Current and Previous Green Technologies, Their Efficiency, Associated Problems, and Success Rates to Mitigate M. aeruginosa in Aquatic Environments
Abstract
:1. Introduction
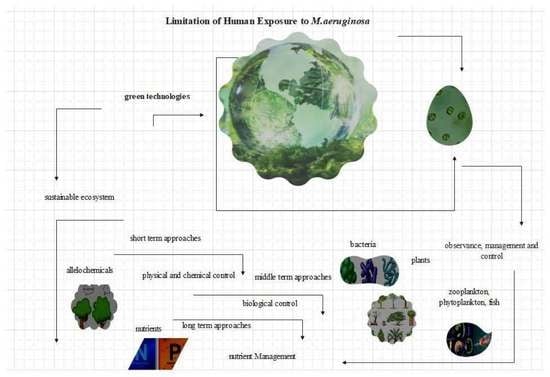
2. Green Technologies to Control Microcystis aeruginosa
- Physical methods
- Chemical methods
- Biological methods.
2.1. Phyical Control
Disadvantages of Physical Control
2.2. Chemical Control
2.2.1. Natural and Modified Clays
Disadvantages of Natural Clays
2.2.2. Modified Clays
Disadvantages of Modified Clays
2.2.3. Eco-Friendly Chemicals
Disadvantages of Eco-Friendly Chemicals
2.3. Biological Control
2.3.1. Microorganisms Control
Bacteria
- (1)
- Disadvantages of Bacteria
Fungi
- (1)
- Disadvantages of Fungi
Virus
- (1)
- Disadvantages of Virus
Phytoplankton and Zooplankton
- (1)
- Disadvantages of Phytoplankton and Zooplankton
2.3.2. Fish
Disadvantages of Fish
2.3.3. Plants
Disadvantages of Plants
3. Summary of Limitations of Green Technologies
- Physical methods are preferred to chemical methods, but they are expensive and are not easy to adapt in field conditions.
- Chemical methods are efficient in M. aeruginosa removal, but they are a source of secondary pollution.
- For the mitigation of M. aeruginosa, many biological control agents existed that includes bacteria, fungi, phages, zooplankton, plants, fish, etc. Many reports of laboratory success have been reported, but when it comes to field management, the success rate appears quite low.
4. Conclusions and Future Solutions
- There is a need to further explore the use of natural clays because of their abundance, cost effectiveness, and easy application. The only problem which has been mentioned in literature is their lower removal efficiency, which has been dealt by using modified clay but still data regarding the application of natural clays on M. aeruginosa is scarce.
- Combined application of ecofriendly chemicals and biological agents should be studied to evaluate their efficiency in M. aeruginosa blooms removal.
- Effect of physical, ecofriendly chemicals and biological agents on nutrient concentrations is also required to understand control mechanism deeply
- Further research is required regarding the effects of all these green technologies, i.e., physical, ecofriendly chemical, and biological methods on non-target organisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agathokleous, E.; Peñuelas, J.; Azevedo, R.A.; Rillig, M.C.; Sun, H.; Calabrese, E.J. Low Levels of Contaminants Stimulate Harmful Algal Organisms and Enrich Their Toxins. Environ. Sci. Technol. 2022, 56, 11991–12002. [Google Scholar] [CrossRef]
- Qin, B.; Zhu, G.; Gao, G.; Zhang, Y.; Li, W.; Paerl, H.W.; Carmichael, W.W. A Drinking Water Crisis in Lake Taihu, China: Linkage to Climatic Variability and Lake Management. Environ. Manag. 2009, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Global Nature Fund. Threatened Lake of the Year 2013: Lake Winnipeg in Canada. 2013. Available online: https://www.globalnature.org/35753/Living-Lakes/Threatened-Lake-2016/Threatened-Lake-2013/resindex.aspx (accessed on 1 April 2023).
- Wassenaar, L.I.; Rao, Y.R. Lake Winnipeg: The forgotten great lake. J. Great Lakes Res. 2012, 38, 1–5. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Mishra, S.; Kane, D.D.; Bade, D.L.; Stanislawczyk, K.; Slodysko, K.N.; Jones, K.W.; Parker, E.M.; Fox, E.L. Cyanobacterial blooms in the central basin of Lake Erie: Potentials for cyanotoxins and environmental drivers. J. Great Lakes Res. 2019, 45, 277–289. [Google Scholar] [CrossRef]
- Francy, D.S.; Brady, A.M.; Stelzer, E.A.; Cicale, J.; Hackney, C.; Dalby, H.D.; Struffolino, P.; Dwyer, D.F. Predicting microcystin concentration action-level exceedances resulting from cyanobacterial blooms in selected lake sites in Ohio. Environ. Monit. Assess. 2020, 192, 513. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Warner, R.A.; Tester, P.A.; Dyble, J.; Fahnenstiel, G.L. Relating spectral shape to cyanobacterial blooms in the Laurentian Great Lakes. Int. J. Remote Sens. 2008, 29, 3665–3672. [Google Scholar] [CrossRef]
- Moreno, I.; Cameán, A.; Tavares, M.J.; Pereira, P.; Franca, S. Toxicity of Cyanobacteria Isolated from the Guadiana River. Aquat. Ecosyst. Health Manag. 2003, 6, 409–413. [Google Scholar] [CrossRef]
- Bowling, L.C.; Merrick, C.; Swann, J.; Green, D.; Smith, G.; Neilan, B.A. Effects of hydrology and river management on the distribution, abundance and persistence of cyanobacterial blooms in the Murray River, Australia. Harmful Algae 2013, 30, 27–36. [Google Scholar] [CrossRef]
- Ke, M.; Feng, L.; Huang, S.; Lu, T.; Yu, Z.; Yang, Y.; Hu, H.; Peijnenburg, W.J.; Feng, L.; Qian, H. Development of a Potentially New Algaecide for Controlling Harmful Cyanobacteria Blooms Which is Ecologically Safe and Selective. J. Agric. Food Chem. 2022, 70, 10134–10143. [Google Scholar] [CrossRef]
- Preston, T.; Stewart, W.D.P.; Reynolds, C.S. Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface. Nature 1980, 288, 365–367. [Google Scholar] [CrossRef]
- Peng, J.; Jin, C.; Wu, Y.; Hou, Z.; Gao, S.; Chu, Z.; Zheng, B. Modeling Non-Point Source Nutrient Loads with Different Cropping Systems in an Agricultural Lake Watershed in Southwestern China: From Field to Watershed Scale. Mathematics 2022, 10, 4047. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, M.; Tsang, D.C.W.; Geng, N.; Lu, D.; Zhu, L.; Igalavithana, A.D.; Dissanayake, P.D.; Rinklebe, J.; Yang, X. Recent advances in control technologies for non point source pollution with nitrogen and phosphorous from agricultural runoff: Current practices and future prospects. Appl. Biol. Chem. 2020, 63, 8. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived Intensification in Harmful Algal Blooms Is a Wave of Cumulative Threat to the Aquatic Ecosystems. Biology 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, S.; Li, L.; Liao, Q.; Chu, W.; Li, H. Ultrasound-enhanced coagulation for Microcystis aeruginosa removal and disinfection by-product control during subsequent chlorination. Water Res. 2021, 201, 117334. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2018, 11, 661–684. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, Y.; Zhen, Z.; Fu, Y.; Liu, Y.; Li, W.; Luo, C.; Ding, A.; Zhang, D. Application and reactivation of magnetic nanoparticles in Microcystis aeruginosa harvesting. Bioresour. Technol. 2015, 190, 82–88. [Google Scholar] [CrossRef]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa: Part I: The key operating conditions. Sep. Purif. Technol. 2006, 52, 84–94. [Google Scholar] [CrossRef]
- Noor, M.H.M.; Wong, S.; Ngadi, N.; Inuwa, I.M.; Opotu, L.A. Assessing the effectiveness of magnetic nanoparticles coagulation/flocculation in water treatment: A systematic literature review. Int. J. Environ. Sci. Technol. 2021, 19, 6935–6956. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Yu, S. Removal of Microcystis aeruginosa using hydrodynamic cavitation: Performance and mechanisms. Water Res. 2014, 62, 241–248. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Huang, Y.; Kong, H.; Lin, Y.; Li, C.; Zeng, G. Laboratory investigation of reducing two algae from eutrophic water treated with light-shading plus aeration. Chemosphere 2009, 76, 1303–1307. [Google Scholar] [CrossRef]
- Lürling, M.; Faassen, E.J. Controlling toxic cyanobacteria: Effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012, 46, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-B.; Baik, S.; Kim, S.; Choi, J.-W.; Lee, S.-H.; Kim, Y.J. The use of ultrasonic frequencies to control the bloom formation, regrowth, and eco-toxicity in Microcystis aeruginosa. Int. J. Environ. Sci. Technol. 2017, 14, 923–932. [Google Scholar] [CrossRef]
- Pan, G.; Zhang, M.-M.; Chen, H.; Zou, H.; Yan, H. Removal of cyanobacterial blooms in Taihu Lake using local soils. I. Equilibrium and kinetic screening on the flocculation of Microcystis aeruginosa using commercially available clays and minerals. Environ. Pollut. 2006, 141, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.-M.; Liu, P.-R.; Liu, X.-Y.; Hong, Y.; Xie, X. Inactivation and Removal Technologies for Algal-Bloom Control: Advances and Challenges. Curr. Pollut. Rep. 2021, 7, 392–406. [Google Scholar] [CrossRef]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef]
- Lee, T.J.; Nakano, K.; Matsumara, M. Ultrasonic Irradiation for Blue-Green Algae Bloom Control. Environ. Technol. 2001, 22, 383–390. [Google Scholar] [CrossRef]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean. Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef]
- Anderson, D.M. Prevention, control and mitigation of harmful algal blooms: Multiple approaches to HAB management. Harmful Algae Manag. Mitig. 2004, 123–130. Available online: https://www.researchgate.net/publication/255649174_Prevention_control_and_mitigation_of_harmful_algal_blooms_multiple_approaches_to_HAB_management (accessed on 1 April 2023).
