Improved Biological Phosphorus Removal under Low Solid Retention Time Regime in Full-Scale Sequencing Batch Reactor
Abstract
:1. Introduction
- To study the influence of lowering SRT (up to 10 days) on EBPR in SBR;
- To study the influence of lowering SRT (up to 10 days) on SND in SBR;
- Using molecular biological techniques for the identification of microbial communities in SBR (before and after lowering the SRT);
- To study the effect of dewatered sludge characteristics on reducing SRT in SBR.
2. Material and Methods
2.1. SBR technology and Overall Process
2.2. Wastewater Characteristics
2.3. Microscopic Investigations for Intracellular Polymers Development, Protozoa, and Microbial Identification
2.4. 16SrRNA Sequencing and DNA Extraction
2.5. Dewatered Sludge Parameter Analyses
2.6. Energy Consumption
3. Results and Discussion
3.1. Average Temporal Variations at Different SRTs in the SBR Plant
3.2. Operational Sludge Parameters
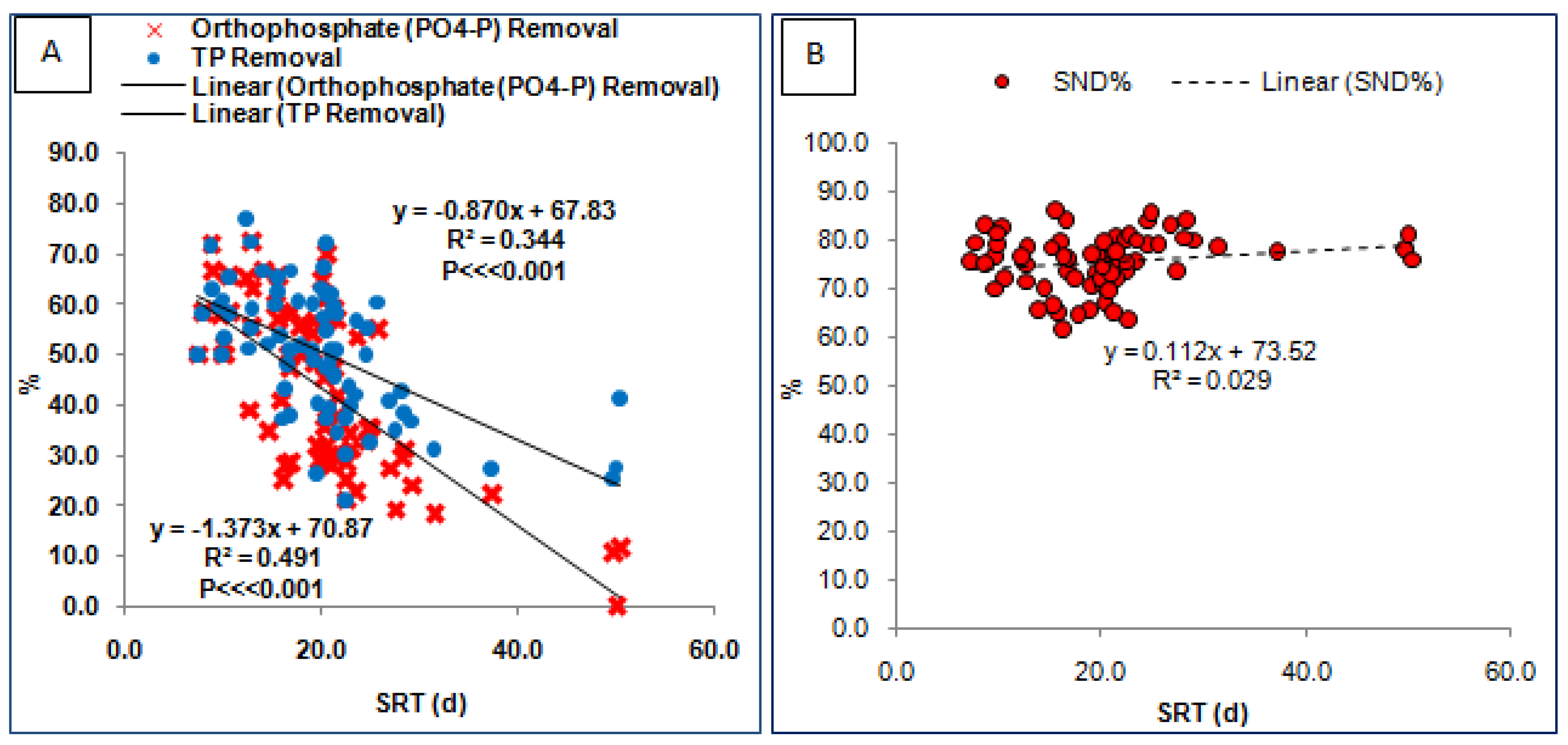
3.3. Biological Nutrient (N and P) Removal at Variable SRT
3.4. Effect of C: P on TP Removal at Low SRT
3.5. Phosphate Release and Uptake at Different SRTs and Runs
3.6. TP Fractions and Their Removal in Different Runs with Variable SRTs
3.7. Light Microscopy and SEM Analysis for Microbiota Identification in Biomass
3.8. Microscopic Investigations for Intracellular Polymers Development: Light Microscopy and STEM Analysis
3.9. 16SrRNA Sequencing and Microbial Community: Before and after Modifications
3.10. Dewatered Sludge Parameters: Before and after Modifications in SRT
3.11. Reduction in Energy-Consumption Rate at Low SRT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furumai, H.; Kazmi, A.A.; Furuya, Y.; Sasaki, K. Effect of sludge retention time (SRT) on nutrient removal in sequencing batch reactors. J. Environ. Sci. Health Part A 1999, 34, 317–328. [Google Scholar] [CrossRef]
- Gu, A.Z.; Saunders, A.; Neethling, J.B.; Stensel, H.D.; Blackall, L.L. Functionally relevant microorganisms to enhanced biological phosphorus removal performance at full-scale wastewater treatment plants in the United States. Water Environ. Res. 2008, 80, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Onnis-Hayden, A.; Majed, N.; Li, Y.; Rahman, S.M.; Drury, D.; Risso, L.; Gu, A.Z. Impact of solid residence time (SRT) on functionally relevant microbial populations and performance in full-scale enhanced biological phosphorus removal (EBPR) systems. Water Environ. Res. 2019, 92, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Roots, P.; Rosenthal, A.; Wang, Y.; Sabba, F.; Jia, Z.; Yang, F.; Zhang, H.; Kozak, J.; Wells, G. Pushing the limits of solids retention time for enhanced biological phosphorus removal: Process characteristics and Accumulibacter population structure. Water Sci. Technol. 2020, 82, 1614–1627. [Google Scholar] [CrossRef]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef]
- Li, W.; Gao, M.; Zeng, F.; Liu, N.; Zhu, X.; Zhang, C. Effect of pH and SRT on denitrifying phosphorus removal in A2N sequencing batch reactor process. Appl. Ecol. Environ. Res. 2019, 17, 5737–5751. [Google Scholar] [CrossRef]
- Li, N.; Wang, X.; Ren, N.; Zhang, K.; Kang, H.; You, S. Effects of Solid Retention Time (SRT) on Sludge Characteristics in Enhanced Biological Phosphorus Removal (EBPR) Reactor. Chem. Biochem. Eng. 2008, 22, 453–458. [Google Scholar]
- Lee, D.; Kim, M.; Chung, J. Relationship between solid retention time and phosphorus removal in anaerobic-intermittent aeration process. J. Biosci. Bioeng. 2007, 103, 338–344. [Google Scholar] [CrossRef]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P.; et al. Side-stream enhanced biological phosphorus removal (S2EBPR) process improves system performance—A full-scale comparative study. Water Res. 2019, 167, 115109. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research Advances of the phosphorus –accumulating organisms of Candidatus Accumulibacter, Dechloromonas, and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef]
- Begmatov, S.; Dorofeev, A.G.; Kadnikov, V.V.; Beletsky, A.V.; Pimenov, N.V.; Ravi, N.V.; Mardanov, A.V. The structure of microbial communities of activated sludge of large-scale wastewater water treatment plants in the city of Moscow. Sci. Rep. 2022, 12, 3458. [Google Scholar] [CrossRef]
- Burkhardt, G. Biological Phosphorus Removal, Operator’s Guide. 2012. Available online: https://www.mi-wea.org/docs/Burkhardt%20Presentation.pdf (accessed on 18 April 2013).
