Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nano-PCM
2.3. Thermal Conductivity Measurement
2.4. Specific Heat Measurement
2.5. Thermal Stability Analysis
3. Results and Discussion
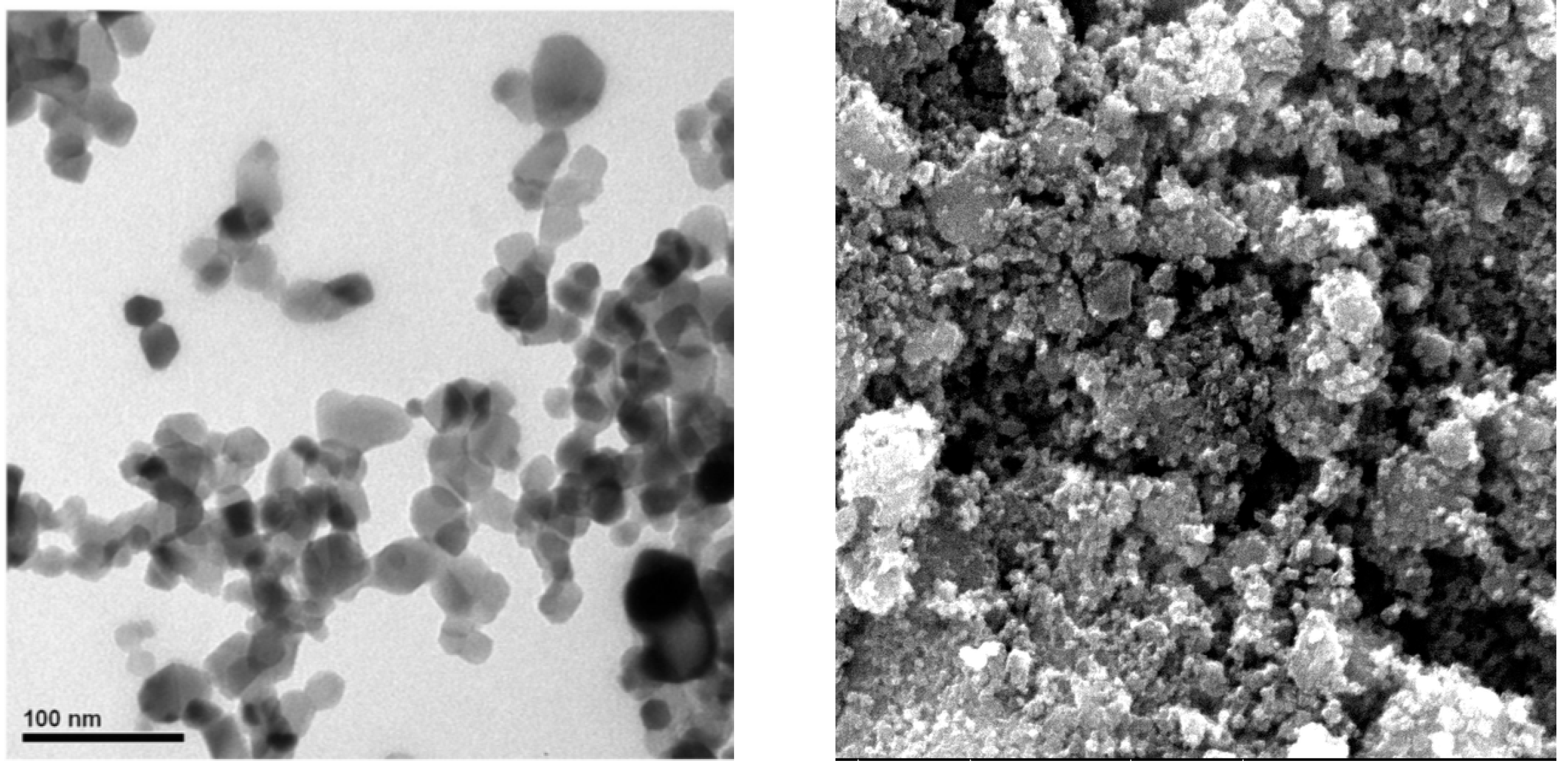
3.1. Morphological Analysis
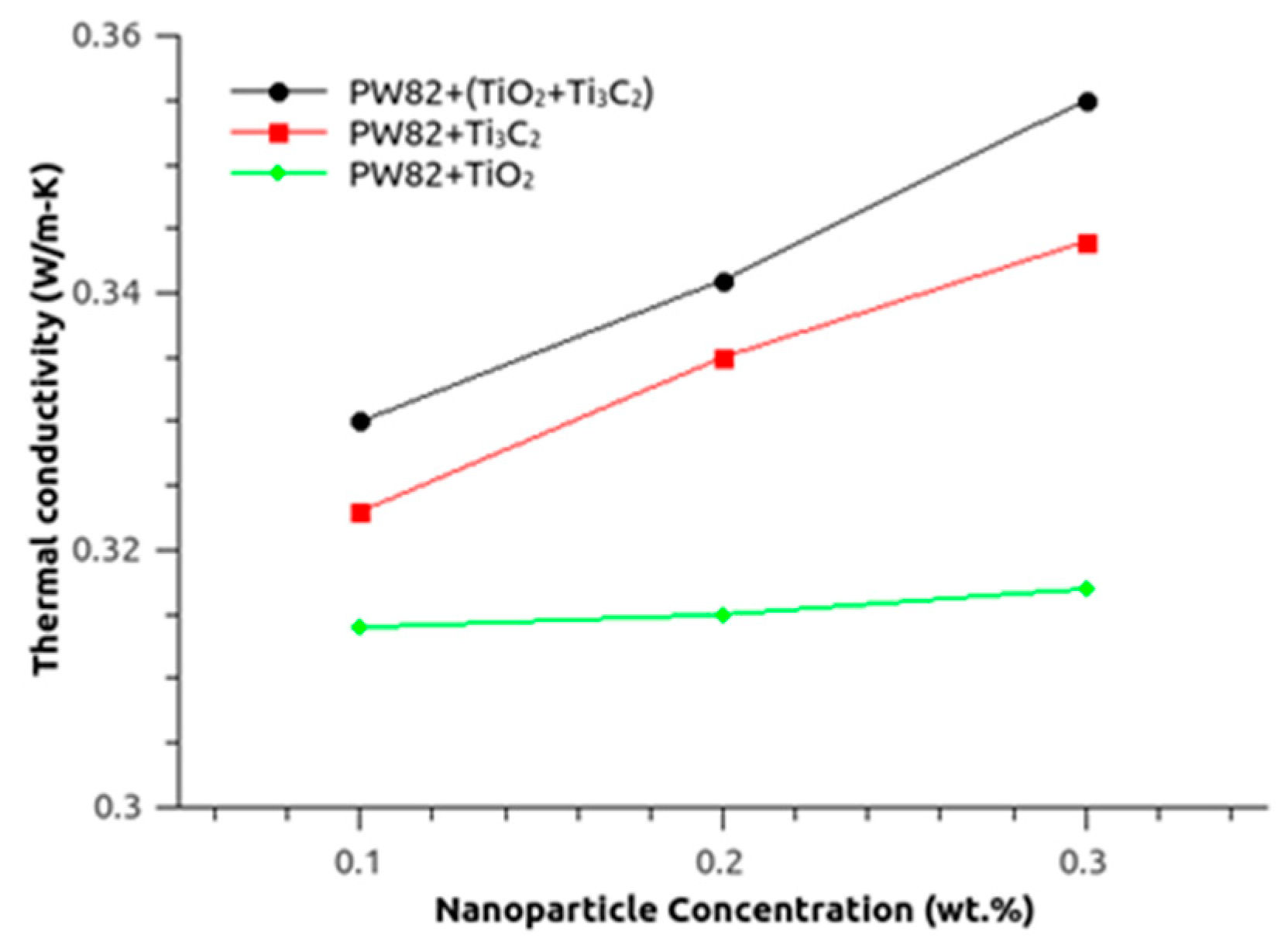
3.2. Thermal Conductivity of MXene and Titania
3.3. Thermal Storage Capacity of Titania and MXene
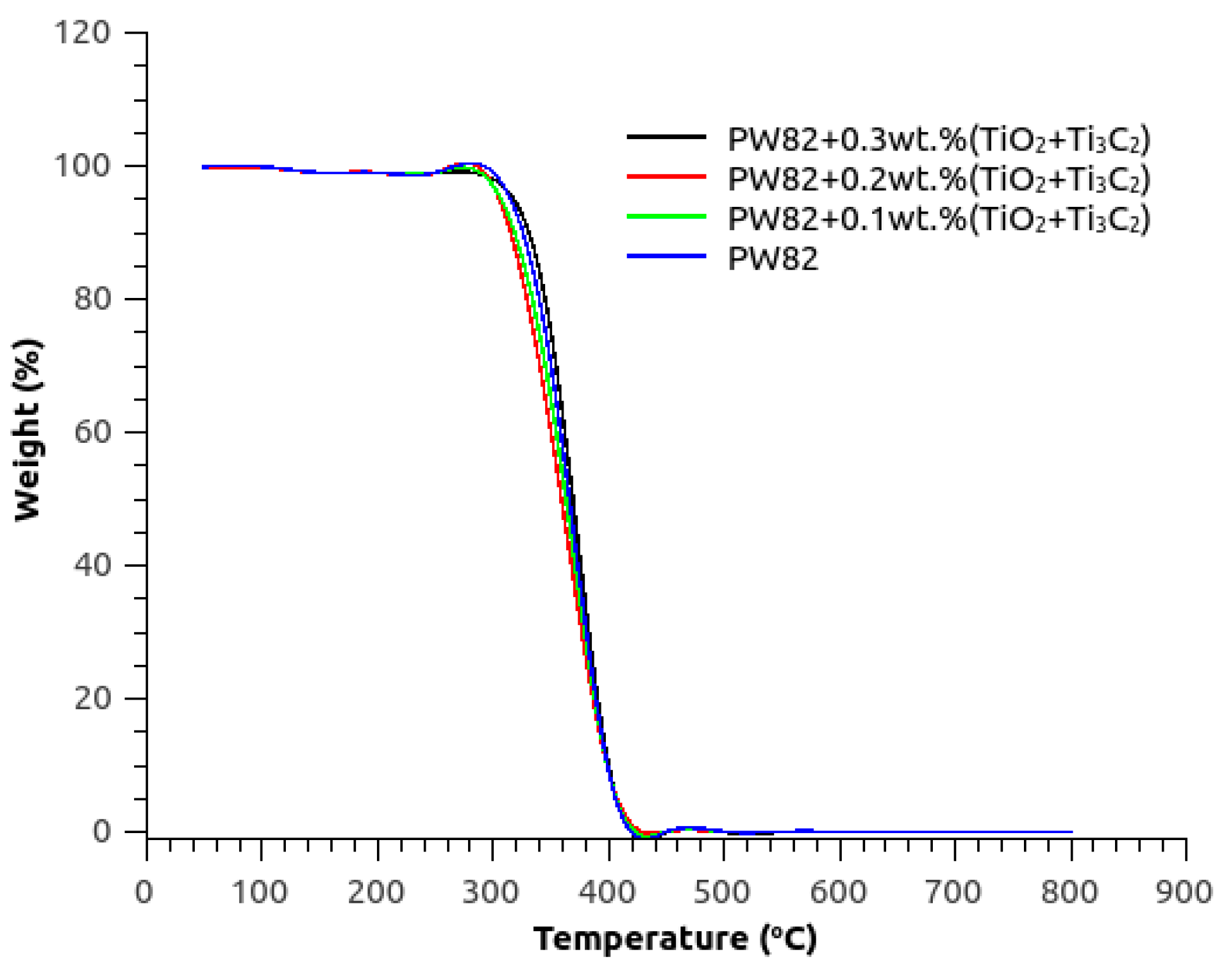
3.4. Thermal Reliability and Stability of PW82 with MXene and Titania
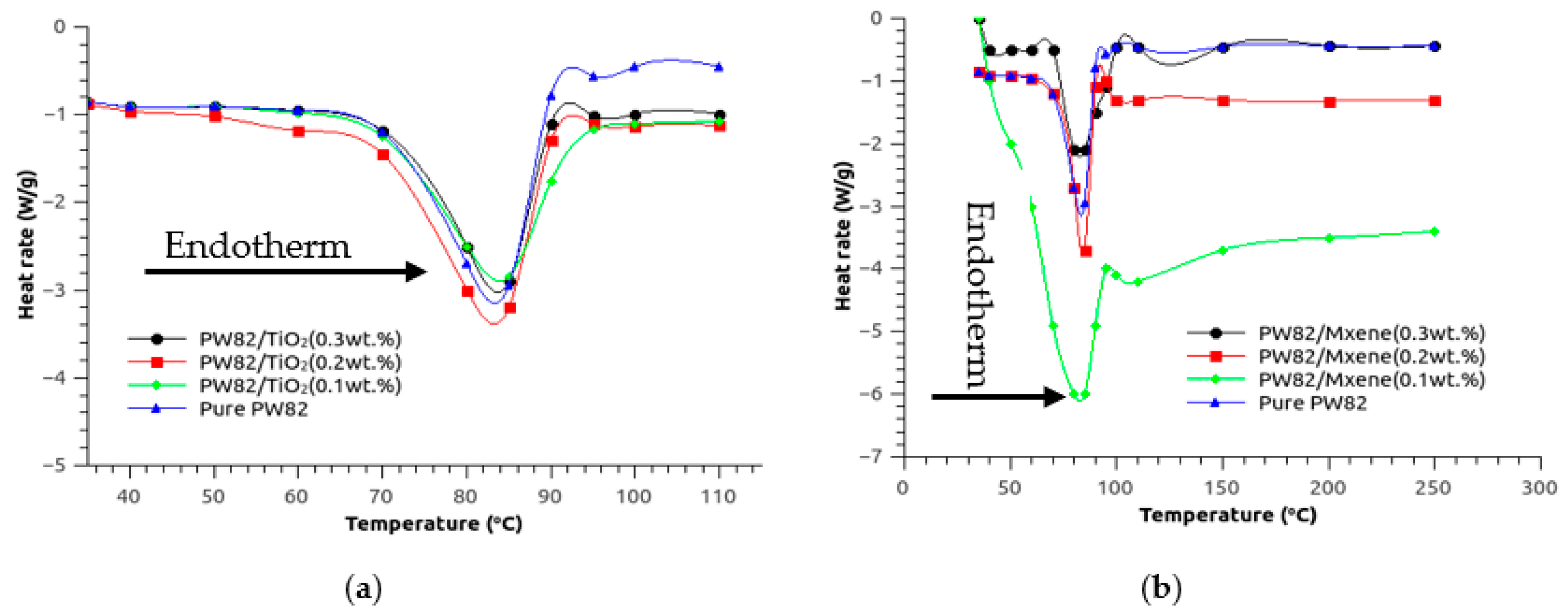
3.5. Estimation of Enthalpy and Melting Point of Various Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dincer, I.; Rosen, M.A. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Nkwetta, D.N.; Haghighat, F. Thermal energy storage with phase change material—A state-of-the art review. Sustain. Cities Soc. 2014, 10, 87–100. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew. Sustain. Energy Rev. 2012, 16, 2118–2132. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M. Application of phase change materials (PCMs) in maintaining comfort temperature inside an automobile. World Acad. Sci. Eng. Technol. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2012, 6, 33–35. [Google Scholar]
- Mondal, S. Phase change materials for smart textiles—An overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Rahimi, M.; Bahrampoury, R. Experimental and computational evolution of a shell and tube heat exchanger as a PCM thermal storage system. Int. Commun. Heat Mass. Transfer. 2014, 50, 128–136. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.J.; Gaspar, A.R.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications, Energy Conversion. Managament 2004, 45, 1597–1615. [Google Scholar]
