Abstract
Silvopastoral system (SPS) has been considered as a sustainable management system contribute to greenhouse gas (GHG) reduction, among other benefits compared with open pasture. However, little research has been conducted on the soil and tree biomass carbon stored in traditional pasture with dispersed trees (PWT) compared with pasture in monoculture (PM). The present study was conducted in the Ecuadorian Amazon Region (EAR), along an elevational gradient from 400 to 2000 masl., within the buffer and transition zone of the Sumaco Biosphere Reserve (SBR), using 71 temporary circular plots of 2826 m2, where 26 plots were stablished in PWT and 45 plots in PM. The main results in PWT show significant differences (p ≤ 0.01) between aboveground carbon biomass (AGCtrees) from 41.1 (lowlands), 26.5 (Middle hills) and 16.7 (high mountains) Mg ha−1 respectively, with an average of 31.0 Mg ha−1 in the whole study area. The total carbon pool along the altitudinal gradient in five components: (AGCtrees), belowground carbon (BGCtrees), pasture carbon (AGClitter+pasture) and carbon in soil components (0–10 and 10–30 cm) for PWT ranged from 112.80 (lowlands) to 91.34 (high mountains) Mg ha−1; while for the PM systems assessing three components (AGClitter+pasture) and carbon in soil components (0–10 and 10–30 cm) ranged from 52.5 (lowlands) to 77.8 (middle zone) Mg ha−1. Finally, the paper shows the main dominant tree species in pasture systems that contribute to carbon storage along elevational gradient and concludes with recommendations for decision-making aimed at improving cattle ranching systems through a silvopastoral approach to mitigate the effects of climate change.
1. Introduction
Currently, silvopastoral systems (SPS) are the subject of multiple economic and conservation initiatives aimed at increasing the percentage of forest cover globally [1,2,3]. This interest is associated with the fact that SPS contribute to greenhouse gas (GHG) reduction [4]. It is estimated that pasture lands account for around 3.87 Pg C in 1.89 billion ha, contributing significantly to mitigating the effects of climate change [1]. Moreover, it provides economic benefits to low-income rural and peri-urban populations [3,5], and the potential related to timber sales, improving soil productive yields, ensuring access to food security in a climatically changing future and reducing deforestation rates [6,7].
However, mean surface temperature has increased by an average of 0.66 °C over the last 60 years [8]. This gradual increase has caused both humans and animals (cattle) to become susceptible to heat stress [9,10], being this exposure strongly negative for the cattle production sector [11]. In this sense, [12] suggests that SPS contribute to temperature regulation through an average cooling between −0.32 °C to −2.4 °C in tropical areas used for these activities. Additionally, it has also been evidenced that cattle raised under a SPS exhibit better animal quality and body weight indexes compared to those managed under a traditional system [13]. This is related to the fact that the SPS allows cattle to have space for shelter in extremely hot days, resulting in low exposure to heat stress and increased grazing and browsing activity [9,13].
This research focuses on the Ecuadorian Amazon Region (EAR) in the Andean Amazon hotspot of biodiversity and endemism [14], with a high potential to provide ecosystem services to local populations [15,16]. Nevertheless, the EAR has experienced significant deforestation process related to land use change for agriculture and pastures [17,18]. Within the boundaries of the EAR, is located the Sumaco Biosphere Reserve (SBR), which is considered a biodiversity hotspot [19]. Large areas of native forests are found within the buffer and transition zones of the SBR, nonetheless, significant areas of pastureland in agricultural systems are also evident [20,21]. Due to the ecological and cultural relevance of this area, it has been suggested to assess the implications of cattle ranching systems related to climate change mitigation, to promote incentives for best management practices (BMP) [21,22].
Therefore, the main objective of this research was carbon stock assessment in silvopastoral systems in Ecuadorian Amazon, with three specific objectives: The first was quantify the soil and tree biomass carbon stored in traditional pasture with dispersed trees, along elevational gradient in the Ecuadorian Amazon. In the second objective the variation of the carbon stock in different components along elevational gradient was determined. Meanwhile, the third objective focused on determine the biomass Important Value (BIV) of dominant tree species in pasture systems along elevational gradient. Finally, the paper concludes with recommendations for decision-making aimed at improving livestock systems through a silvopastoral approach to contribute to nationally determined contributions (NDC) to the Paris Agreement goal of constraining global warming to less than 2 °C.
2. Materials and Methods
2.1. Geographic Setting
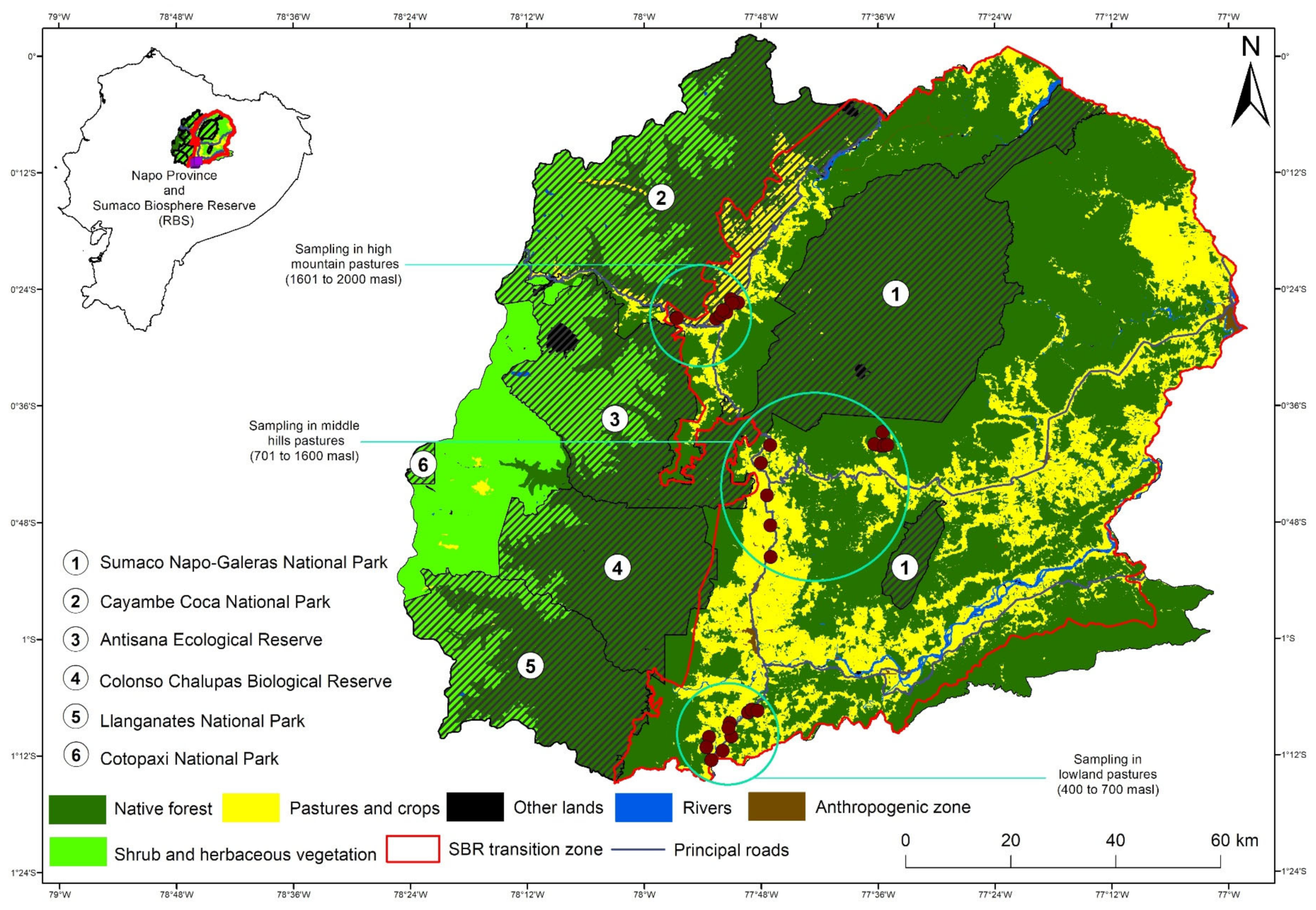
The study was carried out in households involving in the livestock-based livelihood strategy, in an elevational gradient located in the Sumaco Biosphere Reserve (SBR). The SBR has about one million ha [23] and, according to the last multitemporal assessment carried out in 2013, the SBR counted about 53% of primary forest, 28% of secondary forest and 9% of pastureland (81,693 ha) [23]. Three cantons were selected inside the SBR: (a) Arosemena Tola (Lowlands from 400 to 700 masl); (b) Archidona (Middle hills from 701–1600 masl); and (c) Quijos (High mountains from 1600–2000 masl (Figure 1 and Table 1). The complete study area is part of the Uplands Western Amazonia hotspot [14].