- Barnard, J.; Dunlap, P.; Steichen, M. Rethinking the Mechanisms of Biological Phosphorus Removal. Water Environ. Res. 2017, 89, 2043–2054. [Google Scholar] [CrossRef]
- Broughton, A.; Pratt, S.; Shilton, A. Enhanced biological phosphorus removal for high-strength wastewater with a low rbCOD:P ratio. Bioresour. Technol. 2008, 99, 1236–1241. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef]
- Oehmen, A.; Yuan, Z.; Blackall, L.L.; Keller, J. Comparison of acetate and propionate uptake by polyphosphate accumulating organisms and glycogen accumulating organisms. Biotechnol. Bioeng. 2005, 91, 162–168. [Google Scholar] [CrossRef]
- Whang, L.-M.; Park, J.K. Competition between Polyphosphate- and Glycogen-Accumulating Organisms in Enhanced-Biological-Phosphorus-Removal Systems: Effect of Temperature and Sludge Age. Water Environ. Res. 2006, 78, 4–11. [Google Scholar] [CrossRef]
- U.S. EPA. Summary Report: The Causes and Control of Activated Sludge Bulking and Foaming; EPA/625/8-87/012; National Service Center for Environmental Publications (NSCEP): Cincinnati, OH, USA, 1987.
- Li, B.; Wu, G. Effects of Sludge Retention Times on Nutrient Removal and Nitrous Oxide Emission in Biological Nutrient Removal Processes. Int. J. Environ. Res. Public Health 2014, 11, 3553–3569. [Google Scholar] [CrossRef]
- Kim, D.-J.; Kim, S.-H. Effect of nitrite concentration on the distribution and competition of nitrite-oxidizing bacteria in nitratation reactor systems and their kinetic characteristics. Water Res. 2006, 40, 887–894. [Google Scholar] [CrossRef]
- Yu, T.; Qi, R.; Li, D.; Zhang, Y.; Yang, M. Nitrifier characteristics in submerged membrane bioreactors under different sludge retention times. Water Res. 2010, 44, 2823–2830. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J. Role of Solids Retention Time on Complete Nitrification: Mechanistic Understanding and Modeling. J. Environ. Eng. 2014, 140, 48–56. [Google Scholar] [CrossRef]
- Bueno, R.F.; Piveli, R.P.; Campos, F.; Sobrinho, P.A. Simultaneous nitrification and denitrification in the activated sludge systems of continuous flow. Environ. Technol. 2018, 39, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Kazmi, A.A. Intricacies of Enhanced Biological Phosphorus Removal in Full-Scale Sequencing Batch Reactors Based on Wastewater Treatment Plants of India. J. Environ. Eng. 2022, 149, 04022082. [Google Scholar] [CrossRef]
- Srivastava, G.; Rajpal, A.; Khursheed, A.; Nadda, A.K.; Tyagi, V.K.; Kazmi, A.A. Influence of variations in wastewater on simultaneous nutrient removal in pre-anoxic selector attached full-scale sequencing batch reactor. Int. J. Environ. Sci. Technol. 2023, 20, 4355–4372. [Google Scholar] [CrossRef]
- Srivastava, G.; Gani, K.M.; Rajpal, A.; Singh, J.; Bux, F.; Kazmi, A.A. Reduced sludge production in a polyvinyl alcohol gel-based oxic-anoxic-oxic configured pilot-scale plant. Case Stud. Chem. Environ. Eng. 2021, 4, 100136. [Google Scholar] [CrossRef]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Wentzel, M.C.; Mbewe, A.; Lakay, M.T.; Ekama, G.A. Evaluation of a Modified Flocculation Filtration Method to Determine Wastewater Readily Biodegradable COD. WISA, Biennial Conference, Sun City, South Africa. 2000. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.517.5571 (accessed on 30 December 2014).
- Orhon, D.; Genceli, E.A.; Çokgör, E.U. Characterization and Modeling of Activated Sludge for Tannery Wastewater. Water Environ. Res. 1999, 71, 50–63. [Google Scholar] [CrossRef]
- Third, K.A.; Burnett, N.; Cord-Ruwisch, R. Simultaneous nitrification and denitrification using stored substrate (phb) as the electron donor in an SBR. Biotechnol. Bioeng. 2003, 83, 706–720. [Google Scholar] [CrossRef]
- Metcalf and Eddy. Wastewater Engineering Treatment, Disposal, and Reuse; Tata Mc Graw-Hill Publication Co.: New Delhi, India, 2003. [Google Scholar]
- United States Environmental Protection Agency 1978 Method 365.3: Phosphorous, All Forms (Colorimetric, Ascorbic Acid, Two Reagent). Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_365-3_1978.pdf (accessed on 6 December 2015).
- Gu, A.Z.; Liu, L.; Neethling, J.B.; Stensel, H.D.; Murthy, S. Treatability and fate of various phosphorus fractions in different wastewater treatment processes. Water Sci. Technol. 2011, 63, 804–810. [Google Scholar] [CrossRef]
- DAPI Counterstaining Protocols; Thermo Fisher Scientific Inc.: Waltham, MA, USA. Available online: https://www.thermofisher.com/in/en/home/references/protocols/cell-and-tissue-analysis/dapi-protocol/basic-dapi-counterstaining-protocols.html (accessed on 1 April 2023).
- United States Environmental Protection Agency Office of Water (4303-T) EPA-821-R-14-012 September 2014 Method 1682: Salmonella in Sewage Sludge (Biosolids) by Modified Semisolid Rappaport- Vassiliadis (MSRV) Medium. U.S. Environmental Protection Agency Office of Water (4303T) 1200 Pennsylvania Avenue, NW Washington, DC 20460, USA. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1682_2006.pdf (accessed on 1 April 2023).
