The Influence of the COVID-19 Pandemic on Mortality of Patients Hospitalized in Surgical Services in Romania: A Cross-Sectional Study of a National Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment of Participants and Data Collection
2.3. Definition of Variables
2.4. Statistical Methods
3. Results
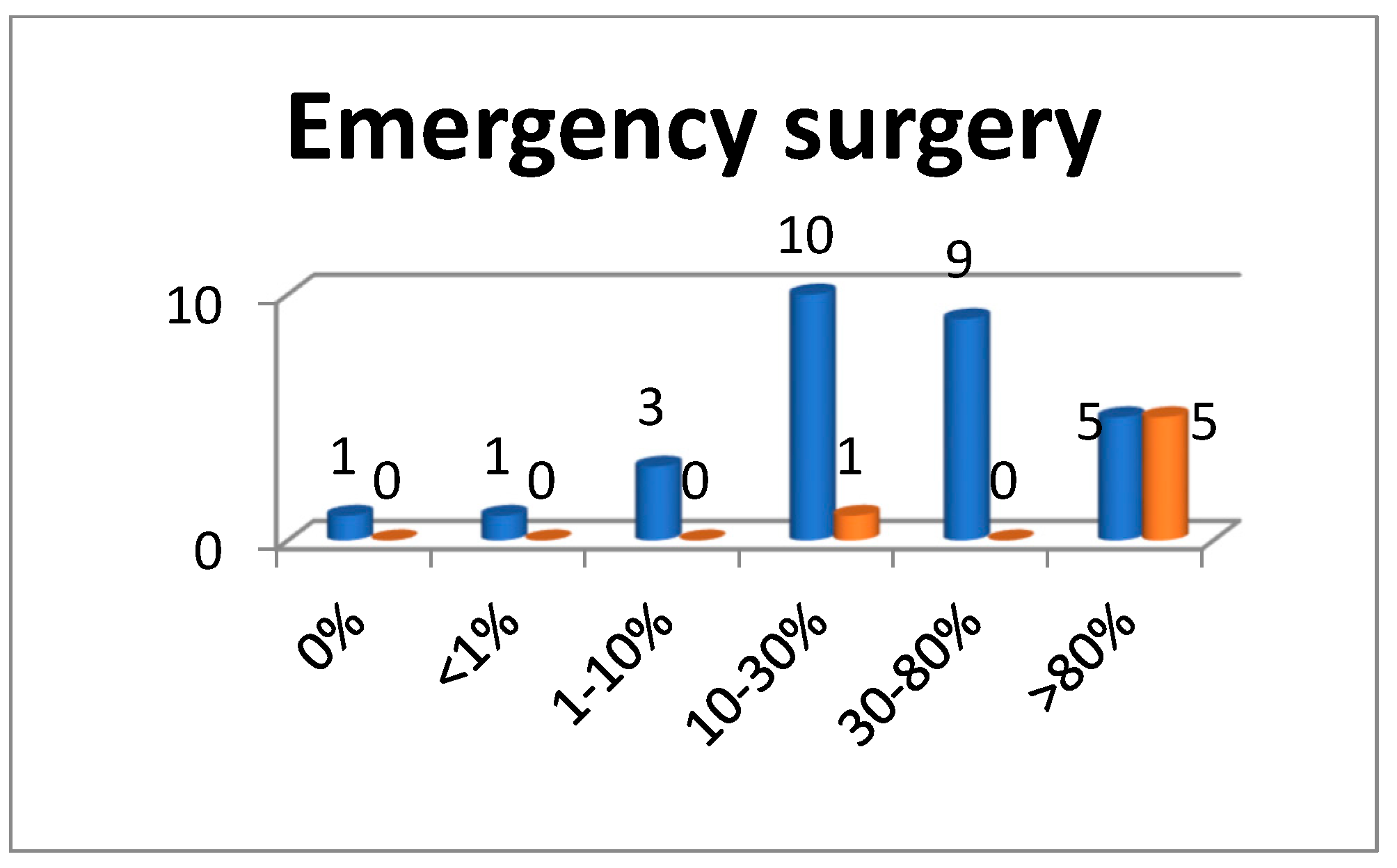
3.1. Hospitalization and Surgical Treatment in Surgical Wards
3.1.1. Surgical Treatment in the Absence of the Patient’s PCR Test Result
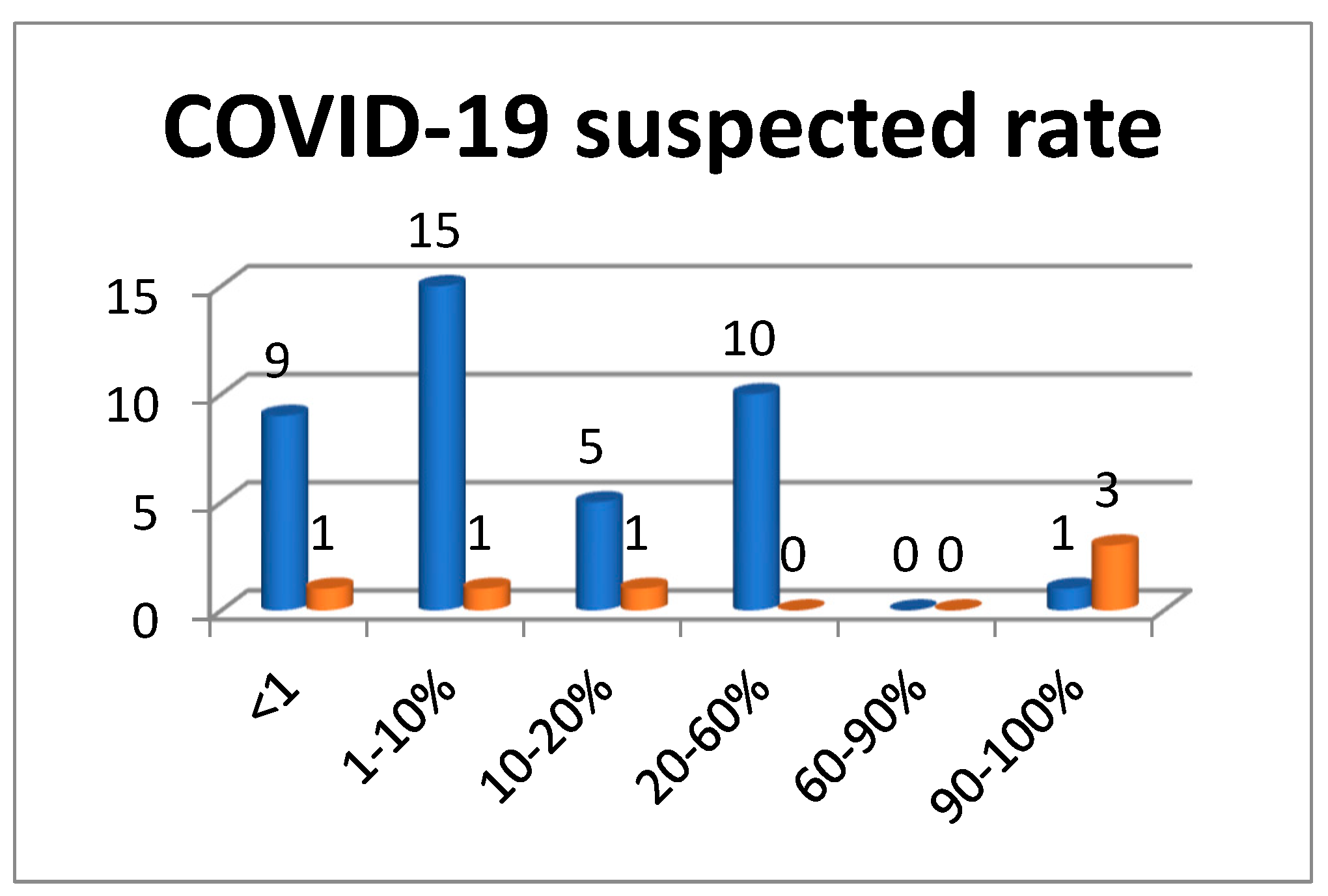
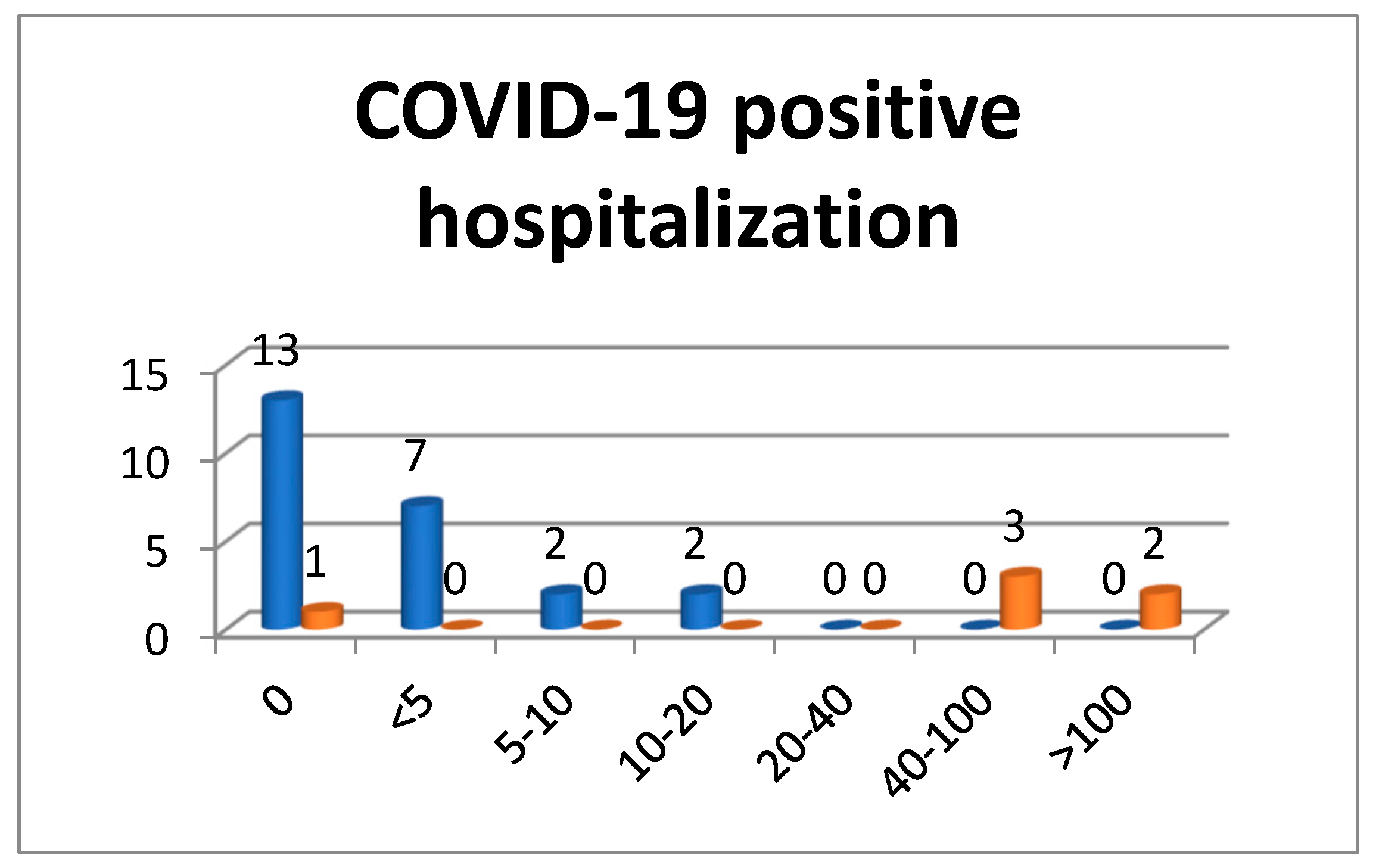
3.1.2. Hospitalization of COVID-19 Positive Patients
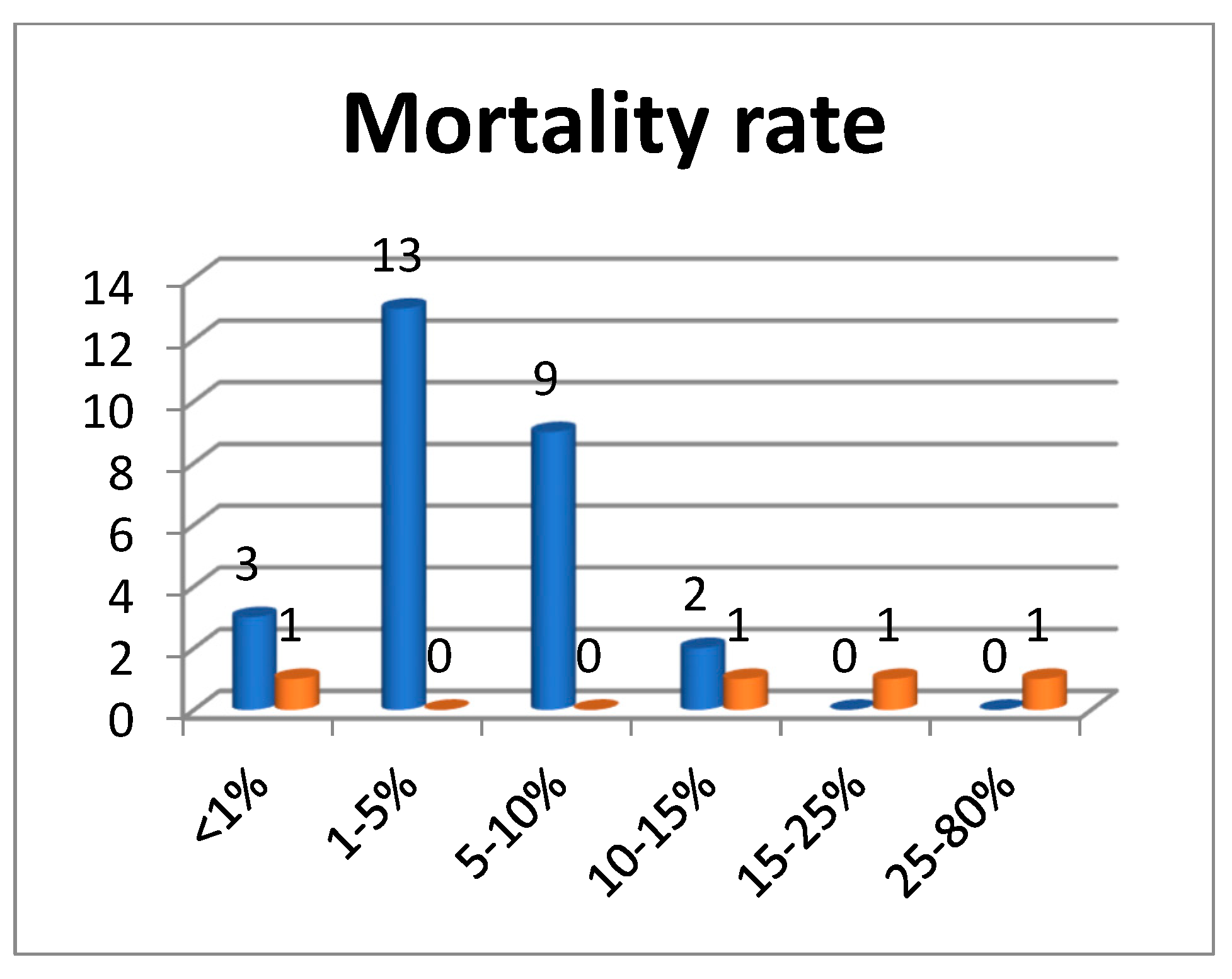
3.2. Mortality in Surgical Services
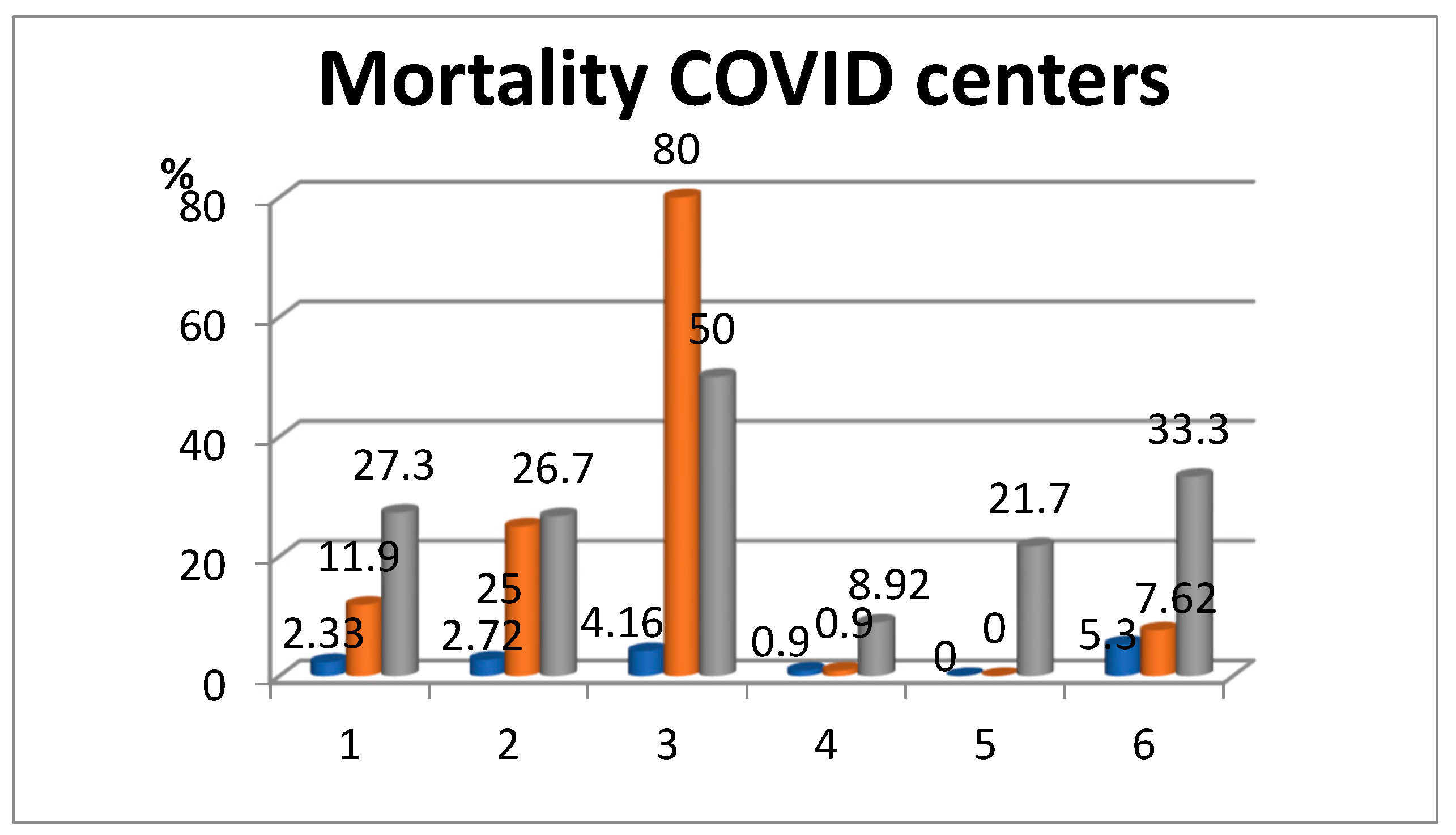
3.3. Deaths of COVID-19-Positive Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Disease 2019 (COVID-19): Situation Report, 103. Available online: https://apps.who.int/iris/handle/10665/332057 (accessed on 1 October 2022).
- Wahed, S.; Chmelo, J.; Navidi, M.; Hayes, N.; Phillips, A.W.; Immanuel, A. Delivering esophago-gastric cancer care during the COVID-19 pandemic in the United Kingdom: A surgical perspective. Dis. Esophagus 2020, 33, doaa091. