Separation of Graphites and Cathode Materials from Spent Lithium-Ion Batteries Using Roasting–Froth Flotation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Flotation Experiments
2.3. Analytical Methods
3. Results and Discussion
3.1. Surface Morphology Analysis of Spent LIB Materials before Roasting
3.2. Thermogravimetric Analysis of Raw Spent LIBs Materials
3.3. SEM-EDS Analysis of Spent LIB Materials at Different Roasting Temperatures
3.4. XPS Analysis
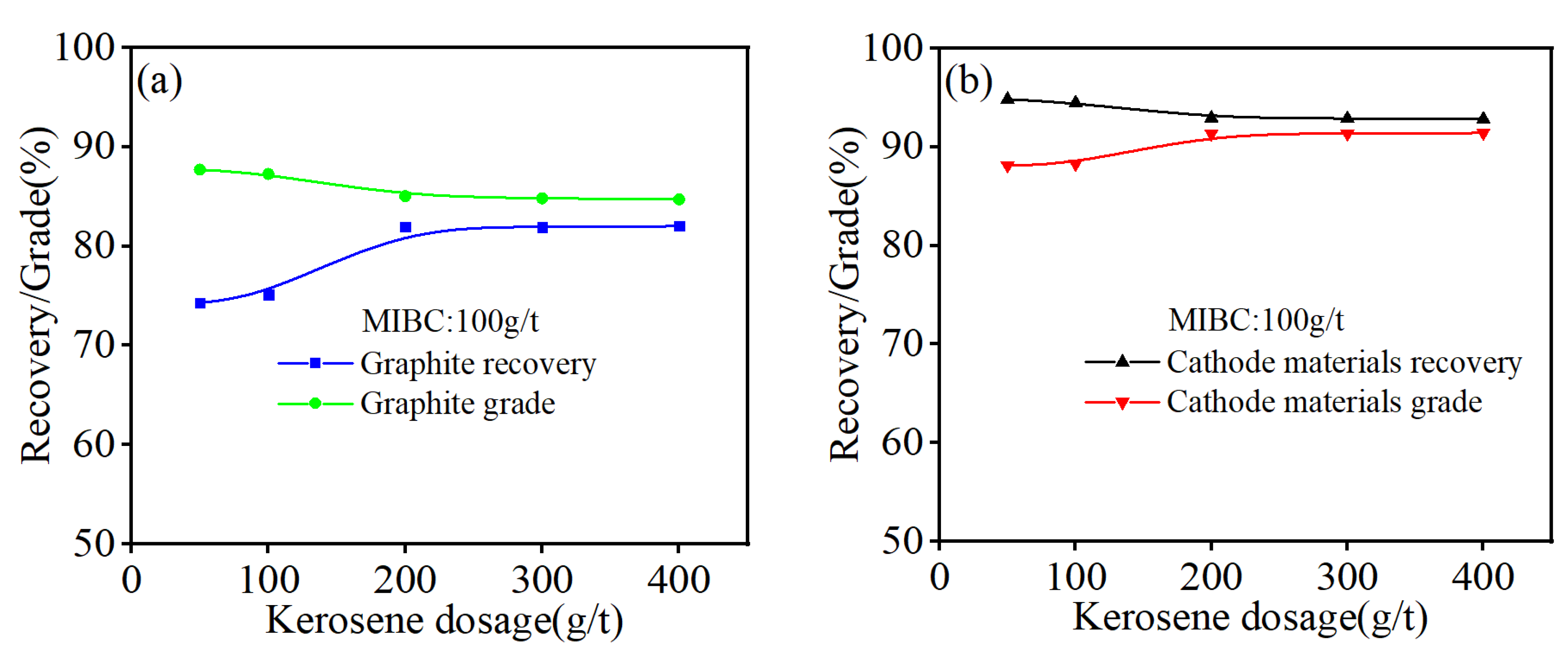
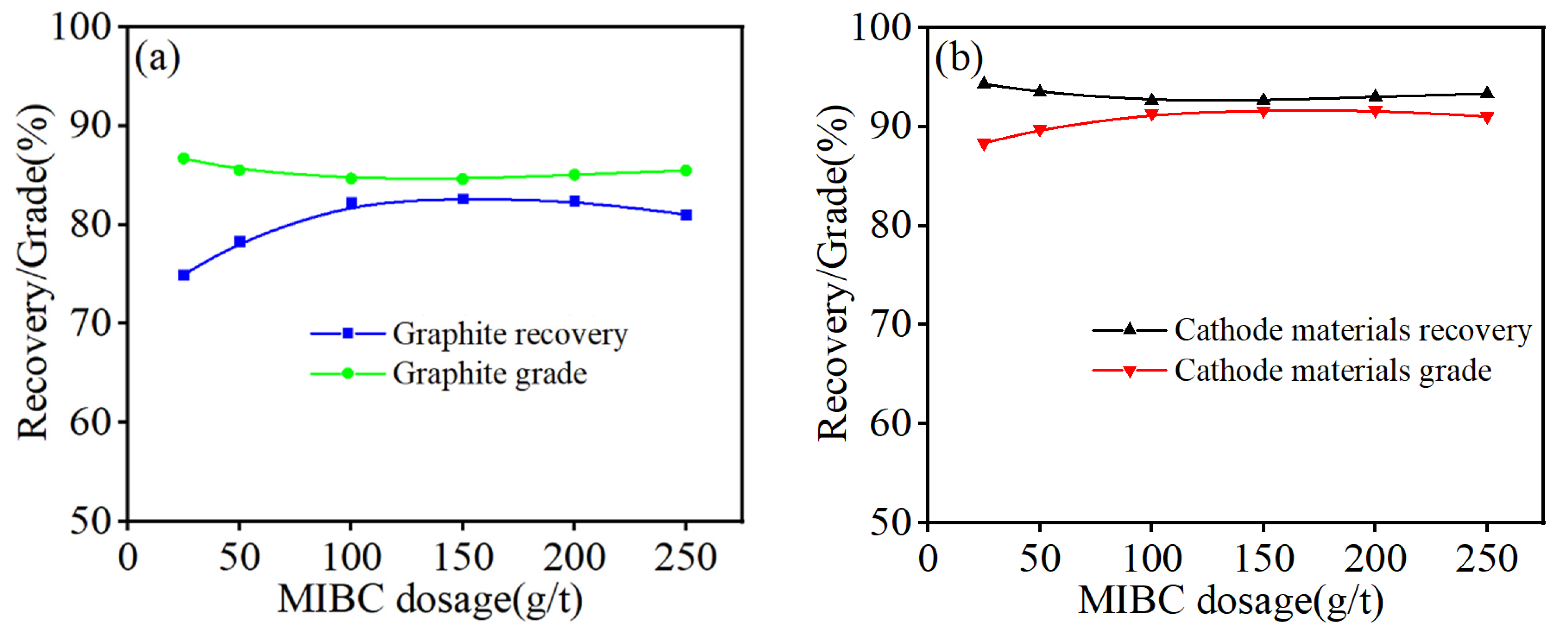
3.5. Flotation Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Feng, Z.L.; Dongrun, Y.; Tao, D.; He, G.; Bin, L.W. The current process for the recycling of spent lithium ion batteries. Front. Chem. 2020, 8, 578044. [Google Scholar]
- Yingjie, Z.; Peichao, N.; Xuan, Y.; Peng, D.; Yan, L.; Qi, M. New research progress on recycling technology of spent ternary lithium ion battery. Chem. Ind. Eng. Prog. 2020, 39, 2828–2840. [Google Scholar]