- Smith, R. Implementation of Solids Retention Time Control in Wastewater Treatment. Implementation of Solids Retention Time Control in Wastewater Treatment (ysi.com). 2018. Available online: https://www.semanticscholar.org/paper/Implementation-of-Solids-Retention-Time-(SRT)-in-Smith-Elger/ecf88db1e7ab565c5452eb89fed8eae5adabd2c2 (accessed on 1 April 2023).
- Ge, H.; Damien, J.; Mouiche, B.M.; Hu, S.; Keller, J. Nutrient removal and energy recovery from high-rate activated sludge processes—Impact of sludge age. Bioresour. Technol. 2017, 245 Pt A, 1155–1161. [Google Scholar] [CrossRef]
- Li, B.; Brett, M.T. The relationship between operational and bioavailable phosphorus fractions in effluents from advanced nutrient removal systems. Int. J. Environ. Sci. Technol. 2015, 12, 3317–3328. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.; Zhu, R.; Cao, T.; Peng, Y. The effect of oxygen supply on nitrogen removal via nitrite using stored substrate (PHB) as the electron donor in SBRs. Biochem. Eng. J. 2015, 103, 130–137. [Google Scholar] [CrossRef]
- Lee, P.-H.; Kwak, W.; Bae, J.; Mccarty, P.L. The effect of SRT on nitrate formation during autotrophic nitrogen removal of anaerobically treated wastewater. Water Sci. Technol. 2013, 68, 1751–1756. [Google Scholar] [CrossRef]
- Khursheed, A.; Ali, M.; Mohammad, F.; Munshi, A.; Fahmi, A.; Kamal, M.A.; Almohana, A.I.; Alrehaili, O.; Gaur, R.Z.; Tyagi, V.K.; et al. Enhanced combined assimilative and bound phosphorus uptake in concurrence with nitrate removal in pre-anoxic cyclic sequencing batch reactor. Environ. Technol. Innov. 2022, 28, 102909. [Google Scholar] [CrossRef]
- Tu, Y.; Schuler, A.J. Low Acetate Concentrations Favor Polyphosphate-Accumulating Organisms over Glycogen-Accumulating Organisms in Enhanced Biological Phosphorus Removal from Wastewater. Environ. Sci. Technol. 2013, 47, 3816–3824. [Google Scholar] [CrossRef]
- Lopez-Vazquez, C.M.; Hooijmansa, C.M.; Brdjanovic, D.; Gijzena, H.J.; van Loosdrecht, M.C.M. Factors affecting the microbial populations at full-scale enhanced biological phosphorus removal (EBPR) wastewater treatment plants in The Netherlands. Water Res. 2008, 42, 2349–2360. [Google Scholar] [CrossRef]
- Mandel, A.; Zekker, I.; Jaagura, M.; Tenno, T. Enhancement of anoxic phosphorus uptake of denitrifying phosphorus removal process by biomass adaption. Int. J. Environ. Sci. Technol. 2019, 16, 5965–5978. [Google Scholar] [CrossRef]
- Maurer, M.; Maurer, M.; Boller, M.; Boller, M.; Maurer, M.; Maurer, M.; Boller, M.; Boller, M. Modelling of phosphorus precipitation in wastewater treatment plants with enhanced biological phosphorus removal. Water Sci. Technol. 1999, 39, 147–163. [Google Scholar] [CrossRef]
- Yu, B.; Luo, J.; Xie, H.; Yang, H.; Chen, S.; Liu, J.; Zhang, R.; Li, Y. Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Yang, L.; Liu, C.; Wang, Q.; Chi, W.; Zhu, H. How Microbes Shape Their Communities? A Microbial Community Model Based on Functional Genes. Genom. Proteom. Bioinform. 2019, 17, 91–105. [Google Scholar] [CrossRef]
- Stokholm-Bjerregaard, M.; McIlroy, S.J.; Nierychlo, M.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. A Critical Assessment of the Microorganisms Proposed to be Important to Enhanced Biological Phosphorus Removal in Full-Scale Wastewater Treatment Systems. Front. Microbiol. 2017, 8, 718. [Google Scholar] [CrossRef]
- Tumlirsch, T.; Sznajder, A.; Jendrossek, D. Formation of polyphosphate by polyphosphate kinases and its relationship to poly-(3-hydroxybutyrate) accumulation in Ralstoniaeutropha strain H16. Appl. Environ. Microbiol. 2015, 81, 8277–8293. [Google Scholar] [CrossRef]
- Mulkerrins, D.; Dobson, A.D.W.; Colleran, E. Parameters affecting biological phosphate removal from wastewaters. Environ. Int. 2004, 30, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mino, T.V.; Loosedrecht, M.; Heijnen, J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Bernat, K.; Wojnowska-Baryla, I. The effect of different nitrogen sources on denitrification with PHB under aerobic condition. Environ. Technol. 2008, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.H.; Wuertz, S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Res. 2019, 149, 496–510. [Google Scholar] [CrossRef]
- Xin, X.; Wang, Z. Rapid Startup of Simultaneous Nitrogen and Phosphorus Removal (SNPR) Process and the Bacterial Community Dynamics in a GSBR. Pol. J. Environ. Stud. 2019, 28, 2931–2940. [Google Scholar] [CrossRef]
- Pandey, P.S.; Yadav, M.K. Enhancement of Energy Efficiency in Wastewater Treatment Plants. Int. J. Innov. Sci. Res. Technol. 2021, 6. Available online: https://ijisrt.com/enhancement-of-energy-efficiency-in-wastewater-treatment-plants (accessed on 1 April 2023).
