Effect of Urea-Calcium Sulfate Cocrystal Nitrogen Fertilizer on Sorghum Productivity and Soil N2O Emissions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
- Soil without N fertilizer input (C0).
- Soil with urea equivalent to 150 kg N ha−1 (UR100).
- Soil with CaSO4⋅4urea equivalent to 60 kg N ha−1 (UC40).
- Soil with CaSO4⋅4urea equivalent to 105 kg N ha−1 (UC70).
- Soil with CaSO4⋅4urea equivalent to 150 kg N ha−1 (UC100).
- Soil with CaSO4⋅4urea equivalent to 195 kg N ha−1 (UC130).
2.2. Plant, Soil, and N2O Flux Measurement and Analysis
2.3. Data Analysis
3. Results
3.1. Soil Properties
3.2. Plant Productivity, N Uptake, and Use Efficiency
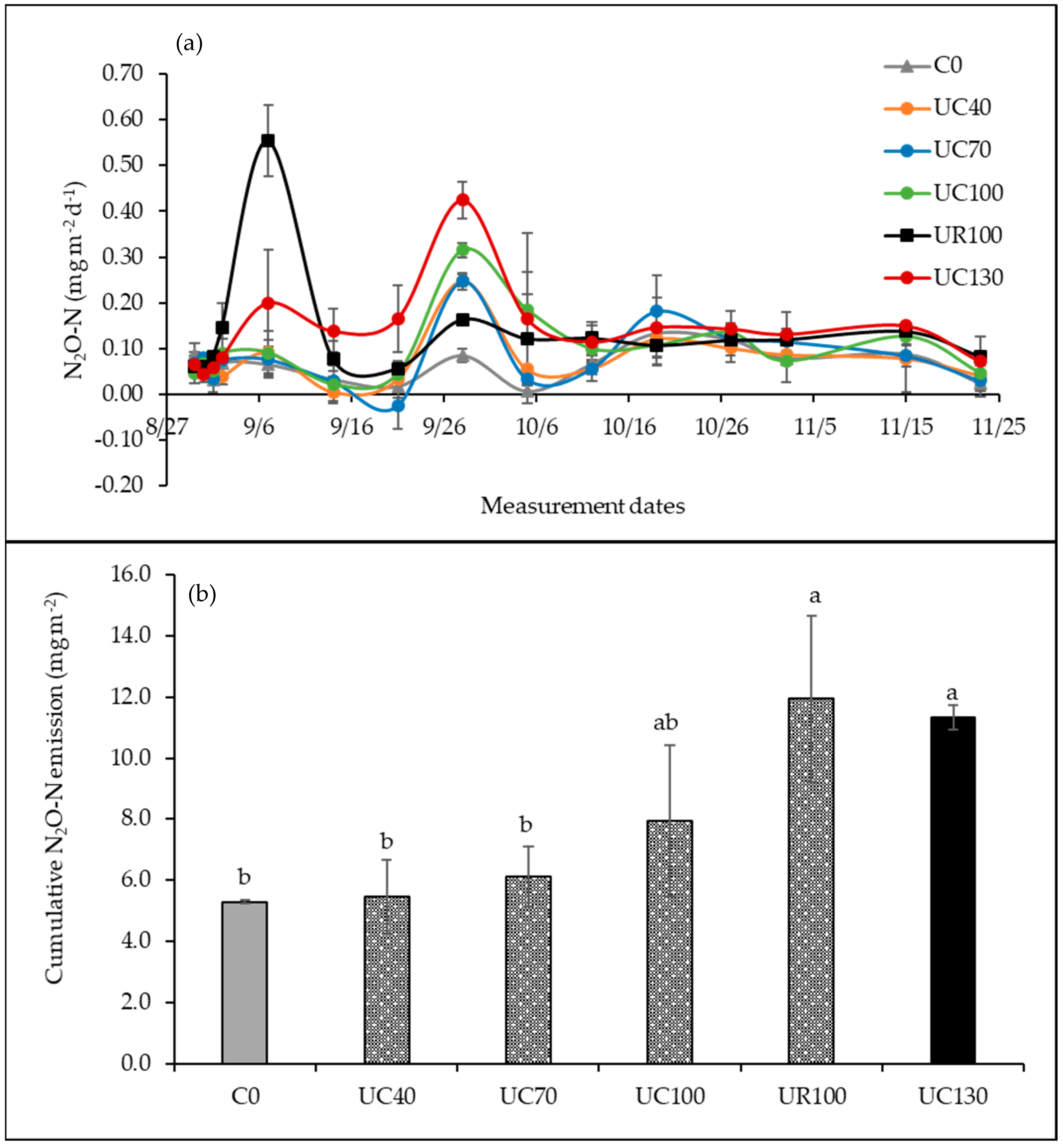
3.3. Soil N2O Emissions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Cowling, E.B. Reactive Nitrogen and The World: 200 Years of Change. AMBIO J. Hum. Environ. 2002, 31, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Cowling, E.B. Reflections on 200 years of Nitrogen, 20 years later. Ambio 2021, 50, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Coyle, M.; Skiba, U.; Sutton, M.A.; Cape, J.N.; Reis, S.; Sheppard, L.J.; Jenkins, A.; Grizzetti, B.; Galloway, J.N.; et al. The global nitrogen cycle in the Twentyfirst century. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130164. [Google Scholar] [CrossRef]
- Tonini, D.; Albizzati, P.F.; Astrup, T.F. Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manag. 2018, 76, 744–766. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure 1999, 7, 205–216. [Google Scholar] [CrossRef]
- Dixon, N.E.; Riddles, P.W.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5. 1.5). V. On the mechanism of action of urease on urea, formamide, acetamide, N-methylurea, and related compounds. Can. J. Biochem. 1980, 58, 1335–1344. [Google Scholar] [CrossRef]
- Mazzei, L.; Cianci, M.; Benini, S.; Ciurli, S. The Structure of the Elusive Urease–Urea Complex Unveils the Mechanism of a Paradigmatic Nickel-Dependent Enzyme. Angew. Chem. Int. Ed. 2019, 58, 7415–7419. [Google Scholar] [CrossRef]
- Valdez, C.E.; Alexandrova, A.N. Why urease is a di-nickel enzyme whereas the CcrA beta-lactamase is a di-zinc enzyme. J. Phys. Chem. B 2012, 116, 10649–10656. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.E.; Horton, E. Studies on Enzyme Action. XV.-Urease: A Selective Enzyme. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1912, 85, 109–127. Available online: http://www.jstor.org/stable/80632 (accessed on 3 March 2023).
- Zantua, M.I.; Bremner, J.M. Stability of urease in soils. Soil Biol. Biochem. 1977, 9, 135–140. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Bio/Technol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; DelSontro, T.; Downing, J.A. Eutrophication will increase methane emissions from lakes and impoundments during the 21st century. Nat. Commun. 2019, 10, 1375. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef]
- Patil, B.S.; Wang, Q.; Hessel, V.; Lang, J. Plasma N2-fixation: 1900–2014. Catal. Today 2015, 256, 49–66. [Google Scholar] [CrossRef]