- Nasser, O.A.; Petranikova, M. Review of achieved purities after li-ion batteries hydrometallurgical treatment and impurities effects on the cathode performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Gavin, H.; Roberto, S.; Emma, K.; Laura, D.; Peter, S.; Rustam, S.; Allan, W.; Paul, C.; Oliver, H.; Simon, L.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar]
- Ruiting, Z.; Zhenzhen, Y.; Ira, B.; Lei, P. Significance of a solid electrolyte interphase on separation of anode and cathode materials from spent li-ion batteries by froth flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Lin, X.; Li, H.; Cao, H. An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries. J. Mater. Cycles Waste Manag. 2013, 15, 420–430. [Google Scholar] [CrossRef]
- Ellingsen, L.A.W.; Majeau-Bettez, G.; Singh, B.; Srivastava, A.K.; Valøen, L.O.; Strømman, A.H. Life cycle assessment of a lithium-ion battery vehicle pack. J. Ind. Ecol. 2014, 18, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.R.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Zhan, R.; Oldenburg, Z.; Pan, L. Recovery of active cathode materials from lithium-ion batteries using froth flotation. Sustain. Mater. Technol. 2018, 17, e00062. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A stepwise recovery of metals from hybrid cathodes of spent Li-ion batteries with leaching-flotation-precipitation process. J. Power Sources 2016, 325, 555–564. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Kwon, O.-S.; Sohn, I. Fundamental thermokinetic study of a sustainable lithium-ion battery pyrometallurgical recycling process. Resour. Conserv. Recycl. 2020, 158, 104809. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Yao, L.; Feng, Y.; Xi, G. A new method for the synthesis of LiNi1/3Co1/3Mn1/3O2 from waste lithium ion batteries. RSC Adv. 2015, 5, 44107–44114. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical separation of aluminium, cobalt, copper and lithium from spent Li-ion batteries. J. Power Sources 2008, 187, 238–246. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of metals from spent lithium-ion batteries with organic acids as leaching reagents and environmental assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries—Process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An atom-economic process for the recovery of high value-added metals from spent lithium-ion batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Pindar, S.; Dhawan, N. Rapid recycling of spent lithium-ion batteries using microwave route. Process Saf. Environ. Prot. 2020, 147, 226–233. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Zhang, L.; Guo, S. Microwave pyrolysis of macadamia shells for efficiently recycling lithium from spent lithium-ion batteries. J. Hazard. Mater. 2020, 396, 122740. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Lin, J.; Wu, J.; Yang, J.; Wu, F.; Chen, R. Low-temperature molten-salt-assisted recovery of valuable metals from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 16144–16150. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Zhou, E.; Hou, P.; Guo, F.; Zhang, L. Recovery and heat treatment of the Li(Ni 1/3 Co 1/3 Mn 1/3 )O2 cathode scrap material for lithium ion battery. J. Power Sources 2013, 232, 348–352. [Google Scholar] [CrossRef]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Zhou, X.; He, W.-Z.; Li, G.-M.; Zhang, X.-J.; Huang, J.-W.; Zhu, S.-G. Recycling of electrode materials from spent lithium-ion batteries. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1–4. [Google Scholar]
- Liu, W.; Zhong, X.; Han, J.; Qin, W.; Liu, T.; Zhao, C.; Chang, Z. Kinetic study and pyrolysis behaviors of spent LiFePO4 batteries. ACS Sustain. Chem. Eng. 2019, 7, 1289–1299. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T. Pretreatment for the recovery of spent lithium ion batteries: Theoretical and practical aspects. J. Clean. Prod. 2020, 263, 121439. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Y.; Xu, Z.; Zhang, X.; Chen, Y.; Yang, E. Separating metal impurities from spent ternary Li-ion batteries materials based on the roasting characteristics. Ionics 2022, 28, 1833–1844. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim. 