- Parlak, M.; Sömek, K.; Temel, Ü.; Yapici, K. Experimental investigation of transient thermal response of phase change material embedded by Graphene nanoparticles in energy storage module. In Proceedings of the 2016 15th IEEE Intersociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems (ITherm), Las Vegas, NV, USA, 31 May–3 June 2016; pp. 645–651. [Google Scholar] [CrossRef]
- Fan, L.W.; Khodadadi, J.M. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew Sustain. Energy Rev. 2011, 15, 24. [Google Scholar] [CrossRef]
- Marin, J.M.; Zalba, B.; Cabeza, L.F.; Mehling, H. Improvement of a thermal energy storage using plates with paraffin–graphite composite. Int. J. Heat Mass. Transf. 2005, 48, 2561. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Ding, J.H.; Wang, X.; Yang, R.; Lin, K.P. Influence of additives on thermal conductivity of shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2006, 90, 1692. [Google Scholar] [CrossRef]
- Wang, J.F.; Xie, H.Q.; Xin, Z.; Li, Y.; Chen, L.F. Enhancing thermal conductivity of palmitic acid based phase change materials with carbon nanotubes as fillers. Sol. Energy 2010, 84, 339. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Gao, W. Preparation and properties studies of halogen-free flame reardant form-stable phase change materials based on paraffin/high density polyethylene composites. Appl. Energy 2008, 85, 765. [Google Scholar] [CrossRef]
- Shatikian, V.; Ziskand, G.; Letan, R. Numerical investigation of a PCM-based heat sink with internal fins. Int. J. Heat. Mass. Transf. 2005, 48, 3689. [Google Scholar] [CrossRef]
- Gharebaghi, M.; Sezai, I. Enhancement of heat transfer in latent heat storage modules with internal fins. Numer. Heat Transf. A 2008, 53, 749–765. [Google Scholar] [CrossRef]