- Leu, S.Y.; Rosso, D.; Larson, L.; Stenstrom, M.K. Real-time monitoring aeration efficiency in the activated sludge process and methods to reduce Energy Consumption. Water Environ. Res. 2009, 81, 2471–2481. [Google Scholar] [CrossRef]
- Leu, S.; Chan, L.; Stenstorm, M.K. Towards long solids retention times of Activated Sludge processes: Benefits in Energy Saving, Effluent Quality, and Stability. Water Environ. Res. 2012, 84, 42–53. [Google Scholar] [CrossRef]
- Schraa, O.; Rieger, L.; Alex, J.; Miletić, I. Ammonia-based aeration control with optimal SRT control: Improved performance and lower energy consumption. Water Sci. Technol. 2019, 79, 63–72. [Google Scholar] [CrossRef]
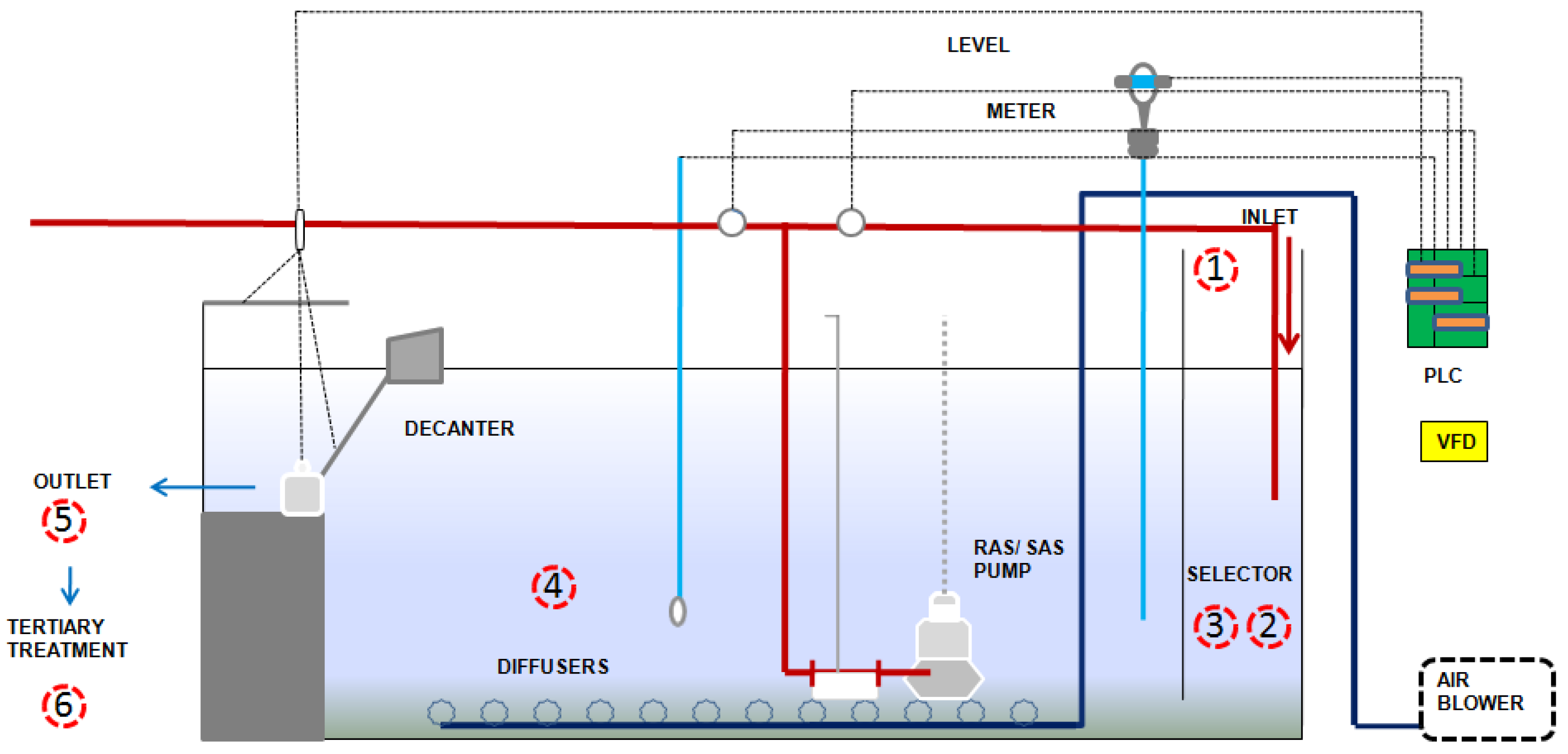

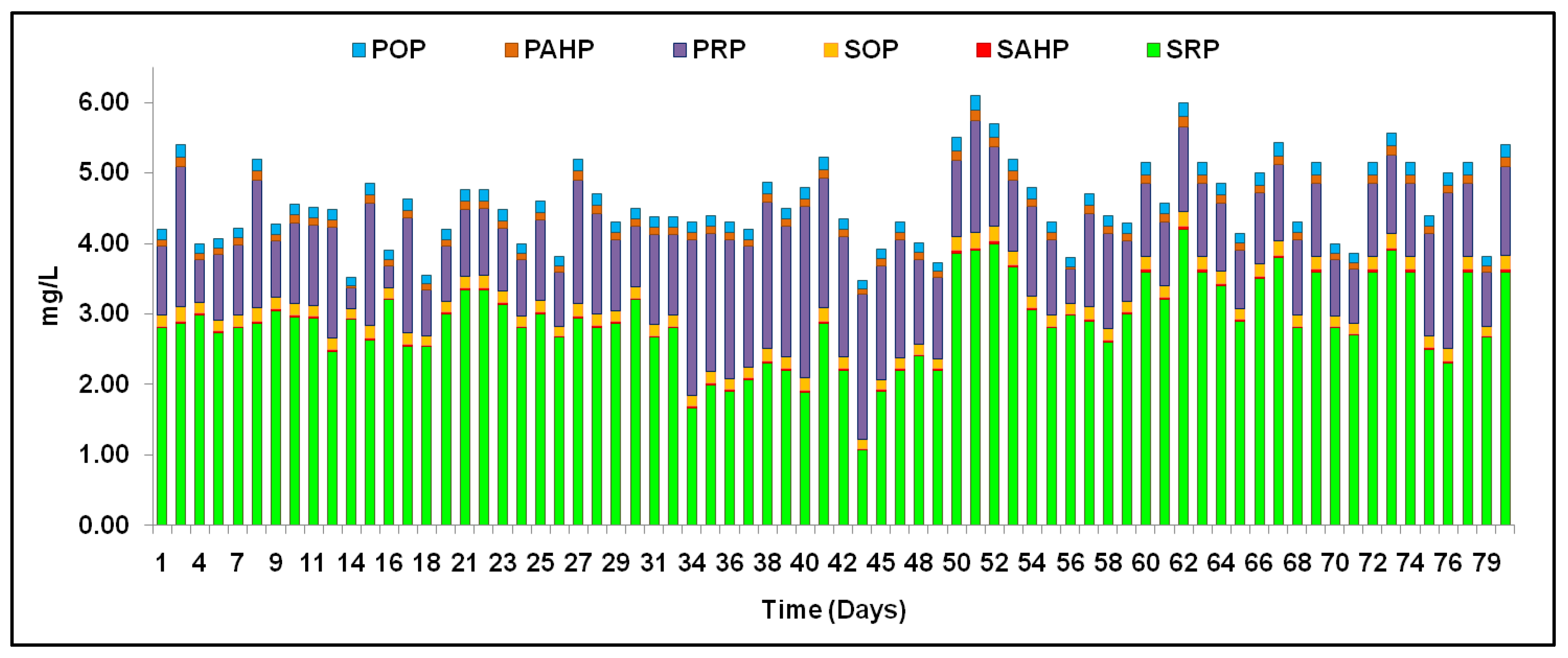
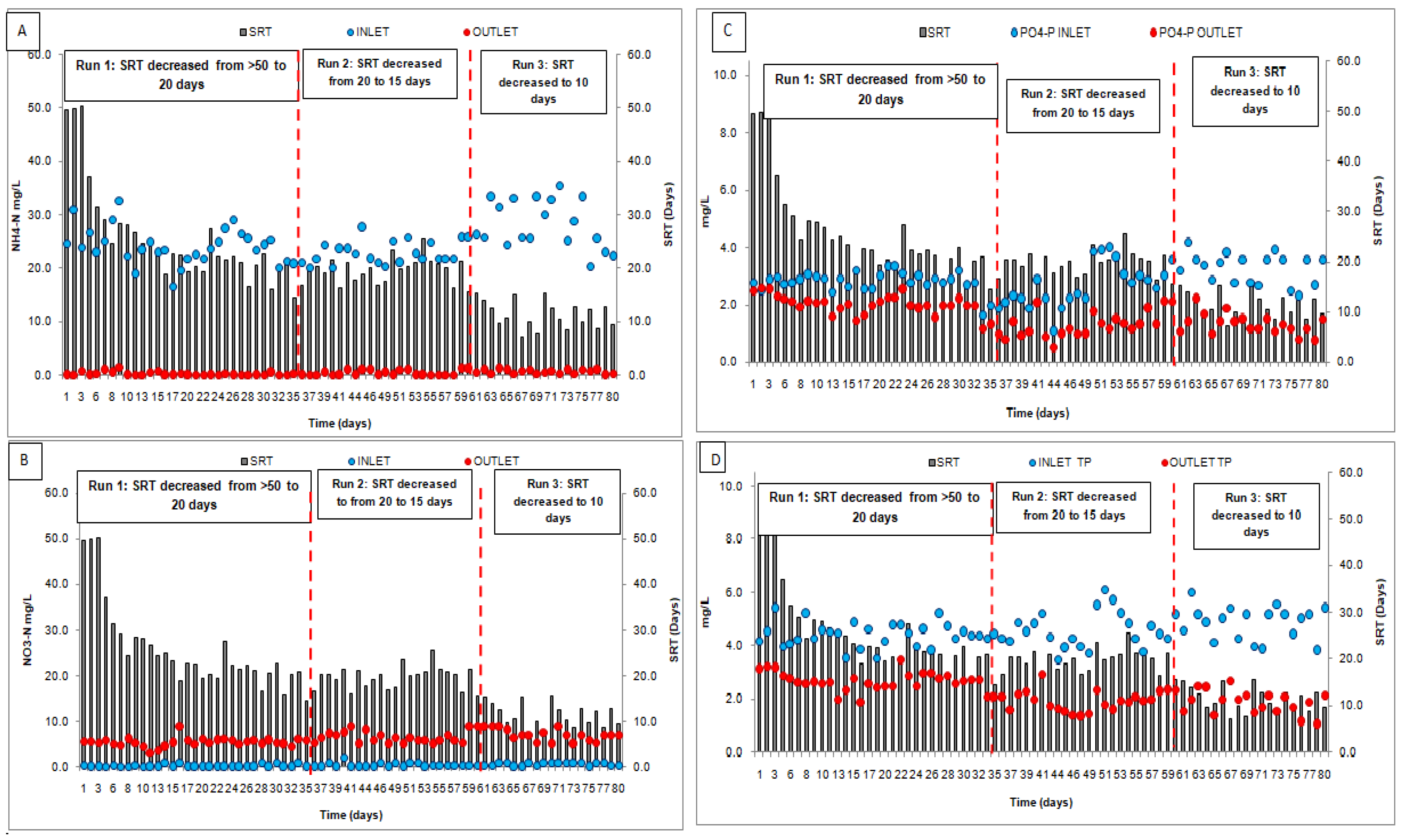
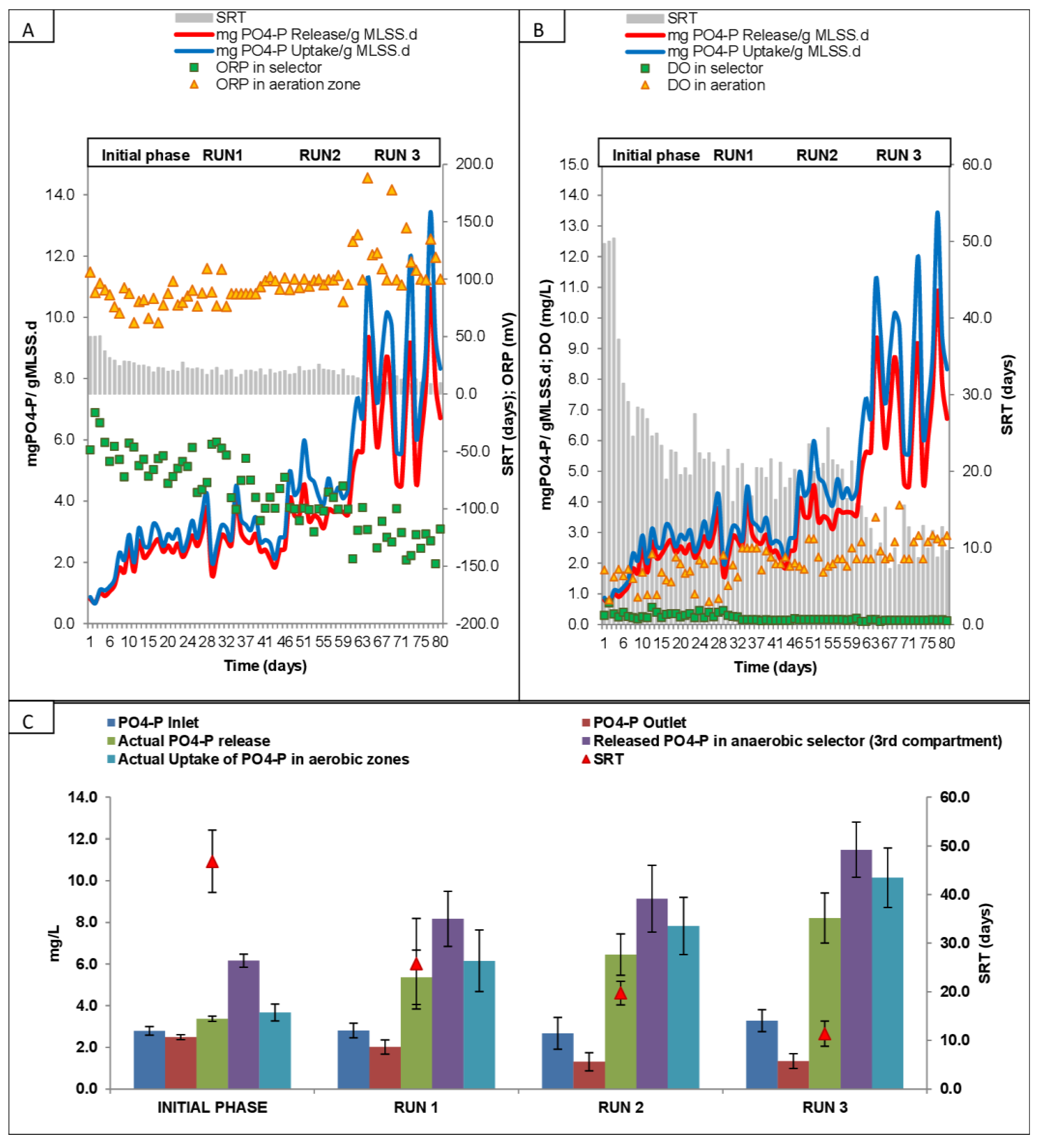

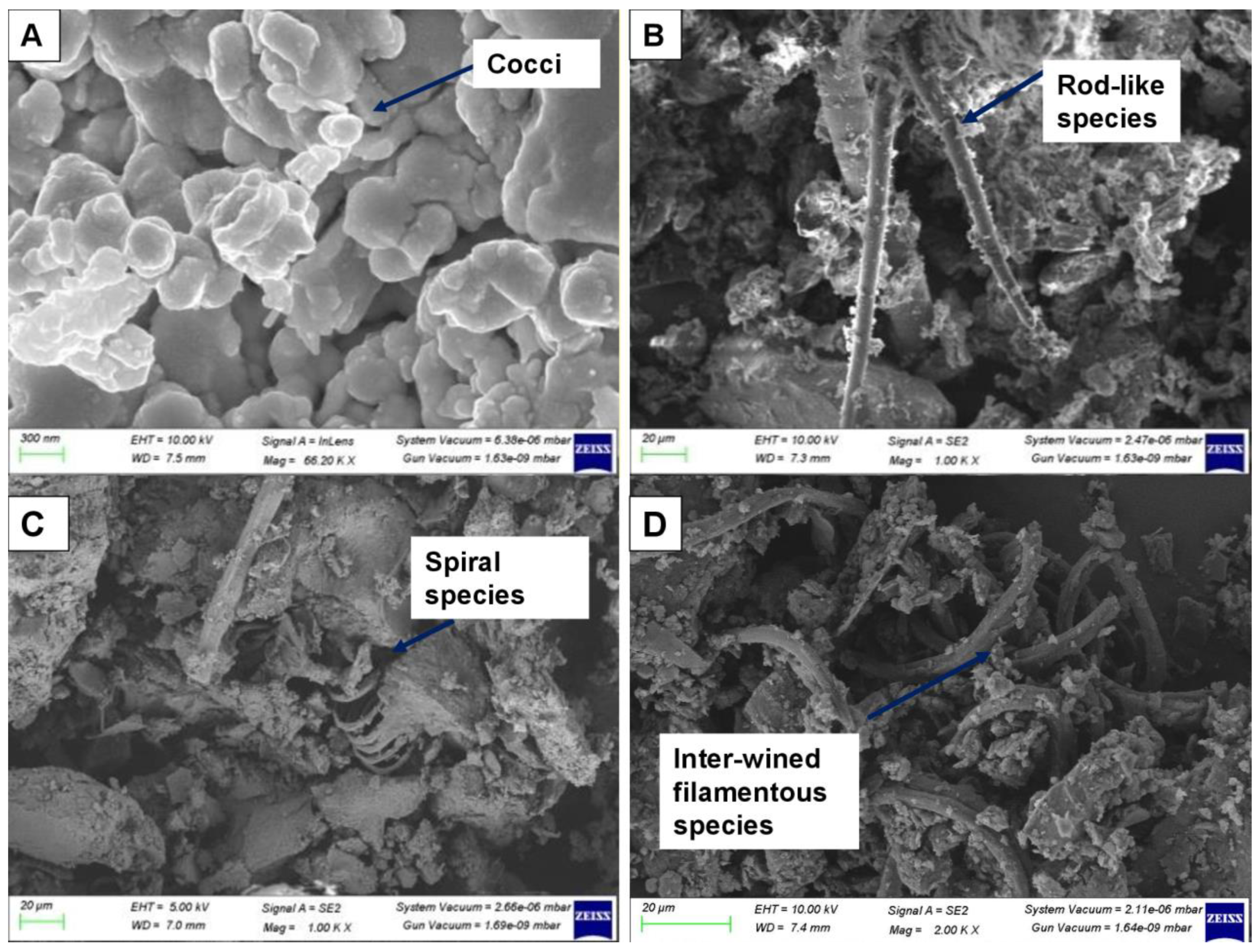

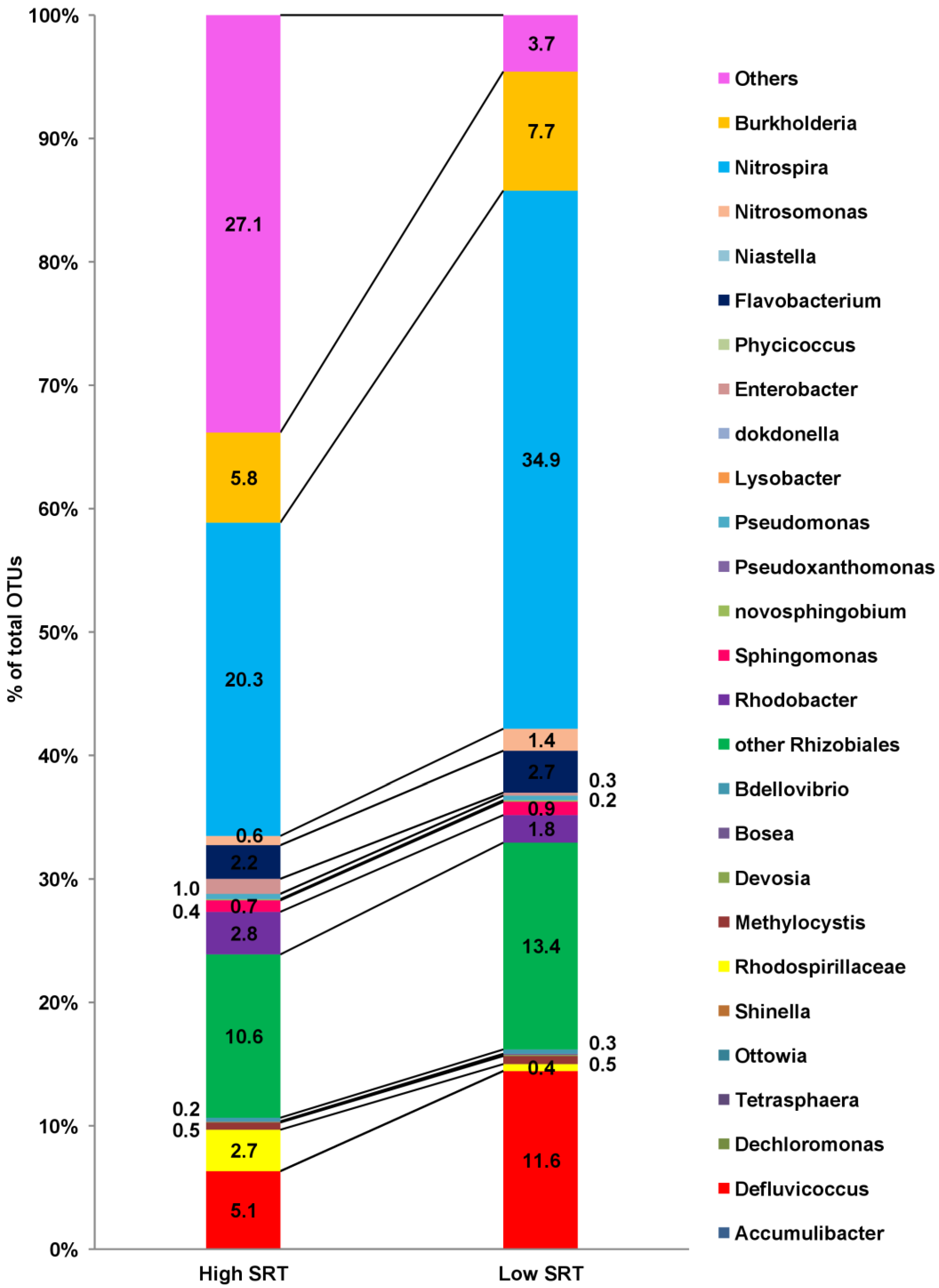

| S. No. | Runs/Phases | Phase Description | Duration | References | |
|---|---|---|---|---|---|
| 1. | Initial phase | SRT > 50 days | 2–3 years | [24,25] | |
| 2. | Run-I | Modifications include lowering the plant’s solid retention times (SRT) from 56 to 20 days | 35 days | Present study | |
| 3. | Run-II | Lowering the diffuser’s running time from 15 min to 10 min in anoxic cum anaerobic selector chambers (DO concentrations reduced to <0.15 mg/L) along with reducing SRT from 20 days to 15 days | 25 days | Present study | |
| 4. | Run-III | Combined effects by lowering the diffuser’s running time from 15 min to 10 min in anoxic cum anaerobic selector chambers (DO concentrations reduced to <0.15 mg/L) with an intensive reduction in SRT from 15 days to ≤10 days | 20 days | Present study | |
