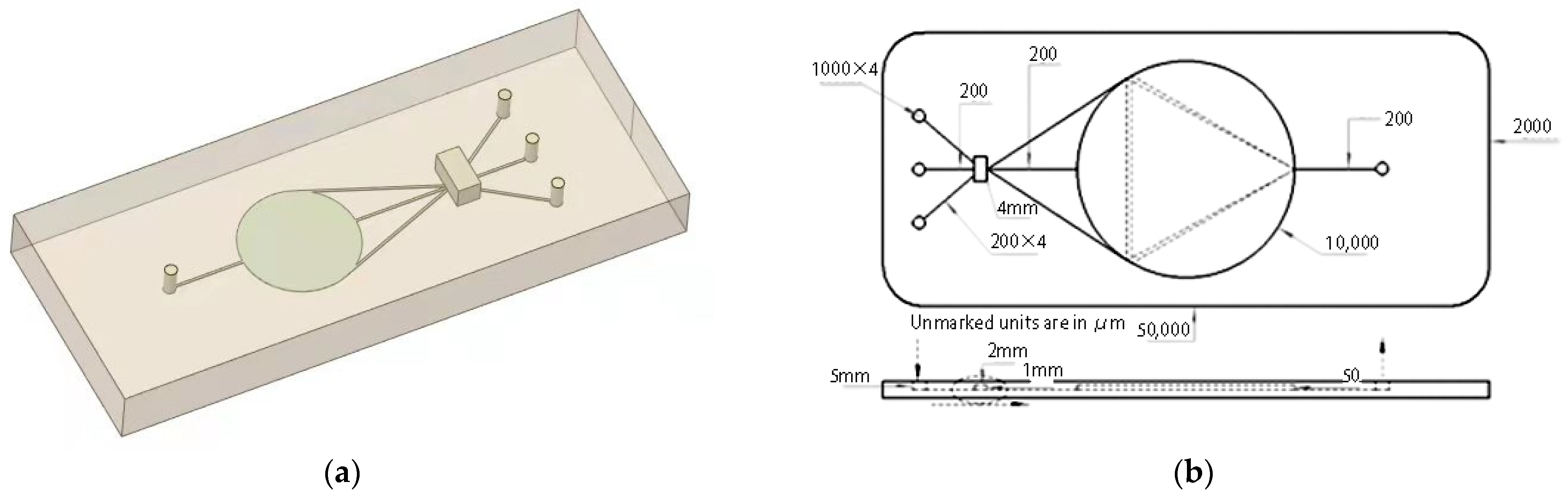
2.2.2. Microfluidic Chip Structure
2.2.3. Calculation of Biomass of Test Solution
2.3. Microfluidic Chip Model and Calculation of Important Parameters Related to CFD
2.3.1. 3D Model of Microfluidic Chip
2.3.2. Boundary Condition Calculation
2.4. Microfluidic Chip CFD Analysis
2.4.1. CFD Condition Setting
2.4.2. Analysis of CFD Results
3. Results and Discussion
3.1. UVA UV Disinfection Test of Residual Liquid
3.1.1. Test System Structure
3.1.2. Test Plan and Content
3.1.3. Test Conditions and Parameters
3.1.4. UVA Disinfection and Microbial Detection
- (1)
- Determine the disinfection time range
- (2)
- UV disinfection test
- (3)
- Microbiological laboratory testing of residual liquid
- (1)
- lb solid medium: Tryptone 10 g, yeast extract 5 g, sodium chloride 10 g, agar 15 g, adjust the pH to 7.0 with NaOH, with the volume diluted to 1000 mL with distilled water. Figure 9 is the test diagram for the preparation of lb solid medium. The medicine was added to a 1000 mL beaker, some distilled water was added and stirred in an electric heating constant temperature water bath until it was fully dissolved. Then, a pH meter was used to test the pH and a liquid dispenser was used to absorb a small amount of 5 mol/L NaOH solution to adjust the pH to 7.0. Finally, the distilled water was used to make up to 1000 mL; the solution needed to be stirred continuously during the process of pH adjustment and constant volume.
- (2)
- pda medium: 200 g potatoes, 20 g sucrose, 15~20 g agar, distilled water to 1000 mL.
- (3)
- The prepared lb and pda medium and petri dish were sterilized in a high temperature sterilization box. Figure 9 shows the sterilized lb and pda solid medium.