- Park, J.; Church, J.; Son, Y.; Kim, K.-T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochem. 2017, 38, 326–334. [Google Scholar] [CrossRef]
- Chambers, L.A.; Harvey, E.N. Some histological effects of ultrasonic waves on cells and tissues of the fish lebistes reticulatus and on the larva of rana sylvatica. J. Morphol. 1931, 52, 155–164. [Google Scholar] [CrossRef]
- Chen, G.; Ding, X.; Zhou, W. Study on ultrasonic treatment for degradation of Microcystins (MCs). Ultrason. Sonochem. 2020, 63, 104900. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Qi, Y.; Ma, C. Imaging mass spectrometry of interspecies metabolic exchange revealed the allelopathic interaction between Microcystis aeruginosa and its antagonist. Chemosphere 2020, 259, 127430. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Wu, R. Research progress of phosphorus adsorption by attapulgite and its prospect as a filler of constructed wetlands to enhance phosphorus removal from mariculture wastewater. J. Environ. Chem. Eng. 2022, 10, 108748. [Google Scholar] [CrossRef]
- Talawar, M.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.; Gandhe, B.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Iqbal, A.; Mohammad-Noor, N.; Razali, R.; Yanto, D.H.Y.; Wilson, L.D.; Mahadi, A.H. A Review on The Biological, Physical and Chemical Mitigation of Harmful Algal Bloom. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2022, 17, 95–110. [Google Scholar] [CrossRef]
- Anderson, D.M. Turning back the harmful red tide—Commentary. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Sengco, M.R.; Li, A.; Tugend, K.; Kulis, D.; Anderson, D.M. Removal of red- and brown-tide cells using clay flocculation. I. Laboratory culture experiments with Gymnodinium breve and Aureococcus anophagefferens. Mar. Ecol. Prog. Ser. 2001, 210, 41–53. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Troeger, B.W.; Reed, L.W. Mutual Flocculation of Algae and Clay: Evidence and Implications. Science 1982, 216, 63–65. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; Volume 10. [Google Scholar]
- Pan, G. A Method for Simultaneously Clearing up Harmful Algal Blooms and Harnessing Organic Pollutants to Promote the Primary Productivity in the Sea. UK Patent GB2337749, 11 March 1975. [Google Scholar]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Yu, Z. An eco-environmental assessment of harmful algal bloom mitigation using modified clay. Harmful Algae 2021, 107, 102067. [Google Scholar] [CrossRef]
- Shirota, A. Red tide problem and countermeasures. II. Int. J. Aqua. Fish. Technol. 1989, 1, 195–223. [Google Scholar]
- Anderson, D.M.; Andersen, P.; Bricelj, V.M.; Cullen, J.J.; Rensel, J.J. Monitoring and Management Strategies for Harmful Algal Blooms in Coastal Waters; UNESCO: Paris, France, 2001. [Google Scholar]
- Zeng, G.; Zhang, R.; Liang, D.; Wang, F.; Han, Y.; Luo, Y.; Gao, P.; Wang, Q.; Wang, Q.; Yu, C.; et al. Comparison of the Advantages and Disadvantages of Algae Removal Technology and Its Development Status. Water 2023, 15, 1104. [Google Scholar] [CrossRef]
- Getchis, T.; Shumway, S. Harmful Algae: An Executive Summary. Connecticut Sea Grant College Program; CTSG-17-08; Background and Purpose: Groton, CT, USA, 2017; 16p. [Google Scholar]
- Yu, Z.; Sengco, M.R.; Anderson, D.M. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. J. Appl. Phycol. 2004, 16, 101–110. [Google Scholar] [CrossRef]
- Xia, T.; Wang, S.-Q.; Yan, H.; Gao, Z.-Z.; Qiu, Y.; Yuan, F.; Huang, G.-Y.; Zhou, J. Effect of hydrodynamic condition on algae control based on montmorillonite modified lime-ceramic sand-lake sediments. Water Qual. Res. J. 2022, 57, 200–214. [Google Scholar] [CrossRef]
- Kang, L.; Mucci, M.; Lürling, M. Compounds to mitigate cyanobacterial blooms affect growth and toxicity of Microcystis aeruginosa. Harmful Algae 2022, 118, 102311. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, P.; Xu, B.; Wang, L.; Ma, G.; Chen, S.; Tan, F.; Shao, Y.; Zhang, L.; Yang, Z.; et al. A novel technology using iron in a coupled process of moderate preoxidation–hybrid coagulation to remove cyanobacteria in drinking water treatment plants. J. Clean. Prod. 2022, 342, 130947. [Google Scholar] [CrossRef]
- Noyma, N.P.; de Magalhães, L.; Furtado, L.L.; Mucci, M.; van Oosterhout, F.; Huszar, V.L.M.; Marinho, M.M.; Lürling, M. Controlling cyanobacterial blooms through effective flocculation and sedimentation with combined use of flocculants and phosphorus adsorbing natural soil and modified clay. Water Res. 2015, 97, 26–38. [Google Scholar] [CrossRef]
- Liu, G.; Fan, C.; Zhong, J.; Zhang, L.; Ding, S.; Yan, S.; Han, S. Using hexadecyl trimethyl ammonium bromide (CTAB) modified clays to clean the Microcystis aeruginosa blooms in Lake Taihu, China. Harmful Algae 2010, 9, 413–418. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Liu, X.; Cai, D.; Feng, H.; Miao, C.; Wang, X.; Wu, Z.; Yu, Z. Flocculation of harmful algal blooms by modified attapulgite and its safety evaluation. Water Res. 2011, 45, 2855–2862. [Google Scholar] [CrossRef]
- Miao, C.; Tang, Y.; Zhang, H.; Wu, Z.; Wang, X. Harmful algae blooms removal from fresh water with modified vermiculite. Environ. Technol. 2014, 35, 340–346. [Google Scholar] [CrossRef]
- Jin, Y.; Pei, H.; Hu, W.; Zhu, Y.; Xu, H.; Ma, C.; Sun, J.; Li, H. A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci. Total Environ. 2017, 583, 496–504. [Google Scholar] [CrossRef]
- Xie, P.; Liu, J. Practical Success of Biomanipulation using Filter-Feeding Fish to Control Cyanobacteria Blooms: A Synthesis of Decades of Research and Application in a Subtropical Hypereutrophic Lake. Sci. World J. 2001, 1, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; O’brien, A.M.; Lins, T.F.; Shahmohamadloo, R.S.; Almirall, X.O.; Rochman, C.M.; Sinton, D. Effects of Hydrogen Peroxide on Cyanobacterium Microcystis aeruginosa in the Presence of Nanoplastics. ACS ES&T Water 2021, 1, 1596–1607. [Google Scholar] [CrossRef]
- Gao, L.; Pan, X.; Zhang, D.; Mu, S.; Lee, D.-J.; Halik, U. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res. 2015, 69, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Long, J.; Song, G.; Mi, W.; Bi, Y. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, J.M. Phytoplankton and episodic suspended sediment loading: Phosphate partitioning and mechanisms for survival. Limnol. Oceanogr. 1992, 37, 974–988. [Google Scholar] [CrossRef]
- Huang, I.S.; Zimba, P.V. Hydrogen peroxide, an ecofriendly remediation method for controlling Microcystis aeruginosa toxic blooms. J. Appl. Phycol. 2020, 32, 3133–3142. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, S.; Kim, H.S.; Park, C.; Choi, Y.-E. Adsorption Strategy for Removal of Harmful Cyanobacterial Species Microcystis aeruginosa Using Chitosan Fiber. Sustainability 2020, 12, 4587. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, S.; Wang, P.; Chen, H.; Zhang, X.; Liu, L.; Li, X.; Ding, Y. A possible environmental-friendly removal of Microcystis aeruginosa by using pyroligneous acid. Ecotoxicol. Environ. Saf. 2020, 205, 111159. [Google Scholar] [CrossRef] [PubMed]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Xian, X.; Li, X.; Ye, C.; Wan, K.; Feng, M.; Luo, C.; Yu, X. Higher sensitivity to Cu2+ exposure of Microcystis aeruginosa in late lag phase is beneficial to its control. Water Res. 2022, 214, 118207. [Google Scholar] [CrossRef]
