Investigating the Potential of Biobinder for Bottom Ash Solidification/Stabilization: Leaching Behaviour and pH Dependence
Abstract
:1. Introduction
2. Materials and Experiments
2.1. Solid Waste
2.2. Biobinder Preparation
2.3. Preparation of Sample
2.4. Toxicity Characteristic Leaching Test
2.5. pH Dependence Test
2.6. Sequential Extraction
2.7. Characterization Methods
2.8. Microstructural Analysis
3. Results and Discussion
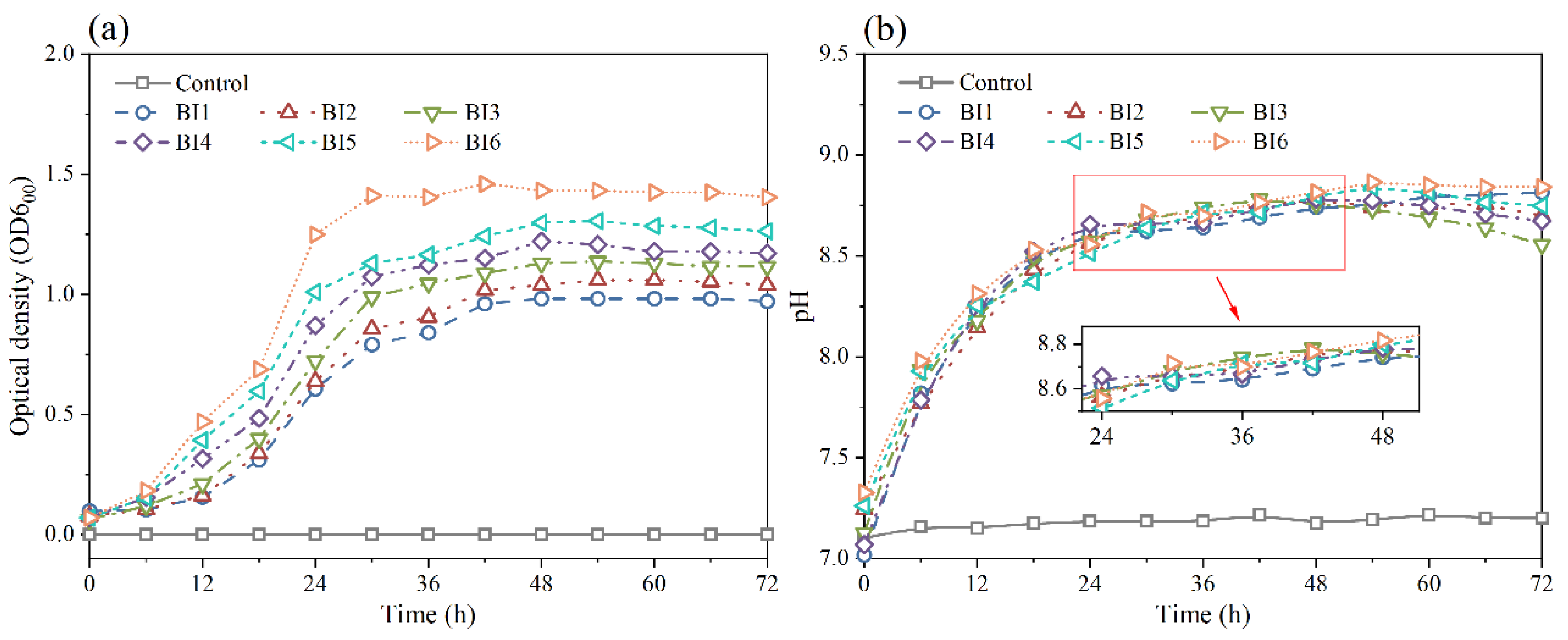
3.1. Characteristics of Bacterial Growth and Untreated IBA
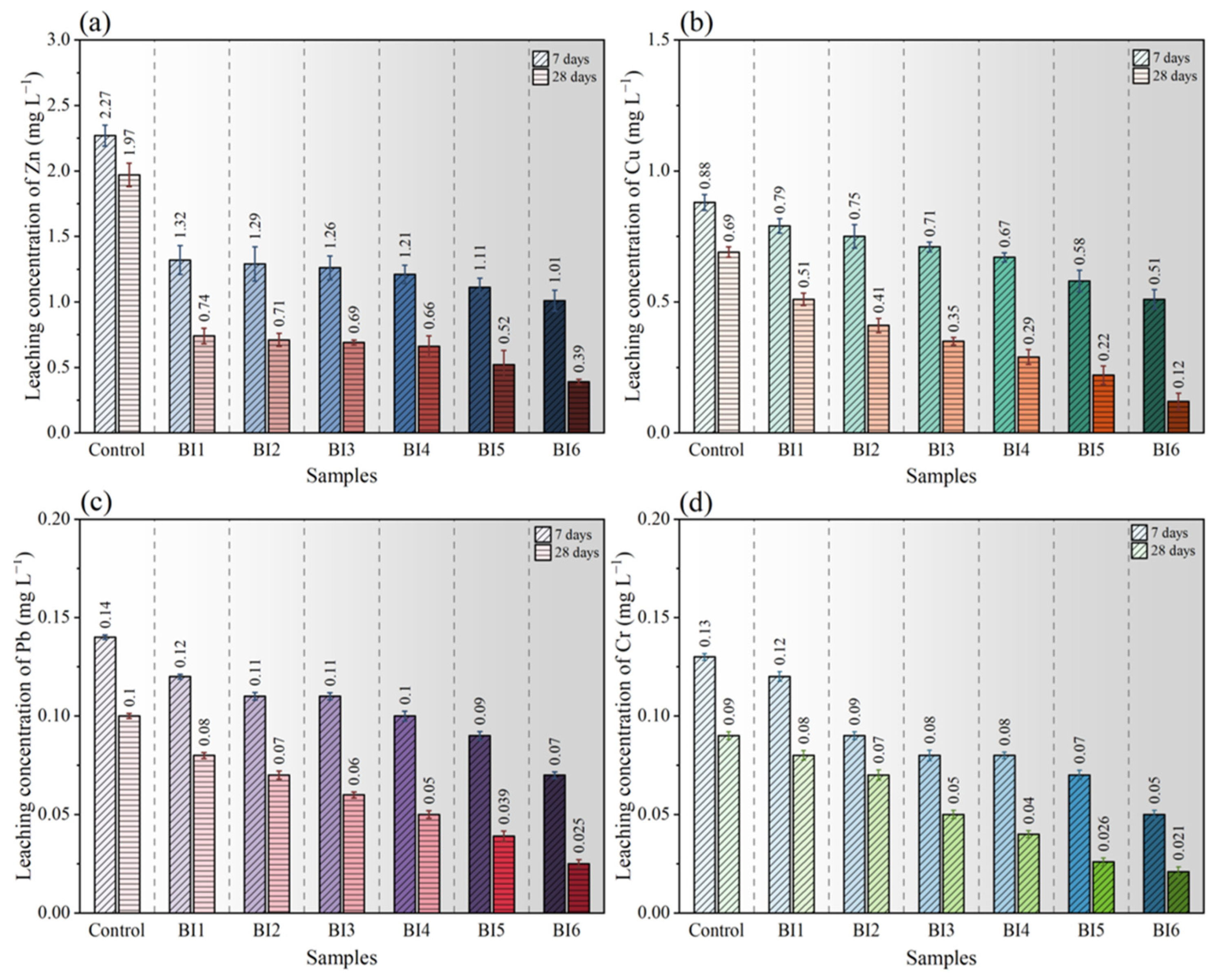
