High-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine
Abstract
1. Introduction
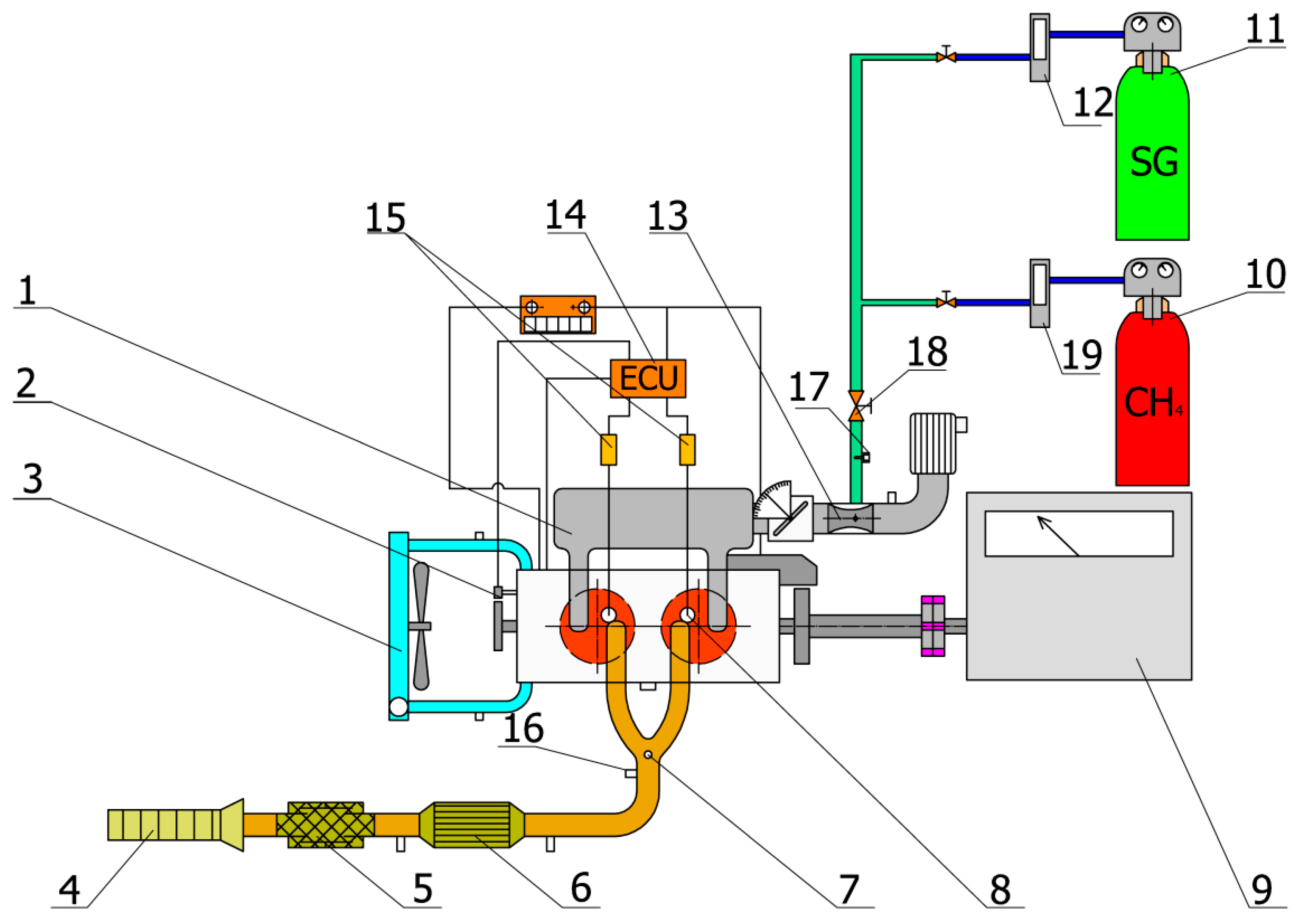
2. Experimental Methods
Pressure Analysis
- dU—differential of internal energy of matter in the system.
- dQ—differential of heat delivered to the system.
- dW—differential of the work produced by the system.
- hi × dmi—i-th component of enthalpy of mass flow across system boundaries (during combustion, this term is assumed to be zero).
- dQch—differential of the released chemical energy from the fuel.
- κ—specific heat ratio.
- u—specific internal energy.
- r—mass specific gas constant.
- T—mean thermodynamic temperature.
- dmc—total mass of charge.
- dQht—differential of the heat transfer to the chamber walls.
3. Experimental Results
3.1. Integral Parameters of Combustion Engine
3.2. Internal Parameters of Combustion Engine
4. Short Discussion
5. Conclusions
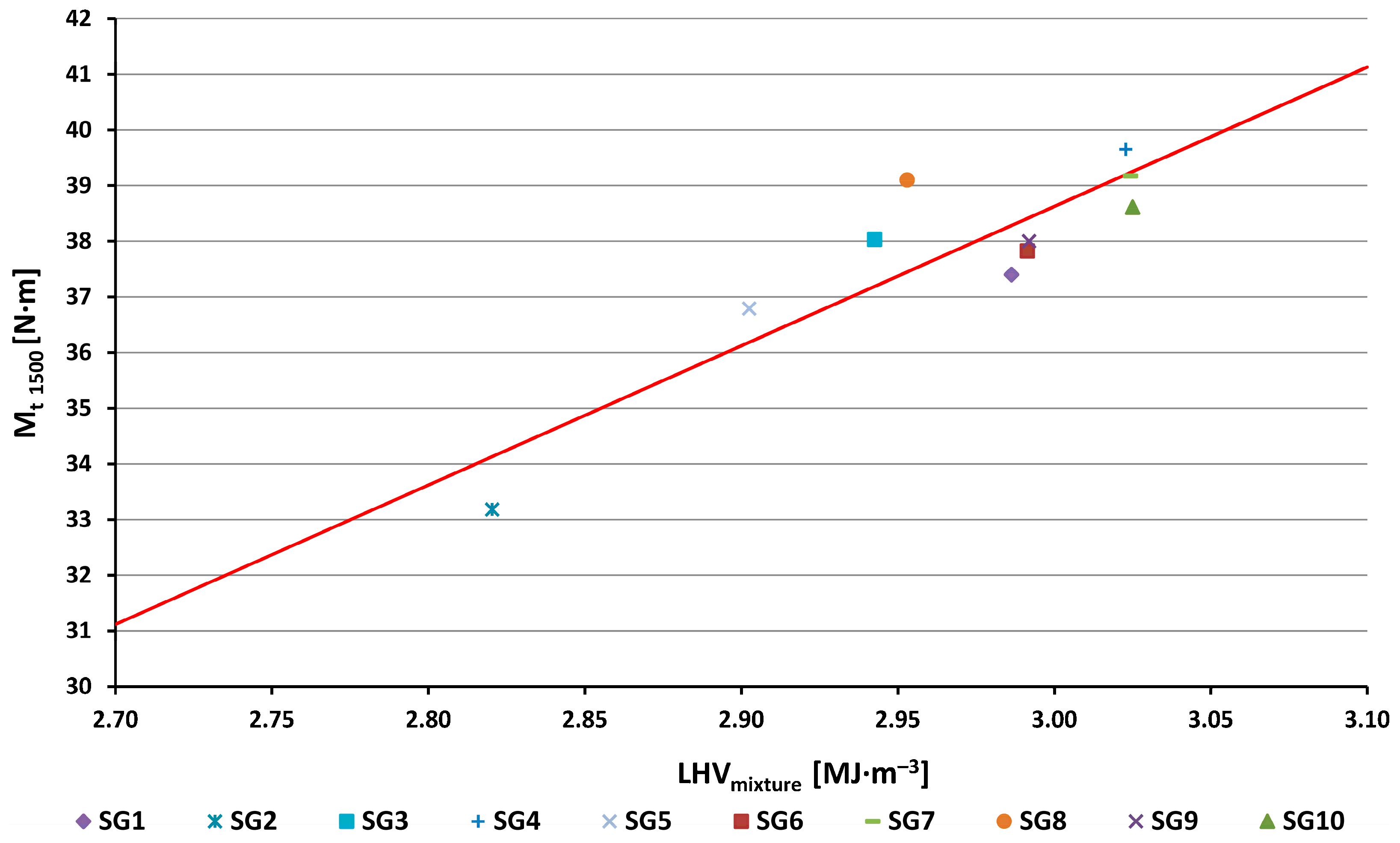
- At the speed of 1500 rpm the torque value achieved for methane was 43.2 N·m. For the investigated synthesis gases (Figure 3, Figure 4 and Figure 13), this value was lower (from 33.2 N·m for the SG2 to 39.7 N·m for the SG4) due to lower achieved values of the volumetric LHV of the stoichiometric mixture as compared to methane. The linear trend line (red line in Figure 13) drawn through the measured moment values shows that the deviations of the measured data from the trend line in the graph are within ±5%.
