Cupressus sempervirens Essential Oil, Nanoemulsion, and Major Terpenes as Sustainable Green Pesticides against the Rice Weevil
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Insect
2.3. EO Extraction
2.4. Analysis of EO and Identification of Constituents
2.5. Isolation of Main Terpenes
2.6. Nanoemulsion Formulation and Characterization
2.7. Contact Insecticidal Activity
2.8. Fumigation
2.9. Repellence Bioactivity
2.10. AChE Inhibition and Estimation of IC50
2.11. Phytotoxicity
2.12. Toxicity on Earthworm
2.13. Statistical Analysis
3. Results
3.1. Composition of EO
| a,b Components | c RI exp. | d RI lit. | Concentration (%) |
|---|---|---|---|
| 2-Hexanal | 860 | 862 | 0.2 |
| Tricyclene | 918 | 916 | 0.1 |
| a-Thujene | 920 | 921 | 0.4 |
| α-Pinene | 928 | 930 | 46.3 |
| Camphene | 930 | 932 | 1.2 |
| a-Fenchene | 941 | 942 | 0.1 |
| Sabinene | 966 | 967 | 0.6 |
| ß-Pinene | 980 | 980 | 0.9 |
| ß-Myrcene | 988 | 988 | 0.1 |
| α-Phellandrene | 1006 | 1008 | 0.2 |
| δ-3-Carene | 1010 | 1010 | 22.7 |
| α-Terpinene | 1016 | 1018 | 1.3 |
| p-Cymene | 1021 | 1020 | 0.6 |
| Limonene | 1032 | 1029 | 1.6 |
| β-Phellandrene | 1034 | 1032 | 0.2 |
| Z-ß-Ocimene | 1038 | 1037 | 0.2 |
| E-ß-Ocimene | 1044 | 1044 | 0.1 |
| γ-Terpinene | 1054 | 1055 | 0.3 |
| cis-Sabinene hydrate | 1067 | 1066 | 0.4 |
| p-Cymenene | 1070 | 1072 | 0.9 |
| α-Terpinolene | 1085 | 1086 | 1.3 |
| Linalool | 1096 | 1095 | 0.4 |
| trans-Sabinene hydrate | 1099 | 1097 | 1.1 |
| Pinocarveol | 1138 | 1140 | 0.1 |
| Camphor | 1142 | 1144 | 0.2 |
| Pinocarvone | 1158 | 1162 | 1.6 |
| Borneol | 1162 | 1165 | 0.2 |
| Terpinen-4-ol | 1176 | 1174 | 1.1 |
| p-Cymen-8-ol | 1180 | 1181 | 0.4 |
| trans-Pinocarveol | 1182 | 1184 | 0.6 |
| α-Terpineol | 1188 | 1186 | 0.2 |
| Myrtenol | 1192 | 1195 | 0.2 |
| Pulegone | 1235 | 1233 | 0.3 |
| Carvacrol methyl ether | 1241 | 1241 | 0,2 |
| cis-Chrysanthenyl acetate | 1244 | 1242 | 0.1 |
| cis-Piperitone epoxide | 1247 | 1248 | 0.4 |
| trans-Piperitone epoxide | 1251 | 1252 | 0.7 |
| Carvone | 1254 | 1258 | 1.1 |
| Carvenone oxide | 1260 | 1260 | 0.2 |
| Bornyl acetate | 1285 | 1186 | 0.4 |
| Thymol | 1289 | 1288 | 0.3 |
| trans-Sabinyl acetate | 1290 | 1292 | 0.2 |
| Carvacrol | 1296 | 1298 | 0.3 |
| α-Cedrene | 1295 | 1294 | 0.1 |
| α-Copaene | 1372 | 1374 | 0.2 |
| β-Bourbonene | 1382 | 1384 | 0.1 |
| α-Gurjunene | 1408 | 1408 | 0.3 |
| β-Caryophyllene | 1414 | 1417 | 0.1 |
| β-Gurjunene | 1430 | 1432 | 0.2 |
| α-Humulene | 1450 | 1452 | 0.2 |
| Alloaromadendrene | 1472 | 1474 | 0.3 |
| Germacrene D | 1480 | 1478 | 0.3 |
| Bicyclogermacrene | 1496 | 1495 | 0.1 |
| β-Bisabolene | 1508 | 1510 | 0.2 |
| cis-Calamenene | 1544 | 1443 | 0.2 |
| Spathulenol | 1574 | 1576 | 0.6 |
| α-Cedrol | 1596 | 1591 | 5.8 |
| α-Acorenol | 1632 | 1630 | 0.3 |
| β-Acorenol | 1635 | 1637 | 0.2 |
| γ-Cadinol | 1648 | 1649 | 0.1 |
| Cadalene | 1674 | 1674 | 0.1 |
| Manool | 1990 | 1989 | 0.3 |
| Grouped compounds (%) | - | - | |
| Monoterpene hydrocarbons | - | - | 77.1% |
| Oxygenated monoterpenes | - | - | 12.9% |
| Sesquiterpene hydrocarbons | - | - | 9.7% |
| % peaks identified | - | - | 99.7 |
| Total yield % (mL/100 g) | - | - | 0.74 |
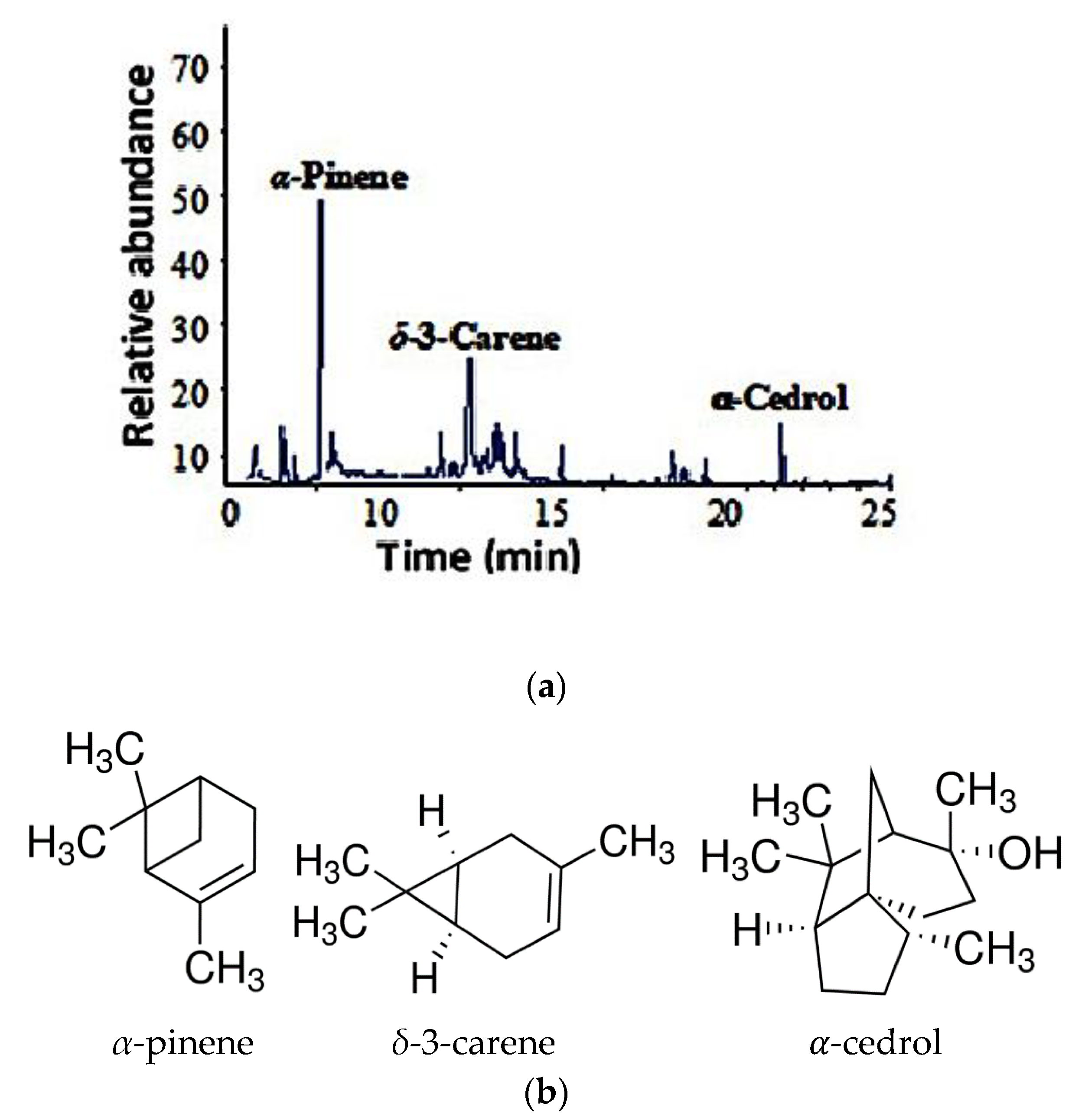
3.2. Nanoemulsion Characterization
3.3. Contact Bioactivity
3.4. Fumigation Bioactivity
3.5. The Dose-Response Mortality
3.6. Repellence Bioactivity