3.2. Toxicity Characteristic Leaching Procedure
3.3. pH Dependence Analysis
3.4. X-ray Diffraction and Thermogravimetric Analysis
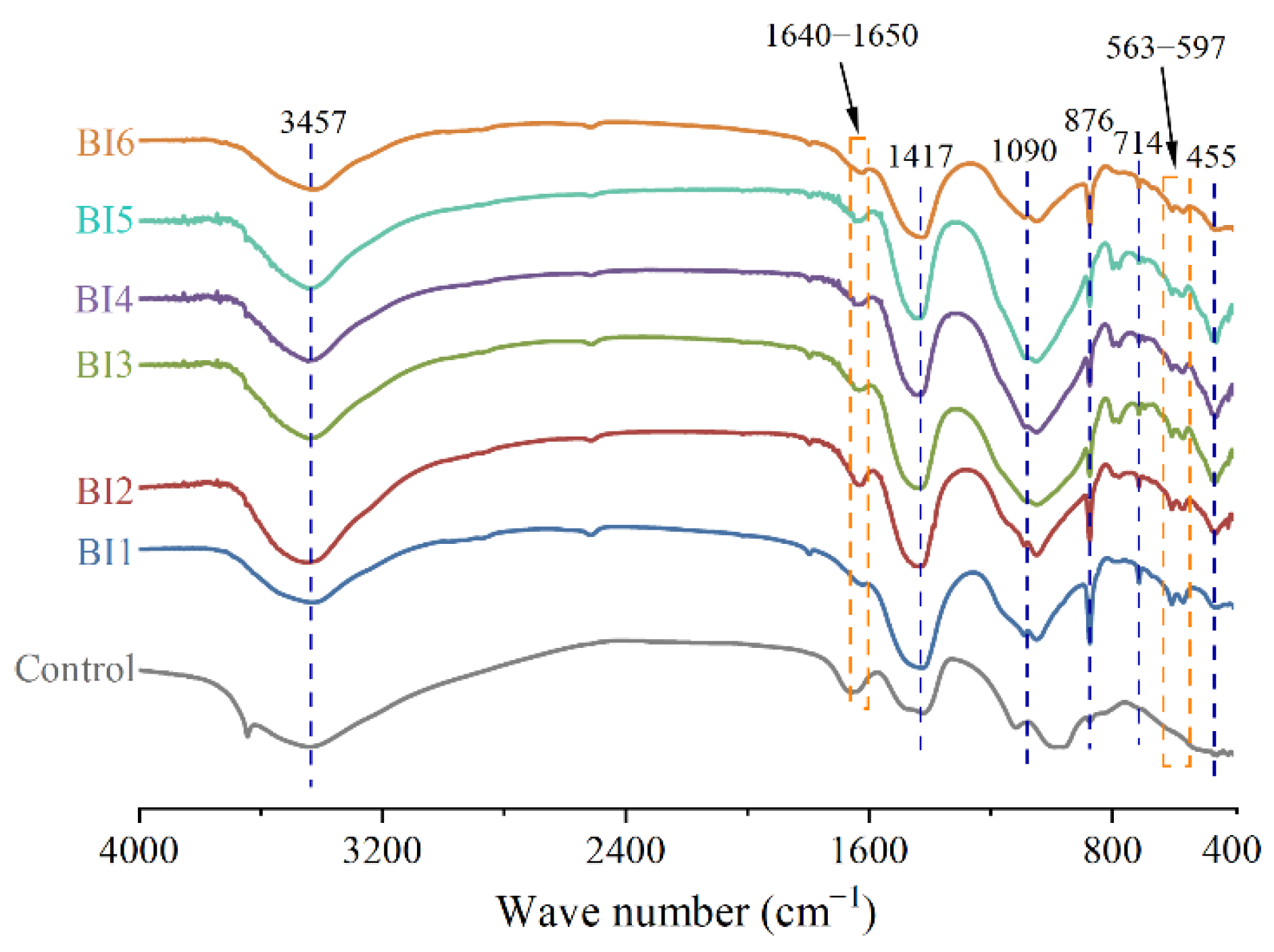
3.5. Fourier Transform Infrared
3.6. Unconfined Compressive Strength
3.7. Scanning Electron Microscope Analysis
3.8. Distribution of Pore and Chemical Speciation
4. Conclusions
- (i)
- Biotreatment can effectively reduce the leaching concentrations of Zn, Cu, Pb, and Cr in IBA. At 28 days, the leaching concentrations of samples from BI1 to BI6 are all below the national standard.
- (ii)
- The higher the bacterial concentration, the lower the leaching concentration of the heavy metals. Especially in the samples with high bacterial concentration, the average immobilization ratios among Zn, Cu, Pb, and Cr reached 65.3%–76.4%, respectively.
- (iii)
- A high concentration of bacteria can improve biomineralization and increase the production of the biogel and bioprecipitate, as well as forming a compact cementing structure.
- (iv)
- The bivalent heavy metal ions were precipitated as heavy metal carbonate by induction of the bacteria, which increased the concentration of carbonate-bound speciation.