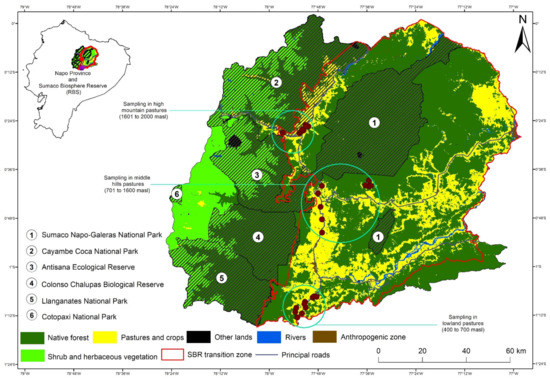
Figure 1.
Location of the study area and the study plots at each elevation site.

Table 1.
Characteristics of the three elevational gradients of the studied scattered tree on cattle farmland in the SBR, Ecuadorian Amazon.
2.2. Bioclimatic Characteristics
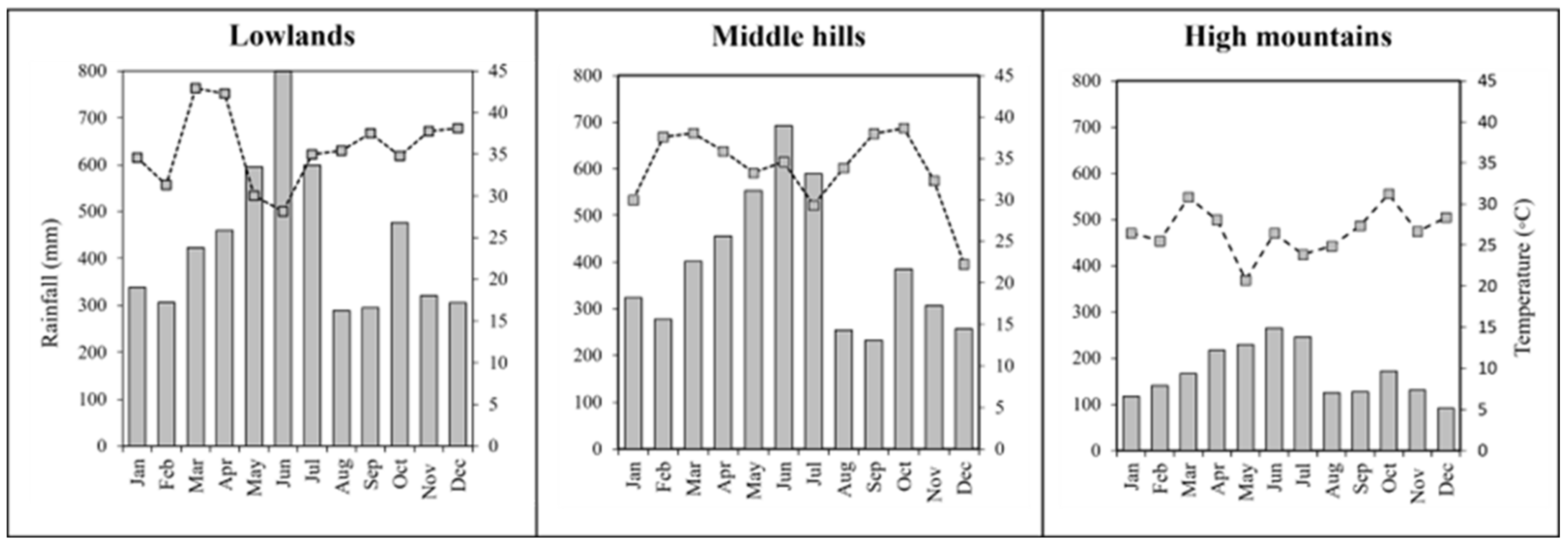
The predominant bioclimatic conditions vary along the elevational gradients, with a mean annual temperature of 35.67 °C and annual rainfall of 5209 mm in Lowlands zone, 33.65 °C and 4728 mm in Middle hills; 26.70 °C and 2205 mm in High mountains zone respectively (Figure 2).

Figure 2.
Climatic variations in three zones (2012–2021) along altitudinal gradient (lowlands, middle hills, and high mountains). Source: authors’ own elaboration based on the information available in [24].
2.3. Field Methods
The criteria for the farm selection were pasture area ≥0.5 hectare, with at least a patch of pasture with dispersed trees, in a crown cover ≥10%. Thus, we installed a 26 circular temporary plot of 2826 m2, in pasture with dispersed trees, distributed among the three zones.
2.3.1. Estimation of Aboveground Biomass and Carbon Content
Aboveground biomass and total carbon content was estimated directly from field data live stems with DBH larger than 10 cm to calculate the total basal area for each of the plots. Basal area is expressed in m2/ha and estimated by the following equation:
where DBH = Diameter at breast height (m).
Aboveground biomass was calculated for each plot using allometric equations developed by [25] for wet tropical forest:
where AGB stands for aboveground biomass in kg dry mass, ρ is wood specific gravity in g/cm3, and DBH is diameter at breast height in centimeters and include all trees having DBH1.30 ≥ 10 cm. Wood specific gravity data for each species were based on [26]. In some cases where the specific gravity was not available, the suggested mean (ρ) for tropical secondary forests [27,28] was used (0.47 g/cm3). Total carbon content of the aboveground biomass (Mg ha−1) was estimated by multiplying the average aboveground biomass estimated for each plot by a wood carbon value of 47% [29].Belowground biomass (BGB) (coarse roots) was indirectly estimated as 30% of aboveground biomass [28].