- Facey, J.A.; Violi, J.P.; King, J.J.; Sarowar, C.; Apte, S.C.; Mitrovic, S.M. The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production. Toxins 2022, 14, 812. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Huang, Y.; Ma, J.; Zhang, S.; Liu, J.; Li, T.; Song, L. Toxicity of the disinfectant benzalkonium chloride (C14) towards cyanobacterium Microcystis results from its impact on the photosynthetic apparatus and cell metabolism. J. Environ. Sci. 2024, 135, 198–209. [Google Scholar] [CrossRef]
- Tsai, K.-P. Effects of two copper compounds on Microcystis aeruginosa cell density, membrane integrity, and microcystin release. Ecotoxicol. Environ. Saf. 2015, 120, 428–435. [Google Scholar] [CrossRef]
- Wu, H.; Wei, G.; Tan, X.; Li, L.; Li, M. Species-dependent variation in sensitivity of Microcystis species to copper sulfate: Implication in algal toxicity of copper and controls of blooms. Sci. Rep. 2017, 7, 40393. [Google Scholar] [CrossRef]
- Iwinski, K.J.; Calomeni, A.J.; Geer, T.D.; Rodgers, J.H., Jr. Cellular and aqueous microcystin-LR following laboratory exposures of Microcystis aeruginosa to copper algaecides. Chemosphere 2016, 147, 74–81. [Google Scholar] [CrossRef]
- García-Villada, L.; Rico, M.; Altamirano, M.; Sánchez-Martín, L.; López-Rodas, V.; Costas, E. Occurrence of copper resistant mutants in the toxic cyanobacteria Microcystis aeruginosa: Characterisation and future implications in the use of copper sulphate as algaecide. Water Res. 2004, 38, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Aragão, M.C.; dos Reis, K.C.; Rocha, M.A.M.; Guedes, D.d.O.; dos Santos, E.C.; Capelo-Neto, J. Removal of Dolichospermum circinale, Microcystis aeruginosa, and their metabolites using hydrogen peroxide and visible light. Aquat. Toxicol. 2021, 232, 105735. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, H.; Wen, Y. Effect of hydrogen peroxide on Microcystic aeruginosa: Role of cytochromes P450. Sci. Total Environ. 2018, 626, 211–218. [Google Scholar] [CrossRef]
- Li, D.; Kang, X.; Chu, L.; Wang, Y.; Song, X.; Zhao, X.; Cao, X. Algicidal mechanism of Raoultella ornithinolytica against Microcystis aeruginosa: Antioxidant response, photosynthetic system damage and microcystin degradation. Environ. Pollut. 2021, 287, 117644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, X.; Jiang, X.; Liu, L.; Wang, H. Algicidal activity of Aspergillus niger induced by calcium ion as signal molecule on Microcystis aeruginosa. Algal Res. 2021, 60, 102536. [Google Scholar] [CrossRef]
- Kong, Y.; Ji, L.; Wang, Y.; Li, J.; Lu, H.; Mo, S.; Wang, X.; Zhu, L.; Xu, X.; Zheng, X. Combined Effect of NZVI and H2O2 on the Cyanobacterium Microcystis aeruginosa: Performance and Mechanism. Nanomaterials 2022, 12, 3017. [Google Scholar] [CrossRef] [PubMed]
- Anam, G.B.; Guda, D.R.; Ahn, Y.-H. Impact of melatonin on the hydrogen peroxide treatment efficacy in Microcystis aeruginosa: Cell growth, oxidative stress response, and gene transcription. Chemosphere 2022, 307, 136036. [Google Scholar] [CrossRef] [PubMed]
- Kansole, M.M.R.; Lin, T.-F. Impacts of Hydrogen Peroxide and Copper Sulfate on the Control of Microcystis aeruginosa and MC-LR and the Inhibition of MC-LR Degrading Bacterium Bacillus sp. Water 2017, 9, 255. [Google Scholar] [CrossRef]
- Ding, Y.; Gan, N.; Li, J.; Sedmak, B.; Song, L. Hydrogen peroxide induces apoptotic-like cell death in Microcystis aeruginosa (Chroococcales, Cyanobacteria) in a dose-dependent manner. Phycologia 2012, 51, 567–575. [Google Scholar] [CrossRef]
- Huo, X.; Chang, D.-W.; Tseng, J.-H.; Burch, M.D.; Lin, T.-F. Exposure of Microcystis aeruginosa to Hydrogen Peroxide under Light: Kinetic Modeling of Cell Rupture and Simultaneous Microcystin Degradation. Environ. Sci. Technol. 2015, 49, 5502–5510. [Google Scholar] [CrossRef]
- Lürling, M.; Meng, D.; Faassen, E.J. Effects of Hydrogen Peroxide and Ultrasound on Biomass Reduction and Toxin Release in the Cyanobacterium, Microcystis aeruginosa. Toxins 2014, 6, 3260–3280. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Jeon, M.-S.; Choi, Y.-E.; Jung, J. Toxicity Reduction of Microcystis Aeruginosa Using Microbubble Ozonation. Ozone Sci. Eng. 2022, 45, 182–190. [Google Scholar] [CrossRef]
- Coral, L.A.; Zamyadi, A.; Barbeau, B.; Bassetti, F.J.; Lapolli, F.R.; Prévost, M. Oxidation of Microcystis aeruginosa and Anabaena flos-aquae by ozone: Impacts on cell integrity and chlorination by-product formation. Water Res. 2013, 47, 2983–2994. [Google Scholar] [CrossRef]
- Gu, J.; Huang, Z.; Fan, H.; Jin, Z.; Yan, Z.; Zhang, J. Mineralogy, geochemistry, and genesis of lateritic bauxite deposits in the Wuchuan–Zheng’an–Daozhen area, Northern Guizhou Province, China. J. Geochem. Explor. 2013, 130, 44–59. [Google Scholar] [CrossRef]
- Nam, G.; Mohamed, M.M.; Jung, J. Novel treatment of Microcystis aeruginosa using chitosan-modified nanobubbles. Environ. Pollut. 2021, 292, 118458. [Google Scholar] [CrossRef]
- Pei, H.-Y.; Ma, C.-X.; Hu, W.-R.; Sun, F. The behaviors of Microcystis aeruginosa cells and extracellular microcystins during chitosan flocculation and flocs storage processes. Bioresour. Technol. 2014, 151, 314–322. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, H.; Dong, Z.; Yang, Z. Allelopathic effect of Chitosan Fiber on the growth of Microcystis aeruginosa. E3S Web Conf. 2020, 206, 02012. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, S.; Feng, H.; Wang, Y.; Li, S.; Zhou, X.; Wang, M.; Rei, L. Antifouling performance of in situ synthesized chitosan-zinc oxide hydrogel film against alga M. aeruginosa. Int. J. Biol. Macromol. 2022, 200, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hu, W.; Pei, H.; Xu, H.; Pei, R. Enhancing integrated removal of Microcystis aeruginosa and adsorption of microcystins using chitosan-aluminum chloride combined coagulants: Effect of chemical dosing orders and coagulation mechanisms. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 258–267. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Levchuk, I.; Rueda-Márquez, J.J.; Manzano, M. Combination of solar disinfection (SODIS) with H2O2 for enhanced disinfection of marine aquaculture effluents. Sol. Energy 2019, 177, 144–154. [Google Scholar] [CrossRef]
- Folt, C.L.; Chen, C.Y.; Moore, M.V.; Burnaford, J. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 1999, 44, 864–877. [Google Scholar] [CrossRef]
- Jackson, M.C.; Loewen, C.J.; Vinebrooke, R.D.; Chimimba, C.T. Net effects of multiple stressors in freshwater ecosystems: A meta-analysis. Glob. Chang. Biol. 2016, 22, 180–189. [Google Scholar] [CrossRef]
- Schinegger, R.; Palt, M.; Segurado, P.; Schmutz, S. Untangling the effects of multiple human stressors and their impacts on fish assemblages in European running waters. Sci. Total Environ. 2016, 573, 1079–1088. [Google Scholar] [CrossRef]
- Gumbo, R.J.; Ross, G.; Cloete, E.T. Biological control of Microcystis dominated harmful algal blooms. Afr. J. Biotechnol. 2008, 7, 4765–4773. [Google Scholar]
- Kim, B.-H.; Sang, M.; Hwang, S.-J.; Han, M.-S. In situ bacterial mitigation of the toxic cyanobacterium Microcystis aeruginosa: Implications for biological bloom control. Limnol. Oceanogr. Methods 2008, 6, 513–522. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Z.; Zhang, J.; Roger, S.-F.; Luo, X. Allelopathically inhibitory effects of eucalyptus extracts on the growth of Microcystis aeruginosa. Chemosphere 2019, 225, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, P.; Wu, X.-P. Grazing on toxic and non-toxic Microcystis aeruginosa PCC7820 by Unio douglasiae and Corbicula fluminea. Limnology 2009, 10, 1–5. [Google Scholar] [CrossRef]