| Parameters | At High SRT | Run 1 | Run 2 | Run 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Influent | Effluent | Influent | Effluent | Influent | Effluent | Influent | Effluent | |
| SRT (d) | 56.8 ± 6.4 | 25.8 ± 9.3 | 19.7 ± 2.4 | 11.4 ± 2.6 | ||||
| pH | 7.2 ± 0.1 | 7.3 ± 0.1 | 7.0 ± 0.3 | 7.4 ± 0.1 | 7.1 ± 0.4 | 7.4 ± 0.2 | 7.0 ± 0.2 | 7.4 ± 0.2 |
| Alkalinity (mg/L) | 320 ± 40 | 220 ± 40 | 340 ± 60 | 240 ± 50 | 320 ± 40 | 210 ± 60 | 340 ± 50 | 230 ± 80 |
| COD (mg/L) | 401 ± 51 | 17 ± 3.8 | 387 ± 45 | 20 ± 5.6 | 377 ± 52 | 18.9 ± 5.1 | 356 ± 60 | 21.5 ± 4.0 |
| sCOD (mg/L) | 121 ± 31 | 8.8 ± 4.0 | 111 ± 25 | 9.0 ± 3.5 | 96 ± 40 | 9.2 ± 3.1 | 124 ± 32 | 9.8 ± 1.3 |
| BOD (mg/L) | 168 ± 22 | 7.9 ± 2.1 | 156 ± 31 | 7.1 ± 3.0 | 187 ± 25 | 6.5 ± 1.8 | 175 ± 30 | 6.6 ± 1.8 |
| sBOD (mg/L) | 51 ± 23 | 3.1 ± 1.2 | 48 ± 31 | 3.2 ± 2.0 | 49 ± 11 | 2.8 ± 1.1 | 56 ± 14 | 3.1 ± 1.0 |
| NH4-N (mg/L) | 22 ± 3.0 | 0.3 ± 0.3 | 24 ± 3.5 | 0.4 ± 0.3 | 23 ± 2.1 | 0.5 ± 0.4 | 28 ± 4.5 | 0.7 ± 0.3 |
| NO3-N (mg/L) | 0.2 ± 0.1 | 5.7 ± 0.2 | 0.4 ± 0.2 | 5.5 ± 1.0 | 0.52 ± 0.5 | 6.6 ± 1.3 | 0.7 ± 0.4 | 7.1 ± 1.3 |
| TKN (mg/L) | 31 ± 3.3 | 4.0 ± 1.2 | 33 ± 4.0 | 3.4 ± 2.8 | 32.9 ± 1.3 | 3.3 ± 2.3 | 37.6 ± 3.5 | 2.7 ± 2.2 |
| TN (mg/L) | 31.2 ± 3.3 | 9.7 ± 1.5 | 33.4 ± 4.1 | 8.9 ± 3.0 | 33.4 ± 3.3 | 9.9 ± 2.1 | 38.3 ± 4.0 | 9.8 ± 3.2 |
| PO4-P (mg/L) | 2.8 ± 0.2 | 2.5 ± 0.1 | 2.8 ± 0.3 | 2.0 ± 0.3 | 2.7 ± 0.8 | 1.3 ± 0.4 | 3.1 ± 0.5 | 1.1 ± 0.3 |
| TP (mg/L) | 4.5 ± 0.6 | 3.1 ± 0.1 | 4.4 ± 0.4 | 2.7 ± 0.4 | 4.6 ± 0.7 | 2.0 ± 0.4 | 4.8 ± 0.6 | 1.7 ± 0.4 |