The biomass importance value (BIV) based on the methodology proposed by [30] was calculated as follows: (N + BA + AGB)/3, where N is relative density, BA is relative basal area and AGB relative above ground biomass.
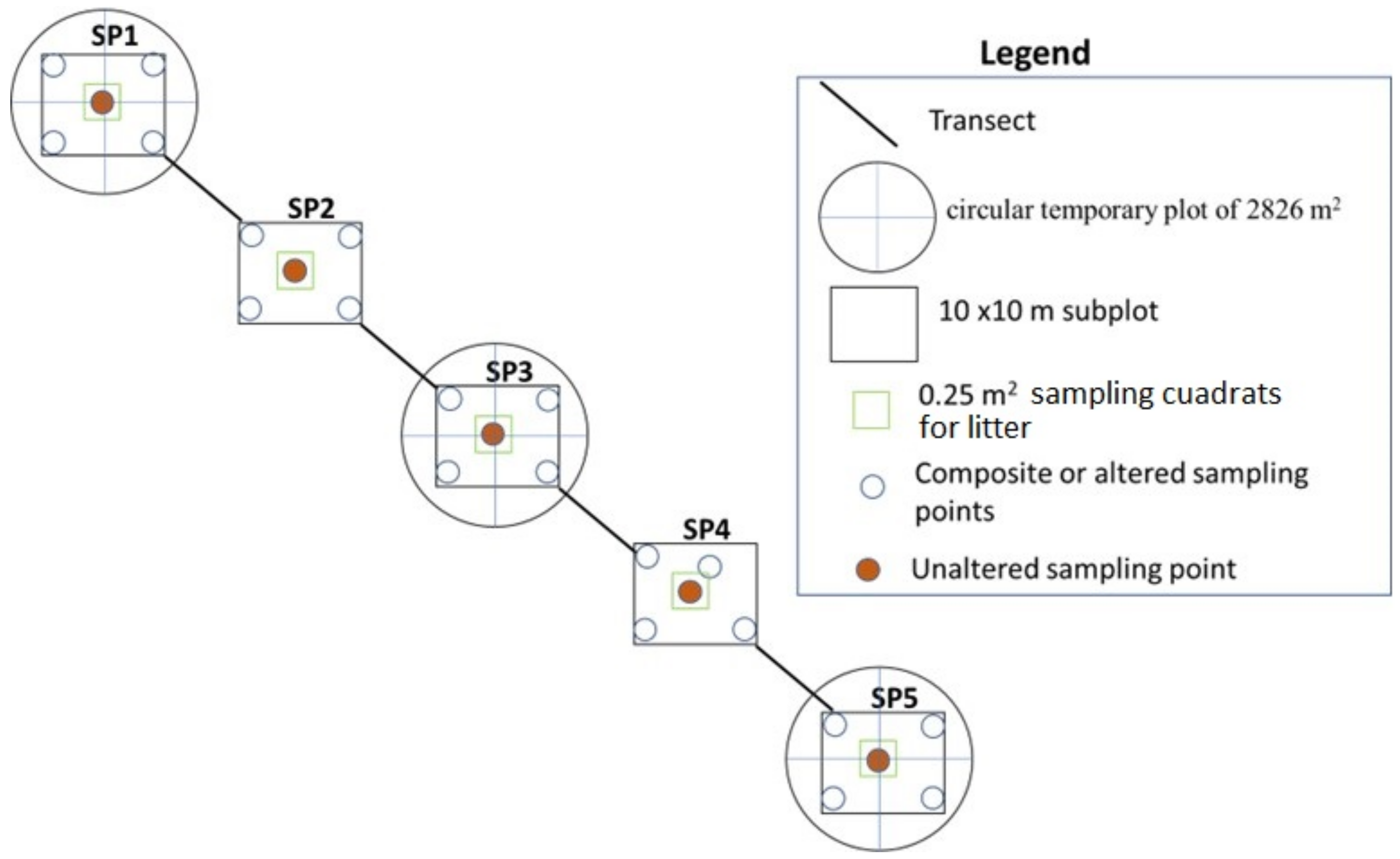
The Leaf litter (LL) was calculated within the subplots of 10 × 10 m with the help of a 0.25 m2 quadrat; all the material corresponding to dead plant material (such as leaves, stems, stems, needles, and twigs) that have fallen to the soil and remains located within was collected. The quadrat was placed in the center of the subplot (Figure 3). The collected material was weighed and placed in bags for drying at 105 °C for 24 h, until a constant weight was obtained. The dry matter was calculated in megagrams per hectare.
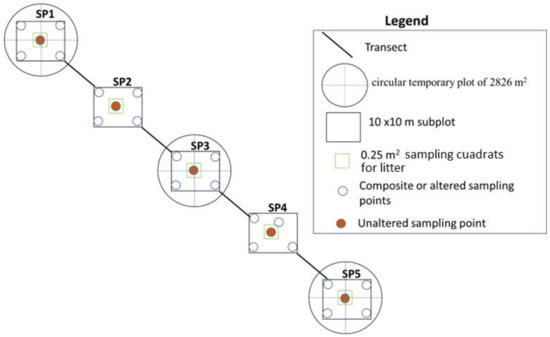
Figure 3.
Sample collection procedure.
2.3.2. Soil Carbon Stocks
We used a systematic sampling method (Figure 3), identifying of traditional pasture with dispersed trees, along the elevational gradient and establishment of plots with five subplots. From each plot, we collected five soil cores from 0–10 and 10–30 cm depth and aggregate them into a composite sample for carbon analysis.
Soil organic carbon (SOC) was calculated using the following equation:
where BD is the bulk density in Mg m−3, TOC is the total organic carbon by percentage and D is the depth in m.
Mg C ha−1 = (BD *(TOC/100)*D*1000)
The bulk density (BD) of samples were determined by the cylinder method [31], with cylinders 5 cm high and 5 cm in diameter collected with an Uhland-type sampler. In the laboratory, the samples were weighed and dried in a stove at 105 °C for 24 h to obtain the dry weight [32]. TOC was determined by the Walkley-Black wet digestion method[33].
Table 2 show the main characteristics of the cattle systems among the elevational gradients, as relevant information to understand the study zone.

Table 2.
Means and standard deviations of floristics composition and forest structure parameters in 26 plots−1 (2826 m2) along the studied gradient of the SBR, Ecuadorian Amazon.
The estimated carbon values in all its components were calculated per hectare. The mean stem density and basal area for one hectare at a specific elevation site was extrapolated, as well as the structural characteristics. Finally, all the resulting values from: (1) floristics composition and forest structure parameters and (2) aboveground biomass (AGB), aboveground carbon (AGC), belowground biomass (BGB), belowground carbon (BGC), aboveground biomass of pasture (AGBPasture), aboveground carbon of pasture (AGCPasture), and total carbon pools along the gradient were compared using one-way ANOVA with SPSS version 22.