- Han, Y.; Zheng, J.; Jiang, C.; Zhang, F.; Wei, L.; Zhu, L. Hydrochloric acid-modified algal biochar for the removal of Microcystis aeruginosa: Coagulation performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 108903. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kang, Y.-H.; Choi, H.-J.; Ka, S.-K.; Han, M.-S. Environment. Antialgal interactions of biological control agents on cyanobacterium and diatom blooms in vitro. Korean J. Ecol. Environ. 2005, 38, 494–502. [Google Scholar]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Gerphagnon, M.; Macarthur, D.J.; Latour, D.; Gachon, C.M.M.; Van Ogtrop, F.; Gleason, F.H.; Sime-Ngando, T. Microbial players involved in the decline of filamentous and colonial cyanobacterial blooms with a focus on fungal parasitism. Environ. Microbiol. 2015, 17, 2573–2587. [Google Scholar] [CrossRef]
- Park, B.S.; Park, C.-S.; Shin, Y.; Yoon, S.; Han, M.-S.; Kang, Y.-H. Different Algicidal Modes of the Two Bacteria Aeromonas bestiarum HYD0802-MK36 and Pseudomonas syringae KACC10292T against Harmful Cyanobacteria Microcystis aeruginosa. Toxins 2022, 14, 128. [Google Scholar] [CrossRef]
- Das Nishu, S.; Kang, Y.; Han, I.; Jung, T.Y.; Lee, T.K. Nutritional status regulates algicidal activity of Aeromonas sp. L23 against cyanobacteria and green algae. PLoS ONE 2019, 14, e0213370. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, H.; Tang, S.; Peng, H.; Yin, D.; Yang, Y.; Liu, Z.; Dang, Z. Physiological responses of Microcystis aeruginosa against the algicidal bacterium Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2016, 127, 214–221. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, Y.; Li, P.; Zhang, K.; Jiang, X.; Zheng, T.; Wang, H. Stress of algicidal substances from a bacterium Exiguobacterium sp. h10 on Microcystis aeruginosa. Lett. Appl. Microbiol. 2017, 64, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Y.; Tang, S.; Liang, J.; Lin, W.; Luo, L. Isolation and Identification of Algicidal Compound from Streptomyces and Algicidal Mechanism to Microcystis aeruginosa. PLoS ONE 2013, 8, e76444. [Google Scholar] [CrossRef]
- Caiola, M.G.; Pellegrini, S. Lysis of microcystis aeruginosa (kütz.) By bdellovibrio-like bacteria. J. Phycol. 1984, 20, 471–475. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, J.; Yang, L.; Chen, H.; Zhang, S.; Zhao, H.; Zhang, N. Allelopathic control of cyanobacterial blooms by periphyton biofilms. Environ. Microbiol. 2011, 13, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Jianhong, Q.; Shaobin, L. The growth of Bacillus sp. and Microcystis aeruginosa and their competition for resources. J. Zhanjiang Ocean. Univ. 2002, 22, 13–18. [Google Scholar]
- Zhang, C.; Massey, I.Y.; Liu, Y.; Huang, F.; Gao, R.; Ding, M.; Xiang, L.; He, C.; Wei, J.; Li, Y.; et al. Identification and characterization of a novel indigenous algicidal bacterium Chryseobacterium species against Microcystis aeruginosa. J. Toxicol. Environ. Health Part A 2019, 82, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ye, Q.; Zhang, F.; Shao, X.; Fan, Y.; Zhu, X.; Li, Y.; Yao, L.; Tian, Y.; Zheng, T.; et al. Flocculating properties and potential of Halobacillus sp. strain H9 for the mitigation of Microcystis aeruginosa blooms. Chemosphere 2019, 218, 138–146. [Google Scholar] [CrossRef]
- Gumbo, J.; Cloete, T. The mechanism of Microcystis aeruginosa death upon exposure to Bacillus mycoides. Phys. Chem. Earth Parts A/B/C 2011, 36, 881–886. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Hu, Z.; Chen, D.; Li, F.; Huang, X.; Li, C. Transcriptome Analysis Reveals the Algicidal Mechanism of Brevibacillus laterosporus against Microcystis aeruginosa through Multiple Metabolic Pathways. Toxins 2022, 14, 492. [Google Scholar] [CrossRef]
- Jing, W.; Sui, G.; Liu, S. Characteristics of a Microcystin-LR Biodegrading Bacterial Isolate: Ochrobactrum sp. FDT5. Bull. Environ. Contam. Toxicol. 2014, 92, 119–122. [Google Scholar] [CrossRef]
- Mu, R.; He, Y.; Liu, S.; Wang, X.; Fan, Z. The Algicidal Characteristics of One Algae-Lysing FDT5 Bacterium on Microcystis aeruginosa. Geomicrobiol. J. 2009, 26, 516–521. [Google Scholar] [CrossRef]
- Sun, P.; Lin, H.; Wang, G.; Zhang, X.; Zhang, Q.; Zhao, Y. Wheat Bran Enhances the Cytotoxicity of Immobilized Alcaligenes aquatilis F8 against Microcystis aeruginosa. PLoS ONE 2015, 10, e0136429. [Google Scholar] [CrossRef]
- Xuan, H.; Dai, X.; Li, J.; Zhang, X.; Yang, C.; Luo, F. A Bacillus sp. strain with antagonistic activity against Fusarium graminearum kills Microcystis aeruginosa selectively. Sci. Total Environ. 2017, 583, 214–221. [Google Scholar] [CrossRef]
- Benegas, G.R.S.; Bernal, S.P.F.; de Oliveira, V.M.; Passarini, M.R.Z. Antimicrobial activity against Microcystis aeruginosa and degradation of microcystin-LR by bacteria isolated from Antarctica. Environ. Sci. Pollut. Res. 2021, 28, 52381–52391. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Zhu, J.P.; Li, M.; Xue, Q.Q.; Zeng, Y.; Wang, Z.P. Effects of freshwater bacterial siderophore on Microcystis and Anabaena. Biol. Control. 2014, 78, 42–48. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Huang, Q.; Xiao, X.; Wang, X.; Zhang, F.; Wang, X.; Liu, Y.; Hu, C. Isolation, identification and characterization of phytoplankton-lytic bacterium CH-22 against Microcystis aeruginosa. Limnologica 2011, 41, 70–77. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Yang, C.; Ding, M.; Hamilton, P.B.; Zhang, X.; Yang, C.; Zhnag, L.; Dai, X. A Streptomyces globisporus strain kills Microcystis aeruginosa via cell-to-cell contact. Sci. Total Environ. 2021, 769, 144489. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Purohit, H.J.; Qureshi, A. Genomic insight for algicidal activity in Rhizobium strain AQ_MP. Arch. Microbiol. 2021, 203, 5193–5203. [Google Scholar] [CrossRef]
- Manage, P.M.; Kawabata, Z.; Nakano, S.-I. Algicidal effect of the bacterium Alcaligenes denitrificans on Microcystis spp. Aquat. Microb. Ecol. 2000, 22, 111–117. [Google Scholar] [CrossRef]
- Yang, L.; Maeda, H.; Yoshikawa, T.; Zhou, G.-Q. Algicidal effect of bacterial isolates of Pedobacter sp. against cyanobacterium Microcystis aeruginosa. Water Sci. Eng. 2012, 5, 375–382. [Google Scholar]
- Zhang, B.-H.; Chen, W.; Li, H.-Q.; Zhou, E.-M.; Hu, W.-Y.; Duan, Y.-Q.; Mohamad, O.A.; Gao, R.; Li, W.-J. An antialgal compound produced by Streptomyces jiujiangensis JXJ 0074T. Appl. Microbiol. Biotechnol. 2015, 99, 7673–7683. [Google Scholar] [CrossRef] [PubMed]
- Su, J.F.; Shao, S.C.; Ma, F.; Lu, J.S.; Zhang, K. Bacteriological control by Raoultella sp. R11 on growth and toxins production of Microcystis aeruginosa. Chem. Eng. J. 2016, 293, 139–150. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, C.; Pan, Z.; Han, S. Synergistic removal of Microcystis aeruginosa by a novel H2O2 pre-oxidation enhanced pressurization method: Performance and mechanism. J. Clean. Prod. 2022, 379, 134745. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Park, C.-S.; Han, M.-S. Pseudomonas aeruginosa UCBPP-PA14 a useful bacterium capable of lysing Microcystis aeruginosa cells and degrading microcystins. J. Appl. Phycol. 2012, 24, 1517–1525. [Google Scholar] [CrossRef]