| TSS (mg/L) | 261 ± 31 | 9.9 ± 4.1 | 252 ± 34 | 9.4 ± 3.5 | 251 ± 31 | 9.2 ± 6.0 | 237 ± 33 | 9.1 ± 5.2 |
| VSS (mg/L) | 149 ± 13 | 5.2 ± 3.1 | 141 ± 32 | 4.9 ± 3.2 | 145 ± 14 | 5.4 ± 1.3 | 134 ± 31 | 5.2 ± 3.1 |
| Total Coliforms (MPN/100 mL) | 240,000 ± 5200 | 1712 ± 150 | 210,000 ± 9100 | 1500 ± 240 | 340,000 ± 12,900 | 1209 ± 50 | 480,000 ± 9500 | 990 ± 60 |
| Fecal Coliforms (MPN/100 mL) | 1300 ± 78 | 79 ± 21 | 1100 ± 54 | 55 ± 22 | 2100 ± 43 | 47 ± 11 | 1800 ± 60 | 49 ± 13 |
| Sludge Parameters | At High SRT | RUN 1 | RUN 2 | RUN 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Aeration | Selector | Aeration | Selector | Aeration | Selector | Aeration | Selector | |
| pH | 7.3 ± 0.1 | 7.4 ± 0.2 | 7.4 ± 0.1 | 7.6 ± 0.1 | 7.2 ± 0.2 | 7.5 ± 0.1 | 7.4 ± 0.2 | 7.8 ± 0.2 |
| ORP (mV) | 95.3 ± 7.9 | −33.1 ± 15.0 | 84.6 ± 12 | −59.1 ± 18.4 | 95 ± 6.2 | −93 ± 14.7 | 121.2 ± 27 | −129.4 ± 22.1 |
| DO (mg/L) | 1.5 ± 0.5 | 0.4 ± 0.2 | 1.6 ± 0.5 | 0.3 ± 0.1 | 2.1 ± 0.3 | 0.2 ± 0.02 | 2.5 ± 0.5 | 0.1 ± 0.02 |
| Temperature (°C) | 29.5 ± 0.5 | 29.4 ± 0.4 | 29.1 ± 0.7 | 29.2 ± 0.4 | 29.0 ± 0.6 | 28.8 ± 0.5 | 28.5 ± 1.0 | 28.6 ± 1.2 |
| MLSS (mg/L) | 5761 ± 1787 | - | 3389 ± 756 | - | 2766 ± 319 | - | 1682 ± 352 | - |
| MLVSS/MLSS | 0.50 ± 0.02 | - | 0.52 ± 0.02 | - | 0.59 ± 0.04 | - | 0.63 ± 0.03 | - |
| SVI (mL/g) | 55.3 ± 15.7 | - | 65.0 ± 8.4 | - | 71.1 ± 6.5 | - | 74.5 ± 7.8 | - |
| HRT (h) | 20.5 ± 0.9 | - | 18.5 ± 1.4 | - | 18.2 ± 1.2 | - | 17.7 ± 1.1 | - |
| S. No. | Regression Equations with Std Err. For Coefficient | Factor x | Factor y | F Value | p Value of F Test | R2 | Adjusted R2 | p Value of t Test for Intercept | p Value of t Test for x |
|---|---|---|---|---|---|---|---|---|---|