3. Results
The following sections describe the resulting values of (1) Floristic composition in pastures with dispersed trees, (2) Carbon stock in pastures with dispersed trees and pastures in monoculture system and (3) Biomass Importance Value (BIV) of the dominant tree species in the pasture systems, distributed along the elevational gradients.
3.1. Floristic Composition in Pasture with Dispersed Trees
Table 2 shows that mean tree species richness decreased significantly with increasing elevation, but significant differences (p < 0.01) were only detected between the lowlands and high mountains. The average tree density (Trees ≥ 10 cm DBH per ha−1) along gradients ranged from 193 (lowlands) to 83.25 (middle hills), with significant differences (p < 0.01) registered between lowlands and middle hills, high mountains (Table 2). Both basal area and average DBH showed no significant variation along the gradient. In the lowlands basal area was the highest (8.67 m2) and high mountains average DBH was 22.85.
3.2. Carbon Stock in Pasture with Dispersed Trees and Pasture in Monoculture System
In the pastures with trees scenario (Table 3), there were significant differences at p < 0.01 between lowlands and high mountains, in the variables AGBtrees, AGCtrees, BGBtres, BGCtrees y CSoil 10–30 cm, with values of 51.84 (Mg ha−1), 24.35 (Mg ha−1), 15.56 (Mg ha−1), 7.31 (Mg ha−1) and 11.97, respectively, and there were no significant differences (0.01%) in lowlands and middle hills for the variables AGBPasture y AGCPasture; while the variables CSoil (0–10 cm) and total carbon stock showed no significant differences among the altitudinal gradient.

Table 3.
Means (±standard deviation) aboveground biomass (AGB), aboveground carbon (AGC), belowground biomass (BGB), belowground carbon (BGC), aboveground biomass of pasture (AGBPasture), aboveground carbon of pasture (AGCPasture), and total carbon pools along the altitudinal gradient of the SBR, Ecuadorian Amazon.
In the monoculture pasture scenario, there were significant differences (p < 0.01) in the variables AGBlitter+pasture, AGClitter+asture and CSoil (10–30 cm); the average CSoil(0–10 cm) was 33.94 with no significant difference among altitudinal gradients, but total carbon stock showed a significant difference (p < 0.05) of 25.27 between lowlands and middle hills and an overall average of 67.12.
3.3. Variation in Carbon Stock in Different Components along the Elevational Gradient
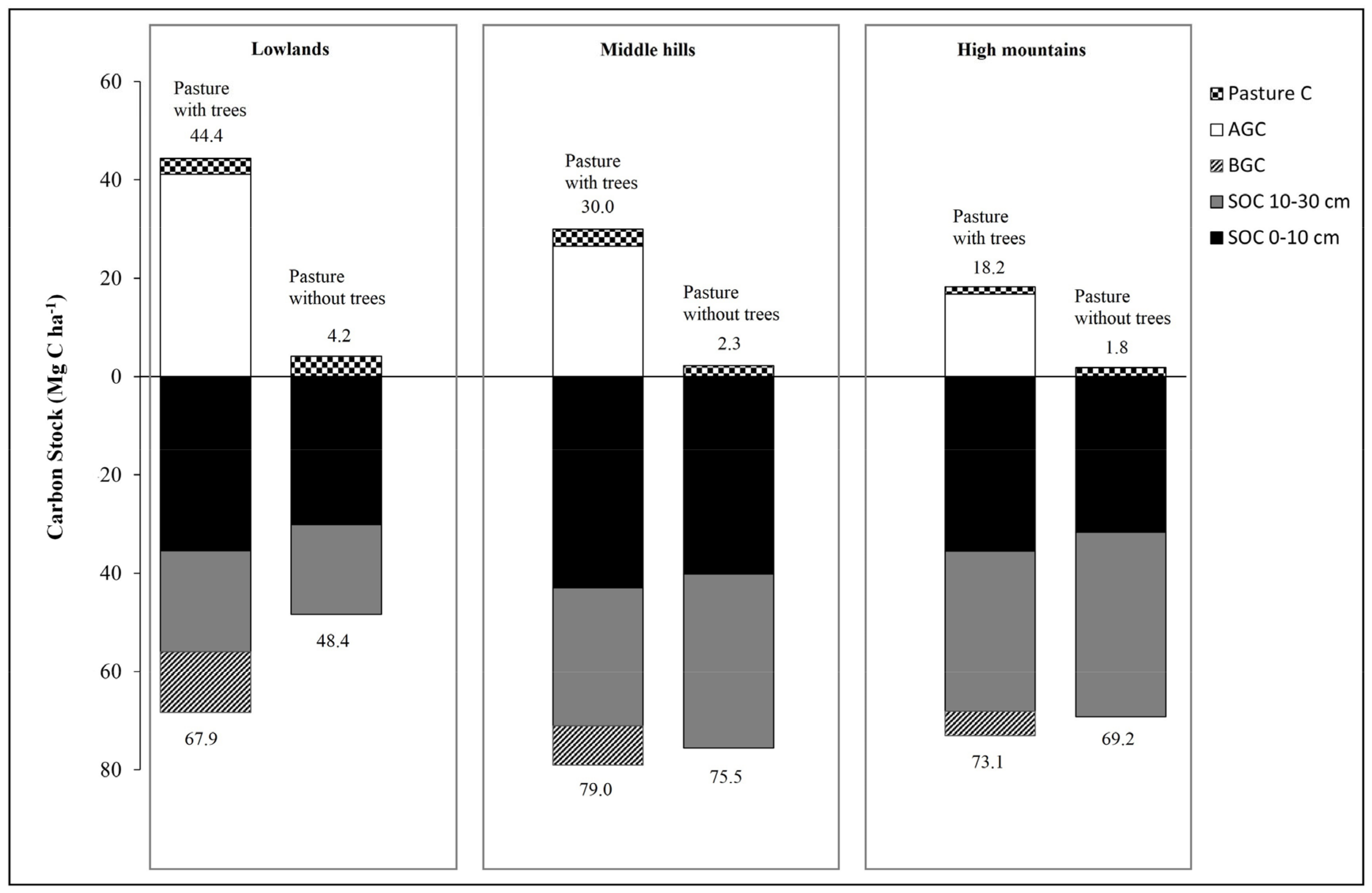
Table 3 and Figure 4 show that in the superficial horizon pastures with trees, the average carbon sequestration ranged from 35.44 to 42.94 Mg C ha−1 along the altitudinal gradient without significant differences (p < 0.05), while for the second horizon (10–30 cm) a decrease was observed with respect to the superficial horizon with significant differences (p < 0.005) along the altitudinal gradient and with higher values in the high zone (32.57 Mg C ha−1).
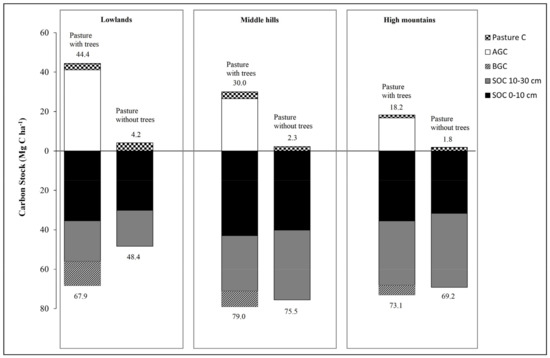
Figure 4.