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Tian, R.; Su, J.; Ma, Z.; Song, D.; Shi, X.; Zhang, H.; Li, C.; Zhang, L. Influences of surface Al concentration on the structure and electrochemical performance of core-shell LiNi 0.8 Co 0.15 Al 0.05 O2 cathode material. Electrochim. Acta 2020, 337, 135769. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Wang, F.; Li, H.; Duan, C.; Wu, C. Surface analysis of cobalt-enriched crushed products of spent lithium-ion batteries by X-ray photoelectron spectroscopy. Sep. Purif. Technol. 2014, 138, 21–27. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.-I. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Paulson, O.; Pugh, R.J. Flotation of inherently hydrophobic particless in aqueous solutions of inorganic electrolytes. Langmuir 1996, 12, 4808–4813. [Google Scholar] [CrossRef]
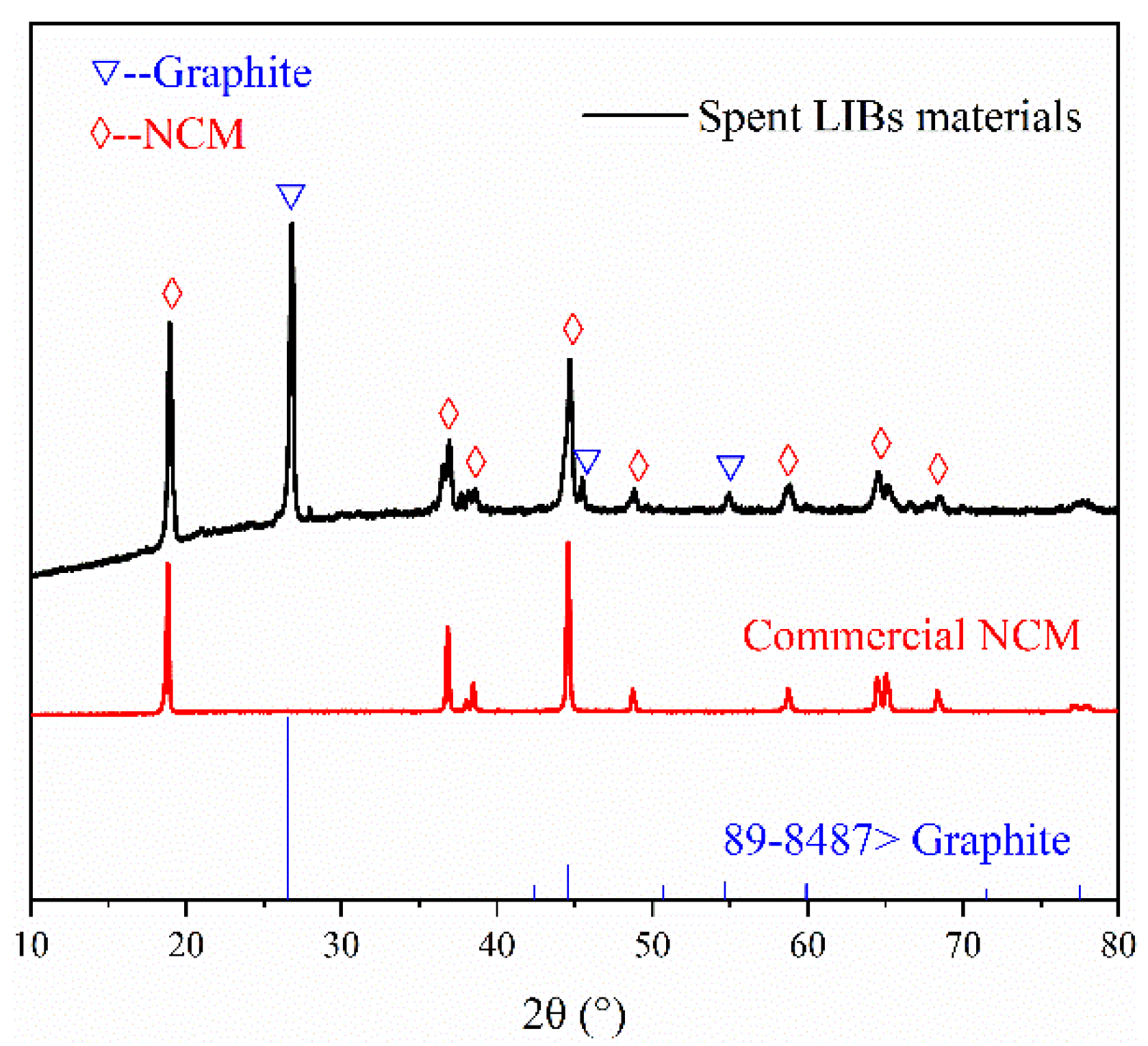


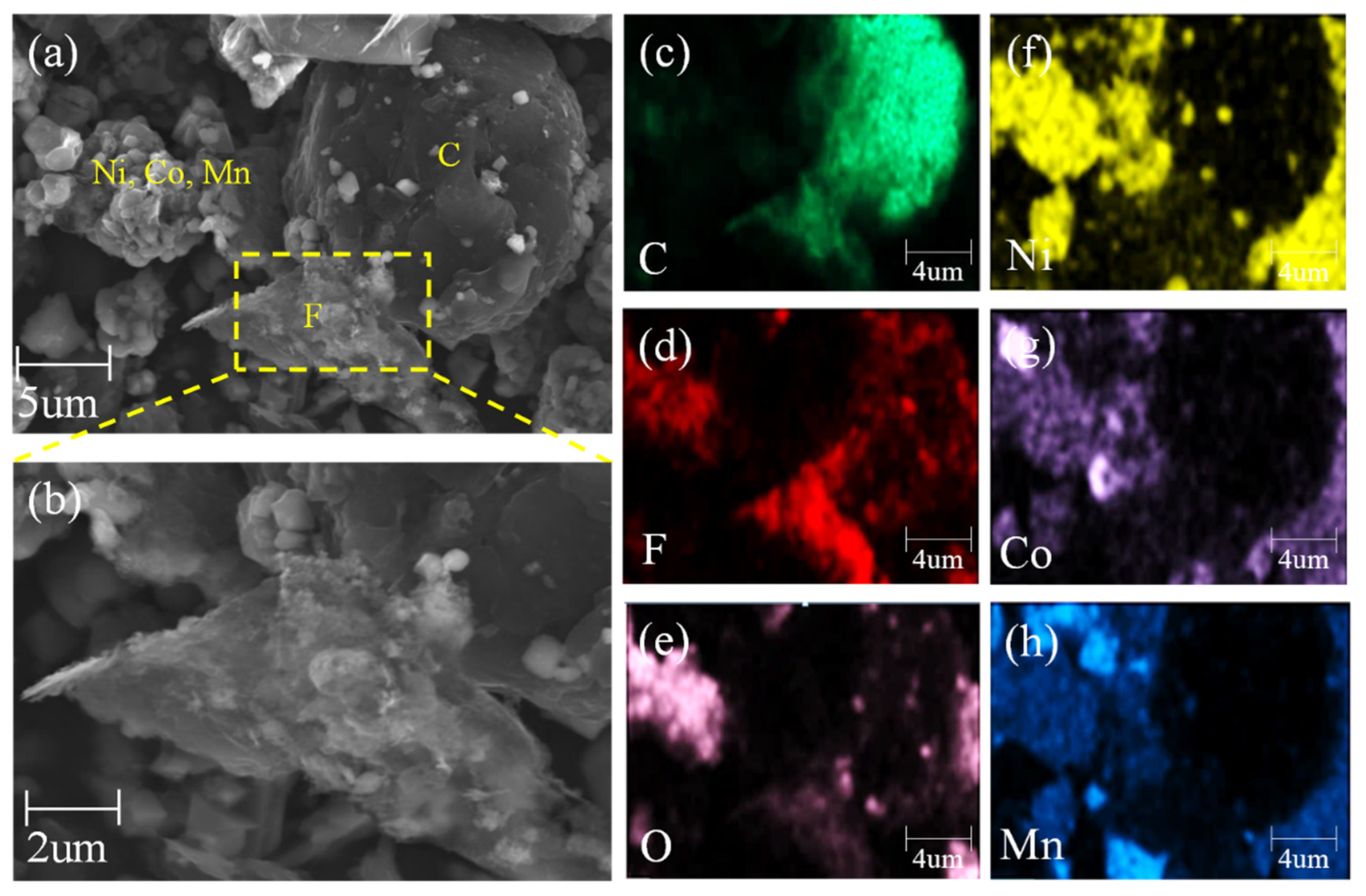
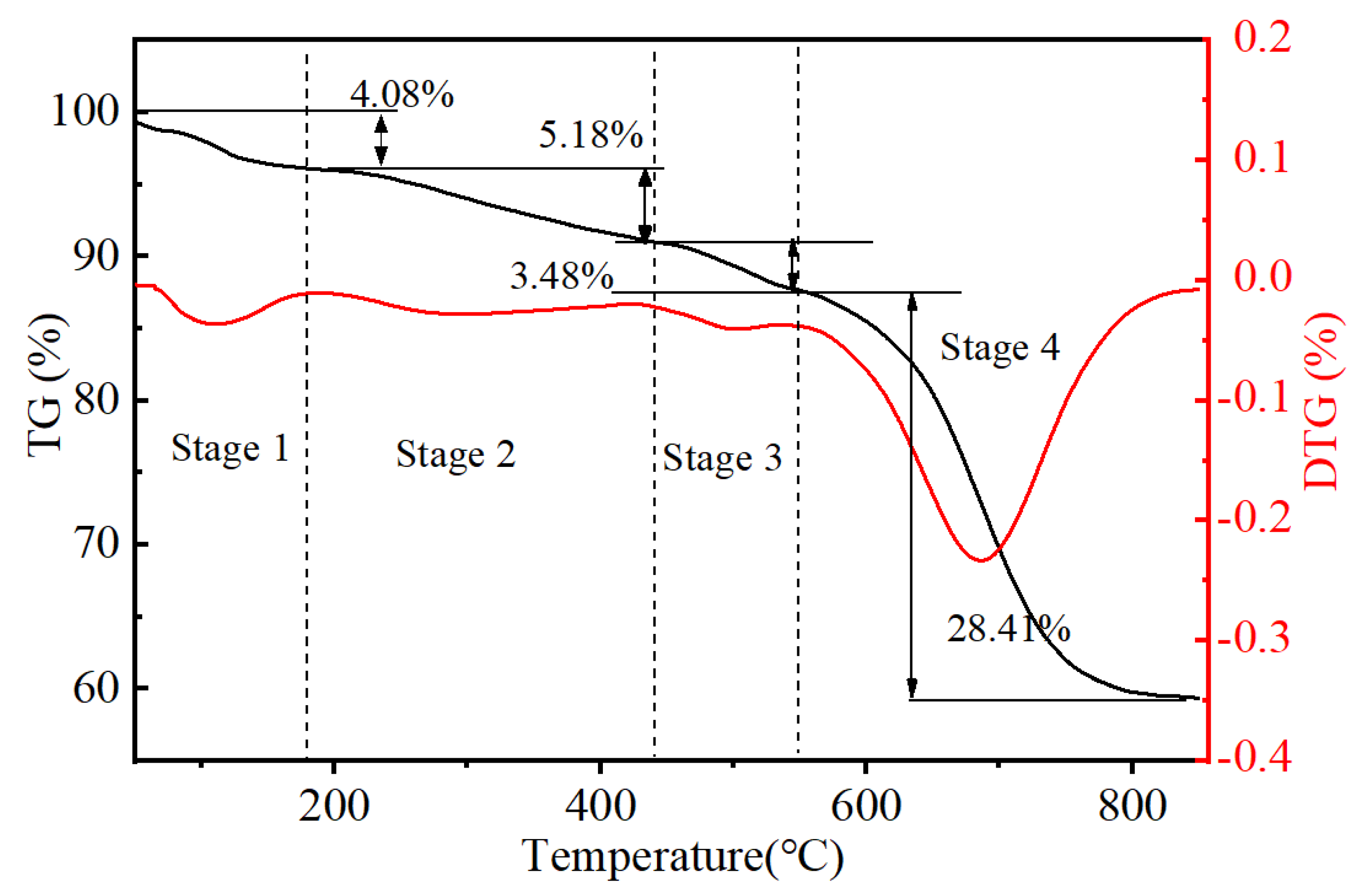
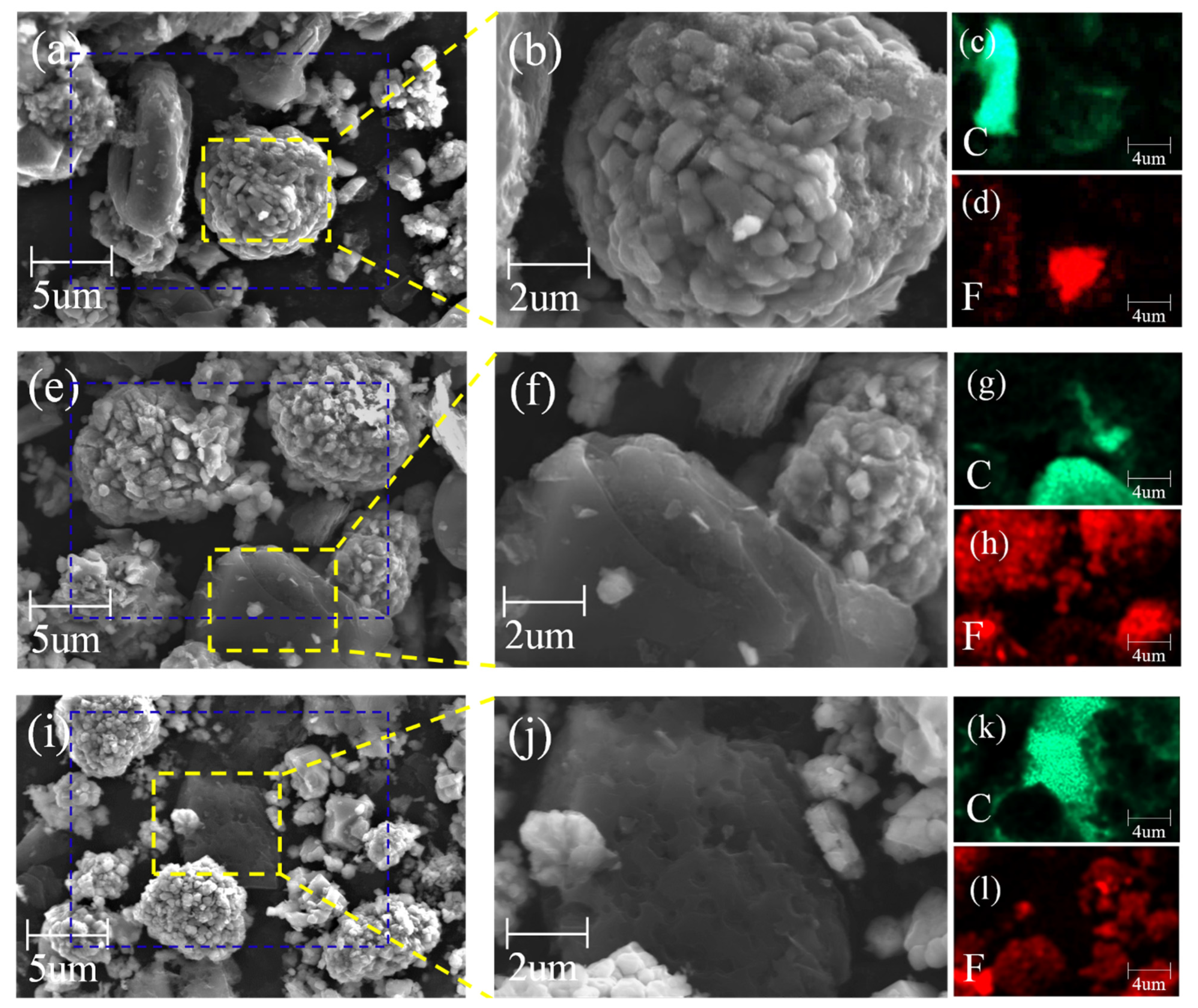
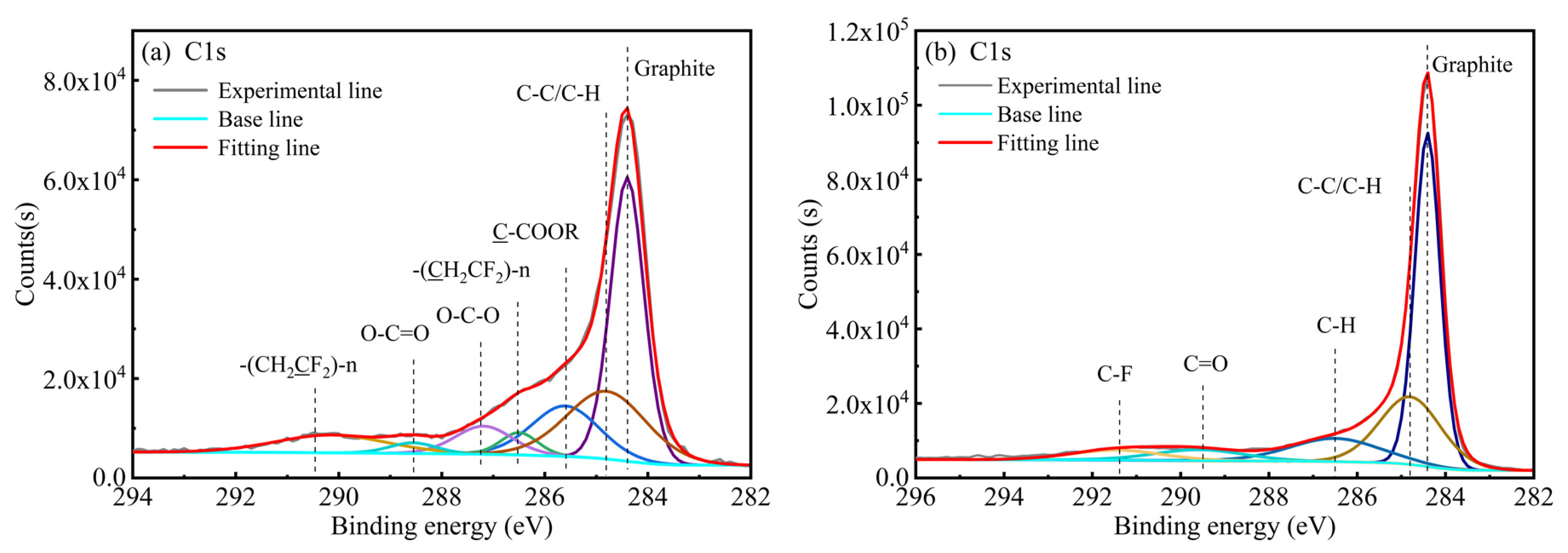

| Elements | Li | Ni | Co | Mn | Others (Graphite + Organics) |
|---|---|---|---|---|---|