3.7. AChE Inhibition
3.8. Phytotoxicity Assessment
3.9. Toxicity against Earthworms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Sparks, T.C.; Bryant, R.J. Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest Manag. Sci. 2021, 78, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kumar, A.; Singh, P.; Das, S.; Dubey, N.K. Prospects of plant products in the management of insect pests of food grains: Current status and future perspectives. In Natural Bioactive Compounds; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 317–335. [Google Scholar] [CrossRef]
- Hossain, F.; Boddupalli, P.M.; Sharma, R.K.; Pradyumn Kumar, P.; Bahadur Singh, B. Evaluation of quality protein maize genotypes for resistance to stored grain weevil Sitophilus oryzae (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2007, 27, 114–121. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crops Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Okpile, C.; Zakka, U.; Nwosu, L.C. Susceptibility of ten rice brands to weevil, Sitophilus oryzae L. (Coleoptera: Curculionidae), and their influence on the insect and infestation rate. Bull. Natl. Res. Cent. 2021, 45, 2. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Innovation in insecticide discovery: Approaches to the discovery of new classes of insecticides. Pest Manag. Sci. 2022, 78, 3226–3247. [Google Scholar] [CrossRef]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Naturforsch. 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Shawir, M.S.; Abou-Taleb, H.K. Chemical composition, insecticidal and biochemical effects of essential oils of different plant species from Northern Egypt on the rice weevil, Sitophilus oryzae L. J. Pest Sci. 2016, 89, 219–229. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) R.K. Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Bonacucina, G.; Nika, E.P.; Skourti, A.; Georgakopoulou, S.K.C.; Filintas, C.S.; Panariti, A.M.E.; Maggi, F.; Petrelli, R.; Ferrati, M.; et al. The Type of Grain Counts: Effectiveness of Three Essential Oil-Based Nanoemulsions against Sitophilus oryzae. Plants 2023, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Green synthesis of nanomaterials and their biological applications. Nanomaterials 2021, 11, 2842. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bhargava, A. Applications of biosynthesized nanoparticles. In Green Nanoparticles: The Future of Nanobiotechnology; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Almadiy, A.A.; Al-Assiuty, B.A.; Mahnashi, M.H. The essential oil of Schinus terebinthifolius and its nanoemulsion and isolated monoterpenes: Investigation of their activity against Culex pipiens with insights into the adverse effects on non-target organisms. Pest Manag. Sci. 2022, 78, 1035–1047. [Google Scholar] [CrossRef]
- Giunti, G.; Palermo, D.; Laudani, F.; Algeri, G.M.; Campolo, O.; Palmeri, V. Repellence and acute toxicity of a nano-emulsion of sweet orange essential oil toward two major stored grain insect pests. Ind. Crops Prod. 2019, 142, 111869. [Google Scholar] [CrossRef]
- Rawat, P.; Khan, M.F.; Kumar, M.; Tamarkar, A.K.; Srivastava, A.K.; Arya, K.R.; Maurya, R. Constituents from fruits of Cupressus sempervirens. Fitoterapia 2010, 81, 162–166. [Google Scholar] [CrossRef]
- Grunewald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicine, 4th ed.; Medical Economics Co.: Montreal, QC, Canada, 2007; pp. 248–249. [Google Scholar]