Total carbon stock (Mg ha−1) for each of the three elevational gradient studied pasture systems.
Regarding the pasture without trees or monoculture, the carbon stored both in litter and pasture shows a slight decrease as the altitudinal gradient increases. While carbon sequestration in the first horizon (0–10 cm) reached 30.08, 40.13 and 30.61 Mg C ha−1 for lowlands, middle hills, and high mountains, respectively. In the second horizon (10–30 cm depth), the middle hills and high mountains showed the significantly higher values with respect to the lowlands with ranges of carbon stored from 20 to 32 Mg C ha−1 (Table 3).
Furthermore, it was found that the carbon stored in the soil (0–10 and 10–30 depth) in pastures with trees represents between 49 and 74% of the total carbon stored (TCS), while for pastures without trees it represents between 92 and 97%, evidencing the role of the soil resource in providing regulating ecosystem services.
3.4. Biomass Important Value (BIV) of Dominant Tree Species in Pasture Systems
Along the grandient, Jacaranda copaia (lowlands), Nectandra spp. (middle hills), Ficus sp. (high mountains); were the most abundant species and the BIV values were ascending (11.66%, 12.41% and 22.96%), with respect to elevation (Table 4).

Table 4.
Density, basal area, live above-ground biomass (AGB) and Biomass Importance Value (BIV) of the most frequent tree species in pasture with trees along the study gradient. Napo, RBS, Ecuadorian Amazon.
Some of the less abundant tree species contributed a high percentage of the AGB (carbon stock): Cordia alliodora 11.47% (400–700 masl), Ficus maxima 18.45% (701–1600 masl) and Ficus sp. 27.35% (1601–2000 masl) (Table 4). The results also showed that the 10 species with the highest BIV contributed between 69.59% (lowlands), 75.81% (middle hills) and 85.34% (high mountains) of the total AGB stored in the altitudinal gradient studied (Table 4).
4. Discussion
Regardless of the altitudinal gradient, the results of this study reflect the existence and permanence of the main tree species (Table 4) and the strong potential of silvopastures throughout the EAR for carbon sequestration in both soil and biomass, which is associated with different factors such as climate (tropical hyper-humid) [34,35], vegetation [36], biogenic macroaggregates [37] and some land uses prevailing in this area such as traditional agroforestry systems (chakra and pasture with trees) that maintain high concentrations of organic carbon in the soil [21,30,32,38]. However, it is important to consider that the altitudinal gradient studied presents high precipitation with average values ranging from 2025 to 5209 mm (Figure 2) without any seasonality. This situation, unlike other typical lowland tropical ecosystems, ensures that fire in this area is not used as a pasture management practice [28], which is considered an advantage in the ecosystems studied, since fire minimizes carbon stocks mainly in soils [39,40].
4.1. Carbon Biomass
We documented high richness and density of tree species in Lowland zone with respect to the Middle hill and High mountain zones. With the exception of the lower zone where we obtained the highest richness, this was similar with the species richness found in four communities in Southeastern Ecuador [28]. But this decreasing pattern of tree species richness is opposite to the patterns found in a forest in the same area specifically in the Ecuadorian Amazonian Andean evergreen forest [30]. This could be due to management activities [41], and the fact that in the high zones cattle ranching started over 70 years ago and ranchers have smaller parcel sizes using more intensive cattle ranching, leaving few trees for cattle shade; in comparison to the middle and low zones where cattle ranching started around 40 and 50 years ago respectively and producers experiment a very extensive cattle ranching allowing more trees in pastures [21].
The difference in species richness and tree density along the altitudinal gradient has resulted in significant differences in the amount of AGCtrees in the studied silvopastoral systems (Table 3), showing similar patterns to tree abundance, with the highest carbon stocks (41.14 Mg ha−1) in the lower zone compared to 26.54 and 16.79 Mg ha−1 in the Middle and High mountain zones respectively, these quantities are similar to those found in SPS in Southeastern Ecuador between 6.8 to 40.8 Mg ha−1 [28], as well as in Mexico (29.1 Mg ha−1) by López-Santiago et al. [42] and in Colombia (31 Mg ha−1) [43]. AGCtrees sequestration potential of silvopastoral systems depends on the plant characteristics (tree species, age, crops, biodiversity, and tree density), structural characteristics and management factors such as regeneration and harvesting regimen, etc. [44,45], and for this case the historical and current land use surrounding the silvopasture also plays an important role in carbon sequestration. Thus, the carbon stored in these SPS, especially in lowland and middle hill zones, corresponds to approximately 28% and 18% respectively of the total carbon stored in a primary forest in the same area, reported in a range of 124 to 160 Mg ha−1 [30].
This study has found that the transition from pasture monoculture to silvopasture has great potential for accumulate and sequester carbon in all components of the system. This benefit for increasing carbon stocks is very clear for above and bleove ground carbon, where further research is needed, given that our study was carried out in a single determined period, it is recommended to perform longitudinal studies in order to determine how much time tree species need to regenerate in these systems, as well as the carbon accumulation rates. These systems must be reinforced with technological alternatives and best management practices (BMPs) [22] to reduce deforestation in tropical areas [46] and might also bring important benefits in terms of climate change adaptation [44].
4.2. Dominant Tree Species
Using the biomass importance value index (BIV) proposed by [30], it is evident that the species Jacaranda copaia, Cordia alliodora, Vochysia braceliniae, and Psidium guajava obtained the highest BIV in the lowland zone, the first three species are of high commercial value and the fourth are of high nutritional value [47,48,49]. In the middle hills zone, the species with the highest BIV were Nectandra spp. Ficus maxima, Cordia alliodora and Ocotea spp., the four species are of high commercial value and the last one high potential for the extraction of essential oils [50,51,52] as well. In the High mountain zone, the species with the highest VIB were Ficus sp., Nectandra spp., Inga spp., and Ocotea spp. (Table 4).
These are also species of high commercial value, while the Inga spp. provide food and incorporate nitrogen into the soil [53,54,55]; evidencing that the most common tree species found throughout the altitudinal gradient are of interest to provide shade, food and commercial timber similar to the findings of [56], and they are part of the native tree diversity, this could be due to the fact that the producers in the study area still have between 17% and 40% of remaining forests in the surroundings of the pastures [21].
Our study however indicated that the least abundant tree species in silvopastoral systems, such as Ficus sp., Cedrela odorata, Cedrela montana, Cordia alliodora, Nectandra spp., Brosimum sp. at the various elevations, could significantly contribute to the aboveground biomass and consequently to carbon storage along the elevation gradient.
4.3. Soil Organic Carbon Pools
Landscape variability in the evaluated gradient also shows different levels of biodiversity and carbon stored, which is associated with organic matter content, climatic conditions, soil texture, site management, vegetation type, land use history, etc. [57]. In this study, pastures with trees presented a greater amount of leaf litter, which is associated with a greater contribution of organic matter, which reaffirms the theory that the quantification of the organic matter cycle is an important indicator of the agricultural potential of soils [58], given the identification of soil quality, structural indices such as bulk density, hydraulic conductivity and aeration porosity [32].
