Hazardous Elements in Sediments Detected in Former Decommissioned Coal Mining Areas in Colombia: A Need for Environmental Recovery
Abstract
:1. Introduction
2. Materials and Methods
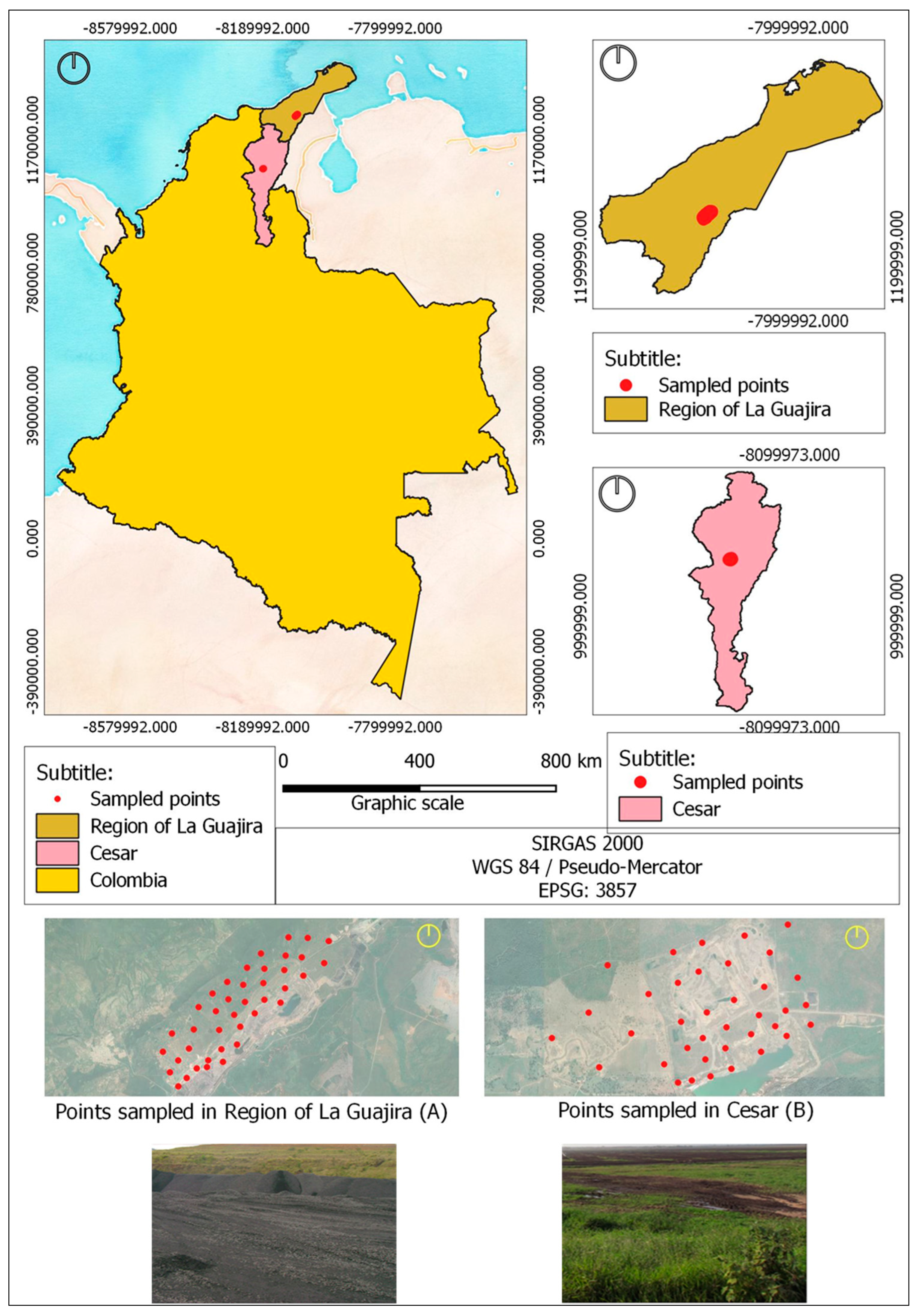
2.1. Studied Areas
2.2. Procedures Used in Sample Collection
2.3. Analytical Methods
3. Results and Discussion
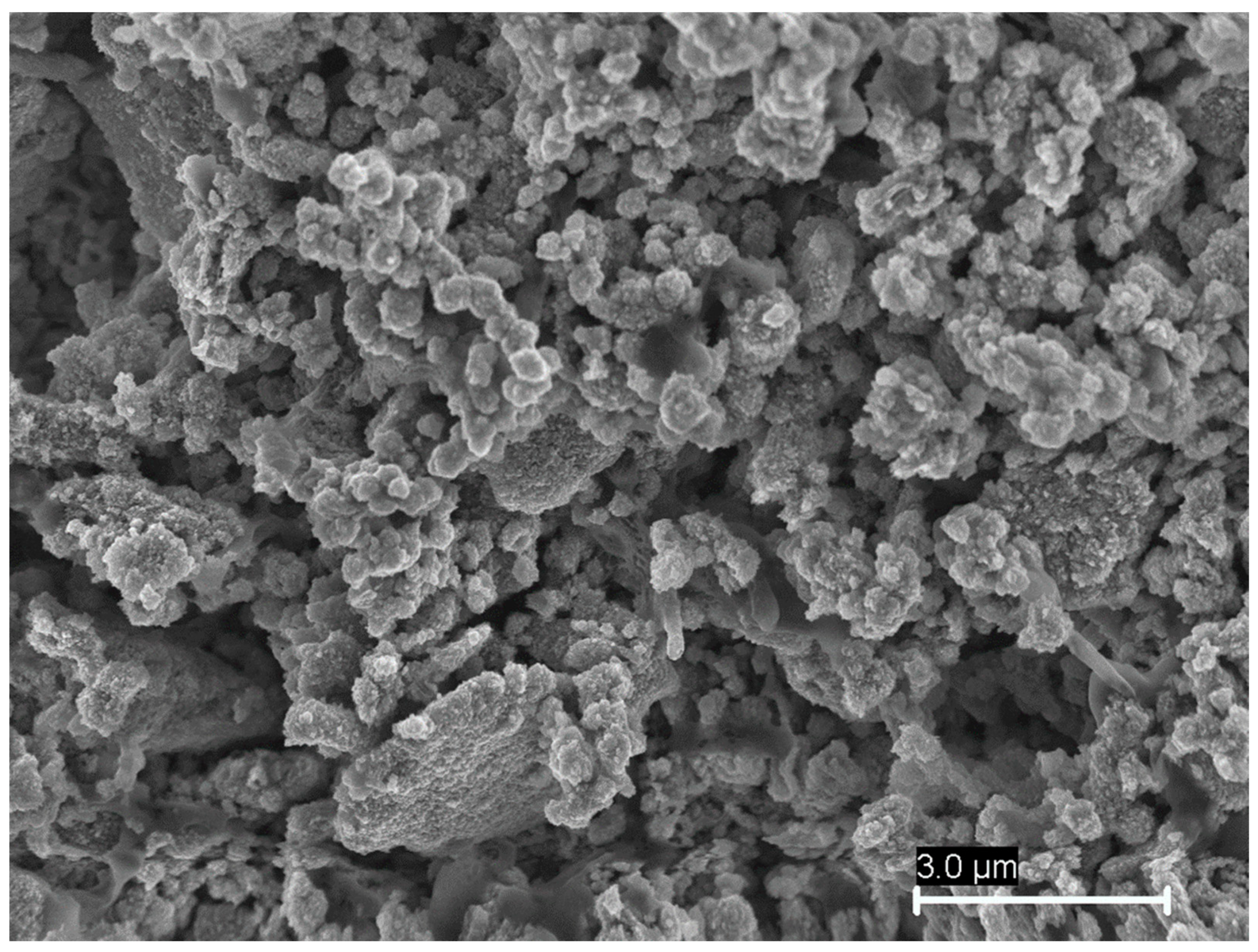
3.1. Characteristics Identified in the Analyzed Samples
3.2. Identification of Chemical Compounds with High-Added Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Qi, C.; Chen, Q.; Liu, H. Evaluating the metal recovery potential of coal fly ash based on sequential extraction and machine learning. Environ. Res. 2013, 224, 115546. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, P.; Maity, S.; Mishra, S.; Chaudhary, D.K.; Dusane, C.; Pillai, A.S.; Kumar, A.V. Estimation of rare earth elements in Indian coal fly ashes for recovery feasibility as a secondary source. J. Hazard. Mater. 2023, 10, 100257. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, S.; Wang, H. Using P–Cl inorganic ultrafine aerosol particles to prevent spontaneous combustion of low-rank coal in an underground coal mine. Fire Saf. J. 2020, 115, 103140. [Google Scholar] [CrossRef]
- Moghadam, M.J.; Ajalloeian, R.; Hajiannia, A. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, X.; Jiang, J.; Li, C. Life cycle assessment of coal-fired solar-assisted carbon capture power generation system integrated with organic Rankine cycle. J. Clean. Prod. 2022, 356, 131888. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Z.; Shen, X. Combined effects of water content and primary air volume on performance of lignite-fired boiler. Fuel 2019, 244, 580–591. [Google Scholar] [CrossRef]