- Yi, Y.-L.; Yu, X.-B.; Zhang, C.; Wang, G.-X. Growth inhibition and microcystin degradation effects of Acinetobacter guillouiae A2 on Microcystis aeruginosa. Res. Microbiol. 2015, 166, 93–101. [Google Scholar] [CrossRef]
- Santos, A.A.; Soldatou, S.; de Magalhães, V.F.; Azevedo, S.M.F.O.; Camacho-Muñoz, D.; Lawton, L.A.; Edwards, C. Degradation of Multiple Peptides by Microcystin-Degrader Paucibacter toxinivorans (2C20). Toxins 2021, 13, 265. [Google Scholar] [CrossRef]
- Crettaz-Minaglia, M.; Fallico, M.; Aranda, O.; Juarez, I.; Pezzoni, M.; Costa, C.; Andrinolo, D.; Giannuzzi, L. Effect of temperature on microcystin-LR removal and lysis activity on Microcystis aeruginosa (cyanobacteria) by an indigenous bacterium belonging to the genus Achromobacter. Environ. Sci. Pollut. Res. 2020, 27, 44427–44439. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Tan, J.; Lin, S.; Li, D.; Yang, H. Isolation, identification and characterization of an algicidal bacterium from Lake Taihu and preliminary studies on its algicidal compounds. J. Environ. Sci. 2012, 24, 1823–1831. [Google Scholar] [CrossRef]
- Shao, J.; He, Y.; Chen, A.; Peng, L.; Luo, S.; Wu, G.; Zou, H.; Li, R. Interactive effects of algicidal efficiency of Bacillus sp. B50 and bacterial community on susceptibility of Microcystis aeruginosa with different growth rates. Int. Biodeterior. Biodegrad. 2015, 97, 1–6. [Google Scholar] [CrossRef]
- Yang, C.; Hou, X.; Wu, D.; Chang, W.; Zhang, X.; Dai, X.; Du, H.; Zhang, X.; Igarashi, Y.; Luo, F. The characteristics and algicidal mechanisms of cyanobactericidal bacteria, a review. World J. Microbiol. Biotechnol. 2020, 36, 188. [Google Scholar] [CrossRef]
- Yang, J.; Qiao, K.; Lv, J.; Liu, Q.; Nan, F.; Xie, S.; Feng, J. Isolation and Identification of Two Algae-Lysing Bacteria against Microcystis aeruginosa. Water 2020, 12, 2485. [Google Scholar] [CrossRef]
- Sun, P.; Esquivel-Elizondo, S.; Zhao, Y.; Wu, Y. Glucose triggers the cytotoxicity of Citrobacter sp. R1 against Microcystis aeruginosa. Sci. Total Environ. 2017, 603–604, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Li, H.; Zheng, X.; Zhang, H.; Wang, C.; Qin, Y.; Xia, B.; Wang, D.; Xu, S.; Zhuang, X. Oxidative stress of Microcystis aeruginosa induced by algicidal bacterium Stenotrophomonas sp. KT48. Appl. Microbiol. Biotechnol. 2022, 106, 4329–4340. [Google Scholar] [CrossRef]
- Zhang, S.; He, X.; Cao, L.; Tong, Y.; Zhao, B.; An, W. A Novel Wide-Range Freshwater Cyanophage MinS1 Infecting the Harmful Cyanobacterium Microcystis aeruginosa. Viruses 2022, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, X. High-Cell-Density Cultivation and Algicidal Activity Assays of a Novel Algicidal Bacterium to Control Algal Bloom Caused by Water Eutrophication. Water Air Soil Pollut. 2014, 225, 2120. [Google Scholar] [CrossRef]
- Xue, G.; Wang, X.; Xu, C.; Song, B.; Chen, H. Removal of harmful algae by Shigella sp. H3 and Alcaligenes sp. H5: Algicidal pathways and characteristics. Environ. Technol. 2022, 43, 4341–4353. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Han, Z.; Ye, J.; Liu, Z. The influence of aquatic macrophytes on Microcystis aeruginosa growth. Ecol. Eng. 2012, 42, 130–133. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Pan, J.; Yang, H. Synergistic algicidal effect and mechanism of two diketopiperazines produced by Chryseobacterium sp. strain GLY-1106 on the harmful bloom-forming Microcystis aeruginosa. Sci. Rep. 2015, 5, 14720. [Google Scholar] [CrossRef]
- Furusawa, G.; Iwamoto, K. Removal of Microcystis aeruginosa cells using the dead cells of a marine filamentous bacterium, Aureispira sp. CCB-QB1. PeerJ 2022, 10, e12867. [Google Scholar] [CrossRef]
- Phankhajon, K.; Somdee, A.; Somdee, T. Algicidal activity of an actinomycete strain, Streptomyces rameus, against Microcystis aeruginosa. Water Sci. Technol. 2016, 74, 1398–1408. [Google Scholar] [CrossRef]
- Somdee, T.; Sumalai, N.; Somdee, A. A novel actinomycete Streptomyces aurantiogriseus with algicidal activity against the toxic cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2013, 25, 1587–1594. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, Y.; Li, J.; Yang, C.; Zhang, X.; Luo, F.; Dai, X. An algicidal Streptomyces amritsarensis strain against Microcystis aeruginosa strongly inhibits microcystin synthesis simultaneously. Sci. Total Environ. 2019, 650, 34–43. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Chen, W.; Li, H.-Q.; Yang, J.-Y.; Zha, D.-M.; Duan, Y.-Q.; Hozzein, N.W.; Xiao, M.; Gao, R.; Li, W.-J. L-valine, an antialgal amino acid from Streptomyces jiujiangensis JXJ 0074T. Appl. Microbiol. Biotechnol. 2016, 100, 4627–4636. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-H.; Peng, P.; Liu, Y.-M.; Jia, R.-B.; Li, L. Algicidal activity of a dibenzofuran-degrader Rhodococcus sp. J. Microbiol. Biotechnol. 2013, 23, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Kovalerchick, D.; Lieman-Hurwitz, J.; Murik, O.; De Philippis, R.; Carmeli, S.; Sukenik, A.; Kaplan, A. Increased algicidal activity of Aeromonas veronii in response to Microcystis aeruginosa: Interspecies crosstalk and secondary metabolites synergism. Environ. Microbiol. 2019, 21, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.-M.; Fan, Z.-Q.; Pei, H.-Y.; Yuan, X.-L.; Liu, S.-X.; Wang, X.-R. Isolation and algae-lysing characteristics of the algicidal bacterium B5. J. Environ. Sci. 2007, 19, 1336–1340. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chi, Y.; Wu, D.; Dai, X.; Zhang, X.; Igarashi, Y.; Luo, F. Algicidal characterization and mechanism of Bacillus licheniformis Sp34 against Microcystis aeruginosa in Dianchi Lake. J. Basic Microbiol. 2019, 59, 1112–1124. [Google Scholar] [CrossRef]
- Sun, P.; Hui, C.; Wang, S.; Khan, R.A.; Zhang, Q.; Zhao, Y.-H. Enhancement of algicidal properties of immobilized Bacillus methylotrophicus ZJU by coating with magnetic Fe3O4 nanoparticles and wheat bran. J. Hazard. Mater. 2016, 301, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, M.; Hong, M.; Park, W. Killing effect of deinoxanthins on cyanobloom-forming Microcystis aeruginosa: Eco-friendly production and specific activity of deinoxanthins. Environ. Res. 2021, 200, 111455. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Alamri, S.; Hashem, M.; Mostafa, Y. Growth inhibition of Microcystis aeruginosa and adsorption of microcystin toxin by the yeast Aureobasidium pullulans, with no effect on microalgae. Environ. Sci. Pollut. Res. 2020, 27, 38038–38046. [Google Scholar] [CrossRef]
- Christoffersen, K.; Lyck, S.; Winding, A. Microbial activity and bacterial community structure during degradation of microcystins. Aquat. Microb. Ecol. 2002, 27, 125–136. [Google Scholar] [CrossRef]
- Kinley, C.M.; Iwinski-Wood, K.J.; Geer, T.D.; Hendrikse, M.; McQueen, A.D.; Calomeni, A.J.; Liang, J.; Friesen, V.; Simair, M.C.; Rodgers, J.H. Microcystin-LR Degradation Following Copper-Based Algaecide Exposures. Water Air Soil Pollut. 2018, 229, 62. [Google Scholar] [CrossRef]
- Shibata, K.; Amemiya, T.; Itoh, K. Activities of Algicidal Bacteria and Their Influences on Microbial Communities. 2008. Available online: https://www.researchgate.net/publication/37570727_Activities_of_Algicidal_Bacteria_and_Their_Influences_on_Microbial_Communities (accessed on 1 April 2023).