- Galovičová, L.; Čmiková, N.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Obradovic, A.D.; et al. Biological Activity of Cupressus sempervirens Essential Oil. Plants 2023, 12, 1097. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Adler, C.; Fontem, D.A.; Bouda, H.; Rechmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Hasnaoui, F.; Zouaoui, I.; Dallali, S.; Dibouba, F. Insecticidal effects of essential oils from six aromatic and medicinal plants on the pine processionary caterpillar (Thaumetopoea pityocampa Schiff). Adv. Med. Plant Res. 2020, 8, 81–88. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Linstorm, P. NIST Chemistry Webbook, NIST Standard Reference Database Number 69. J. Phys. Chem. Ref. Data Monogr. 1998, 9, 1–1951. [Google Scholar]
- Matysik, E.; Wofniak, A.; Paduch, R.; Rejdak, R.; Polak, B.; Donica, H. The new TLC method for separation and determination of multicomponent mixtures of plant extracts. J. Anal. Methods Chem. 2016, 6, 1813581. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M., Jr. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Development (OECD). Guideline for Testing of Chemicals No. 207. Earthworm, Acute Toxicity Tests; OECD—Guideline for Testing Chemicals; OECD: Paris, France, 1984.
- Pavela, R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University: London, UK, 1971; pp. 68–78. [Google Scholar]
- Lee, S.-G. α-Pinene and myrtenol: Complete 1H NMR assignment. Magn. Res. Chem. 2002, 40, 311–312. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Borin Scutti, J.A.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Largo, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Baldwin, S.J.; Clarke, S.E.; Chenery, R.J. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br. J. Clin. Pharmacol. 1999, 48, 424–432. [Google Scholar] [CrossRef]
- Dvorakova, M.; Valterova, I.; Saman, D.; Vanek, T. Biotransformation of (1S)-2-carene and (1S)-3-carene by Picea abies suspension culture. Molecules 2011, 16, 10541–10555. [Google Scholar] [CrossRef]
- Shanker, P.S.; Rao, G.S. Synthesis based on cyclohexadienes. Part 25. Total synthesis of (+/−)-allo-cedrol (khusiol). J. Chem. Soc. Perkin Trans. 1998, 1, 539–548. [Google Scholar]
- Kim, K.H.; Moon, E.; Kim, S.Y.; Choi, S.U.; Son, M.W.; Choi, S.Z.; Lee, K.R. Bioactive sesquiterpenes from the essential oil of Thuja orientalis. Planta Med. 2013, 79, 1680–1684. [Google Scholar] [CrossRef]
- Perez-Hernandez, N.; Gordillo-Roman, B.; Arrieta-Baez, D.; Cerda-Garcia-Rojas, C.M.; Joseph-Nathan, P. Complete 1H NMR assignment of cedranolides. Magn. Res. Chem. 2017, 55, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Naderi, G.A.; Shams Ardekani, M.R.; Sahebkar, A.; Airin, A.; Aslani, S.; Kasher, T.; Emami, S.A. Chemical analysis and biological activities of Cupressus sempervirens var. horizontalis essential oils. Pharm. Biol. 2013, 51, 137–144. [Google Scholar] [CrossRef]
- Selim, S.A.; Adam, M.E.; Hassan, S.M.; Albalawi, A.R. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.). BMC Complement. Altern. Med. 2014, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Akaberi, M.; Khayyat, M.H.; Emami, S.A. Chemical Composition and Antioxidant Activity of Essential Oils from Cupressus sempervirens. var. sempervirens, C. sempervirens. cv. Cereiformis and C. sempervirens var. horizentalis. J. Essent. Oil Bear. Plants 2019, 22, 917–931. [Google Scholar]