3.2. Sterilization Test Results and Analysis
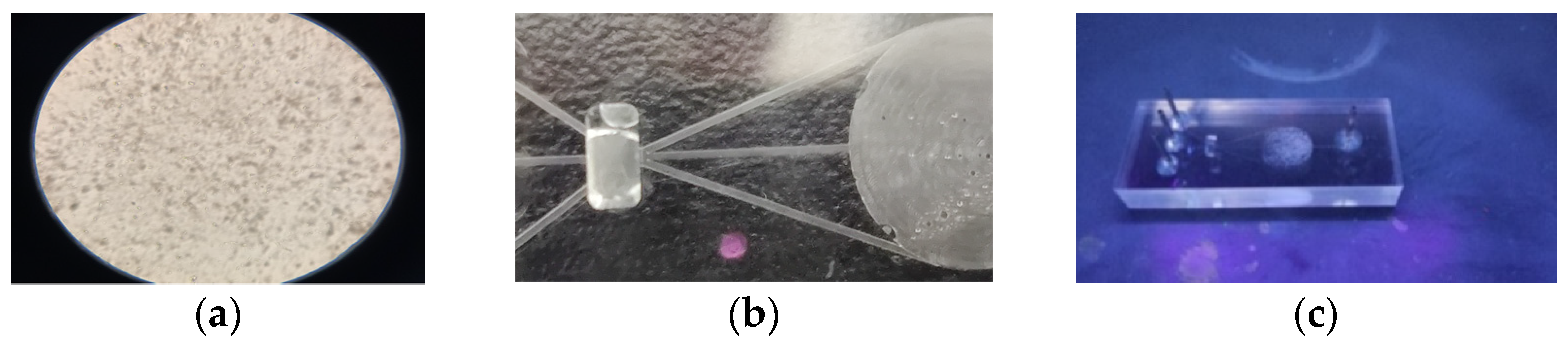
3.3. Microfluidic Chip Bioluminescence Detection Test
Biofluorescence Labeling and Detection
- (1)
- Sterilization absorbance detection of residual liquid
- (2)
- Bioluminescent labeling and detection
3.4. Results and Analysis of the Online Monitoring Test of Microorganisms in the Residual Liquid of the Nutrient Solution
3.5. Discussion of the Results
4. Conclusions
- (1)
- When the central wavelength of the UVA ultraviolet sterilizer is 439 nm, the working power is 30 W, 25 W and 16 W, respectively, and the UV penetration rate of the remaining liquid is 70%, the disinfection time range is controlled between 10~100 s, and the irradiation dose range is 5.09~90.54 mJ/cm2. When other conditions are the same, the relationship between the working power P of the UVA ultraviolet sterilizer used and its irradiance Ee is .
- (2)
- The microbial culture test of the residual liquid before and after disinfection was carried out. When the power of ultraviolet sterilization is 30 W, 25 W and 16 W, as the disinfection time increases, the sterilization rate keeps increasing. When the working power of the UV sterilizer is 30 W, the sterilization rate of the disinfection time is 60.67% for 30 s, 78.67% for 50 s, and 94% for 70 s. It takes about 100 s for the sterilization rate to reach 100%. With the increase in the time that the residual liquid flows through the UV sterilizer cavity, the number of microorganisms in the residual liquid continues to decrease. When the residual liquid flows through the ultraviolet sterilizer cavity for 10 s, the maximum flow rate is 0.158 m3·h−1, When the time is 100 s, the minimum flow rate is 0.018 m3·h−1. Under the condition that other conditions remain unchanged, the direct influencing factor of the ultraviolet disinfection rate of the nutrient solution residual liquid is the ultraviolet light irradiation dose.
- (3)
- The simulation analysis results of the channel performance of the microfluidic chip show that when t = 4 s, the fluid evenly covers the test cell and keeps the velocity close to 0 m/s, and the fluid velocity at the inlet and outlet of the channel is between 0.3 m/s and 0.45 m/s. This is consistent with the calculation results. The designed microfluidic glass chip meets expectations.