- Takamura, Y.; Yamada, T.; Kimoto, A.; Kanehama, N.; Tanaka, T.; Nakadaira, S.; Yagi, O. Growth Inhibition of Microcystis Cyanobacteria by L-Lysine and Disappearance of Natural Microcystis Blooms with Spraying. Microbes Environ. 2004, 19, 31–39. [Google Scholar] [CrossRef]
- Zeng, G.; Gao, P.; Wang, J.; Zhang, J.; Zhang, M.; Sun, D. Algicidal Molecular Mechanism and Toxicological Degradation of Microcystis aeruginosa by White-Rot Fungi. Toxins 2020, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.A.; Hashem, M.; Alamri, S.A. Growth inhibition of the cyanobacterium Microcystis aeruginosa and degradation of its microcystin toxins by the fungus Trichoderma citrinoviride. Toxicon 2014, 86, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Du, J.; Song, F.; Zhao, G.; Tian, X. A Fungus Capable of Degrading Microcystin-LR in the Algal Culture of Microcystis aeruginosa PCC7806. Appl. Biochem. Biotechnol. 2012, 166, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ma, H.; Ren, S.; Gao, X.; He, X.; Zhu, S.; Deng, R.; Zhang, S. Insights into the mechanism of cyanobacteria removal by the algicidal fungi Bjerkandera adusta and Trametes versicolor. Microbiologyopen 2020, 9, e1042. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Feng, X.; Jia, Y.; Wang, C.; He, X.; Zhou, Q.; Tian, X. Isolation and evaluation of terrestrial fungi with algicidal ability from Zijin Mountain, Nanjing, China. J. Microbiol. 2011, 49, 562–567. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- Gao, X.; Wang, C.; Dai, W.; Ren, S.; Tao, F.; He, X.; Han, G.; Wang, W. Proteomic analysis reveals large amounts of decomposition enzymes and major metabolic pathways involved in algicidal process of Trametes versicolor F21a. Sci. Rep. 2017, 7, 3907. [Google Scholar] [CrossRef]
- Jia, Y.; Han, G.; Wang, C.; Guo, P.; Jiang, W.; Li, X.; Tian, X. The efficacy and mechanisms of fungal suppression of freshwater harmful algal bloom species. J. Hazard. Mater. 2010, 183, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, Q.; Lilje, O.; Xu, W.; Zhu, Y.; van Ogtrop, F.F. Inhibition mechanism of Penicillium chrysogenum on Microcystis aeruginosa in aquaculture water. J. Clean. Prod. 2021, 299, 126829. [Google Scholar] [CrossRef]
- Gerphagnon, M.; Latour, D.; Colombet, J.; Sime-Ngando, T. Fungal Parasitism: Life Cycle, Dynamics and Impact on Cyanobacterial Blooms. PLoS ONE 2013, 8, e60894. [Google Scholar] [CrossRef] [PubMed]
- Ibelings, B.W.; De Bruin, A.; Kagami, M.; Rijkeboer, M.; Brehm, M.; Van Donk, E. Host parasite interactions between freshwater phytoplankton and chytrid fungi (chytridiomycota). J. Phycol. 2004, 40, 437–453. [Google Scholar] [CrossRef]
- Mountfort, D.O.; Atkinson, M.; Ponikla, K.; Burke, B.; Todd, K. Lysis of Gymnodinium Species by the Fungus Verticillium lecanii. Bot. Mar. 1996, 39, 159–166. [Google Scholar] [CrossRef]
- Canter, H.M.; Lund, J. Studies on plankton parasites: II. The parasitism of diatoms with special reference to lakes in the English Lake District. Trans. Br. Mycol. Soc. 1953, 36, 13–37. [Google Scholar] [CrossRef]
- Van Donk, E. The role of fungal parasites in phytoplankton succession. Plankton Ecol. Succession Plankton Communities 1989, 171–194. [Google Scholar] [CrossRef]
- Kudoh, S.; Tokahashi, M. Fungal control of population changes of the planktonic diatom asterionella formosa in a shallow eutrophic lake. J. Phycol. 1990, 26, 239–244. [Google Scholar] [CrossRef]
- Sime-Ngando, T. Phytoplankton chytridiomycosis: Fungal parasites of phytoplankton and their imprints on the food web dynamics. Front. Microbiol. 2012, 3, 361. [Google Scholar] [CrossRef]
- Tucker, S.; Pollard, P. Identification of Cyanophage Ma-LBP and Infection of the Cyanobacterium Microcystis aeruginosa from an Australian Subtropical Lake by the Virus. Appl. Environ. Microbiol. 2005, 71, 629–635. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Jaskulska, A.; Pawełczyk, J.; Gągała, I.; Serwecińska, L.; Dziadek, J. Cyanophages infection of Microcystis bloom in lowland dam reservoir of Sulejów, Poland. Microb. Ecol. 2016, 71, 315–325. [Google Scholar] [CrossRef]
- Pollard, P.C.; Young, L.M. Lake viruses lyse cyanobacteria, Cylindrospermopsis raciborskii, enhances filamentous-host dispersal in Australia. Acta Oecol. 2010, 36, 114–119. [Google Scholar] [CrossRef]
- Yoshida, T.; Takashima, Y.; Tomaru, Y.; Shirai, Y.; Takao, Y.; Hiroishi, S.; Nagasaki, K. Isolation and Characterization of a Cyanophage Infecting the Toxic Cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 2006, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Manage, P.M.; Kawabata, Z.; Nakano, S. Dynamics of cyanophage-like particles and algicidal bacteria causing Microcystis aeruginosa mortality. Limnology 2001, 2, 73–78. [Google Scholar] [CrossRef]
- Wang, F.; Li, D.; Cai, R.; Pan, L.; Zhou, Q.; Liu, W.; Qian, M.; Tong, Y. A Novel Freshwater Cyanophage Mae-Yong1326-1 Infecting Bloom-Forming Cyanobacterium Microcystis aeruginosa. Viruses 2022, 14, 2051. [Google Scholar] [CrossRef]
- Long, A.M. Persistence of Algal Viruses and Cyanophages in Freshwater Environments; University of Toronto (Canada): Toronto, ON, Canada, 2017. [Google Scholar]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Xie, W.; He, W.; Xie, J.; Liu, W. Identifying Algicides of Enterobacter hormaechei F2 for Control of the Harmful Alga Microcystis aeruginosa. Int. J. Environ. Res. Public Health 2022, 19, 7556. [Google Scholar] [CrossRef]
- Suttle, C.A. The Ecological, Evolutionary and Geochemical Consequences of Viral Infection of Cyanobacteria and Eukaryotic Algae. Available online: https://www.researchgate.net/publication/258503632_The_Ecological_Evolutionary_and_Geochemical_Consequences_of_Viral_Infection_of_Cyanobacteria_and_Eukaryotic_Algae (accessed on 1 April 2023).
- Short, S.M. The ecology of viruses that infect eukaryotic algae. Environ. Microbiol. 2012, 14, 2253–2271. [Google Scholar] [CrossRef]
- Ben Gharbia, H.; Kéfi-Daly Yahia, O.; Cecchi, P.; Masseret, E.; Amzil, Z.; Herve, F.; Rovillon, G.; Nouri, H.; M’rabet, C.; Couet, D. New insights on the species-specific allelopathic interactions between macrophytes and marine HAB dinoflagellates. PLoS ONE 2017, 12, e0187963. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine—A mini review. Carbohydr. Polym. 2021, 271, 118408. [Google Scholar] [CrossRef] [PubMed]
- Asselman, J.; Hochmuth, J.; De Schamphelaere, K. A comparison of the sensitivities of Daphnia magna and Daphnia pulex to six different cyanobacteria. Harmful Algae 2014, 39, 1–7. [Google Scholar] [CrossRef]
- Ger, K.A.; Hansson, L.-A.; Lürling, M. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw. Biol. 2014, 59, 1783–1798. [Google Scholar] [CrossRef]
- Wilson, A.E.; Sarnelle, O.; Tillmanns, A.R. Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: Meta-analyses of laboratory experiments. Limnol. Oceanogr. 2006, 51, 1915–1924. [Google Scholar] [CrossRef]
- Savic, G.B.; Edwards, C.; Briand, E.; Lawton, L.; Wiegand, C.; Bormans, M. Daphnia magna Exudates Impact Physiological and Metabolic Changes in Microcystis aeruginosa. Toxins 2019, 11, 421. [Google Scholar] [CrossRef]
- Ger, K.A.; Panosso, R.; Lürling, M. Consequences of acclimation to Microcystis on the selective feeding behavior of the calanoid copepod Eudiaptomus gracilis. Limnol. Oceanogr. 2011, 56, 2103–2114. [Google Scholar] [CrossRef]
- Ger, K.A.; Urrutia-Cordero, P.; Frost, P.C.; Hansson, L.-A.; Sarnelle, O.; Wilson, A.E.; Lürling, M. The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 2016, 54, 128–144. [Google Scholar] [CrossRef]
- Jang, M.-H.; Jung, J.-M.; Takamura, N. Changes in microcystin production in cyanobacteria exposed to zooplankton at different population densities and infochemical concentrations. Limnol. Oceanogr. 2007, 52, 1454–1466. [Google Scholar] [CrossRef]