- Schrock, R.R. Reduction of dinitrogen. Proc. Natl. Acad. Sci. USA 2006, 103, 17087. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Yin, J.; Chadwick, D.; Norse, D.; Lu, Y.; Liu, X.; Chen, X.; Zhang, F.; Powlson, D.; et al. Enhanced-efficiency fertilizers are not a panacea for resolving the nitrogen problem. Glob. Chang. Biol. 2018, 24, e511–e521. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-Efficiency Nitrogen Fertilizers: Potential Role in Nitrous Oxide Emission Mitigation. Agron. J. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Lam, S.K.; Wille, U.; Hu, H.-W.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H.; et al. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; He, W.; Jiang, R.; Song, D.; Zou, G.; Chen, Y.; Cao, B.; Wang, J.; Wang, X. Enhanced-Efficiency Fertilizers Impact on Nitrogen Use Efficiency and Nitrous Oxide Emissions from an Open-Field Vegetable System in North China. Plants 2022, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Honer, K.; Kalfaoglu, E.; Pico, C.; McCann, J.; Baltrusaitis, J. Mechanosynthesis of Magnesium and Calcium Salt–Urea Ionic Cocrystal Fertilizer Materials for Improved Nitrogen Management. ACS Sustain. Chem. Eng. 2017, 5, 8546–8550. [Google Scholar] [CrossRef]
- Honer, K.; Pico, C.; Baltrusaitis, J. Reactive Mechanosynthesis of Urea Ionic Cocrystal Fertilizer Materials from Abundant Low Solubility Magnesium- and Calcium-Containing Minerals. ACS Sustain. Chem. Eng. 2018, 6, 4680–4687. [Google Scholar] [CrossRef]
- Sandhu, B.; Sinha, A.S.; Desper, J.; Aakeröy, C.B. Modulating the physical properties of solid forms of urea using cocrystallization technology. Chem. Commun. 2018, 54, 4657–4660. [Google Scholar] [CrossRef]
- Brekalo, I.; Martinez, V.; Karadeniz, B.; Orešković, P.; Drapanauskaite, D.; Vriesema, H.; Stenekes, R.; Etter, M.; Dejanović, I.; Baltrusaitis, J.; et al. Scale-Up of Agrochemical Urea-Gypsum Cocrystal Synthesis Using Thermally Controlled Mechanochemistry. ACS Sustain. Chem. Eng. 2022, 10, 6743–6754. [Google Scholar] [CrossRef]
- Adassooriya, N.M.; Mahanta, S.P.; Thakuria, R. Mechanochemistry as an emerging tool for the preparation of sustained release urea cocrystals as a nitrogen source. CrystEngComm 2022, 24, 1679–1689. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Brazienė, Z.; Avižienytė, D.; Silva, M.; Drapanauskaite, D.; Honer, K.; Gvildienė, K.; Slinksiene, R.; Jancaitiene, K.; Mazeika, R.; et al. Mechanochemically synthesized gypsum and gypsum drywall waste cocrystals with urea for enhanced environmental sustainability fertilizers. J. Environ. Chem. Eng. 2020, 8, 103965. [Google Scholar] [CrossRef]
- Malinowski, P.; Biskupski, A.; Głowiński, J.; Przemysław, M.; Andrzej, B.; Józef, G. Preparation methods of calcium sulphate and urea adduct. Pol. J. Chem. Technol. 2007, 9, 111–114. [Google Scholar] [CrossRef]
- Borowik, M.; Malinowski, P.; Biskupski, A.; Dawidowicz, M.; Schab, S.; Rusek, P.; Igras, J. Production technology of nitrogen-sulphur-calcium fertilizers on the base of urea and phosphogypsum. Chemik 2012, 66, 525–534. Available online: https://www.infona.pl/resource/bwmeta1.element.baztech-article-BPP3-0001-0023 (accessed on 15 March 2023).
