Fate and Transport of Lead and Copper in Calcareous Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analytical Methods
2.1.1. Soil Physical Characteristics
2.1.2. Soil Chemical Analyses
2.1.3. Soil Calcium Carbonate Removal
2.2. Heavy Metals Kinetics and Sorption Isotherms
2.2.1. Sorption Kinetics
- Fractional power model:
- Pseudo-second-order model:
- Elovich model:
- Intra-particle diffusion:
2.2.2. Sorption Isotherms
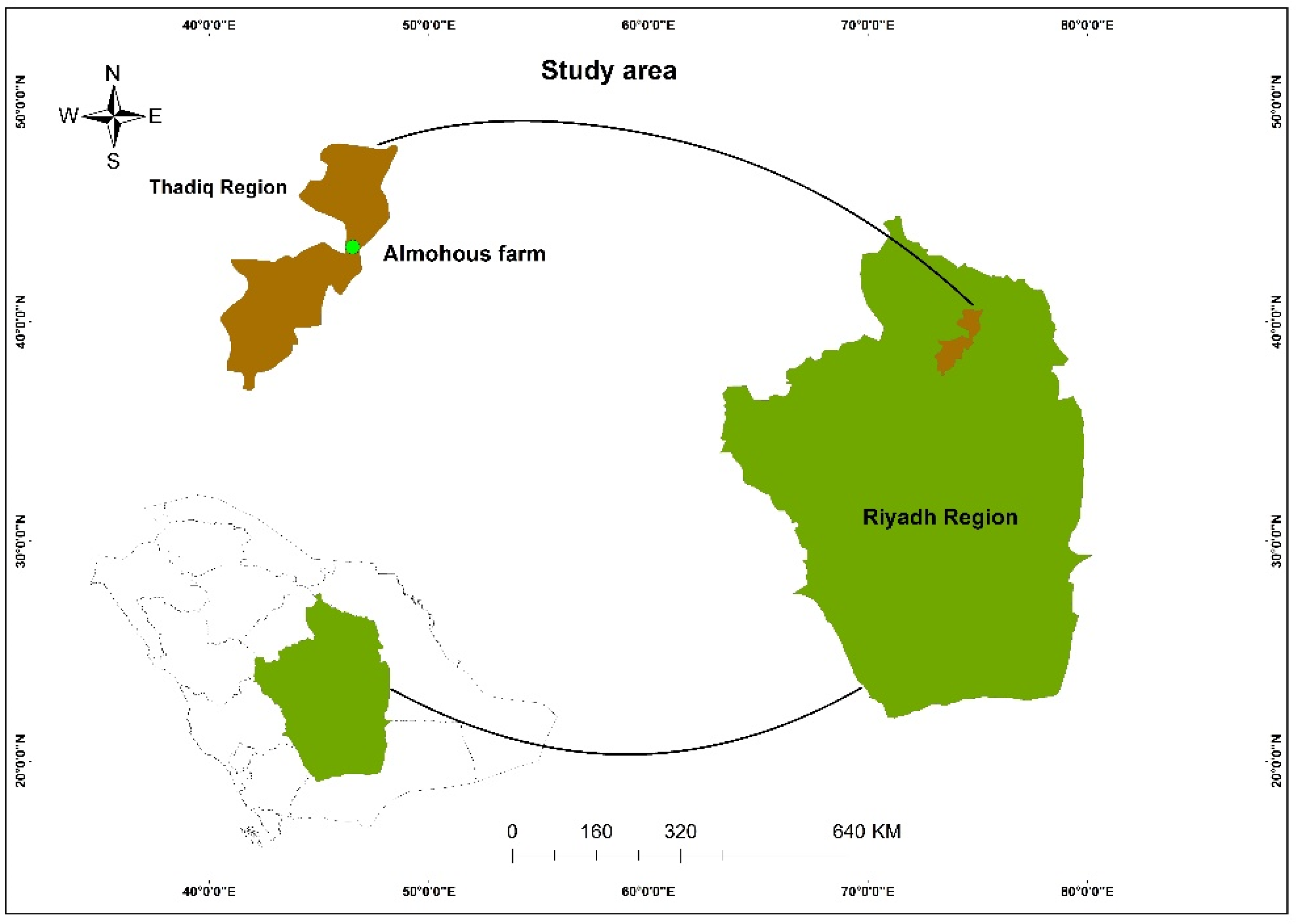
2.3. Column Experiment Setup
3. Results and Discussion
3.1. Lead and Copper Kinetics and Sorption Isotherms
3.1.1. Lead and Copper Adsorption Kinetics
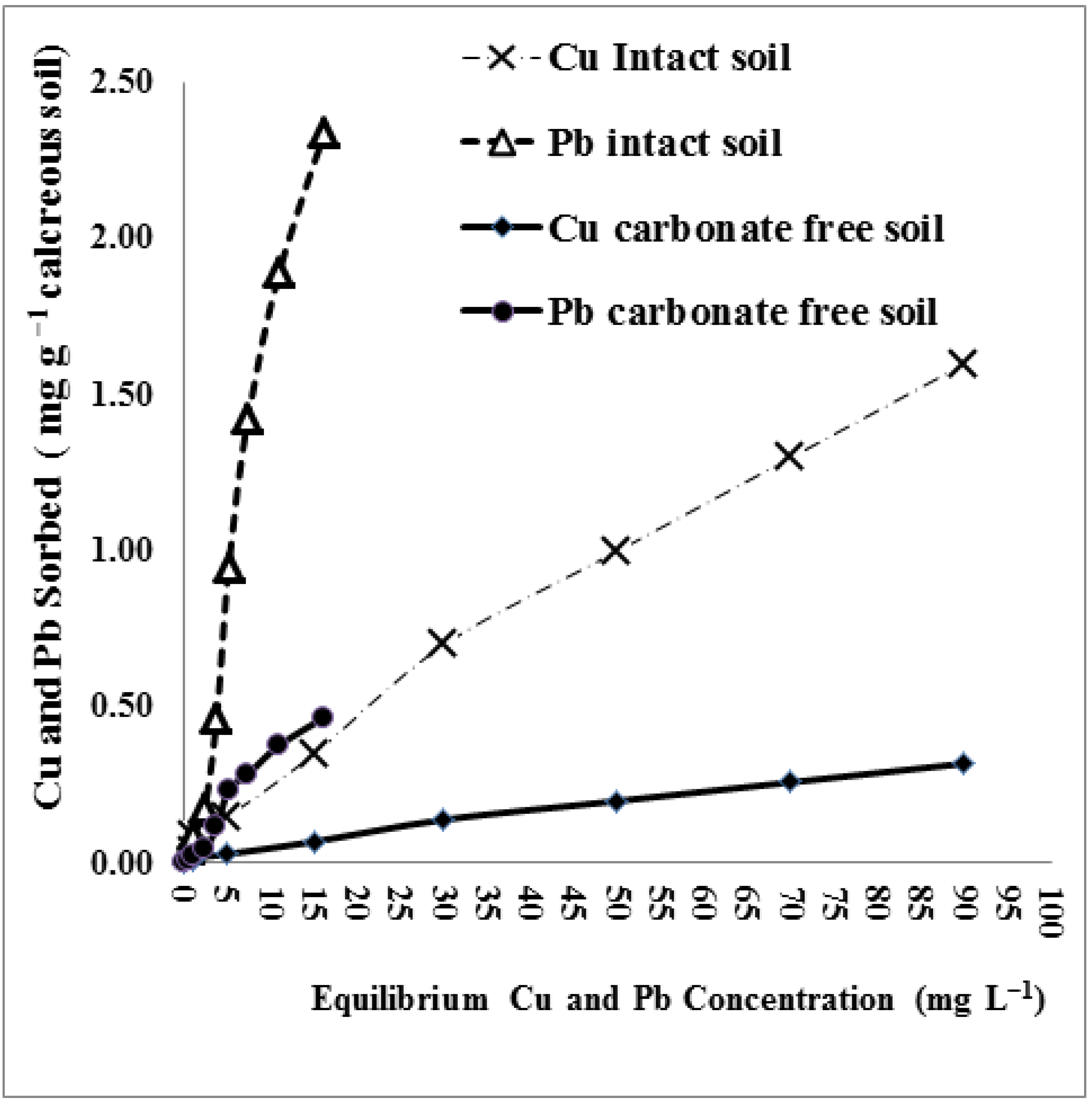
3.1.2. Lead and Copper Sorption Isotherms
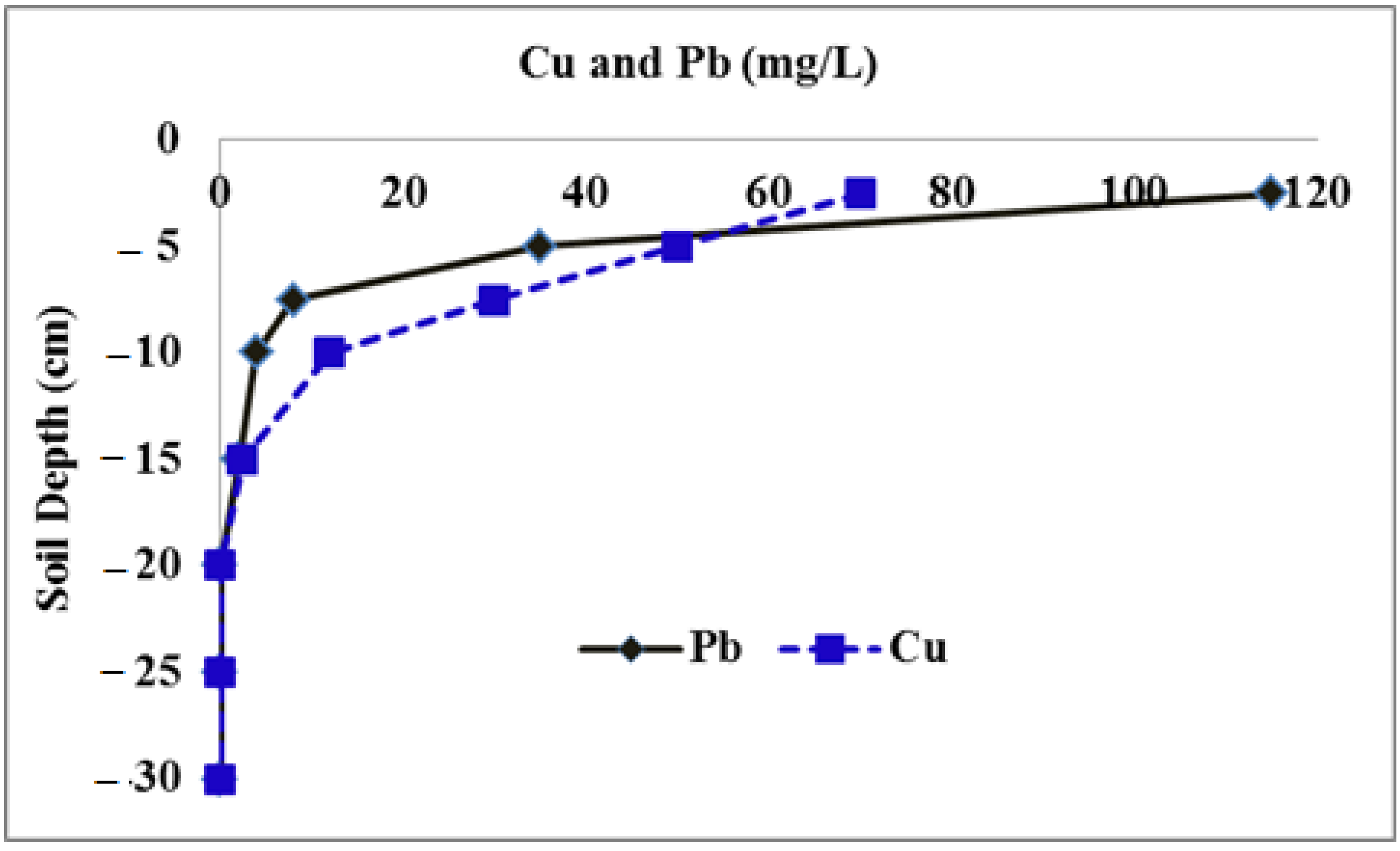
3.2. Lead and Copper Transport in Soil Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305 . [Google Scholar] [CrossRef] [Green Version]
- Chin, N.P. Environmental Toxins: Physical, Social, and Emotional. Breastfeed Med. 2010, 5, 223–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yan, L.; Liu, J.; Zhang, Z.; Tan, C. Removal of Different Kinds of Heavy Metals by Novel PPG-nZVI Beads and Their Application in Simulated Stormwater Infiltration Facility. Appl. Sci. 2019, 9, 4213. [Google Scholar] [CrossRef] [Green Version]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Ehsan, A.; Danish, S.; Ali, M.A.; Fahad, S.; Dawar, K.; Taban, S.; Akça, H.; Shah, A.A.; Ansari, M.J.; et al. Mitigation of lead (Pb) tox-icity in rice cultivated with either ground water or wastewater by application of acidified carbon. J. Environ. Manag. 2022, 307, 114521. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Ibrahim, H.M. Assessment of contamination by potentially toxic trace elements of groundwater and soil surrounding municipal soil waste landfill: A case study from Saudi Arabia. Arab. J. Geosci. 2021, 14, 350–362. [Google Scholar] [CrossRef]