| 1 | y = −1.373 × x + 70.874 (0.166, 3.622) | SRT | PO4-P Removal% (SRP Removal%) | F (1,72) = 68.647 | 4.95 × 10−12 | 0.491 | 0.484 | 2.59 × 10−30 | 4.95 × 10−12 |
| 2 | y = −0.871 × x + 67.831 (0.143, 3.118) | SRT | TP Removal% | F (1,72) = 37.229 | 5 × 10−8 | 0.344 | 0.335 | 4 × 10−33 | 5 × 10−8 |
| 3 | y = −0.947 × x + 77.130 (0.148, 3.232) | SRT | SOP Removal% | F (1,72) = 41.060 | 1.4 × 10−8 | 0.366 | 0.357 | 1.22 × 10−35 | 1.4 × 10−8 |
| 4 | y = −0.816 × x + 90.580 (0.199, 4.345) | SRT | SAHP Removal% | F (1,72) = 16.900 | 0.00011 | 0.192 | 0.180 | 5.51 × 10−32 | 0.00011 |
| 5 | y = −0.231 × x + 64.701(0.334, 7.093) | SRT | PRP Removal% | F (1,72) = 0.534 | 0.467 | 0.007 | −0.0065 | 5.06 × 10−14 | 0.467 |
| 6 | y = −0.746 × x +98.140 (0.152, 3.314) | SRT | PAHP Removal% | F (1,72) = 24.230 | 5.4 × 10−6 | 0.254 | 0.244 | 1.08 × 10−41 | 5.4 × 10−6 |
| 7 | y = −1.109 × x + 63.773 (0.171, 2.733) | SRT | POP Removal% | F (1,72) = 42.175 | 9.8 × 10−9 | 0.372 | 0.364 | 7.35 × 10−27 | 9.8 × 10−9 |
| Parameters | FCO Composts Standards (2009) | US EPA Class a Sludge | USEPA Class b Sludge | CPHEEO-2013 | 3 MLD (at High SRT~50 Days) Dewatered Sludge | 3 MLD (at a Low SRT~10 Days) Dewatered Sludge |
|---|---|---|---|---|---|---|
| pH value | 6.5–7.5 | - | - | - | 6.8 ± 0.3 | 7.3 ± 0.1 |
| Moisture Content (%) | 15–25 | - | - | - | 75.0 ± 1.5 | 82.7 ± 1.3 |
| Color | Dark brown to black | - | - | - | Dark brown to black | Dark brown to black |
| Odor | Absence of foul odor | - | - | - | Absence of foul odor | Absence of foul odor |
| Bulk Density (g/cm3) | <1 | - | 1.3 ± 0.2 | 1.3 ± 0.1 | ||
| Conductivity (ds/m) | Not more than 4 | - | - | - | 0.9 ± 0.2 | 0.8 ± 0.3 |