- Malinowski, P.; Olech, M.; Sas, J.; Wantuch, W.; Biskupski, A.; Urbańczyk, L.; Borowik, M.; Kotowicz, J. Production of compound mineral fertilizers as a method of utilization of waste products in chemical company Alwernia SA. Pol. J. Chem. Technol. 2010, 12, 6–9. [Google Scholar] [CrossRef]
- Swify, S.; Avizienyte, D.; Mazeika, R.; Braziene, Z. Comparative Study Effect of Urea-Sulfur Fertilizers on Nitrogen Uptake and Maize Productivity. Plants 2022, 11, 3020. [Google Scholar] [CrossRef] [PubMed]
- Swify, S.; Avizienyte, D.; Mazeika, R.; Braziene, Z. Influence of Modified Urea Compounds to Improve Nitrogen Use Efficiency under Corn Growth System. Sustainability 2022, 14, 14166. [Google Scholar] [CrossRef]
- Ghimire, U.; Shrestha, N.K.; Biswas, A.; Wagner-Riddle, C.; Yang, W.; Prasher, S.; Rudra, R.; Daggupati, P. A Review of Ongoing Advancements in Soil and Water Assessment Tool (SWAT) for Nitrous Oxide (N2o) Modeling. Atmosphere 2020, 11, 450. [Google Scholar] [CrossRef]
- Smith, P.; Clark, H.; Dong, H.; Elsiddig, E.A.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; Masera, O.; Mbow, C.; et al. Chapter 11—Agriculture, forestry and other land use (AFOLU). In Climate Change 2014: Mitigation of Climate Change; IPCC Working Group III Contribution to AR5; Cambridge University Press: Cambridge, UK, 2014; pp. 811–922. [Google Scholar]
- Tubiello, F.N.; Salvatore, M.; Rossi, S.; Ferrara, A.; Fitton, N.; Smith, P. The FAOSTAT database of greenhouse gas emissions from agriculture. Environ. Res. Lett. 2013, 8, 015009. [Google Scholar] [CrossRef]