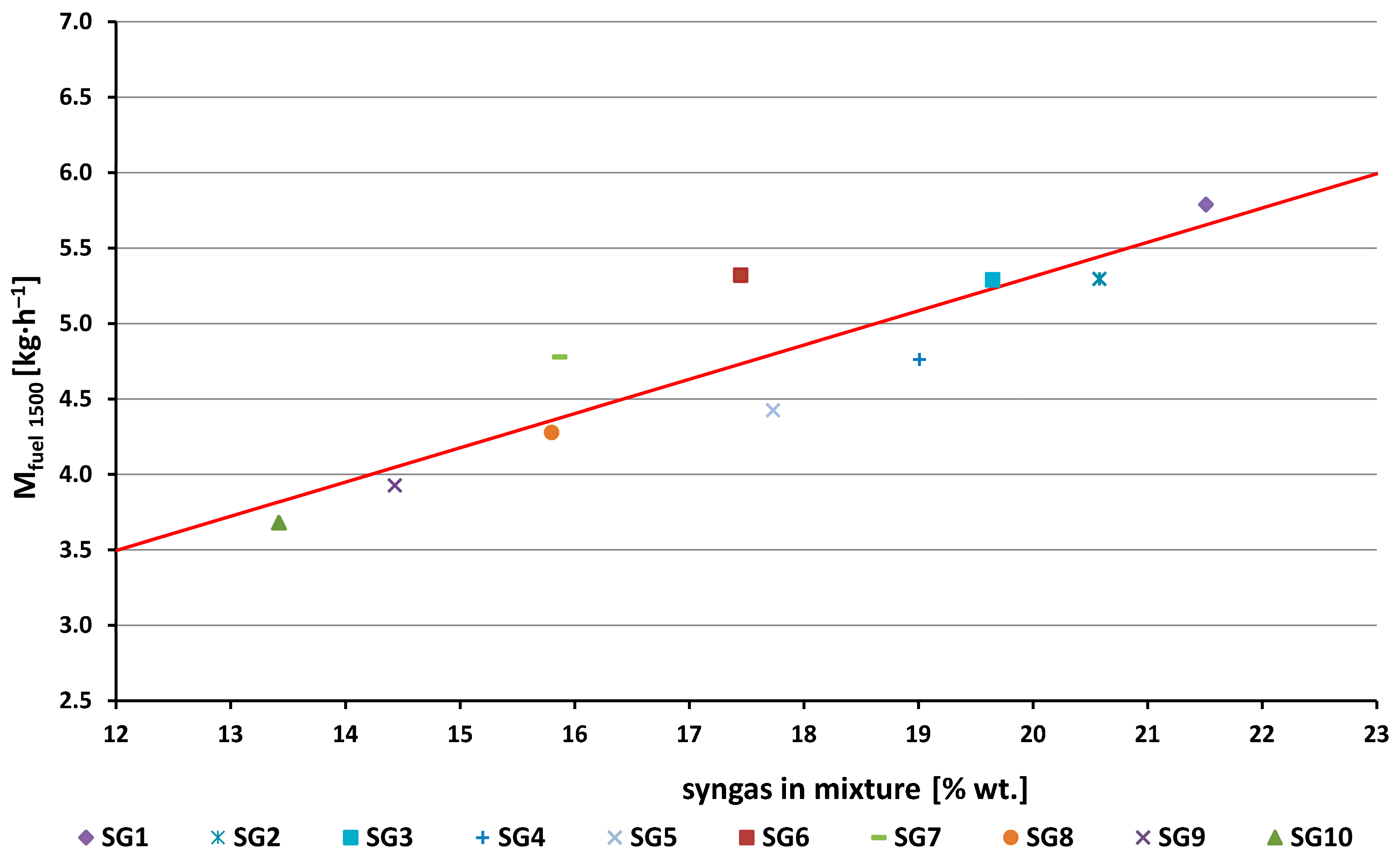
- The synthesis gas consumption (Figure 3, Figure 4 and Figure 14) was 2.4 to 3.7 times higher than the methane consumption (1.56 kg/h). This is due to the fact that when operating on syngas, a smaller amount of air is consumed to create a stoichiometric mixture (from 3.65 kg/kg for the SG1 to 6.45 kg/kg for the SG10, see the A/F ratio in Table 1) compared to methane (17.12 kg/kg). The hourly fuel consumption increases linearly with the increasing mass representation of a synthesis gas in the stoichiometric mixture (Figure 14).