Concerning EAR, some studies in pasture with and without trees report values ranging from 36 to 49 Mg C ha−1 [59] in depths up to 30 cm, although some studies found that soil organic carbon stocks ranged from 85.0 to 97.6 Mg ha−1 [28], which is associated with the historical use of the forest where high biomass content has been generated, fertility improvement that has allowed a high accumulation of organic matter [30,32]. Despite the background of forested areas in the EAR, the conversion of forests to livestock systems represents a decrease in soil carbon stocks, with a higher proportion in those pasture systems without trees (29%) with respect to pastures with trees (4%) [59]. Similar results have been reported by other researchers who found a decrease in soil carbon stock from 8 to 42% when conversion occurs from forest to livestock and cropping systems [32].
Globally, it has been noted that soil carbon sequestration shows a negative correlation with initial carbon stocks and the effects of climatic factors (mean annual temperature and mean annual precipitation) on C sequestration may vary among land use conversion types [57]. In this regard, it has been noted that the critical level of C input requirements to maintain SOC at levels above 10 Mg C ha−1 ranges from 1.1 to 3.5 Mg C/ha/yr and differs according to soil type and production systems [60].
Importantly, that similarities in underlying parent materials, topography, soil textures, bulk densities, as well as the fact that differences were most pronounced in the shallow horizon (0–10 cm depth), support the notion that management activities constitute the most relevant factor for the observed differences in soil carbon [45].
The soils of the Ecuadorian Amazon region are relatively undeveloped with a predominance of the Inceptisols and Andisols orders, with high organic matter content [59], the climate exerts on edaphogenesis a primary influence that favors the leaching of bases (Ca2+, Mg2+, K+, Na+), which induces a predominance of poorly alterable minerals and simple clays such as quartz, kaolinite, gibbsite and iron oxides, conferring them certain morphological characteristics and the decrease of parameters associated to fertility with low cation exchange capacity, poor in phosphorus and mainly acid pH with high potential for aluminum toxicity [32,61,62]. These soil conditions are also characteristic of highly weathered Oxisols and Ultisols that dominate the Neotropics [28].
In this context, given the size of soil organic carbon stocks in these systems, a better understanding of how human activities influence soil carbon concentrations and stability would be essential to manage carbon balances more accurately.
5. Conclusions
The main findings indicate that along the altitudinal gradient, traditional pasture systems with dispersed trees reflect a high potential for carbon sequestration in the Ecuadorian Amazon. Therefore, considering the capacity of these systems to absorb and store carbon in vegetation and soils, they can be considered a fundamental component for climate change mitigation strategies in tropical countries.
Additionally, the traditional pasture with scattered trees system of the EAR offers a high potential to contribute to climate change mitigation (Table 3, Figure 3) as well as to the adaptation of small farmers to the conditions of a changing climate. These systems should be managed by applying the best livestock management practices (BMPs) to avoid deforestation caused by the advance of the agricultural frontier, and in this sense could be linked to the REDD+ approach of Ecuador, contributing to nationally determined contributions (NDCs for the AFOLU sector) to the Paris Agreement goal of constraining global warming to less than 2 °C. However, understanding tree structure and diversity is important to promote practices that contribute to carbon sequestration and provision of other economic services, in this regard it is necessary encourage further research on tree structure, as well as diversity indices and the ecological importance of the species present in pasture with dispersed trees, using long term methodological approaches, to observe carbon accumulation rates and regeneration potential.
Author Contributions
Conceptualization, B.T. and A.G.; methodology, B.T. and C.B. (Cecilio Barba); software, B.T. and C.T.-T.; validation, C.B. (Carlos Bravo), J.C.V. and A.T.; formal analysis, B.T.; investigation, B.T., C.T.-T. C.B. (Carlos Bravo) and A.T.; data curation B.T., C.T.-T. and C.B. (Cecilio Barba); writing—original draft preparation, B.T., C.B. (Cecilio Barba), A.T., C.T.-T., M.H.-R., R.J.H.-F., C.B. (Carlos Bravo) and J.C.V.; writing—review and editing, A.G., B.T. and A.T.; supervision B.T., C.B. (Carlos Bravo) and A.G. All authors have been involved in developing, writing, commenting, editing, and reviewing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This is not applicable as the data are not in any data repository of public access, however if editorial committee needs access, we will happily provide them, please use this email: btorres@uea.edu.ec.
Acknowledgments
This work is part of the results of a joint research agreement between the Amazon State University (UEA) and Rainforest Alliance Inc. The authors thank Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ), through the REDD + Early Movers Program (REM) and Rainforest Alliance Inc., for all support. We also thank the MAG, MAATE, UEA, and ECONGEST AGR267 Group at Cordoba University for their support during the fieldwork stage, as well as the households in the three zones that shared valuable information about their livestock activities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chapman, M.; Walker, W.S.; Cook-Patton, S.C.; Ellis, P.W.; Farina, M.; Griscom, B.W.; Baccini, A. Large Climate Mitigation Potential from Adding Trees to Agricultural Lands. Glob. Chang. Biol. 2020, 26, 4357–4365. [Google Scholar] [CrossRef] [PubMed]
- Munsell, J.F.; Fike, J.H.; Pent, G.J.; Frey, G.E.; Addlestone, B.J.; Downing, A.K. Thinning Forests or Planting Fields? Producer Preferences for Establishing Silvopasture. Agrofor. Syst. 2022, 96, 553–564. [Google Scholar]
- Smith, M.M.; Bentrup, G.; Kellerman, T.; MacFarland, K.; Straight, R.; Ameyaw, L.; Stein, S. Silvopasture in the USA: A Systematic Review of Natural Resource Professional and Producer-Reported Benefits, Challenges, and Management Activities. Agric. Ecosyst. Environ. 2022, 326, 107818. [Google Scholar] [CrossRef]