- Gavlak, R.; Horneck, D.; Miller, R.O. Soil, Plant and Water Reference Methods for the Western Region; Western Region Extension Publication (WREP)-125; WCC-103 Publication: Fort Collins, CO, USA, 2005. [Google Scholar]
- De Villiers, J.P.R.; Boeyens, J.C.A. Crystal structure of a calcium sulfate-urea complex. J. Cryst. Mol. Struct. 1975, 5, 215–226. [Google Scholar] [CrossRef]
- Jones, J.M.; Rollinson, A.N. Thermogravimetric evolved gas analysis of urea and urea solutions with nickel alumina catalyst. Thermochim. Acta 2013, 565, 39–45. [Google Scholar] [CrossRef]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Pathak, R.R.; Ahmad, A.; Lochab, S.; Raghuram, N. Molecular physiology of plant nitrogen use efficiency and biotechnological options for its enhancement. Curr. Sci. 2008, 94, 1394–1403. [Google Scholar]
- Andrews, M.; Raven, J.A.; Lea, P.J. Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol. 2013, 163, 174–199. [Google Scholar] [CrossRef]
- Giguere, A.T.; Taylor, A.E.; Suwa, Y.; Myrold, D.D.; Bottomley, P.J. Uncoupling of ammonia oxidation from nitrite oxidation: Impact upon nitrous oxide production in non-cropped Oregon soils. Soil Biol. Biochem. 2017, 104, 30–38. [Google Scholar] [CrossRef]
- Venterea, R.T.; Rolston, D.E. Mechanisms and kinetics of nitric and nitrous oxide production during nitrification in agricultural soil. Glob. Chang. Biol. 2000, 6, 303–316. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Ostmeyer, T.J.; Bahuguna, R.N.; Kirkham, M.B.; Bean, S.; Jagadish, S.V.K. Enhancing Sorghum Yield Through Efficient Use of Nitrogen—Challenges and Opportunities. Front. Plant Sci. 2022, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Ketterings, Q.M.; Cherney, J.H. Effect of Nitrogen Application on Yield and Quality of Silage Corn after Forage Legume-Grass. Agron. J. 2008, 100, 73–79. [Google Scholar] [CrossRef]