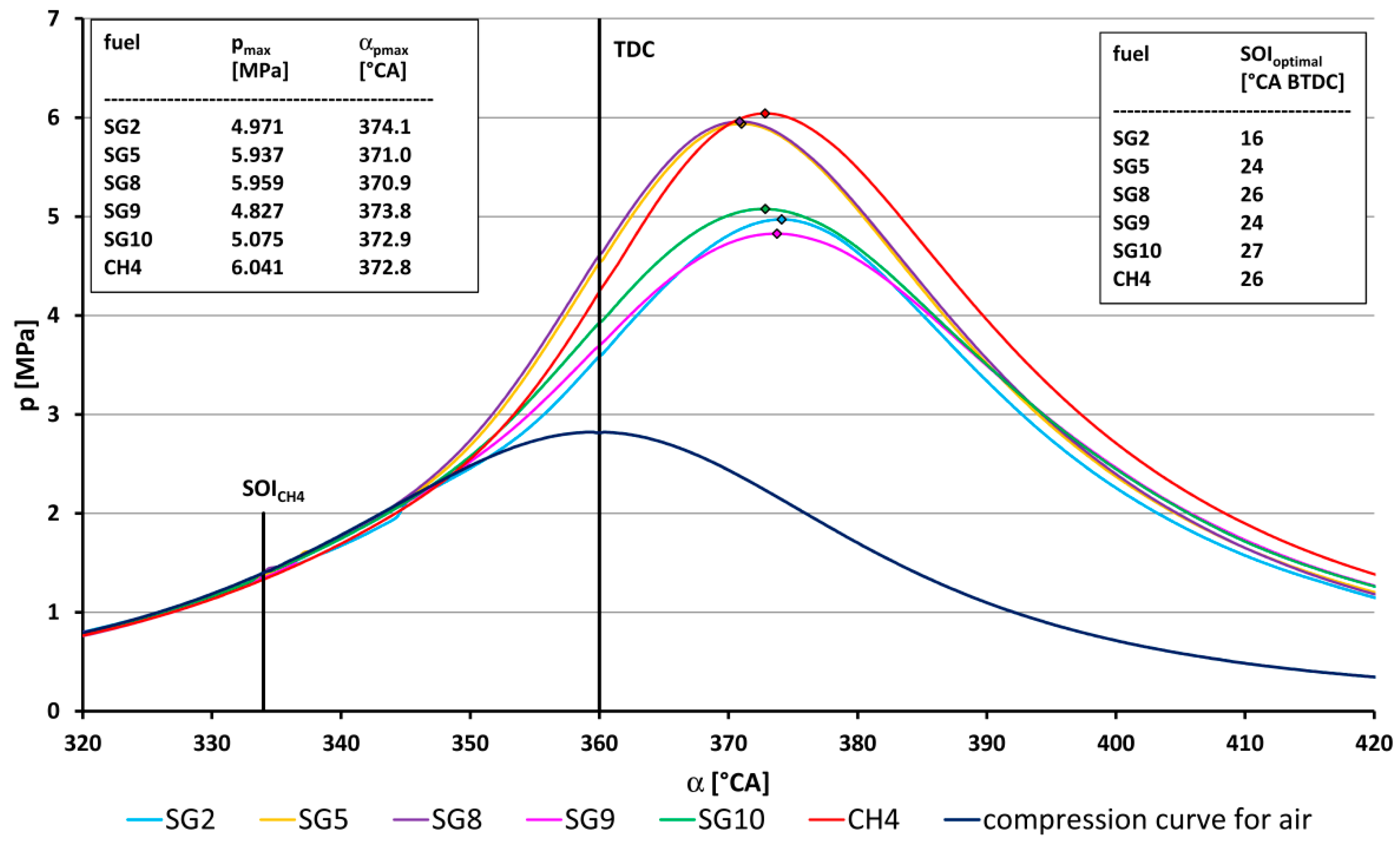
- The highest value of the maximum combustion pressure (5.96 MPa) out of all measured synthesis gases was achieved for SG8 (Figure 7) and, conversely, the lowest combustion pressure (4.83 MPa) was achieved by the SG9 gas. The coefficient of variation of the maximum pressure was the lowest (5.4%) for the synthesis gas SG2, and, conversely, the highest value (8.2%) was when burning the SG9 gas.
- The lowest pressure rise rate value (0.151 MPa/1 °CA) was achieved for the SG9, which contained 50% vol. of methane. Gases with a high hydrogen content (40% vol. in the SG5 and 30% vol. in the SG8) achieved the highest values of the pressure rise rate (0.217 MPa/1 °CA) of the engine.
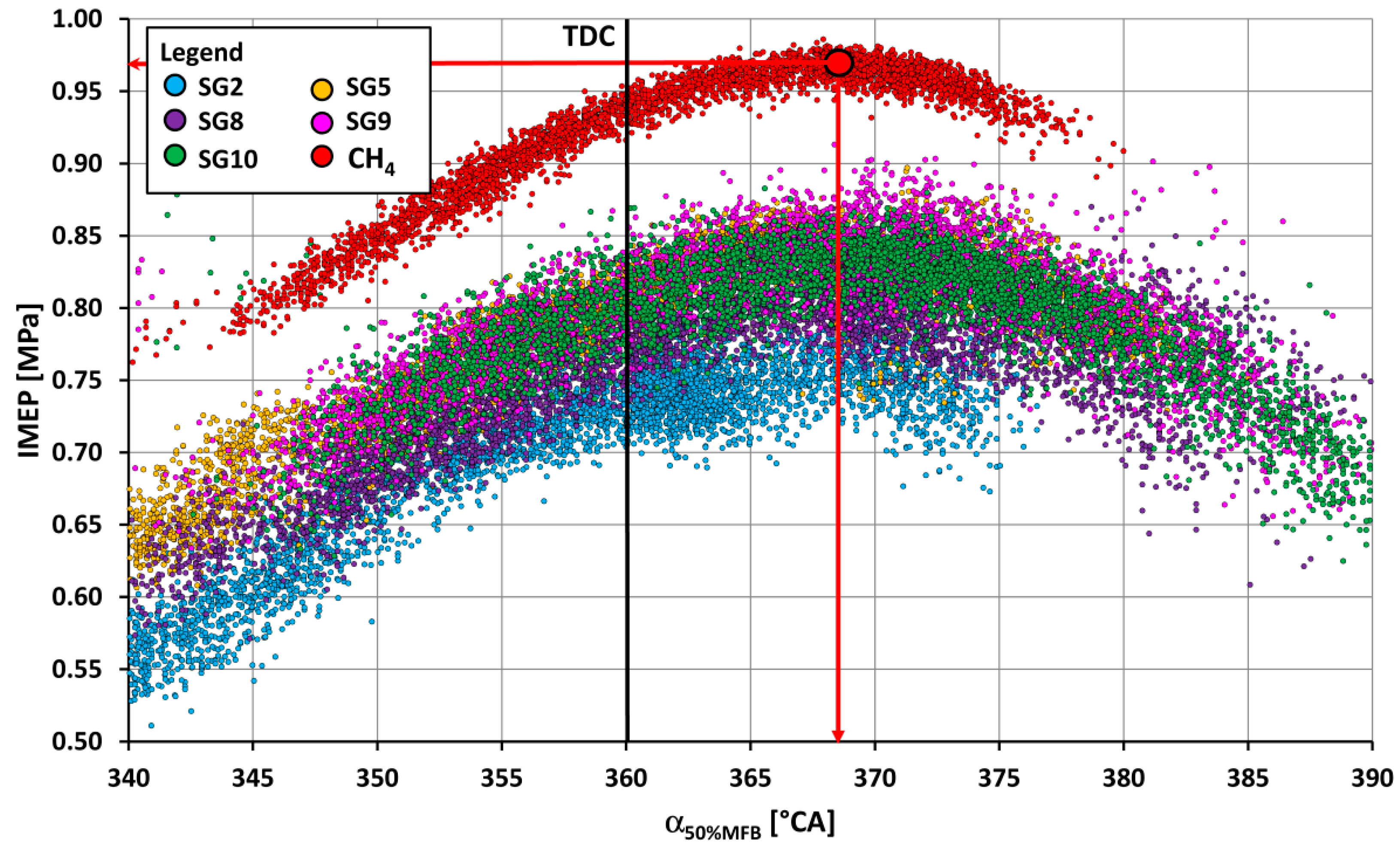
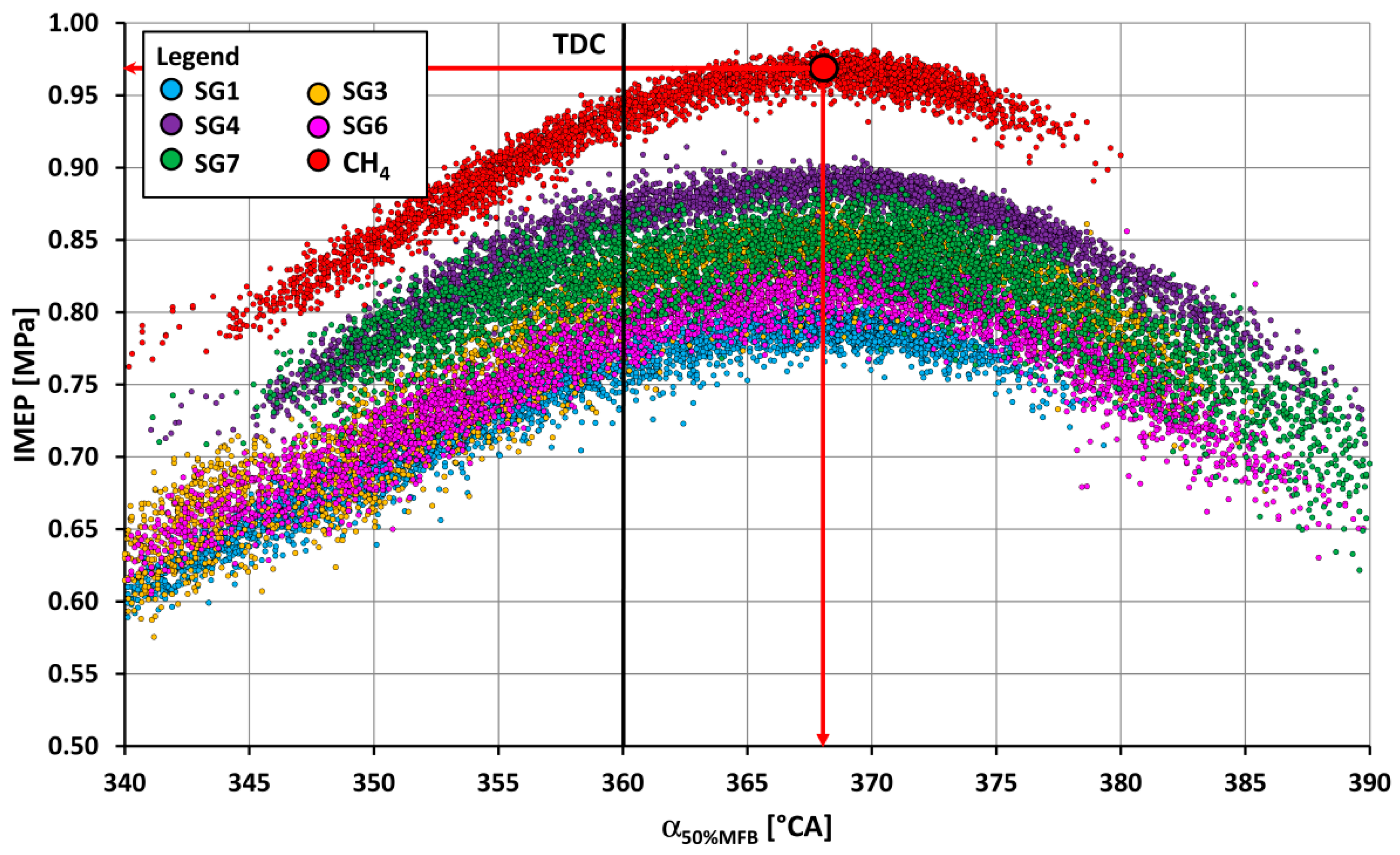
- The SG2 gas, which, in its composition, contains up to 50% vol. of hydrogen (Table 1), burned the fastest out of all combustible components of SGs, and had the least optimum start of ignition angle, namely, 16 °CA BTDC. On the other hand, the gas SG10, which had the highest methane content, had the largest start of ignition angle, namely, 27 °CA BTDC. In