- Van Gremberghe, I.; Vanormelingen, P.; Vanelslander, B.; Van der Gucht, K.; D’hondt, S.; De Meester, L.; Vyverman, W. Genotype-dependent interactions among sympatric Microcystis strains mediated by Daphnia grazing. Oikos 2009, 118, 1647–1658. [Google Scholar] [CrossRef]
- Van Donk, E. Chemical information transfer in freshwater plankton. Ecol. Inform. 2007, 2, 112–120. [Google Scholar] [CrossRef]
- Shapiro, J. Biomanipulation: An ecosystem approach to lake restoration. In Proceedings of the Symposium on Water Quality Management through Biological Control, Gainesville, FL, USA, 23–30 January 1975; pp. 85–95. [Google Scholar]
- Starling, F. Control of eutrophication by silver carp in the tropical Pranoa Resevoir, Brazil: A mesocosm experiment. Hydrobiologia 1993, 257, 143–151. [Google Scholar] [CrossRef]
- Turker, H.; Eversole, A.G.; Brune, D.E. Filtration of green algae and cyanobacteria by Nile tilapia, Oreochromis niloticus, in the Partitioned Aquaculture System. Aquaculture 2003, 215, 93–101. [Google Scholar] [CrossRef]
- Lu, K.; Jin, C.; Dong, S.; Gu, B.; Bowen, S.H. Feeding and control of blue-green algal blooms by tilapia (Oreochromis Niloticus). Hydrobiologia 2006, 568, 111–120. [Google Scholar] [CrossRef]
- Torres, G.S.; Silva, L.H.S.; Rangel, L.M.; Attayde, J.L.; Huszar, V.L.M. Cyanobacteria are controlled by omnivorous filter-feeding fish (Nile tilapia) in a tropical eutrophic reservoir. Hydrobiologia 2016, 765, 115–129. [Google Scholar] [CrossRef]
- Görgényi, J.; Boros, G.; Vitál, Z.; Mozsár, A.; Várbíró, G.; Vasas, G.; Borics, G. The role of filter-feeding Asian carps in algal dispersion. Hydrobiologia 2016, 764, 115–126. [Google Scholar] [CrossRef]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Tebaa, L.; Loudiki, M. Use of macrophytes allelopathy in the biocontrol of harmful Microcystis aeruginosa blooms. Water Supply 2019, 19, 245–253. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Growth inhibition of blue-green algae (Microcystis aeruginosa) by Myriophyllum spicatum-releasing four polyphenols. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Tan, K.; Huang, Z.; Ji, R.; Qiu, Y.; Wang, Z.; Liu, J. A review of allelopathy on microalgae. Microbiology 2019, 165, 587–592. [Google Scholar] [CrossRef]
- Nakai, S.; Yamada, S.; Hosomi, M. Anti-cyanobacterial fatty acids released from Myriophyllum spicatum. Hydrobiologia 2005, 543, 71–78. [Google Scholar] [CrossRef]
- Kong, C.H.; Wang, P.; Zhang, C.X.; Zhang, M.X.; Hu, F. Herbicidal potential of allelochemicals from Lantana camara against Eichhornia crassipes and the alga Microcystis aeruginosa. Weed Res. 2006, 46, 290–295. [Google Scholar] [CrossRef]
- Wang, R.; Hua, M.; Yu, Y.; Zhang, M.; Xian, Q.-M.; Yin, D.-Q. Evaluating the effects of allelochemical ferulic acid on Microcystis aeruginosa by pulse-amplitude-modulated (PAM) fluorometry and flow cytometry. Chemosphere 2016, 147, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Shao, J.; Yu, G.; Wang, Z.; Wu, Z.; Peng, X.; Li, R. Towards clarification of the inhibitory mechanism of wheat bran leachate on Microcystis aeruginosa NIES-843 (cyanobacteria): Physiological responses. Ecotoxicology 2010, 19, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-H.; Wu, X.-Q.; Li, R.-H. Physiological responses of Microcystis aeruginosa PCC7806 to nonanoic acid stress. Environ. Toxicol. 2009, 24, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, R.; Lepo, J.E.; Gu, J.-D. Potential for control of harmful cyanobacterial blooms using biologically derived substances: Problems and prospects. J. Environ. Manag. 2013, 125, 149–155. [Google Scholar] [CrossRef] [PubMed]







| Name | Species | Dosage | R % | Field app. | Lab. app. | Ref. |
|---|---|---|---|---|---|---|
| poslock® aqual-PTM | M. aeruginosa PCC 7820) | 0, 50, 100, 300, 600, 1000 mg /L | 42.6% 28.4% | ✘ | ✓ | [50] |
| mmt modified lime-ceramic sand-lake sediments | M. aeruginosa 469 | 0.7 g/L | 88 % | ✘ | ✓ | [57] |
| ctab | M. aeruginosa | 0.3 g/L | 92% | ✘ | ✓ | [53] |
| modified attapulgite | M. aeruginosa (FACHB 905) | 0.37 g/L | 95% | ✘ | ✓ | [54] |
| SnO2-montmorillonite | M. aeruginosa (FACHB-942) | 0.3 g/L | 95% | ✘ | ✓ | [58] |
| montmorillonite–Cu (II)/Fe(III) oxides magnetic material | M. aeruginosa | 1 g/L | 92% | ✘ | ✓ | [59] |
| chitosan/montmorillonite nanocomposite | M. aeruginosa (FACHB-905) | 100–500 mg/L | 94.7% | ✘ | ✓ | [60] |
| chitosan modified kaolinite (CMK) | M. aeruginosa (NIES-843) | 0, 40, 80 and 160 mg/L | NA | ✘ | ✓ | [60] |
| Name | Field app. | Lab. app. | Parameters Studied | Ref. |
|---|---|---|---|---|
| copper ethanolamine complex, CuSO4·5H2O, CuSO4 | ✘ | ✓ | chlorophyll-a, photosystem II efficiency (PSII), soluble reactive phosphorus (SRP) and intracellular and extracellular microcystin (MC) concentrations, total organic carbon content (TOC), membrane integrity | [50,66] |
| CoCl2·6H2O, FeCl3·6H2O, FeCl3·6H2O and Na2EDTA·2H2O MnCl2·4H2O Na2MoO4·2H2O | ✘ | ✓ | growth, toxin production, cell morphology, iron accumulation | [67] |
| benzalkonium chloride (BAC-14) | ✘ | ✓ | growth inhibition, photosynthesis endpoints, microcystin, multi-platform metabolomics | [68] |
| copper sulfate pentahydrate, thanolamine-chelated copper compound | ✘ | ✓ | ell density, total microcystins, cell membrane integrity | [69] |
| copper sulfate | ✘ | ✓ | cell counting, Fv: Fm, TTC, SOD, and MDA, microcystin-LR, | [70,71] |
| copper sulfate | ✘ | ✓ | superoxide dismutase, catalase, and peroxidase, Fv/Fm chlorophyll fluorescence value and chlorophyll a content, transcriptome analysis, | [60,72] |
| H2O2 | ✘ | ✓ | cell density, chlorophyll, phycocyanin, organic matter, true color, intracellular microcystin, geosmin, total pheophytin, ROS, CAT and SOD, chlorophyll a, carotenoid, TDN, TDP, dissolved organic matter, phytoplankton community analysis, cell lysis, caspase-3 activity, terminal deoxynucleotidyl transferase labeling (TUNEL) assay, RNA analysis | [60,73,74] |
| novel H2O2 pre-oxidation | ✘ | ✓ | chlorophyll a, turbidity, algal removal efficiency, TOC, TN, TP, cell membrane assay, SOD, CAT, microcystin, | [75,76] |
| combined process of nanoscale zero-valent iron (NZVI) and H2O2 | ✘ | ✓ | chl a, phycocyanobilin (PC), allophycocyanin, phycoerythrin, zeta potential, MDA, SOD, CAT, POD, total organic carbon, | [77] |
| N-acetyl-5-methoxytryptamine | ✘ | ✓ | cell density, chl a, SOD, CAT, MDA, MC-LR, mcyB and mcyD genes | [78] |
| H2O2 and copper sulfate | ✘ | ✓ | cell density, MC-LR, inhibition of Bacillus sp. | [79] |
| H2O2 | ✘ | ✓ | effects of EPS on the killing activity of H2O2 | [59] |
| H2O2H2O2 under light H2O2 and ultrasound | ✘ | ✓ | cell count, cell integrity, microcystins, chlorophyll | [80,81,82] |
| Ozone | ✘ | ✓ | algae removal, microcystins, cell morphology, DOC | [63,83,84,85,86] |
| chitosan-modified nanobubbles Chitosan fiber Chitosan chitosan quaternary ammonium salt chitosan fiber chitosan-zinc oxide hydrogel film chitosan-aluminum chloride combined coagulants | ✘ | ✓ | cell intact rate, cell lysis rate, cell inactivation rate, OH radical production, ROS, MC-LR, cell density, phosphorus, chlorophyll a, carotenoids, phycocyanin, allophycocyanin, phycoerythrin, total protein content | [56,63,87,88,89,90] |
| Strain Name | Species | Mode of Action | RE % | Ref. |
|---|---|---|---|---|
| Bacillus mycoides | M. aeruginosaPCC7806 | shadowing and photo-inhibition | NA | [113] |
| Brevibacillus laterosporus | M. aeruginosa FACHB 905 | efflux pump transporters, hydrolytic enzymes, antibiotics, proteases, and other secondary metabolites | 92.30% | [114] |
| Ochrobactrum sp. FDT5 | M. aeruginosa | active cellular Components | 34–58.6% | [115,116] |
| Alcaligenes aquatilis F8 | M. aeruginosa FACHB-905 | cell membrane damage, disappearance of photosynthetic lamellae, cyanelles disorder | 88.45% | [117] |
| Bacillus sp. AF-1 | M. aeruginosa NIES-843, NIES-90, NIES-44 | increased intracellular ROS buildup, cell death, and intracellular component efflux | 93% | [118] |