- Babur, E.; Uslu, Ö.S.; Hakan Yılmaz, C.; Sünbül, M.R. Investigation of the effects of land use on chemical water quality parameters; A case study of Başkonuş-Meydan dam lake in Kahramanmaraş. Aquat. Sci. Eng. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Lu, Y.; Yin, W.; Huang, L.; Zhang, G.; Zhao, Y. Assessment of bioaccessibility and exposure risk of arsenic and lead in urban soils of Guangzhou City, China. Environ. Geochem. Health 2011, 33, 93–102. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Jalali, M.; Latifi, Z. Measuring and simulating effect of organic residues on the transport of cadmium, nickel, and zinc in a calcareous soil. J. Geochem. Explor. 2018, 184, 372–380. [Google Scholar] [CrossRef]
- Lafuente, A.L.; González, C.; Quintana, J.R.; Vázquez, A.; Romero, A. Mobility of heavy metals in poorly developed carbonate soils in the Mediterranean region. Geoderma 2008, 145, 238–244. [Google Scholar] [CrossRef]
- Jalali, M.; Jalili, A. Competitive adsorption of trace elements in calcareous soils as affected by sewage sludge, poultry manure, and municipal waste compost. Environ. Earth Sci. 2011, 63, 731–739. [Google Scholar] [CrossRef]
- Xiao, D.; Cheng, J.; Liang, W.; Sun, L.; Zhao, J. Metal-phenolic coated and prochloraz-loaded calcium carbonate carriers with pH responsiveness for environmentally-safe fungicide delivery. Chem. Eng. J. 2021, 418, 129274. [Google Scholar] [CrossRef]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. J. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Mourid, S.S. Effect of calcium carbonate content on potential toxic heavy metals adsorption in calcareous soils. J. Curr. Sci. Int. 2014, 3, 141–149. [Google Scholar]
- El-Aswad, A.F.; Fouad, M.R.; Badawy, M.E.I.; Aly, M.I. Effect of Calcium Carbonate Content on Potential Pesticide Adsorption and Desorption in Calcareous Soil. Commun. Soil Sci. Plant Anal. 2022, 1–9. [Google Scholar] [CrossRef]