- Hu, J.; Yu, H.; Chen, Y.M.; Zhu, M.F. Study on phase-change characteristics of PET–PEG copolymers. J. Macromol. Sci. B 2006, 45, 615. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201. [Google Scholar] [CrossRef]
- Wang, M.R.; Kang, Q.J.; Pan, N. Thermal conductivity enhancement of carbon fiber composites. Appl. Therm. Eng. 2009, 29, 418. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Huang, Y. Electrospun phase change fibers based on polyethylene glycol/cellulose acetate blends. Appl. Energy 2011, 88, 3133. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Alkan, C. Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem. Eng. J. 2009, 155, 899. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Capric–myristic acid/vermiculite composite as form stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323. [Google Scholar] [CrossRef]
- Kim, S.; Drzal, L.T. High latent heat storage and high thermal conductive phase change materials using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2009, 93, 136. [Google Scholar] [CrossRef]
- Li, J.L.; Xue, P.; Ding, W.Y.; Han, J.M.; Sun, G.L. Micro-encapsulated paraffin/high density polyethylene/wood flour composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 1761. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Lu, W.; Tian, Y. Heat transfer enhancement for thermal energy storage using metal foams embedded within phase change materials (PCMs). Sol. Energy 2010, 84, 1402. [Google Scholar] [CrossRef]