- Cusack, D.F.; Kazanski, C.E.; Hedgpeth, A.; Chow, K.; Cordeiro, A.L.; Karpman, J.; Ryals, R. Reducing Climate Impacts of Beef Production: A Synthesis of Life Cycle Assessments across Management Systems and Global Regions. Glob. Chang. Biol. 2021, 27, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- da Silveira Pontes, L.; Porfírio-da-Silva, V.; Moletta, J.L.; Telles, T.S. Long-Term Profitability of Crop-Livestock Systems, with and without Trees. Agric. Syst. 2021, 192, 103204. [Google Scholar] [CrossRef]
- Conway, A.; Nieman, C. Small-scale Silvopasture: Addressing Urban and Peri-urban Livestock Challenges in the United States with Agroforestry Practices. Urban Agric. Reg. Food Syst. 2022, 7, e20023. [Google Scholar] [CrossRef]
- Ferreiro-Domínguez, N.; Rodríguez-Rigueiro, F.J.; Rigueiro-Rodríguez, A.; González-Hernández, M.P.; Mosquera-Losada, M.R. Climate Change and Silvopasture: The Potential of the Tree and Weather to Modify Soil Carbon Balance. Sustainability 2022, 14, 4270. [Google Scholar] [CrossRef]
- Valipour, M.; Bateni, S.M.; Jun, C. Global Surface Temperature: A New Insight. Climate 2021, 9, 81. [Google Scholar] [CrossRef]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Paiva, S.R. Response to Heat Stress for Small Ruminants: Physiological and Genetic Aspects. Livest. Sci. 2022, 105028. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Increases in Extreme Heat Stress in Domesticated Livestock Species during the Twenty-first Century. Glob. Chang. Biol. 2021, 27, 5762–5772. [Google Scholar] [CrossRef]
- Choudhary, B.B.; Sirohi, S. Economic Losses in Dairy Farms Due to Heat Stress in Sub-Tropics: Evidence from North Indian Plains. J. Dairy Res. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeppetello, L.R.V.; Cook-Patton, S.C.; Parsons, L.A.; Wolff, N.H.; Kroeger, T.; Battisti, D.S.; Bettles, J.; Spector, J.T.; Balakumar, A.; Masuda, Y.J. Consistent Cooling Benefits of Silvopasture in the Tropics. Nat. Commun. 2022, 13, 1–9. [Google Scholar]
- Huertas, S.M.; Bobadilla, P.E.; Alcántara, I.; Akkermans, E.; van Eerdenburg, F.J.C.M. Benefits of Silvopastoral Systems for Keeping Beef Cattle. Animals 2021, 11, 992. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.; Pacheco, P.; Muzo, A.; Torres, B. Smallholders and Timber Extraction in the Ecuadorian Amazon: Amidst Market Opportunities and Regulatory Constraints. Int. For. Rev. 2015, 17, 38–50. [Google Scholar] [CrossRef]
- Raven, P.H.; Gereau, R.E.; Phillipson, P.B.; Chatelain, C.; Jenkins, C.N.; Ulloa Ulloa, C. The Distribution of Biodiversity Richness in the Tropics. Sci. Adv. 2020, 6, eabc6228. [Google Scholar] [CrossRef]
- Sierra, R. Patrones y Factores de Deforestación En El Ecuador Continental, 1990–2010. Y Un Acercamiento A Los Próximos 2013, 10, 57. [Google Scholar]
- Mena, C.F.; Bilsborrow, R.E.; McClain, M.E. Socioeconomic Drivers of Deforestation in the Northern Ecuadorian Amazon. Environ. Manag. 2006, 37, 802–815. [Google Scholar] [CrossRef]
- Trew, B.T.; Maclean, I.M.D. Vulnerability of Global Biodiversity Hotspots to Climate Change. Glob. Ecol. Biogeogr. 2021, 30, 768–783. [Google Scholar] [CrossRef]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- Torres, B.; Andrade, V.; Heredia-R, M.; Toulkeridis, T.; Estupiñán, K.; Luna, M.; Bravo, C.; García, A. Productive Livestock Characterization and Recommendations for Good Practices Focused on the Achievement of the SDGs in the Ecuadorian Amazon. Sustainability 2022, 14, 10738. [Google Scholar] [CrossRef]
- Torres, B.; Eche, D.; Torres, Y.; Bravo, C.; Velasco, C.; García, A. Identification and Assessment of Livestock Best Management Practices (BMPs) Using the REDD+ Approach in the Ecuadorian Amazon. Agronomy 2021, 11, 1336. [Google Scholar] [CrossRef]
- MAATE Interactive Map of MAATE. Available online: http://ide.ambiente.gob.ec/mapainteractivo/ (accessed on 20 September 2022).
- Huntington, J.L.; Hegewisch, K.C.; Daudert, B.; Morton, C.G.; Abatzoglou, J.T.; McEvoy, D.J.; Erickson, T. Climate Engine: Cloud Computing and Visualization of Climate and Remote Sensing Data for Advanced Natural Resource Monitoring and Process Understanding. Bull. Am. Meteorol. Soc. 2017, 98, 2397–2410. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T. Tree Allometry and Improved Estimation of Carbon Stocks and Balance in Tropical Forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef]
- Baker, T.R.; Phillips, O.L.; Malhi, Y.; Almeida, S.; Arroyo, L.; Di Fiore, A.; Erwin, T.; Killeen, T.J.; Laurance, S.G.; Laurance, W.F. Variation in Wood Density Determines Spatial Patterns InAmazonian Forest Biomass. Glob. Chang. Biol. 2004, 10, 545–562. [Google Scholar] [CrossRef]
- Van Breugel, M.; Ransijn, J.; Craven, D.; Bongers, F.; Hall, J.S. Estimating Carbon Stock in Secondary Forests: Decisions and Uncertainties Associated with Allometric Biomass Models. For. Ecol. Manag. 2011, 262, 1648–1657. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Lerner, A.M.; Burbano, D.V.; Schneider, L.C.; Rudel, T.K. Carbon Stocks in Silvopastoral Systems: A Study from Four Communities in Southeastern Ecuador. Biotropica 2015, 47, 407–415. [Google Scholar] [CrossRef]
- IPCC Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to; IPCC: Geneva, Switzerland, 2018.
- Torres, B.; Vasseur, L.; López, R.; Lozano, P.; García, Y.; Arteaga, Y.; Bravo, C.; Barba, C.; García, A. Structure and above Ground Biomass along an Elevation Small-Scale Gradient: Case Study in an Evergreen Andean Amazon Forest, Ecuador. Agrofor. Syst. 2020, 94, 1235–1245. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Particle Density. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 377–382. [Google Scholar]
- Bravo, C.; Goyes-Vera, F.; Arteaga-Crespo, Y.; García-Quintana, Y.; Changoluisa, D. A Soil Quality Index for Seven Productive Landscapes in the Andean-Amazonian Foothills of Ecuador. L. Degrad. Dev. 2021, 32, 2226–2241. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]
- Salinas, N.; Malhi, Y.; Meir, P.; Silman, M.; Roman Cuesta, R.; Huaman, J.; Salinas, D.; Huaman, V.; Gibaja, A.; Mamani, M. The Sensitivity of Tropical Leaf Litter Decomposition to Temperature: Results from a Large-scale Leaf Translocation Experiment along an Elevation Gradient in Peruvian Forests. New Phytol. 2011, 189, 967–977. [Google Scholar] [CrossRef]
- Phillips, O.L.; Sullivan, M.J.P.; Baker, T.R.; Monteagudo Mendoza, A.; Vargas, P.N.; Vásquez, R. Species Matter: Wood Density Influences Tropical Forest Biomass at Multiple Scales. Surv. Geophys. 2019, 40, 913–935. [Google Scholar] [CrossRef]