- Plassard, F.; Winiarski, T.; Petit-Ramel, M. Retention and distribution of three heavy metals in a carbonated soil: Comparison between batch and unsaturated column studies. J. Contam. Hydrol. 2000, 42, 99–111. [Google Scholar] [CrossRef]
- Elzahabi, M.; Yong, R.N. pH influence on sorption characteristics of heavy metal in the vadose zone. Eng. Geol. 2001, 60, 61–68. [Google Scholar] [CrossRef]
- Caetano, M.; Vale, C.; Cesário, R.; Fonseca, N. Evidence for preferential depths of metal retention in roots of salt marsh plants. Sci. Total Environ. 2008, 390, 466–474. [Google Scholar] [CrossRef]
- Fernandez, C.; Monna, F.; Labanowski, J.; Loubet, M.; Van Oort, F. Anthropogenic lead distribution in soils under arable land and permanent grassland estimated by Pb isotopic compositions. Environ. Pollut. 2008, 156, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Sayyad, G.; Afyuni, M.; Mousavi, S.F.; Abbaspour, K.C.; Richards, B.K.; Schulin, R. Transport of Cd, Cu, Pb and Zn in a calcareous soil under wheat and safflower cultivation—A column study. Geoderma 2010, 154, 311–320. [Google Scholar] [CrossRef]
- Jalali, M.; Khanlari, Z.V. Mobility and distribution of zinc, cadmium and lead in calcareous soils receiving spiked sewage sludge. Soil Sediment Contam. 2006, 15, 603–620. [Google Scholar] [CrossRef]
- Vanisree, C.R.; Singh, P.; Jadhav, E.B.; Nair, M.S.; Sankhla, M.S.; Parihar, K.; Awasthi, K.K. Effect of Climate Change and Soil Dynamics on Soil Microbes and Fertility of Soil. In Microbiome under Changing Climate; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–468. ISBN 978-0-323-90571-8. [Google Scholar]
- Hillel, D. Soil and Water Physical Principles and Processes; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Sparks, D.L. Methods of Soil Analysis; Soil Society of American, ASA: Madison, WI, USA, 1996. [Google Scholar]