- Argui, H.; Ben Youchret-Zalleza, O.; Can Suner, S.; Periz, C.D.; Türker, G.; Ulusoy, S.; Ben-Attia, M.; Büyükkaya, F.; Oral, Y.; Said, H. Isolation, Chemical Composition, Physicochemical Properties, and Antibacterial Activity of Cupressus sempervirens L. Essential Oil. J. Essent. Oil Bear. Plants 2021, 24, 439–452. [Google Scholar] [CrossRef]
- Pansera, M.R.; Silvestre, W.P.; Sartori, V.C. Bioactivity of Cupressus sempervirens and Cupressus lusitanica leaf essential oils on Colletotrichum fructicola. J. Essent. Oil Res. 2023, 35, 51–59. [Google Scholar] [CrossRef]
- M’barek, K. Chemical composition and phytotoxicity of Cupressus sempervirens leaves against crops. J. Essent. Oil Bear. Plants 2016, 19, 1582–1599. [Google Scholar] [CrossRef]
- Fadel, H.; Benayache, F.; Chalchat, J.C.; Figueredo, G.; Chalard, P.; Hazmoune, H.; Benayache, S. Essential oil constituents of Juniperus oxycedrus L. and Cupressus sempervirens L. (Cupressaceae) growing in Aures region of Algeria. Nat. Prod. Res. 2021, 35, 2616–2620. [Google Scholar] [CrossRef]
- Miloš, M.; Radonić, A.; Mastelić, J. Seasonal variation in essential oil compositions of Cupressus sempervirens L. J. Essent. Oil Res. 2002, 14, 222–223. [Google Scholar] [CrossRef]
- Nejia, H.; Séverine, C.; Jalloul, B.; Mehrez, R.; Stéphane, C.J. Extraction of essential oil from Cupressus sempervirens: Comparison of global yields, chemical composition and antioxidant activity obtained by hydrodistillation and supercritical extraction. Nat. Prod. Res. 2013, 27, 1795–1799. [Google Scholar] [CrossRef]
- Jain, P.L.B.; Patel, S.R.; Desai, M.A. Patchouli oil: An overview on extraction method, composition and biological activities. J. Essent. Oil Res. 2022, 34, 1–11. [Google Scholar] [CrossRef]
- Landolt, P.J.; Hofstetter, R.W.; Biddick, L.L. Plant essential oils as arrestants and repellents for neonate larvae of the codling moth (Lepidoptera: Tortricidae). Environ. Entomol. 1999, 28, 954–960. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Pitarokili, D.; Papaioannou, F.; Papachristos, D.P.; Koliopoulos, G.; Emmanouel, N.; Tzakou, O.; Michaelakis, A. Essential oil composition, adult repellency and larvicidal activity of eight Cupressaceae species from Greece against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2013, 112, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, J.; Fröhler, C.; Touraud, D.; Kröckel, U.; Geier, M.; Rose, A.; Kunz, W. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flavour Fragr. J. 2009, 24, 160–169. [Google Scholar] [CrossRef]
- Moore, S.J. Plant-based insect repellents. In Insect Repellents Handbook, 2nd ed.; Debboun, M., Frances, S.P., Strickman, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 179–213. [Google Scholar]
- Regnault-Roger, C. The potential of botanical essential oils for insect pest control. Integr. Pest Manag. Rev. 1997, 2, 25–34. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef]
- Khani, M.; Marouf, A.; Amini, S.; Yazdani, D.; Farashiani, M.E.; Ahvazi, M.; Khalighi-Sigaroodi, F.; Hosseini-Gharalari, A. Efficacy of three herbal essential oils against rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). J. Essent. Oil Bear. Plants 2017, 20, 4937–4950. [Google Scholar] [CrossRef]