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Bisagni, P.; Faccincani, R.; Zago, M. COVID-19 outbreak in Northern Italy: Viewpoint of the Milan area surgical community. J. Trauma Acute Care Sur. 2020, 88, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Ti, L.K.; Ang, L.S.; Foong, T.W.; Wei, B.S. What we do when a COVID-19 patient needs an operation: Operating room preparation and guidance. Can. J. Anesth. 2020, 67, 756–758. [Google Scholar] [CrossRef]
- Vilallonga, R.; Garcia Ruiz de Gordejuela, A.; Cossio-Gil, Y.; Domínguez González, J.M.; Martín Sánchez, R.; Armengol Carrasco, M. Transforming a surgical department during the outbreak of new coronavirus pandemic. Clinical implications. Langenbecks Arch. Surg. 2020, 405, 867–875. [Google Scholar] [CrossRef]
- Hojaij, F.C.; Chinelatto, L.A.; Boog, G.H.P.; Kasmirski, J.A.; Lopes, J.V.Z.; Sacramento, F.M. Surgical Practice in the Current COVID-19 Pandemic: A Rapid Systematic Review. Clinics 2020, 75, e1923. [Google Scholar] [CrossRef]
- Wexner, S.D.; Cortés-Guiral, D.; Mortensen, N.; Darzi, A. Lessons Learned and Experiences Shared From the Front Lines: United Kingdom. Am. Surg. 2020, 86, 585–590. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Markar, S.R.; Singh, P.; Griffiths, E.A. Oesophagogastric Anastomosis Audit Group. The influence of the SARS-CoV-2 pandemic on esophagogastric cancer services: An international survey of esophagogastric surgeons. Dis. Esophagus 2020, 33, doaa054. [Google Scholar] [CrossRef]
- Celayir, M.F.; Aygun, N.; Tanal, M.; Koksal, H.M.; Besler, E.; Uludag, M. How should be the Surgical Treatment Approach during the COVID-19 Pandemic in Patients with Gastrointestinal Cancer? Sisli Etfal Hast. Tip Bul. 2020, 54, 136–141. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- Dharmarajan, H.; Anderson, J.L.; Kim, S.; Sridharan, S.; Duvvuri, U.; Ferris, R.L.; Solari, M.G.; Clump, D.A., 2nd; Skinner, H.D.; Ohr, J.P.; et al. Transition to a virtual multidisciplinary tumor board during the COVID-19 pandemic: University of Pittsburgh experience. Head Neck 2020, 42, 1310–1316. [Google Scholar] [CrossRef]