| Arthrobacter sp.443 and UN 383 | M. aeruginosa BCPUSP232 | antimicrobial agents Production | 24.87 and 23.85% | [119] |
| Shewanella maltophilia | M. aeruginosa FACHB-905 | hexahydropyrrolo [1,2-a] pyrazine-1,4-dione, 2,3-indolinedione Secretion | NA | [120] |
| Pseudomonas putida | M. aeruginosa FACHB 905 | extracellular antialgal chemicals are secreted, characterized as anti-heat shock. | 98.8% | [121] |
| Streptomyces globisporus | M. aeruginosa NIES-843, NIES-44, NIES-90 | cell-to-cell contact | 96.7% | [122] |
| Rhizobium AQ_MP | M. aeruginosa | 100% | [123] | |
| Alcaligenes Denitrificans | M. aeruginosa NIES 298 | cell lysis | 96.4% | [124] |
| Streptomyces neyagawaensis | M. aeruginosa NIES-298 | secretion of extracellular antialgal substances | 84.5% | [100] |
| Xanthobacter autotrophicus HYS0201-SM02 (SM02) | M. aeruginosa NIER-100001 | algicidal substance secretion | 95.6% | [96] |
| Stenotrophomonas F6 | M. aeruginosa 9110 | excretion of extracellular algicidal compounds (Cyclo-(Gly-Pro) | 50% | [17] |
| Serratia marcescens | M. aeruginosa TH1, TH2, and FACHB 905 | secretion of a red pigment identified as prodigiosin (C20H25N3O) | 87.7% | [125] |
| Salvia miltiorrhiza | M. aeruginosa FACHB-905 | neo-przewaquinone A oxidative stress, inhibition of three genes involved in photosynthesis (psaB, psbD, and rbcL). | 74.08% | [121,126] |
| Pedobacter sp. | M. aeruginosa NIES-843 | algicidal activity | 50–80% | [125] |
| Acinetobacter sp. J25 | M. aeruginosa | lysing and denitrification | 100% 87.7% | [127] |
| Paucibacter aquatile DH15 | M. aeruginosa KW | oxidative stress, alteration of fatty acid profile, damage to photosynthetic system, carbohydrate, and protein metabolism | 94.9% | [128] |
| Pseudomonas aeruginosa UCBPP-PA14 | M. aeruginosa NIES 298,44 | lysis and toxin Degradation | 92% | [50,129] |
| Acinetobacter guillouiae A2 | M. aeruginosa FACHB-905 | algicidal compound 4-hydroxyphenethyl- amine secretion | 91.6% | [130] |
| Paucibacter toxinivorans 2C20 | M. aeruginosa | toxin degradation | 90% | [131] |
| Achromobacter spp. | M. aeruginosa CAAT 2005-3 | lysis activity | 79.5% | [132] |
| Pseudomonas grimontii | M. aeruginosa FACHB-905 | oxidative stress | 91.81% | [32] |
| Bdellovibrio species | M. aeruginosa Kützing | lysis activity | NA | [108] |
| Exiguobacterium A27 | M. aeruginosa PCC7806 | production of extracellular algicidal compounds | 64.4% | [133] |
| Bacillus sp. B50 | M. aeruginosa FACHB905, FACHB1023 PCC 7806, M. NIES-843, CHAB440, CHAB109, CHAB456, CHAB587, CHAB439, CHAB2162, CHAB2170, CHAB724, CHAB4370 | algicidal activity | 15–71.8% | [134] |
| Aeromonas bestiarum HYD0802-MK36 and Pseudomonas syringae KACC10292T | M. aeruginosa | direct attack and cell-to-cell contact | 100% | [103] |
| Raoultella ornithinolytica | M. aeruginosa FACHB-905 | low-molecular-weight organic acids | 96.2% | [75] |
| Raoultella sp. R11 | M. aeruginosa FACHB 905 | oxidative stress | 94.28%. | [127] |
| Raoultella planticola and Aeromonas sp. | M. aeruginosa FACHB-905 | algae lysis | 90% | [135,136] |
| Halobacillus sp. H9 | M. aeruginosa PCC7806 and TAIHU98 | secretion of active flocculating substance | 95%. | [111,112] |
| Shewanella sp. Lzh-2 | M. aeruginosa 9110 | hexahydropyrrolo [1,2-a] pyrazine-1,4-dione and 2, 3-indolinedione (isatin) secretion | 92.3% | [20] |
| Hahella sp. KA22 | M. aeruginosa TAIHU98 | prodigiosin secretion | 71–88% | [118] |
| Citrobacter sp. R1 | M. aeruginosa FACHB-905 | glycogen synthase gene glgA | 81.6% | [137] |
| Stenotrophomonas sp. KT48 | M. aeruginosa PCC7820 | oxidative stress | 88.47% | [138] |
| Enterobacter hormaechei F2 | M. aeruginosa FACHB-315 | prodigiosin and PQS Secretion | 84.2% | [114,139] |
| Enterobacter sp. NP23 | M. aeruginosa | algicidal activity | 70 % | [140] |
| Shigella sp. H3, Alcaligenes sp. H5 | M. aeruginosa | cells-to-cells direct contact and secretion of algicidal metabolites | 96% and 74% | [141] |
| Aquimarina salinaria sp. Nov | M. aeruginosa MTY01 | phosphatidylethanol- amine, diphosphatidylglycerol | 100% | [142] |
| Chryseobacterium species | M. aeruginosa FACHB 905 | algicidal activity | 80% | [111] |
| Chryseobacterium sp. GLY-1106 | M. aeruginosa 9110. | 1106-A (cyclo(4-OH-Pro-Leu)), 1106-B (cyclo(Pro-Leu)) | 90% | [143] |
| Aureispira sp. CCB-QB1 | M. aeruginosa NISE 102 strain | floculation | 75.39% | [144] |
| Streptomyces rameus | M. aeruginosa KKU-13 | cell lysis | 82% to 95% | [145] |
| Streptomyces aurantiogriseus | M. aeruginosa KKU-13 | production of metabolites | 83.3% | [146] |
| Streptomyces amritsarensis strain HG-16 | M. aeruginosa FACHB-905 | secretion of active Substances | 91.2%. | [147] |
| Streptomyces jiujiangensis JXJ 0074T | M. aeruginosa FACHB-905 | antialgal amino acid: l-Valine 2′-deoxyadenosine | 80% | [126,148] |
| Rhodococcus sp. p52 | M. aeruginosa FACHB927, FACHB 975 | trans-3-indoleacrylic acid, dl-pipecolic acid, and l-pyroglutamic acid secretion | 93.5% | [149] |
| Aeromonas veronii | M. aeruginosa PCC7806 and MGK M. aeruginosa | lumichrome production | NA | [150] |
| Bacillus fusiformis | M. aeruginosa | secretion of metabolites | 90% | [151] |
| Bacillus licheniformis Sp34 | M. aeruginosa DCM3, DCM4 | oxidative stress, lipid Peroxidation, DNA damage, and a malfunction in the DNA-repair system | 75.6% | [152] |
| Bacillus methylotrophicus ZJU | M. aeruginosa | algicidal effect | 89% | [153] |
| Deinococcus metallilatus MA1002 | M. aeruginosa PCC7806 | deinoxanthin Production | 100% | [154] |
| Strain Name | Species | Mode of Action | RE %eff. | Ref. |
|---|---|---|---|---|
| Aureobasidium pullulans KKUY070 | M. aeruginosa DRCK1 | N-β-acetylglucos-aminidase. | 100% | [155] |
| Bjerkandera adusta T1 | M. aeruginosa PCC7806 | Protease, polysaccharide lyases8 (PL8) | 98.27% | [163] |
| Irpex lacteus T2b | M. aeruginosa PCC7806 | Cell-to-cell contact | 99.1% | [164] |
| Lopharia spadicea | M. aeruginosa FACH-918 | Oxidative stress | 100% | [165] |
| Phanerochaete chrysosporium | M. aeruginosa | Release of fungal metabolites | 88.6% | [160] |
| Trametes versicolor F21a | M. aeruginosa PCC7806 | Cellulase, β-glucanase, trypsase, and pepsin | 85% | [166] |
| Trichaptum abietinum 1302BG | M. aeruginosa FACH-918 | Cell-to-cell contact and lytic enzymes release | 100% | [167] |
| Trichoderma citrinoviride kkuf-0955 | M. aeruginosa | Excretion of algicidal compounds | 100% removal | [161] |
| Aspergillus niger 7806F3 | M. aeruginosa 7820, 7806, 1752 | Indirect attack | 80% | [75] |
| Penicillium chrysogenum | Microcystis aeruginosa | Secreting extracellular substances | 69.56% | [168] |
| Aureobasidium pullulans strain KKUY0701 | M. aeruginosa DRCK1 | Cell lysis | 84% | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, Z.; Huang, S.; Bilal, A.; Janjuhah, H.T.; Kontakiotis, G.; Antonarakou, A.; Besiou, E.; Wei, M.; Gao, R.; Zhang, T.; et al. Current and Previous Green Technologies, Their Efficiency, Associated Problems, and Success Rates to Mitigate M. aeruginosa in Aquatic Environments. Sustainability 2023, 15, 8048. https://doi.org/10.3390/su15108048
Khatoon Z, Huang S, Bilal A, Janjuhah HT, Kontakiotis G, Antonarakou A, Besiou E, Wei M, Gao R, Zhang T, et al. Current and Previous Green Technologies, Their Efficiency, Associated Problems, and Success Rates to Mitigate M. aeruginosa in Aquatic Environments. Sustainability. 2023; 15(10):8048. https://doi.org/10.3390/su15108048
Chicago/Turabian StyleKhatoon, Zobia, Suiliang Huang, Ahmer Bilal, Hammad Tariq Janjuhah, George Kontakiotis, Assimina Antonarakou, Evangelia Besiou, Mengjiao Wei, Rui Gao, Tianqi Zhang, and et al. 2023. "Current and Previous Green Technologies, Their Efficiency, Associated Problems, and Success Rates to Mitigate M. aeruginosa in Aquatic Environments" Sustainability 15, no. 10: 8048. https://doi.org/10.3390/su15108048