- (v)
- This work confirms the feasibility of the biobinder for IBA solidification/stabilization. This lays a foundation for the management of MICP in IBA and breaks the traditional idea of using a cement-based binder.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, J.; Qiu, J.; Yuan, J.; Fu, H.; Yang, Y.; Gu, X. Study on denitrifying biogrout to immobilize heavy metals in bottom ash in an anaerobic environment and its immobilization mechanism. J. Environ. Chem. Eng. 2022, 10, 108084. [Google Scholar] [CrossRef]
- Rambaldi, E.; Esposito, L.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Vassura, I. The recycling of MSWI bottom ash in silicate based ceramic. Ceram. Int. 2010, 36, 2469–2476. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Polozhyi, K.; Reiterman, P.; Keppert, M. MSWI Bottom Ash as an Aggregate for a Lightweight Concrete. Adv. Mater. Res. 2014, 1054, 254–257. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernandez, A.I.; Nadal, R.; Espiell, F. Short-term natural weathering of MSWI bottom ash. J. Hazard. Mater. 2000, 79, 287–299. [Google Scholar] [CrossRef]
- Hashemi, S.S.G.; Bin Mahmud, H.; Ghuan, T.C.; Chin, A.B.; Kuenzel, C.; Ranjbar, N. Safe disposal of coal bottom ash by solidification and stabilization techniques. Constr. Build. Mater. 2019, 197, 705–715. [Google Scholar] [CrossRef]
- Anastasiadou, K.; Christopoulos, K.; Mousios, E.; Gidarakos, E. Solidification/stabilization of fly and bottom ash from medical waste incineration facility. J. Hazard. Mater. 2012, 207, 165–170. [Google Scholar] [CrossRef]
- Pecqueur, G.; Crignon, C.; Quenee, B. Behaviour of cement-treated MSWI bottom ash. Waste Manag. 2001, 21, 229–233. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, A. Factors affecting properties of MSWI bottom ash employing cement and fiber for geotechnical applications. Environ. Dev. Sustain. 2020, 22, 6891–6905. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Tian, S.C.; Liu, L.L.; Wang, X.D.; Zhang, Z.T. Recycling ground MSWI bottom ash in cement composites: Long-term environmental impacts. Waste Manag. 2018, 78, 841–848. [Google Scholar] [CrossRef]
- Caprai, V.; Gauvin, F.; Schollbach, K.; Brouwers, H.J.H. MSWI bottom ash as binder replacement in wood cement composites. Constr. Build. Mater. 2019, 196, 672–680. [Google Scholar] [CrossRef]
- Garrabrants, A.C.; Kosson, D.S.; Brown, K.G.; Fagnant, D.P.; Helms, G.; Thorneloe, S.A. Demonstration of the use of test results from the Leaching Environmental Assessment Framework (LEAF) to develop screening-level leaching assessments. Waste Manag. 2021, 121, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, D.; Caviglia, C.; Destefanis, E.; Agostino, A.; Boero, R.; Marinoni, N.; Bonadiman, C.; Pavese, A. Influence of speciation distribution and particle size on heavy metal leaching from MSWI fly ash. Waste Manag. 2022, 138, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Grazulyte, J.; Vaitkus, A.; Sernas, O.; Zalimiene, L. The impact of MSWI bottom ash as aggregate on concrete mechanical performance. Int. J. Pavement Eng. 2021, 23, 2903–2911. [Google Scholar] [CrossRef]
- Xiang, J.; Qiu, J.; Zheng, P.; Sun, X.; Zhao, Y.; Gu, X. Usage of biowashing to remove impurities and heavy metals in raw phosphogypsum and calcined phosphogypsum for cement paste preparation. Chem. Eng. J. 2023, 451, 138594. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Qi, Y.; Liu, Z. Characteristics and leaching behavior of MSWI fly ash in novel solidification/stabilization binders. Waste Manag. 2021, 131, 277–285. [Google Scholar] [CrossRef]