- Curigliano, G.; Banerjee, S.; Cervantes, A.; Garassino, M.C.; Garrido, P.; Girard, N.; Haanen, J.; Jordan, K.; Lordick, F.; Machiels, J.P.; et al. Panel members. Managing cancer patients during the COVID-19 pandemic: An ESMO multidisciplinary expert consensus. Ann. Oncol. 2020, 31, 1320–1335. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Talavera Urquijo, E.; Parise, P.; Nilsson, M.; Reynolds, J.V.; Rosati, R. Esophageal oncologic surgery in SARS-CoV-2 (COVID-19) emergency. Dis Esophagus 2020, 33, doaa028. [Google Scholar] [CrossRef] [PubMed]
- Wexner, S.D.; Nelson, H.; Stain, S.C.; Turner, P.L.; Cortés-Guiral, D. The American College of Surgeons Response to the COVID-19 Pandemic (Part I): Cancer Care, COVID-19 Registry, Surgeon Wellness. Am Surg. 2020, 86, 751–756. [Google Scholar] [CrossRef]
- Howe, J.R.; Bartlett, D.L.; Tyler, D.S.; Wong, S.L.; Hunt, K.K.; DeMatteo, R.P. COVID-19 Guideline Modifications as CMS Announces “Opening Up America Again”: Comments from the Society of Surgical Oncology. Ann. Surg. Oncol. 2020, 27, 2111–2113. [Google Scholar] [CrossRef] [PubMed]
- Revishvili, A.S.; Oloviannyi, V.E.; Sazhin, V.P.; Anishchenko, M.M. Khirurgicheskayapomoshch’ v RossiiskoiFederatsii v period pandemii—osnovnyeitogi 2020 goda [Surgical care in the Russian Federation during the pandemic–the main results of 2020]. Khirurgiia 2021, 12, 5–14. (In Russian) [Google Scholar] [CrossRef]