other words, with the same number of inert gases in the fuel, a larger proportion of hydrogen in the fuel mixture, with its faster burning, affects the reduction in the optimum start of ignition SOI angle before TDC.
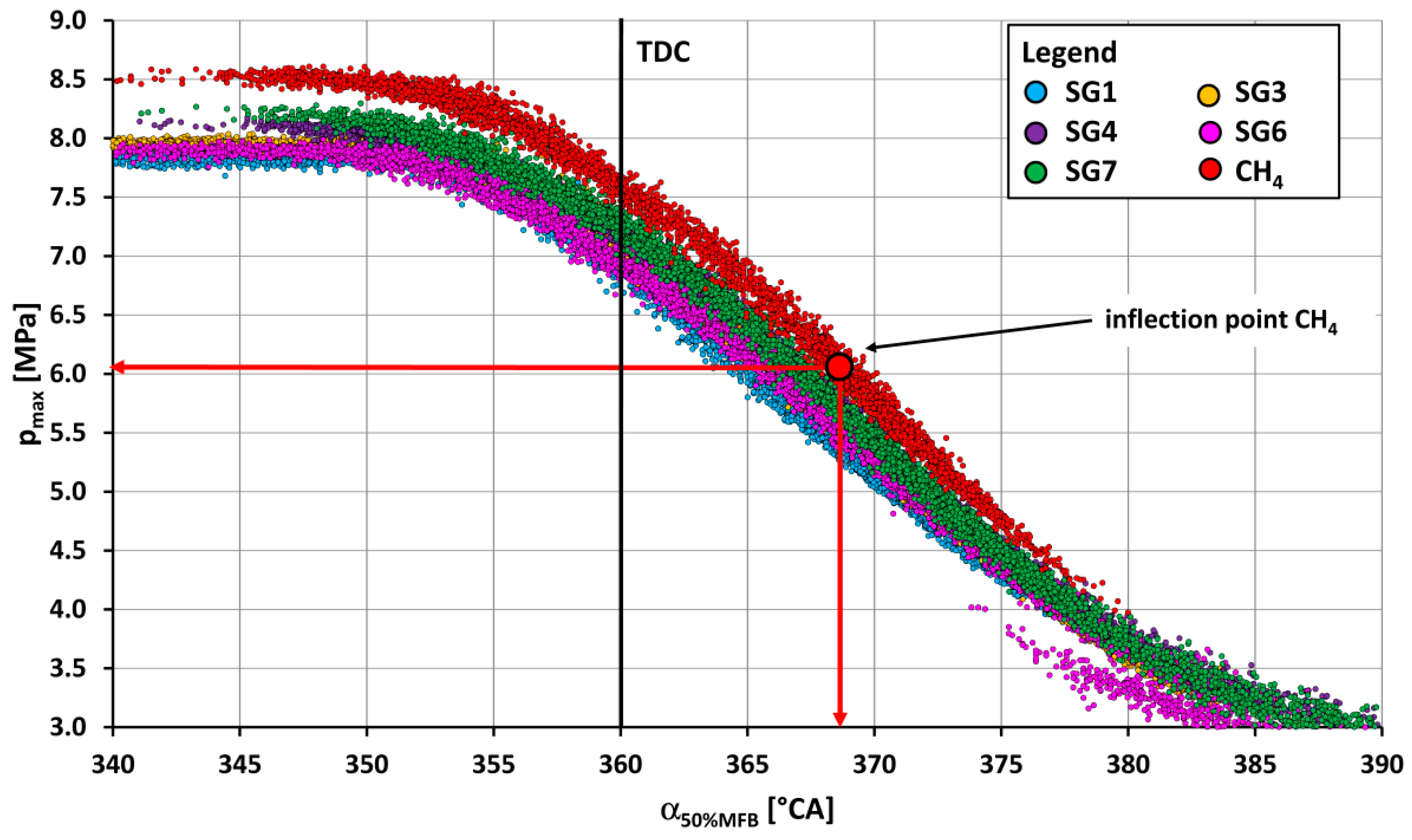
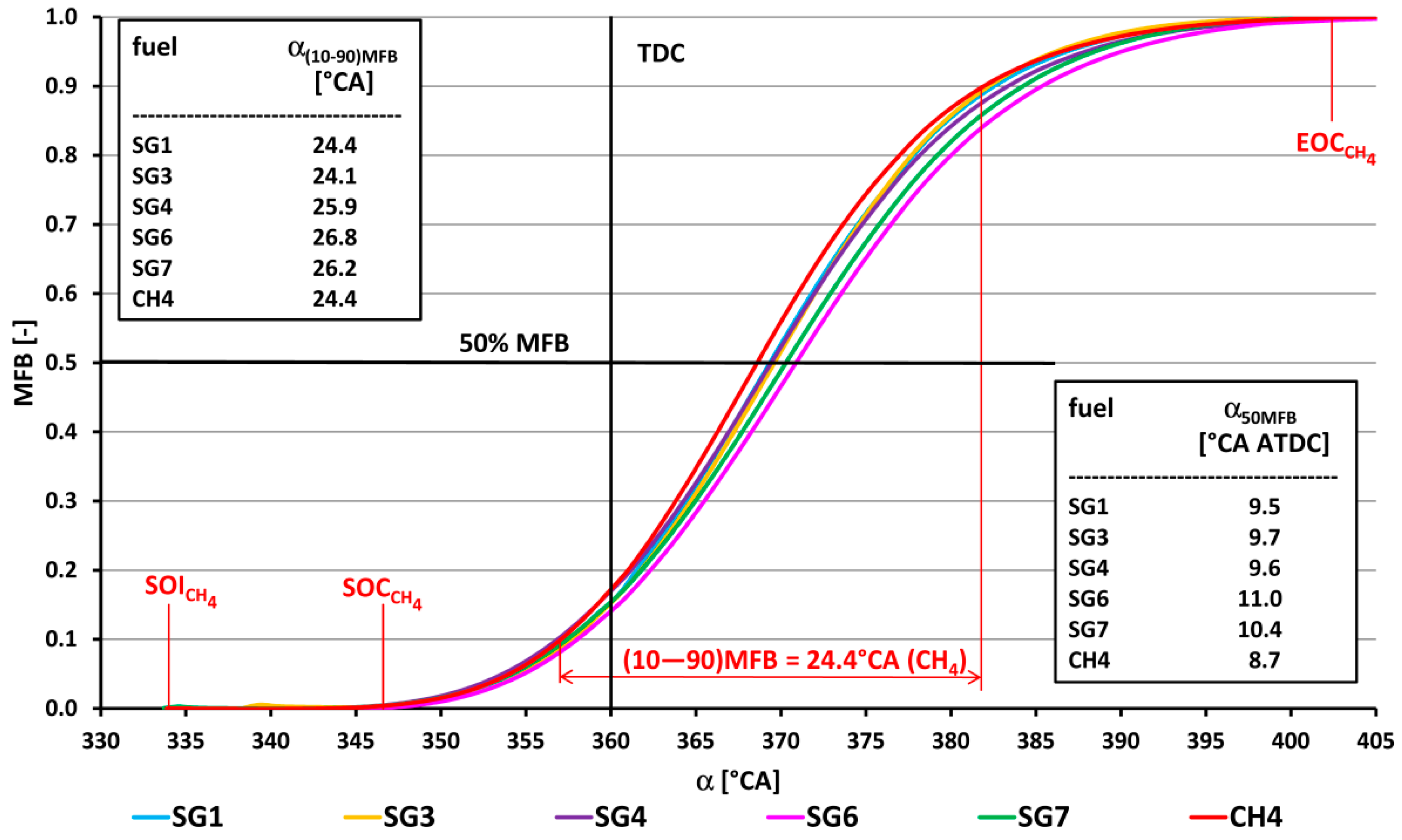
- The course of fuel combustion (Figure 11 and Figure 12) presents the longest main phase of combustion (10–90% MFB) for the synthesis gas SG9 with a value of 27.5 °CA. The SG2 gas with the highest proportion of hydrogen (50% by volume) has the shortest main burning phase (22.6 °CA). The angle with half of the fuel burned varied from 6.9 °CA ATDC for the SG5 or the SG8 to 12.3 °CA ATDC for the SG9. The phase of the start of ignition (SOI—5% MFB) was the shortest (11.6 °CA) for the synthesis gas SG2 with high hydrogen content and, conversely, the longest (21.6 °CA) for the synthesis gas SG10 with the highest methane content.
- The analysis of the effect of hydrogen in synthesis gases on the combustion process in internal combustion engines has shown that the hydrogen content in SGs is one of the main causes of their different behaviour during their combustion in the engine. Increasing the hydrogen content in synthesis gases brings about an increase in the values of the pressure increase rate during the combustion, a decrease in the COV values during the gradual burning-out of fuel, and a shortening of the total combustion period. In other words, synthesis gases with a higher hydrogen content lead to a slight increase in pressure rise rate, but also to a more stable combustion process. The role of hydrogen in synthesis gases, as long as there is no abnormal combustion, is positive. Combustion with a higher H2 content resembles isochoric combustion, which contributes to higher thermal and effective engine efficiency and also to lower fuel consumption. At the same time, it is necessary to consider an important property of hydrogen in a combusted gas mixture [49], namely that a higher proportion of hydrogen in the mixture reduces the emissions of harmful hydrocarbons. A higher hydrogen content in SGs reduces environmentally neutral CO2. On the contrary, when burning SGs, the present hydrogen increases the content of nitrogen oxides and water vapour in the exhaust gases of combustion engines.