- Tabatabai, M.A. Methods of Soil Analysis, Part 3: Chemical Methods. In SSSA Book Series 5; Sparks, D.L., Helmke, P.A., Loeppert, R.H., Eds.; ASA: Madison, WI, USA, 1996. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration methods. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils, Indian Edition; USDA Handbook no. 60; Prinlar: New Delhi, India, 1954. [Google Scholar]
- Kroetsch, D.; Wang, C. Particle size distribution. J. Soil Sampl. Methods Anal. 2008, 2, 713–725. [Google Scholar]
- Cooney, E.L.; Booker, N.A.; Shallcross, D.C.; Stevens, G.W. Ammonia removal from wastewaters using natural Australian zeolite. II. Pilot-Scale study using continuous packed column process. Sep. Sci. Technol. 1999, 34, 2741–2760. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Fatinathan, S. Adsorption of Cu(II) ions in aqueous solution using chitosan beads, chitosan-GLA beads and chitosan-alginate beads. Chem. Eng. J. 2008, 143, 62–72. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Application of Kinetic Models to the Sorption of Copper(II) on to Peat. Adsorpt. Sci. Technol. 2002, 20, 797–815. [Google Scholar] [CrossRef]
- Ho, Y.S.; Porter, J.F.; Mckay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Liu, X.D.; Tokura, S.; Haruki, M.; Nishi, N.; Sakairi, N. Surface modification of nonporous glass beads with chitosan and their adsorption property for transition metal ions. Carbohydr. Polym. 2002, 49, 103–108. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aljohani, B.H.; Aly, A.A. Impacts of Olive Waste-derived Biochar on Hydro-Physical Properties of Sandy Soil. Sustainability 2021, 13, 5493. [Google Scholar] [CrossRef]
- Aly, A.A.; Hasan, N.Y.; Al-Farraj, A.S. Olive mill wastewater treatment using a simple zeolite-based low-cost method. J. Environ. Manag. 2014, 145, 341–348. [Google Scholar] [CrossRef]
- Fifi, U.; Winiarski, T.; Emmanuel, E. Assessing the Mobility of Lead, Copper and Cadmium in a Calcareous Soil of Port-au-Prince, Haiti. Int. J. Environ. Res. Public Health 2013, 10, 5830–5843. [Google Scholar] [CrossRef]