- Parray, A.M.; Chaudhari, V.A.; Bhandare, M.S.; Madhabananda, K.; Muduly, D.K.; Sudhindran, S.; Mathews, J.; Pradeep, R.; Thammineedi, S.R.; Amal, K.; et al. Impact of COVID-19 on gastrointestinal cancer surgery: A National Survey. Langenbecks Arch. Surg. 2022, 407, 3735–3745. [Google Scholar] [CrossRef]
- Prachand, V.N.; Milner, R.; Angelos, P.; Posner, M.C.; Fung, J.J.; Agrawal, N.; Jeevanandam, V.; Matthews, J.B. Medically Necessary, Time-Sensitive Procedures: Scoring System to Ethically and Efficiently Manage Resource Scarcity and Provider Risk During the COVID-19 Pandemic. J. Am. Coll. Surg. 2020, 231, 281–288. [Google Scholar] [CrossRef]
- COVID-19: Elective Case Triage Guidelines for Surgical Care. Available online: https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case (accessed on 15 December 2022).
- Ademuyiwa, A.O.; Bekele, A.; Berhea, A.B.; Borgstein, E.; Capo-Chichi, N.; Derbew, M.; Evans, F.M.; Feyssa, M.D.; Galukande, M.; Gawande, A.A.; et al. COVID-19 preparedness within the surgical, obstetric, and anesthetic ecosystem in subSaharan Africa. Ann. Surg. 2020, 272, e9–e13. [Google Scholar] [CrossRef]