- Bollam, S.; Romana, K.K.; Rayaprolu, L.; Vemula, A.; Das, R.R.; Rathore, A.; Gandham, P.; Chander, G.; Deshpande, S.P.; Gupta, R. Nitrogen Use Efficiency in Sorghum: Exploring Native Variability for Traits Under Variable N-Regimes. Front. Plant Sci. 2021, 12, 643192. [Google Scholar] [CrossRef]
- Mahama, G.Y.; Prasad, P.V.V.; Mengel, D.B.; Tesso, T.T. Influence of Nitrogen Fertilizer on Growth and Yield of Grain Sorghum Hybrids and Inbred Lines. Agron. J. 2014, 106, 1623–1630. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Ottman, M.J.; Kimball, B.A.; Wall, G.W.; Pinter, P.J.; LaMorte, R.L.; Leavitt, S.W. Drought-induced changes in nitrogen partitioning between cyanide and nitrate in leaves and stems of sorghum grown at elevated CO2 are age dependent. Field Crops Res. 2016, 185, 97–102. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Borrell, A.K.; Chapman, S.C.; Broad, I.J.; Hammer, G.L. Functional dynamics of the nitrogen balance of sorghum: I. N demand of vegetative plant parts. Field Crops Res. 2010, 115, 19–28. [Google Scholar] [CrossRef]
- van Oosterom, E.J.; Chapman, S.C.; Borrell, A.K.; Broad, I.J.; Hammer, G.L. Functional dynamics of the nitrogen balance of sorghum. II. Grain filling period. Field Crops Res. 2010, 115, 29–38. [Google Scholar] [CrossRef]