- (4)
- In the liquid-return biofluorescence detection test and residual liquid sterilization absorbance detection test based on the microfluidic chip, when the power of ultraviolet sterilization is 30 W, the absorbance value of different sterilization test samples does not change significantly. In addition, there are weak peaks, which are close to the optimal wavelength (300 nm), and the absorption value is approximately 0.07. The sterilization time has little effect on the absorbance of the detected sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Hu, X.; Hu, C.; Wei, D. Sequential use of ultraviolet light and chlorine for reclaimed water disinfection. J. Environ. Sci. 2011, 23, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, M.; Jiang, H.; Zhang, M.; Zhang, C. Removal of resistance genes in simulated pipeline network by chlorine/chloramine and low-pressure UV sequential disinfection. China Environ. Sci. 2022, 42, 1–10. [Google Scholar]
- Lv, D. Multi-barrier disinfection strategy for drinking water and application of UV disinfection technology. Water Purif. Technol. 2019, 38, 1–6. [Google Scholar]
- Zhang, T.; Hu, Y.; Jiang, L.; Yao, S.; Lin, K.; Zhou, Y.; Cui, C. Removal of antibiotic resistance genes and control of horizontal transfer risk by UV, chlorination and UV/chlorination treatments of drinking water. Chem. Eng. J. 2018, 358, 589–597. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, M.; Zhou, H.; Wang, S.; Wang, Y.; Bai, M.; Zhang, C. Selective antibiotic resistance genes in multiphase samples during biofilm growth in a simulated drinking water distribution system: Occurrence, correlation and low-pressure ultraviolet removal. Sci. Total Environ. 2018, 649, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Dan, Q.; Ma, W.; Yin, B.; Cao, T.; Jiang, C.; Xu, Y. Research on greenhouse nutrient solution recycling device and disinfection effect. Northeast Agric. Sci. 2019, 44, 52–56. [Google Scholar]
- Tsunedomi, A.; Miyawaki, K.; Masamura, A.; Nakahashi, M.; Mawatari, K.; Shimohata, T.; Uebanso, T.; Kinouchi, Y.; Akutagawa, M.; Emoto, T.; et al. UVA-LED device to disinfect hydroponic nutrient solution. J. Med. Investig. 2018, 65, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Wu, S.; Chu, C.; Zhou, D.; Jiang, Z. Research progress on recycling of nutrient solution waste in soilless culture. J. Agron. 2014, 4, 51–53. [Google Scholar]
- Liu, W.; Huang, C. Effects of the circulation pump type and ultraviolet sterilization on nutrient solutions and plant growth in plant factories. HortTechnology 2019, 29, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.-Y.; Ryu, S.-H.; Lim, J.-H. Design and implementation of an integrated management system in a plant factory to save energy. Clust. Comput. 2014, 17, 727–740. [Google Scholar] [CrossRef]
- Settanni, L.; Miceli, A.; Francesca, N.; Cruciata, M.; Moschetti, G. Microbiological investigation of Raphanus sativus L. grown hydroponically in nutrient solutions contaminated with spoilage and pathogenic bacteria. Int. J. Food Microbiol. 2013, 160, 344–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crop. Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D. Emerging Trends in Microfluidics Based Devices. Biotechnol. J. 2020, 15, e1900279. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Fu, Y. Research progress of rapid detection technology of microorganisms in planetary protection. J. Kunming Univ. Sci. Technol. (Nat. Sci. Ed.) 2021, 46, 147–157. [Google Scholar]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Cui, P.; Wang, S. Application of microfluidic chip technology in pharmaceutical analysis: A review. J. Pharm. Anal. 2019, 9, 238–247. [Google Scholar] [CrossRef]
- Tozar, T.; Boni, M.; Andrei, I.R.; Pascu, M.L.; Staicu, A. High performance thin layer chromatography densitometry method based on picosecond laser-induced fluorescence for the analysis of thioridazine and its photoproducts. J. Chromatogr. A 2021, 1655, 462488. [Google Scholar] [CrossRef]
- Hussein, S.A.E.-R.; El-Wadood, H.M.A.; Abdallah, M.A.-E.W.; Khorshed, A.A.E.-H. HPTLC with Fluorescence Densitometry for Simultaneous Determination of Some Angiotensin II Receptor Blockers in Tablets and Plasma. J. AOAC Int. 2015, 98, 354–360. [Google Scholar] [CrossRef]