- Ademe, Y.; Genetu, A.; Laeke, T.; Taye, M.; Bekele, A. Impact of COVID-19 on Surgical Volume: Single-Center Experience from Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2022, 32, 37–44. [Google Scholar] [CrossRef]
- Foo, F.J.; Ho, L.M.L.; Tan, W.J.; Koh, F.H.; Sivarajah, S.S.; Park, S.Y.; Chen, W.T.; Chew, M.H. Colorectal cancer surgery in Asia during the COVID-19 pandemic: A tale of 3 cities. Asian J. Surg. 2022, 45, 1095–1100. [Google Scholar] [CrossRef]
- Knisely, A.; Zhou, Z.N.; Wu, J.; Huang, Y.; Holcomb, K.; Melamed, A.; Advincula, A.P.; Lalwani, A.; Khoury-Collado, F.; Tergas, A.I.; et al. Perioperative Morbidity and Mortality of Patients With COVID-19 Who Undergo Urgent and Emergent Surgical Procedures. Ann. Surg. 2021, 273, 34–40. [Google Scholar] [CrossRef] [PubMed]
- American College of Surgeons. March 24 O, 2020 COVID-19 Guidelines for Triage of Colorectal Cancer Patients. 2020. Available online: https://www.facs.org/covid-19/clinical-guidance/elective-case/colorectal-cancer (accessed on 15 December 2020).