- Habtegebrial, K.; Singh, B.R. Response of Wheat Cultivars to Nitrogen and Sulfur for Crop Yield, Nitrogen Use Efficiency, and Protein Quality in the Semiarid Region. J. Plant Nutr. 2009, 32, 1768–1787. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Wang, L.; Zhou, C.; Jing, J.; Sun, X.; Tian, X. Combined effects of nitrogen and sulfur fertilization on maize growth, physiological traits, N and S uptake, and their diagnosis. Field Crops Res. 2019, 242, 107593. [Google Scholar] [CrossRef]
- Wang, C.; Lai, D.Y.F.; Sardans, J.; Wang, W.; Zeng, C.; Peñuelas, J. Factors Related with CH4 and N2O Emissions from a Paddy Field: Clues for Management implications. PLoS ONE 2017, 12, e0169254. [Google Scholar] [CrossRef]
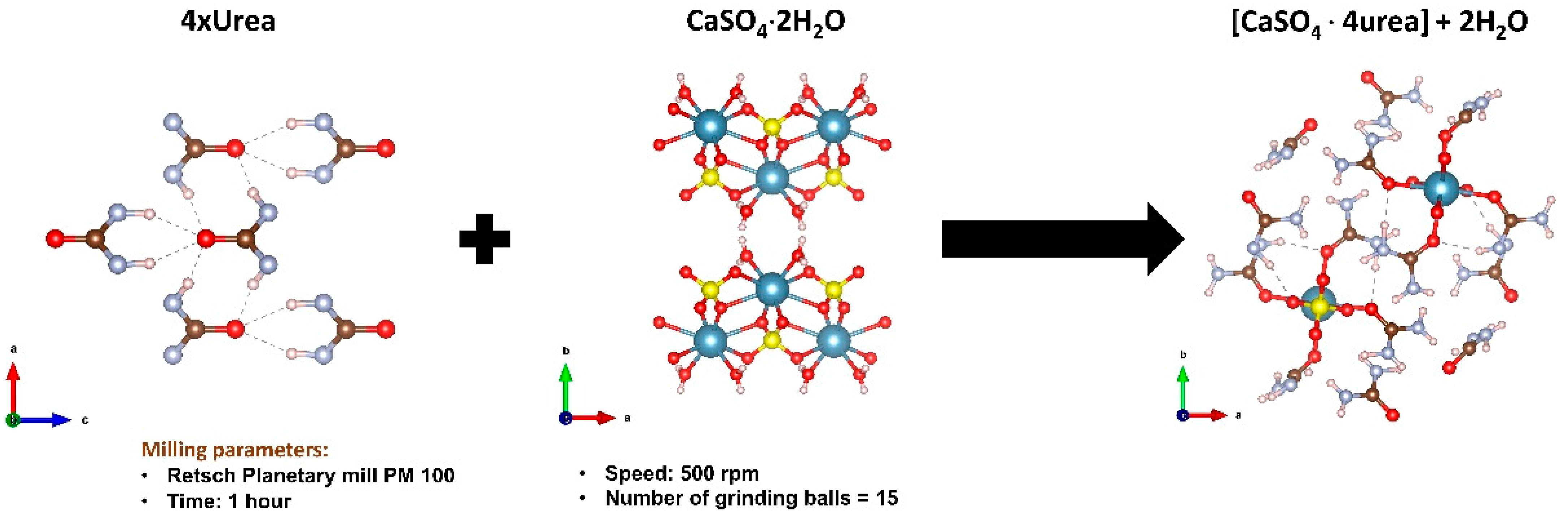
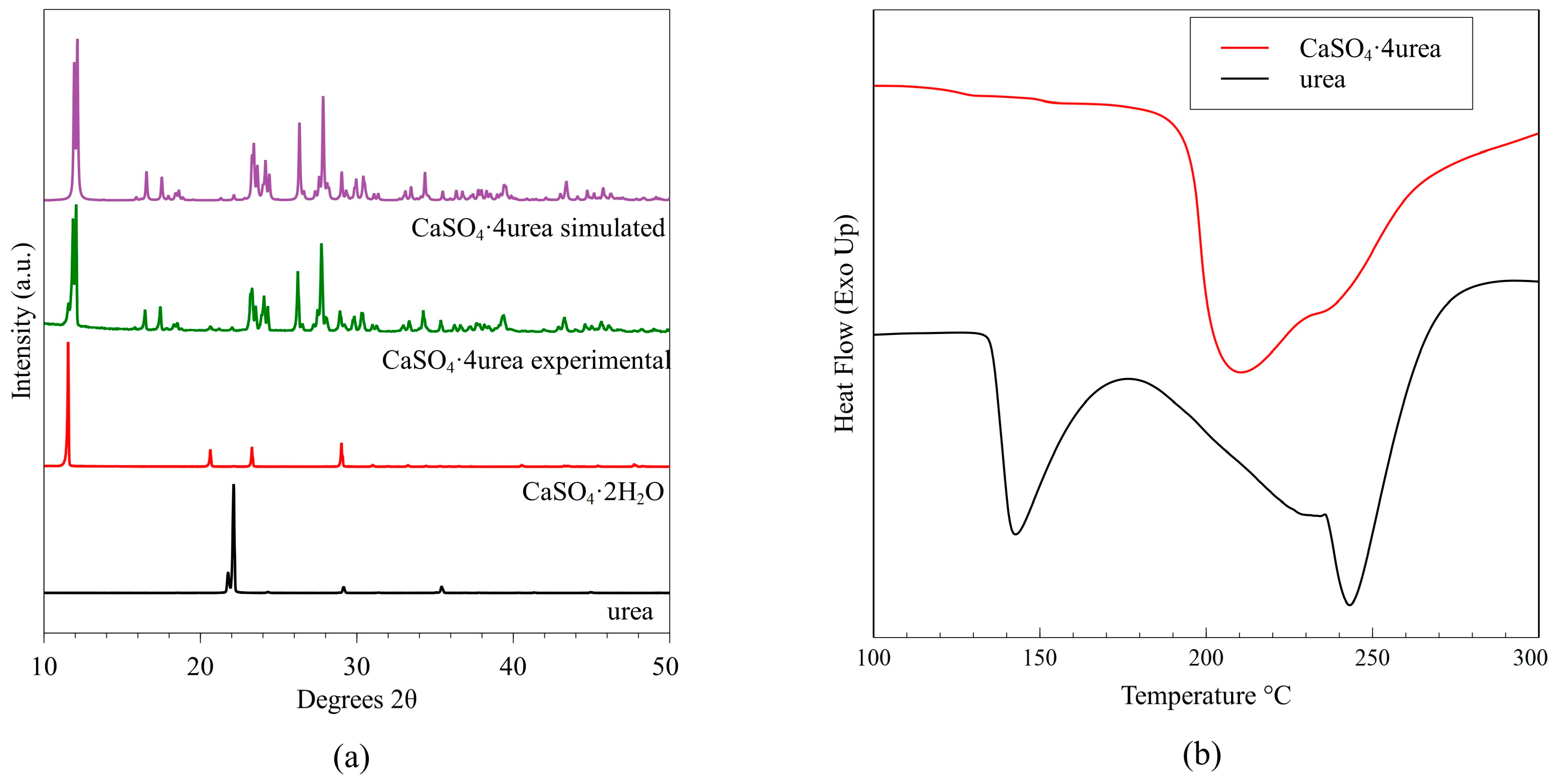

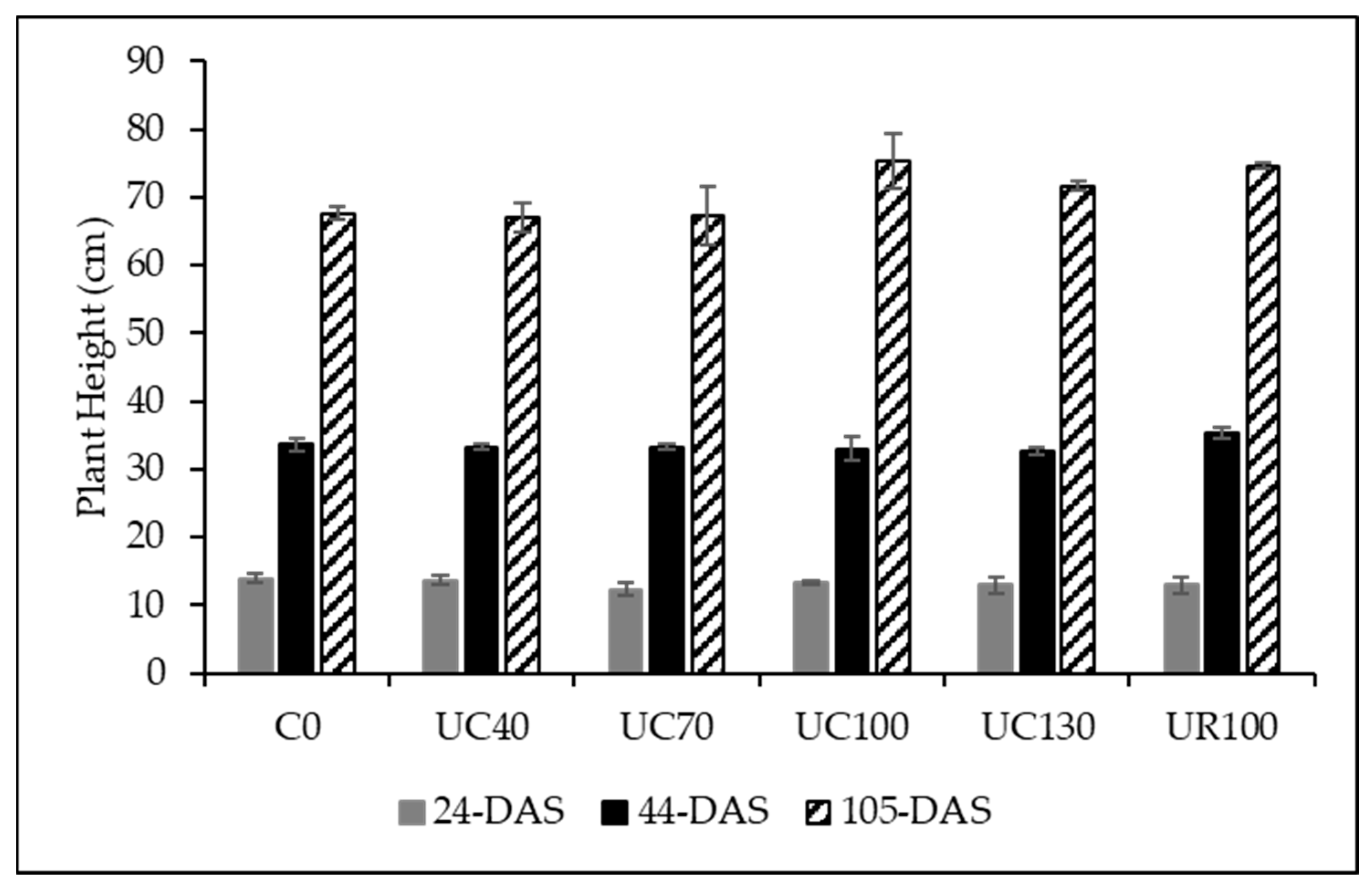
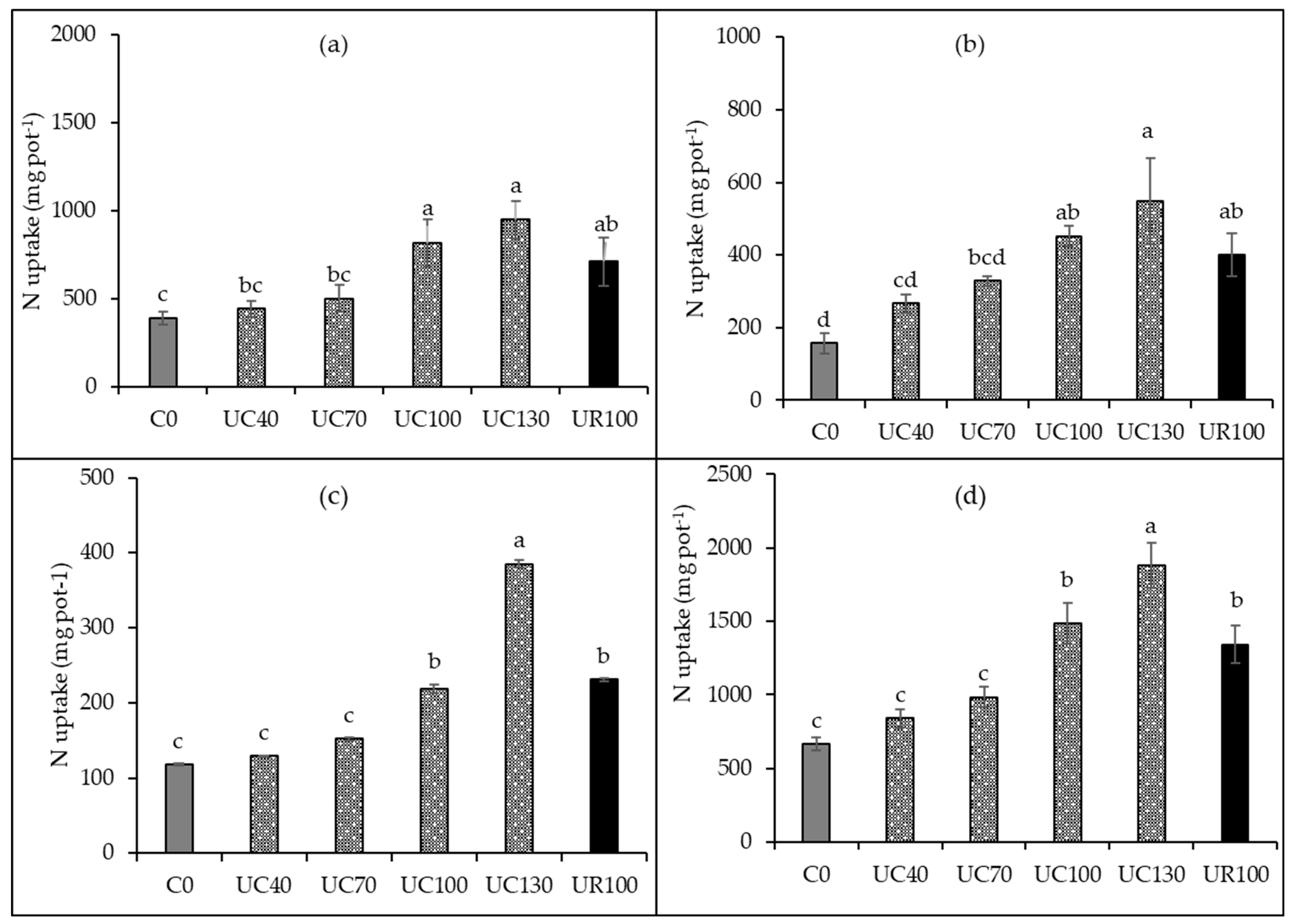
| Soil Properties | Units | Values |
|---|---|---|
| Soil pH (1:1) | - | 7.00 |
| Soil organic matter (SOM) | % | 1.30 |
| Olsen P | μg g−1 | 27.6 |
| Nitrate-N | μg g−1 | 40.0 |
| Potassium | μg g−1 | 517 |
| Calcium | μg g−1 | 2064 |
| Magnesium | μg g−1 | 342 |
| Sodium | μg g−1 | 13.0 |
| Sulfate-S | μg g−1 | 8.60 |
| Zinc | μg g−1 | 0.73 |
| Iron | μg g−1 | 8.30 |
| Manganese | μg g−1 | 9.91 |
| Copper | μg g−1 | 0.97 |
| Cation exchange capacity (CEC) | meq/100 g−1 | 14.5 |
| Treatments † | pH | SOM | Nitrate-N | Phosphorus | Potassium | Calcium | Magnesium | Sulphate-S |
|---|---|---|---|---|---|---|---|---|
| - | mg g−1 | -----------------------------------------μg g−1----------------------------------------- | ||||||
| C0 | 7.77 ± 0.03 | 15.7 ± 0.33 | 4.70 ± 0.17 b | 17.0 ± 0.17 | 488 ± 11.8 | 2092 ± 33.1 | 395 ± 17.1 b | 7.20 ± 0.17 c |