- The results for high-energy synthesis gases provide an idea of the integral parameters of the engine (torque and hourly consumption, Figure 3 and Figure 4), as well as its internal parameters, which are related to the combustion processes of these gases (Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12), to which we paid the most attention in this article. The authors have grouped and summarized the results of the measurements into the following groups. They are similar to the conclusions reached for low- and medium-energy synthesis gases [1,2].
- (a)
- For the use of high-energy synthesis gases in cogeneration units, the most important criterion for obtaining energy from them must be the criterion of low consumption and, at the same time, high efficiency of the use. The results show that for the measured gases at 1500 rpm, the two required conditions are simultaneously fulfilled for gases SG8 (effective efficiency 32%), SG7 (32%) and SG4 (31%) (Figure 14). At the same time, the SG4 gas has achieved the highest performance parameters and also has one of the highest volumetric lower heating values of mixture LHVmixture of all gases (Table 1). The examined gases contain a high proportion of methane (from 30 to 40%) and hydrogen (10% and 30%). Therefore, the general conclusion that we can recommend for the production of high-energy gases is to manage the gasification technology in such a manner that the resulting gases contain as much methane and hydrogen as possible.
- (b)
- In order to avoid abnormal combustion in the form of engine knocking or back-firing of the mixture in the intake manifold, it is necessary to take into account the fact that in the case of gases with a low methane content (e.g., SG2, Table 1), if the hydrogen content in the fuel exceeds 25% vol., then the volume of inert gases must not fall below 25% of the volume [1,2]. During the experiments with SGs, this restriction was observed and we did not measure any signs of abnormal combustion during the experiments.
- (c)
- (d)
- When changing the composition of synthesis gases, the same as with the low and medium-energy synthesis gases [1,2], it is necessary to optimize the engine for the compression ratio, the start of ignition angle (SOI) for individual gases, the ignition system (energy value sparks, thermal value of the spark plug), the geometry of the pipeline system in terms of achieving the maximum filling of the cylinders (valve timing, use of the wave effect), the shape of the combustion chamber or the shape of the channels and valves (flow coefficients), the optimum turbulence of the filling, the use of supercharging, etc. The results of SGs analyses carried out by the authors of this article are directly applicable in practice. The analyses provide several suggestions on how to set up waste gasification technologies to achieve optimal performance and the best economic (hourly fuel consumption, effective efficiency) parameter of the engine or cogeneration units.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polóni, M.; Chríbik, A. Low-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine. SAE Int. J. Engines 2020, 13, 633–648. [Google Scholar] [CrossRef]
- Chríbik, A.; Polóni, M.; Magdolen, Ľ.; Minárik, M. Medium-Energy Synthesis Gases from Waste as an Energy Source for an Internal Combustion Engine. Appl. Sci. 2022, 12, 98. [Google Scholar] [CrossRef]
- Minister of the Environment and Climate Change. Environment and Climate Change Canada Health Canada 2020; Minister of the Environment and Climate Change: New Delhi, India, 2020; ISBN 978-0-660-35897-0.
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- World Economic Forum. The New Plastics Economy. 2016. Available online: https://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 25 April 2023).
- Center for International Environmental Law. Plastic & Health. 2019. Available online: https://www.ciel.org/wp-content/uploads/2019/02/Plastic-and-Health-The-Hidden-Costs-of-a-Plastic-Planet-February-2019.pdf (accessed on 25 April 2023).
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2022, 7, 104–116. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste–A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Utekar, S.; K, S.V.; More, N.; Rao, A. Comprehensive study of recycling of thermosetting polymer composites—Driving force, challenges and methods. Compos. Part B Eng. 2021, 207, 108596. [Google Scholar] [CrossRef]
- What Plastics Can and Cannot Be Recycled? Available online: https://www.slrecyclingltd.co.uk/what-plastics-can-and-cannot-be-recycled/ (accessed on 11 February 2022).
- Pickering, S. Recycling technologies for thermoset composite materials—Current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, D.; Tsui, T.-H.; Loh, K.-C.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Plastic-containing food waste conversion to biomethane, syngas, and biochar via anaerobic digestion and gasification: Focusing on reactor performance, microbial community analysis, and energy balance assessment. J. Environ. Manag. 2022, 306, 114471. [Google Scholar] [CrossRef]
- Lea, W. Plastic incineration versus recycling: A comparison of energy and landfill cost savings. J. Hazard. Mater. 1996, 47, 295–302. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Martín, M.M. Chapter 5—Syngas. In Industrial Chemical Process Analysis and Design; Elsevier: Amsterdam, The Netherland, 2016; ISBN 978-0-08-101093-8. [Google Scholar]
- Adrados, A.; de Marco, I.; Caballero, B.; López, A.; Laresgoiti, M.; Torres, A. Pyrolysis of plastic packaging waste: A comparison of plastic residuals from material recovery facilities with simulated plastic waste. Waste Manag. 2012, 32, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Urionabarrenechea, A.; de Marco, I.; Caballero, B.; Laresgoiti, M.; Adrados, A. Catalytic stepwise pyrolysis of packaging plastic waste. J. Anal. Appl. Pyrolysis 2012, 96, 54–62. [Google Scholar] [CrossRef]
- Namioka, T.; Saito, A.; Inoue, Y.; Park, Y.; Min, T.-J.; Roh, S.-A.; Yoshikawa, K. Hydrogen-rich gas production from waste plastics by pyrolysis and low-temperature steam reforming over a ruthenium catalyst. Appl. Energy 2011, 88, 2019–2026. [Google Scholar] [CrossRef]
- Saad, J.M.; Williams, P.T. Pyrolysis-catalytic dry (CO2) reforming of waste plastics for syngas production: Influence of process parameters. Fuel 2017, 193, 7–14. [Google Scholar] [CrossRef]
- van de Loosdrecht, J.; Niemantsverdriet, J.W. Synthesis Gas to Hydrogen, Methanol, and Synthetic Fuels. In Chemical Energy Storage; Robert Schlogl, X., Ed.; Walter de Gruyter GmbH: Berlin, Germany, 2012; pp. 443–458. ISBN 978-3-11-026407-4. [Google Scholar] [CrossRef]
- Kim, J.-W.; Mun, T.-Y.; Kim, J.-S. Air gasification of mixed plastic wastes using a two-stage gasifier for the production of producer gas with low tar and a high caloric value. Fuel 2011, 90, 2266–2272. [Google Scholar] [CrossRef]
- Ludlow-Palafox, C.; Chase, H.A. Microwave-Induced Pyrolysis of Plastic Wastes. Ind. Eng. Chem. Res. 2001, 40, 4749–4756. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Vargas-Salgado, C.; Águila-León, J.; Alfonso-Solar, D.; Malmquist, A. Simulations and experimental study to compare the behavior of a genset running on gasoline or syngas for small scale power generation. Energy 2021, 244, 122633. [Google Scholar] [CrossRef]
- Wender, I. Synthesis Gas as a Source of Fuels and Chemicals: C-1 Chemistry. Annu. Rev. Energy 1986, 11, 295–314. [Google Scholar] [CrossRef]
- El-Nagar, R.A.; Ghanem, A.A. Syngas Production, Properties, and Its Importance. In Sustainable Alternative Syngas Fuel; IntechOpen: London, UK, 2019; ISBN 978-1-78984-581-5. [Google Scholar]
- Tucki, K.; Orynycz, O.; Wasiak, A.; Świć, A.; Mruk, R.; Botwińska, K. Estimation of Carbon Dioxide Emissions from a Diesel Engine Powered by Lignocellulose Derived Fuel for Better Management of Fuel Production. Energies 2020, 13, 561. [Google Scholar] [CrossRef]
- Puškár, M.; Živčák, J.; Kočišová, M.; Šoltésová, M.; Kopas, M. Impact of bio-renewable energy sources on reduction of emission footprint from vehicles. Biofuels Bioprod. Biorefining 2021, 15, 1385–1394. [Google Scholar] [CrossRef]
- Statistical Review of World Energy. Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 11 February 2022).