- Hawlader, M.N.A.; Uddin, M.S.; Khin, M.M. Microencapsulated PCM thermal energy storage system. Appl. Energy 2003, 74, 195. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Karaipekli, A.; Uzun, O. Microencapsulated n-octacosane as phase change material for thermal energy storage. Sol. Energy 2009, 83, 1757. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yu, F.; Zeng, X.R.; Zhang, Z.G. Preparation, characterization and thermal properties of nanocapsules containing phase change material n-dodecanol by miniemulsion polymerization with polymerizable emulsifier. Appl. Energy 2012, 91, 7. [Google Scholar] [CrossRef]
- Shaikh, S.; Lafdi, K.; Hallinan, K. Carbon nano additives to enhance latent energy storage of phase change materials. J. Appl. Phys. 2008, 103, 094302. [Google Scholar] [CrossRef]
- Wang, J.F.; Xie, H.Q.; Xin, Z. Thermal properties of paraffin based composites containing multi-walled carbon nanotubes. Thermochim. Acta 2009, 488, 39. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behaviour of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208. [Google Scholar] [CrossRef]
- Elgafy, A.; Lafdi, K. Effect of carbon nanofiber additives on thermal behavior of phase change materials. Carbon 2005, 43, 3067. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, T.Y. Preparation and thermophysical properties of nanoparticle-in paraffin emulsion as phase change material. Int. Commun. Heat Mass. Transf. 2009, 36, 467. [Google Scholar] [CrossRef]
- Khan, A.A.; Danish, M.; Rubaiee, S.; Yahya, S.M. Insight into the investigation of Fe3O4/SiO2 nanoparticles suspended aqueous nanofluids in hybrid photovoltaic/thermal system. Clean. Eng. Technol. 2022, 11, 100572. [Google Scholar] [CrossRef]
- Arasu, A.V.; Mujumdar, A.S. Numerical study on melting of paraffin wax with Al2O3 in a square enclosure. Int. Commun. Heat Mass. Transf. 2012, 39, 8. [Google Scholar] [CrossRef]