| Wt/% | 3.28 | 8.82 | 7.14 | 13.05 | 67.71 |
| Components | Raw Spent LIB Materials | Components | Roasted Materials | ||||
|---|---|---|---|---|---|---|---|
| Peak (BE) | FWHM (eV) | Atomic (%) | Peak (BE) | FWHM (eV) | Atomic (%) | ||
| Graphite | 284.4 | 0.93 | 43.05 | Graphite | 284.4 | 0.68 | 50.55 |
| C-C/C-H | 284.8 | 1.73 | 27.53 | C-C/C-H | 284.8 | 1.64 | 25.00 |
| C-COOR | 285.7 | 1.21 | 13.68 | C-H | 286.6 | 2.40 | 12.60 |
| -(CH2CF2)-n | 286.6 | 0.93 | 5.45 | C=O | 288.8 | 2.40 | 5.92 |
| O-C-O | 287.8 | 1.10 | 3.62 | C-F | 291.6 | 2.40 | 5.92 |
| O-C=O | 288.5 | 1.33 | 3.09 | ||||
| -(CF2CH2)-n | 290.5 | 1.31 | 3.44 | ||||
| Components | Raw Spent LIB Materials | Components | Roasted Materials | ||||
|---|---|---|---|---|---|---|---|
| Peak (BE) | FWHM (eV) | Atomic (%) | Peak (BE) | FWHM (eV) | Atomic (%) | ||
| Li-F | 685.67 | 1.41 | 23.43 | Li-F | 685.10 | 1.57 | 29.17 |
| Al-F | 686.97 | 1.69 | 29.56 | Mn-F/Ni-F | 685.90 | 1.45 | 60.87 |
| -(CH2CF2)-n | 687.85 | 1.63 | 33.27 | Al-F/Co-F | 686.89 | 1.61 | 6.91 |