- U.S. Energy Information Administration. International Energy Outlook 2021; U.S. Department of Energy: Washington, DC, USA, 2021.
- Nakyai, T.; Saebea, D. Exergoeconomic comparison of syngas production from biomass, coal, and natural gas for dimethyl ether synthesis in single-step and two-step processes. J. Clean. Prod. 2019, 241, 118334. [Google Scholar] [CrossRef]
- Tucki, K.; Krzywonos, M.; Orynycz, O.; Kupczyk, A.; Bączyk, A.; Wielewska, I. Analysis of the Possibility of Fulfilling the Paris Agreement by the Visegrad Group Countries. Sustainability 2021, 13, 8826. [Google Scholar] [CrossRef]
- Puškár, M.; Kopas, M.; Sabadka, D.; Kliment, M.; Šoltésová, M. Reduction of the Gaseous Emissions in the Marine Diesel Engine Using Biodiesel Mixtures. J. Mar. Sci. Eng. 2020, 8, 330. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaima, S.A. Study of Syngas Combustion Parameters Effect on Internal Combustion Engine. Asian J. Sci. Res. 2013, 6, 187–196. [Google Scholar] [CrossRef]
- Wang, W.; Bai, B.; Wei, W.; Cao, C.; Jin, H. Hydrogen-rich syngas production by gasification of Urea-formaldehyde plastics in supercritical water. Int. J. Hydrogen Energy 2021, 46, 35121–35129. [Google Scholar] [CrossRef]
- WuWu, S.-L.; Kuo, J.-H.; Wey, M.-Y. Highly abrasion and coking-resistance core-shell catalyst for hydrogen-rich syngas production from waste plastics in a two-staged fluidized bed reactor. Appl. Catal. A Gen. 2021, 612, 117989. [Google Scholar] [CrossRef]
- Al-Asadi, M.; Miskolczi, N.; Eller, Z. Pyrolysis-gasification of wastes plastics for syngas production using metal modified zeolite catalysts under different ratio of nitrogen/oxygen. J. Clean. Prod. 2020, 271, 122186. [Google Scholar] [CrossRef]
- Fiore, M.; Magi, V.; Viggiano, A. Internal combustion engines powered by syngas: A review. Appl. Energy 2020, 276, 115415. [Google Scholar] [CrossRef]
- Jamsran, N.; Park, H.; Lee, J.; Oh, S.; Kim, C.; Lee, Y.; Kang, K. Influence of syngas composition on combustion and emissions in a homogeneous charge compression ignition engine. Fuel 2021, 306, 121774. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A. Syngas (H2/CO) in a spark-ignition direct-injection engine. Part 1: Combustion, performance and emissions comparison with CNG. Int. J. Hydrogen Energy 2014, 39, 17884–17895. [Google Scholar] [CrossRef]
- Papagiannakis, R.; Rakopoulos, C.; Hountalas, D.; Giakoumis, E. Study of the performance and exhaust emissions of a spark-ignited engine operating on syngas fuel. Int. J. Altern. Propuls. 2007, 1, 190. [Google Scholar] [CrossRef]
- Merker, G.P.; Schwarz, C.; Teichmann, R. Combustion Engines Development: Mixture Formation, Combustion, Emissions and Simulation; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-02951-6. [Google Scholar] [CrossRef]
- Tazerout, M.; Le Corre, O.; Ramesh, A. A New Method to Determine the Start and End of Combustion in an Internal Combustion Engine Using Entropy Changes. Int. J. Thermodyn. 2000, 3, 49–55. [Google Scholar] [CrossRef]
- Chríbik, A.; Polóni, M.; Lach, J.; Ragan, B. The effect of adding hydrogen on the performance and the cyclic variability of a spark ignition engine powered by natural gas. Acta Polytech. 2014, 54, 10–14. [Google Scholar] [CrossRef]
- Chríbik, A.; Polóni, M.; Minárik, M.; Mitrovič, R.; Miškovič, Z. The Effect of Inert Gas in the Mixture with Natural Gas on the Parameters of the Combustion Engine. In Computational and Experimental Approaches in Materials Science and Engineering; Springer: Cham, Switzerland, 2020; pp. 410–426. ISBN 978-3-030-30852-0. [Google Scholar] [CrossRef]