- Coran, S.; Bartolucci, G.; Bambagiotti-Alberti, M. Selective determination of aloin indifferent matrices by HPTLC den-sitometry in fluorescence mode. J. Pharm. Biomed. Anal. 2011, 54, 422–425. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.-U.; Warren, B.M.; Makris, K.; Evans, G.W.H.; Papadopoulou, E.; Coleman, S.; Niu, X. A Droplet Microfluidic-Based Sensor for Simultaneous in Situ Monitoring of Nitrate and Nitrite in Natural Waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Yang, N.; Wang, P.; Zhang, R.; Xu, P.; Sun, J.; Mao, H. Photoelectric detection method of rice smut spores based on enrichment microfluidic chip. Chin. J. Agric. Eng. 2017, 33, 161–168. [Google Scholar]
- Abdullafva, A.M.; Blinkova, L.P.; Usha, B.V.; Pakhomov, Y.D. Method for Express Detection of Viable Microorganisms in Meat and Meat Products. Patent RU20200126113, 5 August 2020. [Google Scholar]
- Singh, K.R.; Nayak, V.; Singh, J.; Adetunji, C.O.; Singh, R.P. Introduction: Potentialities of bionanomaterials towards the environmental and agricultural domain. In Bionanomaterials for Environmental and Agricultural Applications; IOP Publishing: Bristol, UK, 2021; pp. 1–19. [Google Scholar]









| Parameter | Type | (m/s) | (m/s) | (m/s) | Turbulence Coefficient | (m/s) | (m/s) | (m/s) | Tag Pool Properties |
|---|---|---|---|---|---|---|---|---|---|
| Entrance 22 | speed entry | 0.00432 | 0.00541 | 0.00721 | 1.1 | 0.00393 | 0.00492 | 0.00655 | Turbulence intensity: 1.18% |
| Entrance 23 | 0.00648 | 0.08115 | 0.1080 | 0.00589 | 0.07377 | 0.09818 | |||
| Entrance 24 | 0.00432 | 0.00541 | 0.00721 | 0.00393 | 0.00492 | 0.00655 | |||
| Entrance 27 | speed exit | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Material | Density (kg·m−3) | Specific Heat Capacity (J·kg−1·k−1) | Transmittance | Viscosity | Refractive Index |
|---|---|---|---|---|---|
| PMMA | 1770 | 1464 | 0.92 | / | 1.51 |
| pure water | 1000 | 4200 | 0.99 | 1.01 | 1.10 |
| residual liquid | 1005 | 4080 | 0.98 | 1.32 | 1.15 |
| dye | 1070 | 1950 | 0.75 | 1.47 | 1.89 |
| Power (W) | Wave (nm) | Value (uW/cm2/nm) | Bek (uW/lm) | Ee (mW/cm2) |
|---|---|---|---|---|
| 30 | 439.0 | 48.534 | 1.87 | 1.006 |
| 25 | 439.0 | 43.418 | 1.83 | 0.871 |
| 16 | 439.0 | 28.134 | 1.78 | 0.509 |
| Power (W) | Ee (mW/cm2) | T (s) | ki |
|---|---|---|---|
| 30 | 1.006 | 49.70–99.30 | 29.82 |
| 25 | 0.871 | 57.41–114.81 | 28.71 |
| 16 | 0.509 | 98.23–196.46 | 31.42 |
| Power of Ultraviolet Lamp (Radiation Flux) (W) | Pump Flow q/(m3·h−1) | Time to Flow through the UV Sterilizer Chamber/s | Microbial Sterilization Rate after One Disinfection (%) |
|---|---|---|---|
| 30 | 0.052 | 90 | 94 |
| 0.067 | 70 | 89.33 | |
| 0.094 | 50 | 78.67 | |
| 0.156 | 30 | 60.67 | |
| 25 | 0.036 | 80 | 87.53 |
| 0.048 | 60 | 80.95 | |
| 0.072 | 40 | 65.62 | |
| 0.144 | 20 | 23.79 | |
| 16 | 0.018 | 100 | 81.33 |
| 0.026 | 70 | 62.81 | |
| 0.053 | 40 | 41.36 | |
| 0.158 | 10 | 7.89 |
| Wavelength | Strength U | The Amount of Bacteria after Disinfection V/Individual | Ratio (U/V) | m Value |
|---|---|---|---|---|
| 513 | 423,820.8 | 15,037 | 28.25 | 28.79 |
| 421,442 | 13,877 | 30.37 | ||
| 157,925.1 | 5834 | 27.07 | ||
| 23,230.7 | 777 | 29.91 | ||
| 8528.59 | 301 | 28.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fang, W.; Zhao, Z. Design of UVA Ultraviolet Disinfection System for Nutrient Solution Residual Liquid and Development of Microbial Online Monitoring System. Sustainability 2023, 15, 173. https://doi.org/10.3390/su15010173
Wang X, Fang W, Zhao Z. Design of UVA Ultraviolet Disinfection System for Nutrient Solution Residual Liquid and Development of Microbial Online Monitoring System. Sustainability. 2023; 15(1):173. https://doi.org/10.3390/su15010173
Chicago/Turabian StyleWang, Xinzhong, Weiquan Fang, and Zhongfeng Zhao. 2023. "Design of UVA Ultraviolet Disinfection System for Nutrient Solution Residual Liquid and Development of Microbial Online Monitoring System" Sustainability 15, no. 1: 173. https://doi.org/10.3390/su15010173
APA StyleWang, X., Fang, W., & Zhao, Z. (2023). Design of UVA Ultraviolet Disinfection System for Nutrient Solution Residual Liquid and Development of Microbial Online Monitoring System. Sustainability, 15(1), 173. https://doi.org/10.3390/su15010173