- Pei, R.; Liu, J.; Wang, S.; Yang, M. Use of bacterial cell walls to improve the mechanical performance of concrete. Cem. Concr. Compos. 2013, 39, 122–130. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Kua, H.W.; Gupta, S.; Aday, A.N.; Srubar, W.V. Biochar-immobilized bacteria and superabsorbent polymers enable self-healing of fiber-reinforced concrete after multiple damage cycles. Cem. Concr. Compos. 2019, 100, 35–52. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, H.; Xu, H.; Gao, Y.X.; Ding, X.Q.; Ma, M.L. Microbial induced solidification and stabilization of municipal solid waste incineration fly ash with high alkalinity and heavy metal toxicity. PLoS ONE 2019, 14, e0223900. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Chen, L.; Guo, B.; Tan, Y.; Sasaki, K.; Tsang, D.C.W. Designing novel magnesium oxysulfate cement for stabilization/solidification of municipal solid waste incineration fly ash. J. Hazard. Mater. 2022, 423, 127025. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.P.; Xiang, J.C.; Zhang, W.Q.; Zhao, Y.L.; Sun, X.G.; Gu, X.W. Effect of microbial-cemented on mechanical properties of iron tailings backfill and its mechanism analysis. Constr. Build. Mater. 2022, 318, 10. [Google Scholar] [CrossRef]
- Ma, Q.; Xiang, J.C.; Yang, Y.C.; Xiao, H.L.; Wan, J. Study on the mechanical properties of flax fiber-reinforced silty clay contaminated by zinc-ion solution. Environ. Technol. 2021, 42, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.Z. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Xiang, J.; Qiu, J.; Li, Z.; Chen, J.; Song, Y. Eco-friendly treatment for MSWI bottom ash applied to supplementary cementing: Mechanical properties and heavy metal leaching concentration evaluation. Constr. Build. Mater. 2022, 327, 127012. [Google Scholar] [CrossRef]
- Kahani, M.; Kalantary, F.; Soudi, M.R.; Pakdel, L.; Aghaalizadeh, S. Optimization of cost effective culture medium for Sporosarcina pasteurii as biocementing agent using response surface methodology: Up cycling dairy waste and seawater. J. Clean. Prod. 2020, 253, 120022. [Google Scholar] [CrossRef]
- Lai, Y.M.; Yu, J.; Liu, S.Y.; Liu, J.F.; Wang, R.K.; Dong, B.W. Experimental study to improve the mechanical properties of iron tailings sand by using MICP at low pH. Constr. Build. Mater. 2021, 273, 121729. [Google Scholar] [CrossRef]
- Liu, J.R.; Su, J.F.; Ali, A.; Wang, Z.; Zhang, R.J. Potential of a novel facultative anaerobic denitrifying Cupriavidus sp. W12 to remove fluoride and calcium through calcium bioprecipitation. J. Hazard. Mater. 2022, 423, 126976. [Google Scholar] [CrossRef]
- Skevi, L.; Reeksting, B.J.; Hoffmann, T.D.; Gebhard, S.; Paine, K. Incorporation of bacteria in concrete: The case against MICP as a means for strength improvement. Cem. Concr. Compos. 2021, 120, 104056. [Google Scholar] [CrossRef]
- Proudfoot, D.; Brooks, L.; Gammons, C.H.; Barth, E.; Bless, D.; Nagisetty, R.M.; Lauchnor, E.G. Investigating the potential for microbially induced carbonate precipitation to treat mine waste. J. Hazard. Mater. 2022, 424, 127490. [Google Scholar] [CrossRef]
- Yue, J.W.; Zhao, L.M.; Zhang, B.X.; Kong, Q.M.; Wang, S.Y.; Wang, H. Effect of Glutinous Rice Slurry on the Reinforcement of Silt in the Yellow River Basin by Microbially Induced Carbonate Precipitation (MICP): Mechanical Property and Microcosmic Structure. Adv. Mater. Sci. Eng. 2021, 2021, 5539854. [Google Scholar] [CrossRef]
- Chen, X.Y.; Achal, V. Biostimulation of carbonate precipitation process in soil for copper immobilization. J. Hazard. Mater. 2019, 368, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Poon, C.S.; Sun, H.; Lo, I.M.C.; Kirk, D.W. Heavy metal speciation and leaching behaviors in cement based solidified/stabilized waste materials. J. Hazard. Mater. 2001, 82, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.Y.A.; Yu, W.H.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.E.; Palomo, A.; Fernandez-Jimenez, A. Effect of alkalis on fresh C-S-H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernandez-Jimenez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Schuiling, R.D.; Ding, Z.; Hickey, L.; Wharton, D.; Frost, R.L. Vibrational spectroscopic study of syngenite formed during the treatment of liquid manure with sulphuric acid. Vib. Spectrosc. 2002, 28, 209–221. [Google Scholar] [CrossRef]