- Teng, T.-P.; Yu, C.-C. Characteristics of phase-change materials containing oxide nano-additives for thermal storage. Nanoscale Res. Lett. 2012, 7, 611. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, N.; Bauer, T.; Martin, C.; Eck, M.; Wörner, A. Thermal energy storage—Overview and specific insight into nitrate salts for sensible and latent heat storage. Beilstein. J. Nanotechnol. 2015, 6, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.P.; Lin, B.G.; Yeh, Y.Y. Characterization of heat storage by nanocomposite -enhanced phase change materi-als. Adv. Mater. Res. 2011, 287, 1448. [Google Scholar] [CrossRef]
- Jiao, F.; Bai, D.; Du, J.; Hong, Y. Numerical investigation on melting and thermal performances of a phase change material in partitioned cavities with fins for thermal energy storage. J. Energy Storage 2022, 56, 106022. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S.; Koduru, J.R.; Dehghani, M.H. MXene-based phase change materials for solar thermal energy storage. Energy Convers. Manag. 2022, 273, 116432. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Wei, K.; Si, W.; Kang, X.; Fang, Y.; Zhang, H.; Shi, J.; Zhou, X. Thermal storage properties of polyurethane solid-solid phase-change material with low phase-change temperature and its effects on performance of asphalt binders. J. Energy Storage 2022, 55, 105686. [Google Scholar] [CrossRef]
- Viana, M.M.; Soares, V.F.; Mohallem, N.D.S. Synthesis and characterization of TiO2 nanoparticles. Ceram. Int. 2010, 36, 2047–2053. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Wojciechowski, T.; Ziemkowska, W.; Rozmysłowska, A.; Chlubny, L.; Olszyna, A. The Atomic Structure of Ti2C and Ti3C2 MXenes is Responsible for Their Antibacterial Activity Toward E. coli Bacteria. J. Mater. Eng. Perform. 2018, 28, 1272–1277. [Google Scholar] [CrossRef]