- Zheng, M.H.; Boni, L.; Fingerhut, A. Minimally Invasive Surgery and the Novel Coronavirus Outbreak: Lessons Learned in China and Italy. Ann. Surg. 2020, 272, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- COVID Surgery Collaborative. Global Surgery Collaborative. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef]
- Lei, S.; Jiang, F.; Su, W.; Chen, C.; Chen, J.; Mei, W.; Zhan, L.Y.; Jia, Y.; Zhang, L.; Liu, D.; et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinical Medicine 2020, 21, 100331. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Haffner, M.R.; Le, H.V.; Saiz, A.M.; Han, G.; Fine, J.; Wolinsky, P.; Klineberg, E.O. Postoperative In-Hospital Morbidity and Mortality of Patients With COVID-19 Infection Compared with Patients Without COVID-19 Infection. JAMA Netw. Open 2021, 4, e215697. [Google Scholar] [CrossRef]
- Order nr.555 on date 3 April 2020. (Ordin nr.555 din 3 aprilie 2020). Available online: https://www.cdep.ro/pls/legis/legis_pck.htp_act?ida=164856 (accessed on 1 October 2022).
- Carrier, F.M.; Amzallag, É.; Lecluyse, V.; Côté, G.; Couture, É.J.; D’Aragon, F.; Kandelman, S.; Turgeon, A.F.; Deschamps, A.; Nitulescu, R.; et al. Postoperative outcomes in surgical COVID-19 patients: A multicenter cohort study. BMC Anesthesiol. 2021, 21, 15. [Google Scholar] [CrossRef]
- Finley, C.; Prashad, A.; Camuso, N.; Daly, C.; Earle, C.C. Life saving cancer surgeries need to be managed appropriately during the COVID-19 pandemic. Can. J. Surg./J. Can. Chir. 2020, 63, 1–4. [Google Scholar] [CrossRef]
- Dort, J.; Romanelli, J.; Choudhury, N.; Flink, B.J.; Lak, K.; Levy, S.; Needleman, B.J.; Paget, C.J., 3rd; Telem, D.; Schwarz, E.; et al. SAGES primer for taking care of yourself during and after the COVID-19 crisis. Surg. Endosc. 2020, 34, 2856–2862. [Google Scholar] [CrossRef]
- Francis, N.; Dort, J.; Cho, E.; Feldman, L.; Keller, D.; Lim, R.; Mikami, D.; Phillips, E.; Spaniolas, K.; Tsuda, S.; et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg. Endosc. 2020, 34, 2327–2331. [Google Scholar] [CrossRef]
- Săftoiu, A.; Tomulescu, V.; Tanţău, M.; Gheorghe, C.; Dumitru, E.; Mateescu, B.; Negreanu, L.; Jinga, M.; Seicean, A.; Ciocîrlan, M.; et al. SRED-ARCE Recommendations for Minimally Invasive Interventions During the COVID-19 Pandemic in Romania. Chirurgia 2020, 115, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Zago, M.; Uranues, S.; Chiarelli, M.E.; Grandi, S.; Fumagalli, L.A.; Tavola, M.; Chiarugi, M.; Mariani, D.; Wienerroither, V.; Kurihara, H.; et al. Enhancing safety of laparoscopic surgery in COVID-19 era: Clinical experience with low-cost filtration devices. Eur. J. Trauma Emerg. Surg. 2020, 46, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Lou, Z.; Zhang, W. Several suggestions of operation for colorectal cancer under the outbreak of corona virus disease 2019 in China. Chin. J. Gastrointest. Surg. 2020, 23, 208–211. [Google Scholar] [CrossRef]
- Nekkanti, S.S.; Vasudevan Nair, S.; Parmar, V.; Saklani, A.; Shrikhande, S.; Sudhakar, S.N.; Joshi, A.; Murthy, V.; Patkar, N.; Khattry, N.; et al. Mandatory preoperative COVID-19 testing for cancer patients-Is it justified? J. Surg. Oncol. 2020, 122, 1288–1292. [Google Scholar] [CrossRef]
- Coimbra, R.; Edwards, S.; Kurihara, H.; Bass, G.A.; Balogh, Z.J.; Tilsed, J.; Faccincani, R.; Carlucci, M.; Martínez Casas, I.; Gaarder, C.; et al. European Society of Trauma and Emergency Surgery (ESTES) recommendations for trauma and emergency surgery preparation during times of COVID-19 infection. Eur. J. Trauma Emerg. Surg. 2020, 46, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.; Myśliwiec, P.; Rogula, W.; Solecki, M.; Furtak, J.P.; Kazanowski, M.; Kłęk, S.; Nowakowski, M.; Pędziwiatr, M.; Zawadzki, M.; et al. Guidelines for the management of surgical departments in non-uniform hospitals during the COVID-19 pandemic. Pol. Przegl. Chir. 2020, 92, 48–59. [Google Scholar] [CrossRef]