| UC40 | 7.77 ± 0.03 | 15.3 ± 0.88 | 4.30 ± 0.12 b | 16.6 ± 0.15 | 462 ± 27.0 | 2046 ± 15.9 | 356 ± 23.4 b | 7.43 ± 1.27 bc |
| UC70 | 7.77 ± 0.12 | 15.0 ± 1.00 | 6.10 ± 1.73 b | 14.8 ± 1.13 | 457 ± 18.8 | 2096 ± 136 | 405 ± 31.6 b | 15.5 ± 4.18 bc |
| UC100 | 7.60 ± 0.21 | 16.3 ± 0.33 | 13.4 ± 5.02 b | 16.0 ± 1.18 | 457 ± 14.9 | 2148 ± 187 | 383 ± 26.6 b | 17.0 ± 0.83 b |
| UC130 | 7.13 ± 0.23 | 17.7 ± 0.33 | 31.6 ± 9.05 a | 17.5 ± 1.99 | 491 ± 5.13 | 2352 ± 140 | 487 ± 38.0 a | 52.4 ± 5.13 a |
| UR100 | 7.57 ± 0.15 | 16.3 ± 0.33 | 12.0 ± 4.06 b | 16.3 ± 0.50 | 491 ± 11.3 | 2182 + 116 | 417 ± 19.1 ab | 15.3 ± 2.05 bc |
| p-value | NS | NS | 0.02 | NS | NS | NS | 0.05 | 0.001 |
| Treatments † | Grain Yield (g Pot−1) | Shoot (g Pot−1) | Root (g Pot−1) | Grain Numbers | Grain N (mg g−1 Grain) | Shoot N (mg g−1 Biomass) | Root N (mg g−1 Biomass) | NUE (mg g−1 Biomass) |
|---|---|---|---|---|---|---|---|---|
| C0 | 17.7 ± 0.61 c | 22.9 ± 3.33 b | 13.0 ± 1.30 b | 617 ± 46.0 c | 22.0 ± 1.40 | 6.86 ± 0.60 d | 8.95 ± 0.40 | - |
| UC40 | 18.0 ± 0.33 bc | 28.1 ± 0.77 ab | 12.1 ± 0.33 b | 537 ± 49.2 c | 24.5 ± 2.40 | 9.55 ± 1.12 cd | 10.65 ± 0.80 | 39.2 ± 6.91 c |
| UC70 | 18.6 ± 1.82 bc | 29.8 ± 1.48 ab | 12.9 ± 0.21 b | 580 ± 42.5 c | 27.3 ± 2.36 | 11.1 ± 0.55 bc | 11.8 ± 1.64 | 45.5 ± 6.41 c |
| UC100 | 33.2 ± 5.83 a | 34.4 ± 4.71 a | 18.4 ± 5.23 b | 1119 ± 190 a | 27.3 ± 7.92 | 13.4 ± 0.92 ab | 12.1 ± 0.57 | 82.0 ± 9.53 ab |
| UC130 | 33.9 ± 3.08 a | 37.9 ± 6.06 a | 31.2 ± 2.89 a | 1098 ± 112 ab | 28.8 ± 4.87 | 14.2 ± 0.89 a | 12.4 ± 0.86 | 96.2 ± 7.98 a |
| UR100 | 26.8 ± 0.18 ab | 34.8 ± 1.87 a | 22.0 ± 5.67 ab | 789 ± 42.0 bc | 26.6 ± 5.31 | 11.4 ± 1.14 abc | 11.5 ± 1.94 | 62.7 ± 12.6 bc |