- Tang, S.W.; Wang, Y.; Geng, Z.C.; Xu, X.F.; Yu, W.Z.; Hubao, A.; Chen, J.T. Structure, Fractality, Mechanics and Durability of Calcium Silicate Hydrates. Fractal Fract. 2021, 5, 47. [Google Scholar] [CrossRef]
- Lo, Y.; Lee, H.M. Curing effects on carbonation of concrete using a phenolphthalein indicator and Fourier-transform infrared spectroscopy. Build. Environ. 2002, 37, 507–514. [Google Scholar] [CrossRef]
- Pham, V.P.; Nakano, A.; van der Star, W.R.L.; Heimovaara, T.J.; van Paassen, L.A. Applying MICP by denitrification in soils: A process analysis. Environ. Geotech. 2018, 5, 79–93. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Kesmez, M.; Cocke, D.L. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total Environ. 2004, 325, 255–262. [Google Scholar] [CrossRef]
- Xiang, J.; Qiu, J.; Wang, Y.; Gu, X. Calcium acetate as calcium source used to biocement for improving performance and reducing ammonia emission. J. Clean. Prod. 2022, 348, 131286. [Google Scholar] [CrossRef]
- Tirkolaei, H.K.; Bilsel, H. Statistical Modeling of Environmental Factors on Microbial Urea Hydrolysis Process for Biocement Production. Adv. Mater. Sci. Eng. 2015, 2015, 340930. [Google Scholar]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. Aci Mater. J. 2001, 98, 3–9. [Google Scholar]
- Deng, X.J.; Yuan, Z.X.; Li, Y.; Liu, H.; Feng, J.Y.; de Wit, B. Experimental study on the mechanical properties of microbial mixed backfill. Constr. Build. Mater. 2020, 265, 120643. [Google Scholar] [CrossRef]
- Chen, X.D.; Wu, S.X. Influence of water-to-cement ratio and curing period on pore structure of cement mortar. Constr. Build. Mater. 2013, 38, 804–812. [Google Scholar] [CrossRef]
- Renhe, Y.; Baoyuan, L.; Zhongwei, W. Study on the pore structure of hardened cement paste by SAXS. Cem. Concr. Res. 1990, 20, 385–393. [Google Scholar] [CrossRef]
- Li, M.G.; Sun, C.J.; Gau, S.H.; Chuang, C.J. Effects of wet ball milling on lead stabilization and particle size variation in municipal solid waste incinerator fly ash. J. Hazard. Mater. 2010, 174, 586–591. [Google Scholar] [CrossRef]
- Xue, Z.-F.; Cheng, W.-C.; Wang, L.; Hu, W. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. J. Hazard. Mater. 2022, 426, 128090. [Google Scholar] [CrossRef]
- Kang, C.-H.; Oh, S.J.; Shin, Y.; Han, S.-H.; Nam, I.-H.; So, J.-S. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol. Eng. 2015, 74, 402–407. [Google Scholar] [CrossRef]






| Heavy Metals | Cr | Ni | Cu | Zn | As | Cd | Pb | Sb |
|---|---|---|---|---|---|---|---|---|
| Total content (mg kg−1) | 203.21 | 68.71 | 1006.92 | 3592.63 | 93.18 | 0.24 | 261.15 | 3.83 |
| National standard (mg kg−1) | 100 | 100 | 200 | 1000 | 50 | 10 | 100 | 200 |
| TCLP standard (mg L−1) | 4.5 | 0.5 | 40 | 100 | 0.3 | 0.15 | 0.25 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wu, N.; Song, Y.; Xiang, J. Investigating the Potential of Biobinder for Bottom Ash Solidification/Stabilization: Leaching Behaviour and pH Dependence. Sustainability 2023, 15, 7859. https://doi.org/10.3390/su15107859
Li Z, Wu N, Song Y, Xiang J. Investigating the Potential of Biobinder for Bottom Ash Solidification/Stabilization: Leaching Behaviour and pH Dependence. Sustainability. 2023; 15(10):7859. https://doi.org/10.3390/su15107859
Chicago/Turabian StyleLi, Zhongliu, Nianze Wu, Yuying Song, and Junchen Xiang. 2023. "Investigating the Potential of Biobinder for Bottom Ash Solidification/Stabilization: Leaching Behaviour and pH Dependence" Sustainability 15, no. 10: 7859. https://doi.org/10.3390/su15107859