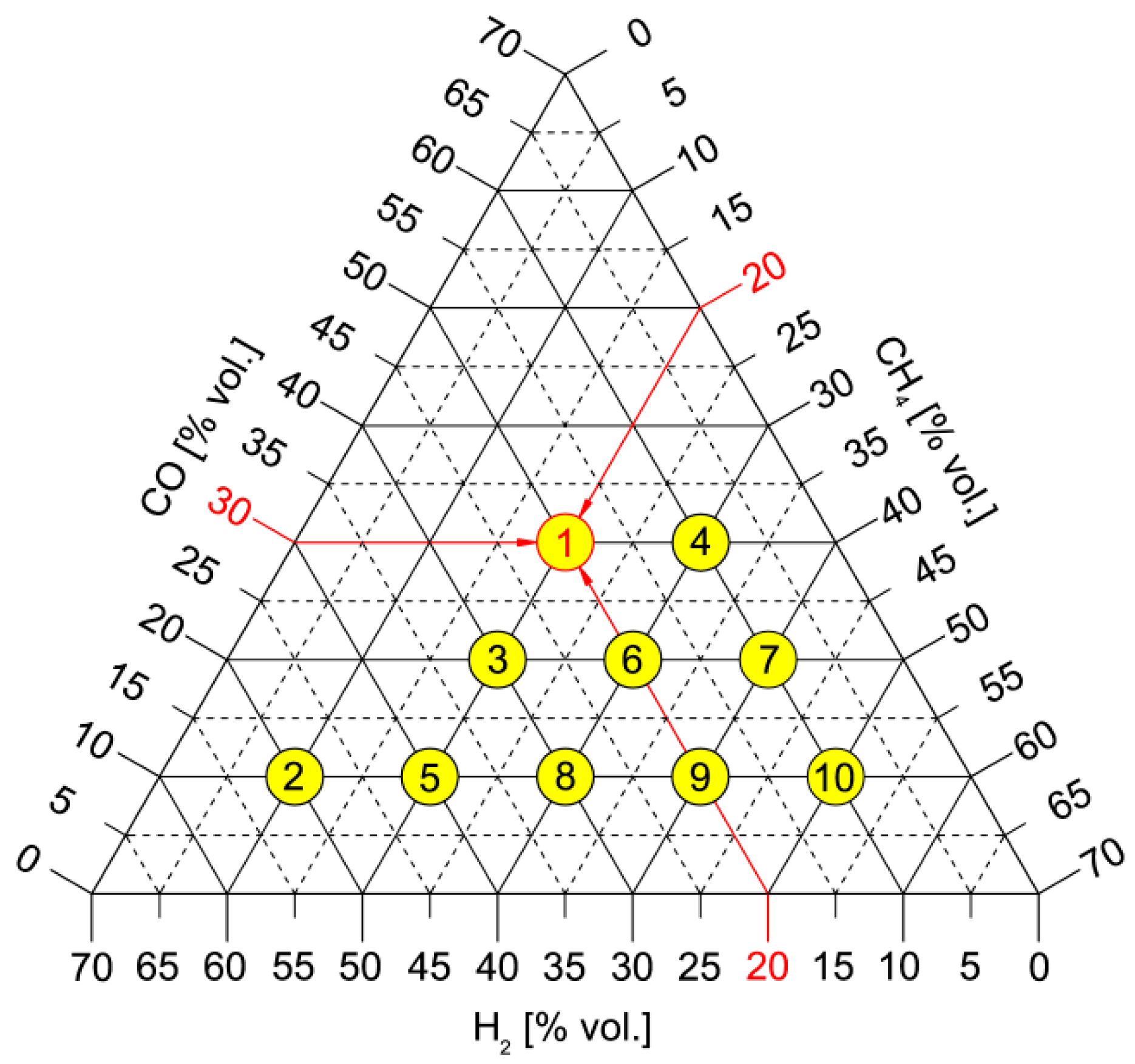





| Name | Unit | CH4 | SG1 | SG2 | SG3 | SG4 | SG5 |
| CH4 | [% vol.] | 100 | 20 | 10 | 20 | 30 | 20 |
| H2 | [% vol.] | 0 | 20 | 50 | 30 | 10 | 40 |
| CO | [% vol.] | 0 | 30 | 10 | 20 | 30 | 10 |
| CO2 | [% vol.] | 0 | 25 | 25 | 25 | 25 | 25 |
| N2 | [% vol.] | 0 | 5 | 5 | 5 | 5 | 5 |
| LHV | [MJ·kg−1] | 50.012 | 12.027 | 12.879 | 13.274 | 13.545 | 14.857 |
| LHV | [MJ·m−3] | 33.358 | 12.209 | 9.540 | 12.041 | 14.540 | 11.871 |
| A/F ratio | [kg·kg−1] | 17.12 | 3.65 | 3.86 | 4.09 | 4.26 | 4.64 |
| M | [kg·kmol−1] | 16.04 | 24.42 | 17.82 | 21.82 | 25.82 | 19.22 |
| ρNTP fuel | [kg·m−3] | 0.667 | 1.015 | 0.741 | 0.907 | 1.073 | 0.799 |
| ρNTP mixture | [kg·m−3] | 1.153 | 1.158 | 1.067 | 1.205 | 1.177 | 1.105 |
| Fuel in mix. | [% vol.] | 9.51 | 24.46 | 29.56 | 24.44 | 20.79 | 24.45 |
| LHVmixture | [MJ·m−3] | 3.172 | 2.986 | 2.820 | 2.943 | 3.023 | 2.902 |
| Name | Unit | CH4 | SG6 | SG7 | SG8 | SG9 | SG10 |
| CH4 | [% vol.] | 100 | 30 | 40 | 30 | 40 | 50 |
| H2 | [% vol.] | 0 | 20 | 10 | 30 | 20 | 10 |
| CO | [% vol.] | 0 | 20 | 20 | 10 | 10 | 10 |
| CO2 | [% vol.] | 0 | 25 | 25 | 25 | 25 | 25 |
| N2 | [% vol.] | 0 | 5 | 5 | 5 | 5 | 5 |
| LHV | [MJ·kg−1] | 50.012 | 14.892 | 16.315 | 16.560 | 18.054 | 19.364 |
| LHV | [MJ·m−3] | 33.358 | 14.376 | 16.702 | 14.197 | 16.530 | 18.859 |
| A/F ratio | [kg·kg−1] | 17.12 | 4.73 | 5.30 | 5.33 | 5.93 | 6.45 |
| M | [kg·kmol−1] | 16.04 | 23.22 | 24.62 | 20.62 | 22.02 | 23.43 |
| ρNTP fuel | [kg·m−3] | 0.667 | 0.965 | 1.024 | 0.857 | 0.916 | 0.974 |
| ρNTP mixture | [kg·m−3] | 1.153 | 1.155 | 1.172 | 1.132 | 1.152 | 1.168 |
| Fuel in mix. | [% vol.] | 9.51 | 20.81 | 18.11 | 20.80 | 18.10 | 16.04 |
| LHVmixture | [MJ·m−3] | 3.172 | 2.991 | 3.025 | 2.953 | 2.992 | 3.025 |
 | Principle of the Work | Spark Ignition |
| Number of cylinders and arrangement | 2 in a row | |
| Crankshaft angle (°) | 360 | |
| Bore/Stroke (mm) | 75/77.6 | |
| Sweep volume (cm3) | 686 | |
| Compression ratio (-) | 12.5:1 | |
| Valve timing, drive | OHC, timing belt | |
| Preparation of mixture | External, in a mixer with electronic control of mixture richness to stoichiometric mixture | |
| Cooling | By liquid, with forced circulation, two-circuit thermostatically controlled, radiator blown by a fan, driven by an electric motor | |
| Regulation | Electronically controlled throttle Brisk10DS- the highest thermal value | |
| Ignition system | Ignition coil Bosch, energy 65 mJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chríbik, A.; Polóni, M.; Majkút, A.; Écsi, L.; Gulan, L. High-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine. Sustainability 2023, 15, 7806. https://doi.org/10.3390/su15107806
Chríbik A, Polóni M, Majkút A, Écsi L, Gulan L. High-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine. Sustainability. 2023; 15(10):7806. https://doi.org/10.3390/su15107806
Chicago/Turabian StyleChríbik, Andrej, Marián Polóni, Andrej Majkút, Ladislav Écsi, and Ladislav Gulan. 2023. "High-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine" Sustainability 15, no. 10: 7806. https://doi.org/10.3390/su15107806
APA StyleChríbik, A., Polóni, M., Majkút, A., Écsi, L., & Gulan, L. (2023). High-Energy Synthesis Gases from Waste as Energy Source for Internal Combustion Engine. Sustainability, 15(10), 7806. https://doi.org/10.3390/su15107806