- Bincy, K.; Remesh, A.V.; Prabhakar, P.R.; Babu, C.S.V. Chemical composition and insecticidal activity of Ocimum basilicum (Lamiaceae) essential oil and its major constituent, estragole against Sitophilus oryzae (Coleoptera: Curculionidae). J. Plant. Dis. Prot. 2022, 130, 529–541. [Google Scholar] [CrossRef]
- Fouad, H.A.; Tavares, W.; Zanuncio, J.C. Toxicity and repellent activity of monoterpene enantiomers to rice weevils (Sitophilus oryzae). Pest. Manag. Sci. 2021, 77, 3500–3507. [Google Scholar] [CrossRef]
- Balasubramani, S.; Rajendhiran, T.; Moola, A.K.; Kumari, R.; Diana, B. Development of nanoemulsion from Vitex negundo L. essential oil and their efficacy of antioxidant, antimicrobial and larvicidal activities (Aedes aegypti L.). Environ. Sci. Pollut. Res. 2017, 24, 15125–15133. [Google Scholar] [CrossRef]
- Dai, L.; Li, W.; Hou, X. Effect of the molecular structure of mixed non-ionic surfactants on the temperature of miniemulsion formation. Colloids Surf. A Physicochem. Eng. Asp. 1997, 125, 27–32. [Google Scholar] [CrossRef]
- Mondal, P.; Laishram, R.; Sarkar, P.; Kumar, R.; Karmakar, R.; Hazra, D.K.; Banerjee, K.; Pal, K.; Choudhury, A. Plant essential oil-based nanoemulsions: A novel asset in the crop protection arsenal. In Agricultural Nanobiotechnology, Biogenic Nanoparticles, Nanofertilizers and Nanoscale Biocontrol Agents; Ghosh, S., Thongmee, S., Kumar, A., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2022; pp. 325–353. [Google Scholar] [CrossRef]
- Reddy, S.R.; Fogler, H.S. Emulsion stability: Determination from turbidity. J. Colloid Interface Sci. 1981, 79, 101–104. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2022, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential Oil-Based Nano-Biopesticides: Formulation and Bioactivity Against the Confused Flour Beetle Tribolium confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Santana, A.S.; Baldin, E.L.L.; dos Santos, T.L.B.; Baptista, Y.A.; dos Santos, M.C.; Lima, A.P.S.; Tanajura, L.S.; Vieira, T.M.; Crotti, A.E.M. Synergism between essential oils: A promising alternative to control Sitophilus zeamais (Coleoptera: Curculionidae). Crop Prot. 2022, 153, 105882. [Google Scholar] [CrossRef]
- Arokiyaraj, C.; Bhattacharyya, K.; Reddy, S. Toxicity and synergistic activity of compounds from essential oils and their effect on detoxification enzymes against Planococcus lilacinus. Front. Plant Sci. 2022, 13, 1016737. [Google Scholar] [CrossRef] [PubMed]
- Tumen, I.; Senol, F.S.; Orhan, I.E. Evaluation of possible in vitro neurobiological effects of two varieties of Cupressus sempervirens (Mediterranean cypress) through their antioxidant and enzyme inhibition actions. Türk. Biyokimya. Dergisi. (Turk. J. Biochem.) 2012, 37, 5–13. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Alimi, D.; Hajri, A.; Jallouli, S.; Sebai, H. Phytochemistry, anti-tick, repellency and anti-cholinesterase activities of Cupressus sempervirens L. and Mentha pulegium L. combinations against Hyalomma scupense (Acari: Ixodidae). Vet. Parasitol. 2022, 303, 109665. [Google Scholar] [CrossRef]
- Almadiy, A.A.; Nenaah, G.E. Bioactivity and safety evaluations of Cupressus sempervirens essential oil, its nanoemulsion and main terpenes against Culex quinquefasciatus Say. Environ. Sci. Pollut. Res. 2022, 29, 13417–13430. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Song, S.Y.; Chang, H.J.; Kim, S.D.; Kwag, E.B.; Park, S.J.; Yoo, H.S. Acute and sub-chronic toxicological evaluation of the herbal product HAD-B1 in Beagle dogs. Toxicol. Rep. 2021, 8, 1819–1829. [Google Scholar] [CrossRef]