- Low, J.; Zhang, L.; Tong, T.; Shen, B.; Yu, J. TiO2/MXene Ti3C2 composite with excellent photocatalytic CO2 reduction activity. J. Catal. 2018, 361, 255–266. [Google Scholar] [CrossRef]
- Saber, D.; El-Aziz, K.; Felemban, B.F.; Alghtani, A.H.; Ali, H.T.; Ahmed, E.M.; Megahed, M. Characterization and performance evaluation of Cu-based/TiO2 nano composites. Sci. Rep. 2022, 12, 1–14. [Google Scholar] [CrossRef]
- Deka, P.P.; Ansu, A.K.; Sharma, R.K.; Tyagi, V.V.; Sarı, A. Development and characterization of form-stable porous TiO2/tetradecanoic acid based composite PCM with long-term stability as solar thermal energy storage material. Int. J. Energy Res. 2020, 44, 10044–10057. [Google Scholar] [CrossRef]
- Tang, B.; Qiu, M.; Zhang, S. Thermal conductivity enhancement of PEG/SiO2 composite PCM by in situ Cu doping. Sol. Energy Mater. Sol. Cells 2012, 105, 242–248. [Google Scholar] [CrossRef]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhag, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical proper-ties. Nanoscale Res. Lett. 2013, 8, 32. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Yan, B.; Ma, H.; Li, N.; Ye, M. One-pot hydrothermal synthesis of Ag-reduced graphene oxide composite with ionic liquid. J. Mater. Chem. 2011, 21, 7795–7801. [Google Scholar] [CrossRef]
- Bayés-García, L.; Ventola, L.; Cordobilla, R.; Benages, R.; Calvet, T.; Cuevas-Diarte, M. Phase change materials (PCM) micro-capsules with different shell compositions: Preparation, char-acterization and thermal stability. Sol. Energy Mater. Sol. Cells 2010, 94, 1235–1240. [Google Scholar] [CrossRef]
- Phase Change Materials; Products Ltd. Company, 2011.