- AL-Farraj, A.S.; Al- Sewailem, M.S.; Aly, A.A.; Al-Wabel, M.I.; El-Maghraby, S.E. Assessment and heavy metals behaviors of industrial waste water on Riyadh City, Saudi Arabia. Proc. Int. Acad. Ecol. Environ. Sci. 2013, 3, 266–277. [Google Scholar]
- Al-Janabi, F.K.; Al-Robiaee, M.A. Kintic of copper adsorption in calcareous soil. Iraqi J. Agric. Sci. 2016, 47, 621–627. [Google Scholar] [CrossRef]
- Usman, R.A.; Sallam, A.S.; Al-Omran, A.; El-Naggar, A.H.; Alenazi, K.H.; Nadeem, M.; Al-Wabel, M.I. Chemically Modified Biochar Produced from Conocarpus Wastes: An Efficient Sorbent for Fe(II) Removal from Acidic Aqueous Solutions. Adsorpt. Sci. Technol. 2013, 31, 625–640. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Raikhy, N.P.; Takkar, P.N. Zinc and copper adsorption by a soil with and without removal of carbonates. J. Indian Soc. Soil Sci. 1983, 31, 611–614. [Google Scholar]
- Rodriguez-Rubio, P.; Morillo, E.; Madrid, L.; Undabeytia, T. Maqueda, C. Retention of copper by a calcareous soil and its textural fractions: Influence of amendment with two agroindustrial residues. Eur. J. Soil Sci. 2003, 54, 401–409. [Google Scholar] [CrossRef]
- Arias, M.; Pérez-Novo, C.; Lopez, E.; Soto, B. Competitive adsorption and desorption of copper and zinc in acids soils. Geoderma 2006, 133, 151–159. [Google Scholar] [CrossRef]
- Tellan, A.C.; Owalude, S.O. Some Langmuir and Freundlich parameters of adsorption studies of chlorpheniramine maleate. Res. J. Appl. Sci. 2007, 2, 875–878. [Google Scholar]
- Gomes, P.C.; Fontes, M.P.F.; da Silva, A.G.; de, S. Mendonça, E.; Netto, A.R. Selectivity sequence and competitive adsorption of heavy metals by Brazilian soils. Soil Sci. Soc. Am. J. 2001, 65, 1115–1121. [Google Scholar] [CrossRef]
- Yong, R.N.; Mohamed, A.M.O.; Warkentin, B.P. Principles of Contaminant Transport in Soils; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 1989. [Google Scholar]