- IUCN Centre for Mediterranean Cooperation. A Guide to Medicinal Plants in North Africa. C. sempervirens; IUCN Centre for Mediterranean Cooperation: Malaga, Spain, 2005; p. 106. [Google Scholar]

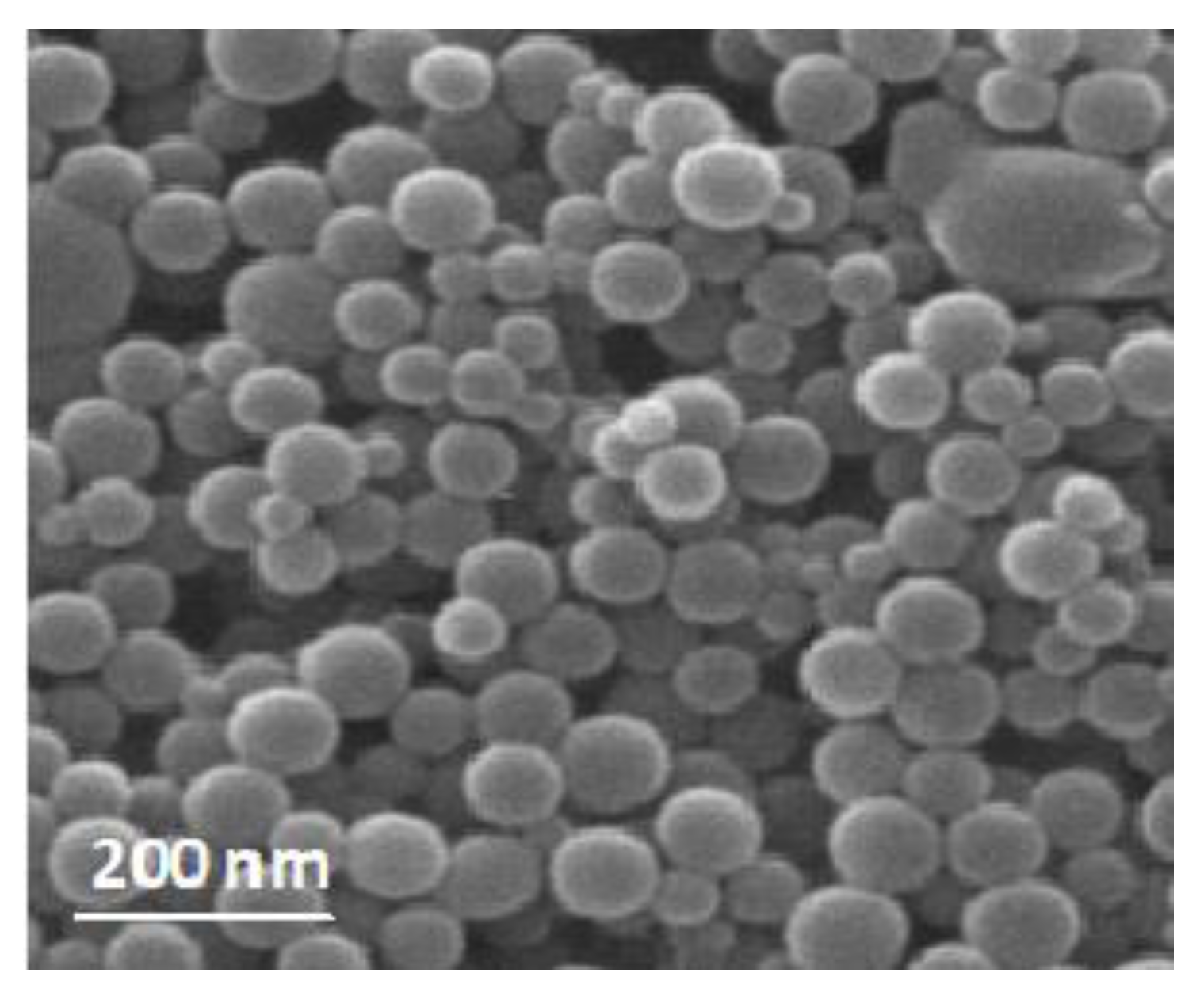


| Storage Period (Days) | Viscosity (mPa·s) | pH | PDI | Size (nm ± S.E.) |
|---|---|---|---|---|
| 0 | 4.1 | 6.1 ± 0.04 c | 0.18 ± 0.03 a | 67.8 ± 3.1 a |
| 1 | 4.1 | 5.8 ± 0.06 b | 0.20 ± 0.05 a | 69.2 ± 3.6 a |
| 10 | 4.4 | 5.6 ± 0.04 b | 0.20 ± 0.02 a | 73.4 ± 4.2 b |
| 20 | 4.8 | 5.1 ± 0.08 a | 0.23 ± 0.03 b | 78.6 ± 5.7 bc |
| 30 | 5.1 | 4.9 ± 0.16 a | 0.25 ± 0.02 bc | 86.1 ± 6.3 c |
| 45 | 5.5 | 4.7 ± 0.14 a | 0.28 ± 0.02 c | 92.1 ± 6.1 d |
| Test Material | Concentration (µL/cm2) | Mortality (% Mean ± S.E.) after Exposure Period | |||
|---|---|---|---|---|---|
| Day1 | Day 2 | Day 4 | Day 7 | ||
| Crude oil | 5.0 | 18.1 ± 2.3 ghi | 25.3 ± 1.1 fg | 44.3 ± 2.6 g | 70.3 ± 2.1 d |
| 10.0 | 27.6 ± 2.1 f | 46.6 ± 2.3 e | 71.1 ± 2.3 d | 84.1 ± 2.2 c | |
| 20.0 | 39.9 ± 2.1 e | 63.6 ± 3.1 d | 88.0 ± 3.2 b | 100.0 ± 0.0 a | |
| 40.0 | 71.3 ± 3.0 b | 93.8 ± 2.6 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Nanoemulsion | 5.0 | 36.3 ± 3.1 e | 48.1 ± 2.1 e | 83.6 ± 3.1 b c | 92.4 ± 2.1 b b |
| 10.0 | 54.4 ± 3.1 d | 70.9 ± 2.1 c | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| 20.0 | 67.8 ± 2.6 bc | 81.3 ± 1.9 b | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| 40.0 | 92.1 ± 3.3 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| α-Cedrol | 5.0 | 15.8 ± 1.1 ijk | 29.3 ± 2.1 f | 36.1 ± 2.1 h | 44.8 ± 2.1 h |
| 10.0 | 25.5 ± 2.1 fg | 41.9 ± 2.3 e | 60.0 ± 2.1 e | 71.0 ± 2.1 d | |
| 20.0 | 36.0 ± 2.3 e | 60.8 ± 2.6 d | 70.9 ± 1.9 d | 83.3 ± 1.7 c | |
| 40.0 | 61.3 ± 3.1 cd | 83.1 ± 3.1 b | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| δ-3-Carene | 5.0 | 11.3 ± 2.0 jk | 16.6 ± 2.1 h | 22.6 ± 3.1 i | 29.3 ± 4.2 j |
| 10.0 | 19.0 ± 3.0 ghi | 30.3 ± 1.9 f | 37.1 ± 2.1 h | 46.7 ± 2.4 h | |
| 20.0 | 27.3 ± 2.3 f | 41.9 ± 1.6 e | 54.3 ± 2.6 ef | 65.9 ± 2.0 f | |
| 40.0 | 38.2 ± 3.3 e | 60.4 ± 3.1 d | 77.3 ± 1.9 cd | 84.3 ± 1.9 c | |
| α-Pinene | 5.0 | 8.3 ± 1.1 k | 14.3 ± 1.1 h | 19.4 ± 1.3 i | 25.4 ± 2.1 k |
| 10.0 | 13.1 ± 1.1 jk | 20.8 ± 1.3 gh | 33.6 ± 1.3 h | 42.6 ± 1.8 hi | |
| 20.0 | 19.6 ± 1.6 ghi | 28.5 ± 2.3 f | 51.3 ± 1.9 f | 56.3 ± 1.7 g | |
| 40.0 | 23.0 ± 2.3 fgh | 44.0 ± 2.9 e | 70.5 ± 2.3 d | 77.5 ± 2.0 e | |
| * F-value | - | 167.90 | 278.00 | 323.96 | 436.45 |
| Test Material | Concentration (µL/L Air) | Mortality (% Mean ± S.E.) after Exposure Period | |||
|---|---|---|---|---|---|
| Day1 | Day 2 | Day 4 | Day 7 | ||
| Crude oil | 2.5 | 15.6 ± 1.3 ijk | 23.1 ± 1.6 fg | 39.3 ± 2.4 e | 53.8 ± 2.1 h |
| 5.0 | 24.1 ± 1.3 hi | 36.0 ± 2.3 e | 63.6 ± 3.2 c | 72.7 ± 2.4 e | |
| 10.0 | 33.6 ± 2.1 efg | 55.3 ± 3.6 cd | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| 20.0 | 55.3 ± 2.3 c | 94.6 ± 2.2 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Nanoemulsion | 2.5 | 27.5 ± 1.1 fgh | 36.1 ± 2.3 e | 66.6 ± 2.1 c | 74.1 ± 2.4 e |
| 5.0 | 49.9 ± 2.3 cd | 60.3 ± 3.6 c | 92.1 ± 2.1 a | 100.0 ± 0.0 a | |