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Abdelrazik, A.S.; Samylingam, L.; Sabri, M.F.M.; Sidik, N.A.C. Improved thermo-physical properties and energy efficiency of hybrid PCM/graphene-silver nanocomposite in a hybrid CPV/thermal solar system. J. Therm. Anal. Calorim. 2020, 147, 1125–1142. [Google Scholar] [CrossRef]
- Hajizadeh, M.R.; Selimefendigil, F.; Muhammad, T.; Ramzan, M.; Babazadeh, H.; Li, Z. Solidification of PCM with nano powders inside a heat exchanger. J. Mol. Liq. 2020, 306, 112892. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical modeling of nano enhanced PCM solidification in an enclosure with metallic fin. J. Mol. Liq. 2018, 259, 424–438. [Google Scholar] [CrossRef]
- Kaviarasu, C.; Prakash, D. Review on Phase Change Materials with Nanoparticle in Engineering Applications. J. Eng. Sci. Technol. Rev. 2016, 9, 26–36. [Google Scholar] [CrossRef]






| Heating/Cooling Cycles | 0 | 10 | 20 | 30 | 40 | 50 | 75 | 100 | 150 |
|---|---|---|---|---|---|---|---|---|---|
| Thermal conductivity (W/m-K) | 0.355 | 0.354 | 0.352 | 0.354 | 0.353 | 0.351 | 0.353 | 0.354 | 0.352 |
| Phase change enthalpy (J/g) | 95.40 | 94.25 | 94.18 | 94.86 | 95.22 | 95.33 | 95.10 | 94.98 | 95.15 |
| PCM Type | Concentration (wt.%) | Tm (°C) | Hm (J/g) | Ts (°C) | Hs (J/g) |
|---|---|---|---|---|---|
| PW82 | --- | 70.8 | 175.6 | 68.9 | 159.4 |
| PW82 + TiO2 | 0.1 | 71.0 | 165.2 | 68.7 | 155.10 |
| 0.2 | 71.2 | 158.4 | 68.1 | 148.25 | |
| 0.3 | 71.3 | 150.5 | 67.6 | 142.62 | |
| PW82 + Ti3C2 | 0.1 | 70.9 | 100.75 | 70.1 | 95.75 |
| 0.2 | 71.5 | 110.15 | 69.5 | 105.10 | |
| 0.3 | 71.8 | 122.35 | 68.2 | 120.13 | |
| PW82/TiO2 + Ti3C2 | 0.1 | 71 | 80.7 | 68.50 | 75.49 |
| 0.2 | 72.5 | 87.8 | 67.33 | 82.75 | |
| 0.3 | 73.1 | 95.4 | 66.43 | 90.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.A.; Yahya, S.M.; Ali, M.A. Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage. Sustainability 2023, 15, 516. https://doi.org/10.3390/su15010516
Khan AA, Yahya SM, Ali MA. Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage. Sustainability. 2023; 15(1):516. https://doi.org/10.3390/su15010516
Chicago/Turabian StyleKhan, Ajiv Alam, Syed Mohd Yahya, and Masood Ashraf Ali. 2023. "Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage" Sustainability 15, no. 1: 516. https://doi.org/10.3390/su15010516
APA StyleKhan, A. A., Yahya, S. M., & Ali, M. A. (2023). Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage. Sustainability, 15(1), 516. https://doi.org/10.3390/su15010516