| Particle Size Distribution | Textural Class | CaCO3 % | O.M % | CEC (meq/100 g Soil) | Surface Area | Ks | ||
|---|---|---|---|---|---|---|---|---|
| Sand % | Silt % | Clay % | (m2 g−1) | (cm min−1) | ||||
| 84 | 6 | 10 | Loamy sand | 11 | 0.08 | 8 | 202 | 0.1 |
| pH | ECe (dSm−1) | Soluble Cations (meq/L) | Soluble Anions (meq/L) | NO3− (mg/L) | Heavy Metals * (mg/Kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca++ | Mg++ | Na+ | K+ | CO3− | HCO3− | Cl− | SO4− | Cu | Pb | |||
| 8.2 | 1.2 | 7 | 3 | 1.8 | 0.5 | 0 | 3.5 | 3 | 6 | 0.8 | ND | ND |
| Sorbents | Initial pH | Pseudo-Second-Order | Elovich | Power Function | Intra-Particle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe | K2 | R2 | α | β | R2 | b | a | R2 | ki | a | R2 | ||
| Pb2+ | 8.2 | 1.03 | 0.03 | 0.9995 | 2.4 | 0.19 | 0.95 | 0.52 | 0.04 | 0.89 | 0.101 | 0.112 | 0.86 |
| Cu2+ | 8.2 | 0.76 | 0.02 | 0.9979 | 24.0 | 0.05 | 0.97 | 0.29 | 0.03 | 0.85 | 0.042 | 0.143 | 0.78 |
| Sorpent | Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|---|
| Kf | R2 | 1/n | n | qo (mg g−1) | KL (Lm g−1) | R2 | |
| Pb2+ | 0.08 | 0.96 | 1.3 | 0.8 | 6.8 | 0.8 | 0.95 |
| Cu2+ | 0.04 | 0.98 | 0.8 | 1.3 | 4.0 | 2.1 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.G.; Alasmary, Z. Fate and Transport of Lead and Copper in Calcareous Soil. Sustainability 2023, 15, 775. https://doi.org/10.3390/su15010775
Alghamdi AG, Alasmary Z. Fate and Transport of Lead and Copper in Calcareous Soil. Sustainability. 2023; 15(1):775. https://doi.org/10.3390/su15010775
Chicago/Turabian StyleAlghamdi, Abdulaziz G., and Zafer Alasmary. 2023. "Fate and Transport of Lead and Copper in Calcareous Soil" Sustainability 15, no. 1: 775. https://doi.org/10.3390/su15010775
APA StyleAlghamdi, A. G., & Alasmary, Z. (2023). Fate and Transport of Lead and Copper in Calcareous Soil. Sustainability, 15(1), 775. https://doi.org/10.3390/su15010775