| 10.0 | 68.4 ± 3.1 b | 94.3 ± 3.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| 20.0 | 91.1 ± 3.3 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| α-Cedrol | 2.5 | 13.3 ± 1.6 jk | 20.6 ± 3.3 fg | 34.3 ± 4.5 ef | 49.5 ± 3.4 i |
| 5.0 | 20.3 ± 2.1 hij | 32.3 ± 3.3 e | 49.3 ± 4.5 d | 58.9 ± 2.7 g | |
| 10.0 | 34.8 ± 2.1 ef | 47.9 ± 3.6 d | 68.6 ± 3.3 c | 76.1 ± 2.3 e | |
| 20.0 | 46.4 ± 2.3 d | 70.3 ± 4.1 b | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| δ-3-Carene | 2.5 | 9.3 ± 1.3 k | 15.3 ± 3.3 fg | 30.3 ± 4.5 fg | 40.8 ± 3.4 j |
| 5.0 | 14.3 ± 2.3 jk | 23.0 ± 3.3 f | 40.0 ± 4.5 e | 52.5 ± 2.8 h | |
| 10.0 | 25.0 ± 2.0 gh | 37.3 ± 3.6 e | 53.3 ± 3.3 d | 64.0 ± 3.2 f | |
| 20.0 | 37.3 ± 2.9 e | 56.3 ± 4.1 c | 77.0 ± 3.9 b | 89.9 ± 2.1 c | |
| α-Pinene | 2.5 | 8.9 ± 1.1 k | 12.9 ± 3.3 g | 26.0 ± 3.2 g | 33.0 ± 3.3 g |
| 5.0 | 12.1 ± 1.1 jk | 19.1 ± 1.8 fg | 36.3 ± 4.5 ef | 47.4 ± 3.6 ef | |
| 10.0 | 19.6 ± 2.1 hij | 30.9 ± 1.5 e | 51.6 ± 3.1 d | 59.7± 3.1 g | |
| 20.0 | 33.1 ± 2.3 efg | 53.3 ± 2.3 cd | 70.1 ±2.9 c | 81.2 ±2.6 d | |
| * F-value | - | 134.04 | 265.03 | 317.22 | 302.08 |
| Test Material | Bioassay | LC50 ** (95% fl) | LC95 ** (95% fl) | Slope (±S.E.) | *** χ2 (df = 4) |
|---|---|---|---|---|---|
| Crude oil | Contact (µL/cm2) | 13.3 (11.1–16.3) | 25.9 (19.3–32.2) | 2.1 ± 0.20 | 1.33 |
| Fumigation (µL/L) | 8.7 (7.5–10.1) | 16.3 (13.8–21.3) | 2.0 ± 0.24 | 1.08 | |
| Nanoemulsion | Contact (µL/cm2) | 5.8 (5.3–7.2) | 10.2 (8.6–13.3) | 1.5 ± 0.18 | 0.94 |
| Fumigation (µL/L) | 4.1 (3.7–4.9) | 7.3 (6.1–8.6) | 1.6 ± 0.14 | 0.91 | |
| α-Cedrol | Contact (µL/cm2) | 15.1 (13.4–18.9) | 27.5 (22.9–35.3) | 2.1 ± 0.28 | 2.04 |
| Fumigation (µL/L) | 12.2 (10.5–15.8) | 22.9 (18.6–27.1) | 2.6 ± 0.26 | 2.18 | |
| δ-3-Carene | Contact (µL/cm2) | 30.7 (27.6–36.6) | 55.3 (48.4–64.8) | 2.9 ± 0.32 | 2.77 |
| Fumigation (µL/L) | 17.2 (15.4–21.3) | 39.6 (34.7–47.6) | 2.8 ± 0.40 | 3.06 | |
| α-Pinene | Contact (µL/cm2) | 53.4 (46.3–63.1) | 114.8 (101.8–119.1) | 3.1 ± 0.30 | 3.12 |
| Fumigation (µL/L) | 19.6 (17.3–24.5) | 42.2 (37.0–50.3) | 2.7 ± 0.41 | 3.22 |
| Test Material | Concentration (µL/cm2) | Repellency (% Mean ± S.E.) after Period (h) | |||
|---|---|---|---|---|---|
| 2 | 6 | 12 | 24 | ||
| Crude oil | 0.11 | 29.6 ± 1.1 gh | 51.3 ± 2.3 efg | 62.3 ± 3.1 f | 73.9 ± 2.6 e |
| 0.22 | 44.9 ± 2.3 e | 72.9 ± 2.3 bcd | 80.1 ± 2.9 c | 100.0 ± 0.0 a | |
| 0.44 | 63.3 ± 3.1 b | 80.6 ± 1.9 abc | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| 0.88 | 82.3 ± 3.1 a | 91.1 ± 2.6 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Nanoemulsion | 0.11 | 20.1 ± 1.3 j | 37.1 ± 1.3 gh | 46.1 ± 2.3 h | 61.3 ± 2.1 h |
| 0.22 | 29.3 ± 1.6 gh | 53.3 ± 2.1 ef | 68.6 ± 2.6 e | 80.1 ± 2.2 d | |
| 0.44 | 44.6 ± 2.1 e | 66.1 ± 3.6 cde | 80.9 ± 3.3 c | 89.6± 0.0 b | |
| 0.88 | 59.3 ± 2.6 c | 83.9 ± 2.6 ab | 90.3 ± 3.1 b | 100.0 ± 0.0 a | |
| α-Cedrol | 0.11 | 15.6 ± 1.3 k | 28.6 ± 2.1 hi | 37.3 ± 2.3 i | 43.9 ± 2.3 i |
| 0.22 | 23.6 ± 2.1 i | 44.6 ± 2.3 fg | 60.3 ± 2.1 f | 70.1 ± 2.1 f | |
| 0.44 | 37.9 ± 2.1 f | 58.9 ± 2.6 def | 72.9 ± 1.9 d | 83.3 ± 1.6 c | |
| 0.88 | 48.3 ± 2.3 d | 71.3 ± 3.1 bcd | 83.6 ± 2.1 c | 100.0 ± 0.0 a | |
| δ-3-Carene | 0.11 | 9.6 ± 1.3 lm | 17.9 ± 1.9 ij | 28.6 ± 3.1 k | 33.3 ± 3.1 k |
| 0.22 | 15.3 ± 2.1 k | 27.6 ± 1.6 hij | 36.1 ± 2.1 i | 41.3 ± 2.6 j | |