- Ayaz, M.; Jehan, N.; Nakonieczny, J.; Mentel, U.; Uz Zaman, Q. Health costs of environmental pollution faced by underground coal miners: Evidence from Balochistan, Pakistan. Resour. Policy 2022, 76, 102536. [Google Scholar] [CrossRef]
- Finkelman, R.B. Potential health impacts of burning coal beds and waste banks. Int. J. Coal Geol. 2004, 59, 19–24. [Google Scholar] [CrossRef]
- Golik, V.I.; Razorenov, I.I.; Vagin, V.S.; Liashenko, V.I. Study and development of hardening mixture composition based on unconventional industrial waste. Izvestiya Vysshikh Uchebnykh Zavedenii. Gorn. Zh. 2021, 1, 13–27. [Google Scholar] [CrossRef]
- Rybak, J.; Gorbatyuk, S.M.; Bujanovna-Syuryun, K.C.; Khairutdinov, A.M.; Tyulyaeva, Y.S.; Makarov, P.S. Utilization of Mineral Waste: A Method for Expanding the Mineral Resource Base of a Mining and Smelting Company. Metall. 2021, 64, 851–861. [Google Scholar] [CrossRef]
- Neckel, A.; Osorio-Martinez, J.; Pinto, D.; Bodah, B.W.; Adelodun, B.; Silva, L.F. Hazardous elements present in coal nanoparticles in a Caribbean port region in Colombia. Sci. Total Environ. 2022, 838, 156363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Ma, Y.; You, C. Sulfur fixation for raw coal with combined microwave irradiation and ultrafine Ca(OH)2 method. Fuel 2022, 330, 125570. [Google Scholar] [CrossRef]