| p-value | 0.01 | 0.1 | 0.01 | 0.01 | NS | 0.01 | NS | 0.05 |
| Sulfate-S | N2O-N | Shoot | Root | Grain | Grain Number | |
|---|---|---|---|---|---|---|
| Nitrate-N | 0.71 *** | 0.70 *** | 0.42 | 0.43 | 0.45 | 0.48 * |
| Sulfate-S | 0.54 * | 0.48 * | 0.74 *** | 0.64 ** | 0.58 * | |
| N2O-N | 0.20 | 0.37 | 0.30 | 0.22 | ||
| Shoot | 0.68 ** | 0.51 * | 0.53 * | |||
| Root | 0.70 *** | 0.64 *** | ||||
| Grain | 0.95 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bista, P.; Eisa, M.; Ragauskaitė, D.; Sapkota, S.; Baltrusaitis, J.; Ghimire, R. Effect of Urea-Calcium Sulfate Cocrystal Nitrogen Fertilizer on Sorghum Productivity and Soil N2O Emissions. Sustainability 2023, 15, 8010. https://doi.org/10.3390/su15108010
Bista P, Eisa M, Ragauskaitė D, Sapkota S, Baltrusaitis J, Ghimire R. Effect of Urea-Calcium Sulfate Cocrystal Nitrogen Fertilizer on Sorghum Productivity and Soil N2O Emissions. Sustainability. 2023; 15(10):8010. https://doi.org/10.3390/su15108010
Chicago/Turabian StyleBista, Prakriti, Mohamed Eisa, Dovilė Ragauskaitė, Sundar Sapkota, Jonas Baltrusaitis, and Rajan Ghimire. 2023. "Effect of Urea-Calcium Sulfate Cocrystal Nitrogen Fertilizer on Sorghum Productivity and Soil N2O Emissions" Sustainability 15, no. 10: 8010. https://doi.org/10.3390/su15108010
APA StyleBista, P., Eisa, M., Ragauskaitė, D., Sapkota, S., Baltrusaitis, J., & Ghimire, R. (2023). Effect of Urea-Calcium Sulfate Cocrystal Nitrogen Fertilizer on Sorghum Productivity and Soil N2O Emissions. Sustainability, 15(10), 8010. https://doi.org/10.3390/su15108010