- Pietsch, K.A.; Ogle, K.; Cornelissen, J.H.C.; Cornwell, W.K.; Bönisch, G.; Craine, J.M.; Jackson, B.G.; Kattge, J.; Peltzer, D.A.; Penuelas, J. Global Relationship of Wood and Leaf Litter Decomposability: The Role of Functional Traits within and across Plant Organs. Glob. Ecol. Biogeogr. 2014, 23, 1046–1057. [Google Scholar] [CrossRef]
- da Silva Neto, E.C.; Pereira, M.G.; Fernandes, J.C.F.; Corrêa Neto, T.A. Aggregate Formation and Soil Organic Matter under Different Vegetation in Atlantic Forest from Southeastern Brazil. Semin. Ci. Agr. 2016, 3927–3940. [Google Scholar] [CrossRef]
- Rodríguez, L.; Suárez, J.C.; Rodriguez, W.; Artunduaga, K.J.; Lavelle, P. Agroforestry Systems Impact Soil Macroaggregation and Enhance Carbon Storage in Colombian Deforested Amazonia. Geoderma 2021, 384, 114810. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, J.; Butnor, J.R.; Coetsee, C.; Boucher, P.B.; Case, M.F.; Hockridge, E.G.; Davies, A.B.; Staver, A.C. Limited Increases in Savanna Carbon Stocks over Decades of Fire Suppression. Nature 2022, 603, 445–449. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A. Los Efectos de Los Incendios Forestales En Los Suelos. Síntesis y Conclusiones. Nuevos Retos En La Investigación y En La Gestión. In Efectos los Incend. For. Sobre los Suelos en España. El Estado La Cuestión Visto por los Científicos Españoles; Universitat València: València, Spain, 2009; pp. 355–383. [Google Scholar]
- Feliciano, D.; Slee, B.; Hunter, C.; Smith, P. Estimating the Contribution of Rural Land Uses to Greenhouse Gas Emissions: A Case Study of North East Scotland. Environ. Sci. Policy 2013, 25, 36–49. [Google Scholar] [CrossRef]
- López-Santiago, J.G.; Casanova-Lugo, F.; Villanueva-López, G.; Díaz-Echeverría, V.F.; Solorio-Sánchez, F.J.; Martínez-Zurimendi, P.; Aryal, D.R.; Chay-Canul, A.J. Carbon Storage in a Silvopastoral System Compared to That in a Deciduous Dry Forest in Michoacán, Mexico. Agrofor. Syst. 2019, 93, 199–211. [Google Scholar] [CrossRef]
- Aynekulu, E.; Suber, M.; van Noordwijk, M.; Arango, J.; Roshetko, J.M.; Rosenstock, T.S. Carbon Storage Potential of Silvopastoral Systems of Colombia. Land 2020, 9. [Google Scholar] [CrossRef]
- Feliciano, D.; Sobenes, A. Stakeholders’ Perceptions of Factors Influencing Climate Change Risk in a Central America Hotspot. Reg. Environ. Chang. 2022, 22, 1–19. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Murthy, I.K.; Gupta, M.; Tomar, S.; Munsi, M.; Tiwari, R.; Hegde, G.T.; Ravindranath, N.H. Carbon Sequestration Potential of Agroforestry Systems in India. J. Earth Sci. Clim. Chang. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Montagnini, F.; Ugalde, L.; Navarro, C. Growth Characteristics of Some Native Tree Species Used in Silvopastoral Systems in the Humid Lowlands of Costa Rica. Agrofor. Syst. 2003, 59, 163–170. [Google Scholar] [CrossRef]
- Jernigan, K. An Ethnobotanical Investigation of Tree Identification by the Aguaruna Jívaro of the Peruvian Amazon. J. Ethnobiol. 2006, 26, 107–125. [Google Scholar] [CrossRef]
- Álvarez, F.; Casanoves, F.; Suárez, J.C. Influence of Scattered Trees in Grazing Areas on Soil Properties in the Piedmont Region of the Colombian Amazon. PLoS One 2021, 16, e0261612. [Google Scholar] [CrossRef]
- Oza, M.J.; Kulkarni, Y.A. Traditional Uses, Phytochemistry and Pharmacology of the Medicinal Species of the Genus Cordia (Boraginaceae). J. Pharm. Pharmacol. 2017, 69, 755–789. [Google Scholar] [CrossRef]
- Salehi, B.; Prakash Mishra, A.; Nigam, M.; Karazhan, N.; Shukla, I.; Kiełtyka-Dadasiewicz, A.; Sawicka, B.; Głowacka, A.; Abu-Darwish, M.S.; Hussein Tarawneh, A. Ficus Plants: State of the Art from a Phytochemical, Pharmacological, and Toxicological Perspective. Phyther. Res. 2021, 35, 1187–1217. [Google Scholar] [CrossRef]
- Passos, B.G.; de Albuquerque, R.D.D.G.; Muñoz-Acevedo, A.; Echeverria, J.; Llaure-Mora, A.M.; Ganoza-Yupanqui, M.L.; Rocha, L. Essential Oils from Ocotea Species: Chemical Variety, Biological Activities and Geographic Availability. Fitoterapia 2021, 105065. [Google Scholar] [CrossRef]
- Palacios Bucheli, V.J.; Cárcamo Mallen, R.W.; Álvarez Macías, A.; Coral, C.; Bokelmann, W. Indigenous Family Labor in Agroforestry Systems in the Context of Global Transformations: The Case of the Inga and Camëntsá Communities in Putumayo, Colombia. Forests 2021, 12, 1503. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Salas-Ruiz, A.; Changoluisa, D.; Bravo-Medina, C. Towards Sustainable Urban Planning for Puyo (Ecuador): Amazon Forest Landscape as Potential Green Infrastructure. Sustainability 2020, 12, 4768. [Google Scholar] [CrossRef]
- Duchicela, J.; Valdivieso, A.; Prado-Vivar, B.; Arévalo-Granda, V.; Hickey-Darquea, A.; Hof, P. Diversity of Arbuscular Mycorrhizal Fungi in the Ecuadorian Amazon Region. In Mycorrhizal Fungi in South America; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–170. [Google Scholar]
- Lerner, A.M.; Rudel, T.K.; Schneider, L.C.; McGroddy, M.; Burbano, D.V.; Mena, C.F. The Spontaneous Emergence of Silvo-Pastoral Landscapes in the Ecuadorian Amazon: Patterns and Processes. Reg. Environ. Chang. 2015, 15, 1421–1431. [Google Scholar] [CrossRef]
- Deng, L.; Zhu, G.; Tang, Z.; Shangguan, Z. Global Patterns of the Effects of Land-Use Changes on Soil Carbon Stocks. Glob. Ecol. Conserv. 2016, 5, 127–138. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The Role of Soil Organic Matter in Sustaining Soil Fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Bravo, C.; Torres, B.; Alemán, R.; Changoluisa, D.; Marín, H.; Reyes, H.; Navarrete, H. Soil Structure and Carbon Sequestration as Ecosytem Services under Different Land Uses in the Ecuadorian Amazon Region. In Proceedings of the Conference on Molecular, Biomedical & Computational Sciences and Engineering, Porto, Portugal, 15–17 June 2017; pp. 1–8. [Google Scholar]
- Lal, R. Sequestering Carbon and Increasing Productivity by Conservation Agriculture. J. Soil Water Conserv. 2015, 70, 55A–62A. [Google Scholar] [CrossRef]
- Gardi, C.; Jones, A.; Montanarella, L.; Vargas, R.; Cruz, C. Soil Atlas of Latin America: An Innovative Tool for Policy Development and Awareness Raising. 한국토양비료학회 학술발표회 초록집 2014, 540. [Google Scholar]
- Custode, E.; Sourdat, M. Paisajes y Suelos de La Amazonía Ecuatoriana: Entre La Conservación y La Explotación. Rev. Banco Cent. Ecuador 1986, 24, 325–339. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).