| Total Organic Carbon (%) | ≥12 | - | - | - | 24.7 ± 1.5 | 31.5 ± 1.3 |
| Total Nitrogen (%) | ≥0.8 | - | - | - | 1.6–3.0 | 1.9–2.4 |
| Total Phosphate as P2O5 (%) | ≥0.4 | - | - | - | 2.2–2.9 | 3.7–5.2 |
| Total Potassium as K2O (%) | ≥0.4 | - | - | - | 0.3 ± 0.03 | 0.4 ± 0.05 |
| C/N ratio | <20 | - | - | - | 9.7 ± 1.2 | 14.6 ± 0.6 |
| Particle Size | 90% materials 4 mm sieve | - | - | - | - | - |
| Fecal Coliforms (MPN/g dry solids) | - | <1000 MPN/g | <2 × 106 MPN/g | <2 × 106 MPN/g | 43,000 ± 570 | 36,000 ± 430 |
| Salmonellaspecies (MPN/4 g dry solids) | - | <3 MPN/4 g | - | - | 8.0 ± 1.0 | 6.0 ± 1.0 |
| Helminth eggs (Numbers/4 g of TS) | - | ≤1 per 4 g of total solids (dry weight basis) | - | - | 64 ± 5 | 55 ± 2 |
| Gross Calorific Value (Kcal/kg) Dry Basis | - | - | - | - | 2250 ± 280 | 2281 ± 190 |
| SOUR (mgO2/g TS.h) Vector Attraction | - | <1.5 | - | - | 1.3 ± 0.3 | 1.2 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, G.; Kapoor, A.; Kazmi, A.A. Improved Biological Phosphorus Removal under Low Solid Retention Time Regime in Full-Scale Sequencing Batch Reactor. Sustainability 2023, 15, 7918. https://doi.org/10.3390/su15107918
Srivastava G, Kapoor A, Kazmi AA. Improved Biological Phosphorus Removal under Low Solid Retention Time Regime in Full-Scale Sequencing Batch Reactor. Sustainability. 2023; 15(10):7918. https://doi.org/10.3390/su15107918
Chicago/Turabian StyleSrivastava, Ghazal, Aparna Kapoor, and Absar Ahmad Kazmi. 2023. "Improved Biological Phosphorus Removal under Low Solid Retention Time Regime in Full-Scale Sequencing Batch Reactor" Sustainability 15, no. 10: 7918. https://doi.org/10.3390/su15107918