| 0.44 | 27.6 ± 2.1 h | 43.9 ± 2.6 fg | 51.3 ± 2.6 g | 60.9 ± 2.3 h | |
| 0.88 | 39.1 ± 2.3 f | 51.3 ± 3.3 efg | 62.3 ± 1.9 f | 66.1 ± 1.9 g | |
| α-Pinene | 0.11 | 7.9 ± 1.1 m | 12.6 ± 1.1 k | 20.9 ± 1.6 m | 22.1 ± 2.3 m |
| 0.22 | 11.3 ± 1.3 l | 16.1 ± 1.3 ij | 24.6 ± 1.9 l | 30.6 ± 1.9 l | |
| 0.44 | 18.9 ± 1.6 j | 23.9 ± 2.3 hij | 32.3 ± 1.9 j | 39.3 ± 2.6 j | |
| 0.88 | 31.1 ± 2.1 g | 37.9 ± 2.9 gh | 44.1 ± 2.1 h | 46.1 ± 2.3 i | |
| Control | - | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| * F-value | - | 961.75 | 61.65 | 1237.03 | 2644.87 |
| Plant Material | * IC50 (mM) | (95% Fiducial Limits) | Slope (±S.E.) | ** χ2(df = 5) | p |
|---|---|---|---|---|---|
| Crude oil | 14.03 | (12.21–16.28) | 1.44 ± 0.22 | 2.41 | 0.243 |
| Nanoemulsion | 9.88 | (7.94–11.71) | 1.09± 0.14 | 2.68 | 0.201 |
| α-Cedrol | 17.21 | (15.20–20.07) | 1.30 ± 0.19 | 2.88 | 0.311 |
| δ-3-Carene | 34.54 | (30.00–39.33) | 1.66 ± 0.25 | 3.82 | 0.355 |
| α-Pinene | 39.83 | (34.44–46.12) | 2.08 ± 0.34 | 4.05 | 0.512 |
| Methomyl | 2.17 × 10−3 | (1.73 × 10−3–3.66 × 10−3) | 1.03 ± 0.18 | 2.62 | 0.377 |
| Plant Material | Concentration (µL/mL) | Germination (%) | RL | SL |
|---|---|---|---|---|
| Crude oil | 50 | 90.6 ± 1.4 a | 9.08 ± 0.31 a | 3.32 ± 0.14 ab |
| 100 | 80.3 ± 1.5 ab | 8.89 ± 0.23 a | 3.12 ± 0.11 ab | |
| 150 | 70.1 ± 1.3 b | 8.07 ± 0.18 a | 2.26 ± 0.15 c | |
| Nanoemulsion | 50 | 88.9 ± 1.3 a | 9.03 ± 0.19 a | 3.16 ± 0.12 ab |
| 100 | 76.3 ± 1.2 ab | 8.70 ± 0.20 a | 3.03 ± 0.14 ab | |
| 150 | 65.2 ± 1.2 bc | 7.08 ± 0.28 a | 2.05 ± 0.12 c | |
| α-Cedrol | 50 | 88.2 ± 1.3 a | 9.11 ± 0.41 a | 3.28 ± 0.15 ab |
| 100 | 83.4 ± 1.9 ab | 9.02 ± 0.26 a | 3.20 ± 0.13 ab | |
| 150 | 74.2 ± 1.4 b | 8.24 ± 0.12 a | 2.59 ± 0.17 bc | |
| δ-3-Carene | 50 | 90.6 ± 1.7 a | 9.20 ± 0.18 a | 3.39 ± 0.16 a |
| 100 | 88.7 ± 1.4 a | 9.12 ± 0.31 a | 3.34 ± 0.11 a | |
| 150 | 88.3 ± 1.9 a | 9.01 ± 0.22 a | 3.09 ± 0.11 ab | |
| α-Pinene | 50 | 91.8 ± 1.1 a | 9.24 ± 0.19 a | 3.41 ± 0.13 a |
| 100 | 91.0 ± 1.3 a | 9.23 ± 0.08 a | 3.31 ± 0.11 ab | |
| 150 | 90.2 ± 1.3 a | 9.19 ± 0.22 a | 3.16 ± 0.12 ab | |
| Control | - | 91.7 ± 1.6 a | 9.22 ± 0.32 a | 3.43 ± 0.18 a |
| F-value | - | 4.28 | 1.16 | 7.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almadiy, A.A.; Nenaah, G.E.; Albogami, B.Z.; Shawer, D.M.; Alasmari, S. Cupressus sempervirens Essential Oil, Nanoemulsion, and Major Terpenes as Sustainable Green Pesticides against the Rice Weevil. Sustainability 2023, 15, 8021. https://doi.org/10.3390/su15108021
Almadiy AA, Nenaah GE, Albogami BZ, Shawer DM, Alasmari S. Cupressus sempervirens Essential Oil, Nanoemulsion, and Major Terpenes as Sustainable Green Pesticides against the Rice Weevil. Sustainability. 2023; 15(10):8021. https://doi.org/10.3390/su15108021
Chicago/Turabian StyleAlmadiy, Abdulrhman A., Gomah E. Nenaah, Bader Z. Albogami, Dalia M. Shawer, and Saeed Alasmari. 2023. "Cupressus sempervirens Essential Oil, Nanoemulsion, and Major Terpenes as Sustainable Green Pesticides against the Rice Weevil" Sustainability 15, no. 10: 8021. https://doi.org/10.3390/su15108021
APA StyleAlmadiy, A. A., Nenaah, G. E., Albogami, B. Z., Shawer, D. M., & Alasmari, S. (2023). Cupressus sempervirens Essential Oil, Nanoemulsion, and Major Terpenes as Sustainable Green Pesticides against the Rice Weevil. Sustainability, 15(10), 8021. https://doi.org/10.3390/su15108021