| P-F | 688.52 | 2.40 | 13.84 | C-F | 687.85 | 1.44 | 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, J.; Wang, Y.; Sun, M.; Wang, L.; Tu, Y. Separation of Graphites and Cathode Materials from Spent Lithium-Ion Batteries Using Roasting–Froth Flotation. Sustainability 2023, 15, 30. https://doi.org/10.3390/su15010030
Zhang J, Li J, Wang Y, Sun M, Wang L, Tu Y. Separation of Graphites and Cathode Materials from Spent Lithium-Ion Batteries Using Roasting–Froth Flotation. Sustainability. 2023; 15(1):30. https://doi.org/10.3390/su15010030
Chicago/Turabian StyleZhang, Jie, Jiapeng Li, Yu Wang, Meijie Sun, Lufan Wang, and Yanan Tu. 2023. "Separation of Graphites and Cathode Materials from Spent Lithium-Ion Batteries Using Roasting–Froth Flotation" Sustainability 15, no. 1: 30. https://doi.org/10.3390/su15010030
APA StyleZhang, J., Li, J., Wang, Y., Sun, M., Wang, L., & Tu, Y. (2023). Separation of Graphites and Cathode Materials from Spent Lithium-Ion Batteries Using Roasting–Froth Flotation. Sustainability, 15(1), 30. https://doi.org/10.3390/su15010030