- Ellisa, R.; Hay-David, A.G.C.; Brennan, P.A. Science Direct Operating during the COVID-19 pandemic: How to reduce medical error. Br. J. Oral Maxillofac. Surg. 2020, 58, 577–580. [Google Scholar] [CrossRef]
- Kasiukiewicz, A.; Wojszel, Z.B.; Kasiukiewicz, A.; Wojszel, Z.B. Assessment of Referrals and Hospitalizations in the Hospital Transformed into COVID-19 Facility in Poland during the “Spring Wave” of the Epidemic in 2020-A Cross-Sectional Study. Int. J. Env. Res. Public Health. 2021, 18, 7143. [Google Scholar] [CrossRef]
- De Simone, B.; Chouillard, E.; Di Saverio, S.; Pagani, L.; Sartelli, M.; Biffl, W.L.; Coccolini, F.; Pieri, A.; Khan, M.; Borzellino, G.; et al. Emergency surgery during the COVID-19 pandemic: What you need to know for practice. Ann. R. Coll. Surg. Engl. 2020, 102, 323–332. [Google Scholar] [CrossRef]
- COVID Surg Collaborative. Outcomes from elective colorectal cancer surgery during the SARS-CoV-2 pandemic. Color. Dis. 2020, 23, 732–749. [Google Scholar] [CrossRef]
- Abate, S.M.; Mantefardo, B.; Basu, B. Postoperative mortality among surgical patients with COVID-19: A systematic review and meta-analysis. Patient Saf. Surg. 2020, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Gebran, A.; El Moheb, M.; Argandykov, D.; Mashbari, H.; Gartland, R.M.; Hwabejire, J.O.; Velmahos, G.C.; Kaafarani, H.M.A. Mesenteric Ischemia in Patients with Coronavirus 2019: A Scoping Review. Surg. Infect. 2022, 23, 781–786. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Sartori, A.; Vitiello, A.; Piatto, G.; Noaro, G.; Olmi, S.; Foschi, D.; De Re, L.; Zappa, M.; Sarro, G.; et al. Complications and mortality in a cohort of patients undergoing emergency and elective surgery with perioperative SARS-CoV-2 infection: An Italian multicenter study. Teachings of Phase 1 to be brought in Phase 2 pandemic. Updates Surg. 2021, 73, 745–752. [Google Scholar] [CrossRef] [PubMed]
- COVID Surg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: An international cohort study. Lancet 2020, 96, 27–38. [Google Scholar] [CrossRef]
- Maliki, I.; Elmsellem, H.; Hafez, B.; EL Moussaoui, A.; Reda Kachmar, M.; Ouahbi, A. The psychological properties of the Arabic BDI-II and the psychological state of the general Moroccan population during the mandatory quarantine due to the COVID-19 pandemic. Casp. J. Environ. Sci. 2021, 19, 139–150. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bîrlă, R.; Panaitescu, E.; Ceban, C.; Radu, A.-V.; Hoară, P.; Constantin, A.; Păduraru, D.N.; Bordianu, A.; Cristian, D.A.; Constantinoiu, S. The Influence of the COVID-19 Pandemic on Mortality of Patients Hospitalized in Surgical Services in Romania: A Cross-Sectional Study of a National Survey. Sustainability 2023, 15, 237. https://doi.org/10.3390/su15010237
Bîrlă R, Panaitescu E, Ceban C, Radu A-V, Hoară P, Constantin A, Păduraru DN, Bordianu A, Cristian DA, Constantinoiu S. The Influence of the COVID-19 Pandemic on Mortality of Patients Hospitalized in Surgical Services in Romania: A Cross-Sectional Study of a National Survey. Sustainability. 2023; 15(1):237. https://doi.org/10.3390/su15010237
Chicago/Turabian StyleBîrlă, Rodica, Eugenia Panaitescu, Cornelia Ceban, Andra-Victoria Radu, Petre Hoară, Adrian Constantin, Dan Nicolae Păduraru, Anca Bordianu, Daniel Alin Cristian, and Silviu Constantinoiu. 2023. "The Influence of the COVID-19 Pandemic on Mortality of Patients Hospitalized in Surgical Services in Romania: A Cross-Sectional Study of a National Survey" Sustainability 15, no. 1: 237. https://doi.org/10.3390/su15010237
APA StyleBîrlă, R., Panaitescu, E., Ceban, C., Radu, A.-V., Hoară, P., Constantin, A., Păduraru, D. N., Bordianu, A., Cristian, D. A., & Constantinoiu, S. (2023). The Influence of the COVID-19 Pandemic on Mortality of Patients Hospitalized in Surgical Services in Romania: A Cross-Sectional Study of a National Survey. Sustainability, 15(1), 237. https://doi.org/10.3390/su15010237