- Wang, G.; Bai, X.; Wu, C.; Li, W.; Liu, K.; Kiani, A. Recent advances in the beneficiation of ultrafine coal particles. Fuel Process. Technol. 2018, 178, 104–125. [Google Scholar] [CrossRef]
- Khayrutdinov, M.M.; Golik, V.I.; Aleksakhin, A.V.; Trushina, E.V.; Lazareva, N.V.; Aleksakhina, Y.V. Proposal of an Algorithm for Choice of a Development System for Operational and Environmental Safety in Mining. Resources 2022, 11, 88. [Google Scholar] [CrossRef]
- Neckel, A.; Pinto, D.; Adelodun, B.; Dotto, G.L. An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete. Sustainability 2022, 14, 3943. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Chou, C.L.; Li, Y.; Wang, Z.; Ge, Y.; Zheng, C. Trace element emissions from spontaneous combustion of gob piles in coal mines, Shanxi, China. Int. J. Coal Geol. 2008, 73, 52–62. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Misz-Kennan, M.; Hower, J.C.; Fabiańska, M.J. Mineralogy and geochemistry of coal wastes from the Starzykowiec coal-waste dump (Upper Silesia, Poland). Int. J. Coal Geol. 2014, 127, 42–55. [Google Scholar] [CrossRef]
- Silva, L.F.; Pinto, D.; Dotto, G.L. A tool for realistic study of nanoparticulate coal rejects. J. Clean. Prod. 2021, 278, 121916. [Google Scholar] [CrossRef]
- Yuan, B.; Cao, H.; Du, P.; Ren, J.; Chen, J.; Zhang, H.; Zhang, Y.; Luo, H. Source-oriented probabilistic health risk assessment of soil potentially toxic elements in a typical mining city. J. Hazard. Mater. 2023, 443, 130222. [Google Scholar] [CrossRef]
- Padhye, L.P.; Jasemizad, T.; Bolan, S.; Tsyusko, O.V.; Unrine, J.M.; Biswal, B.K.; Balasubramanian, R.; Zhang, Y.; Zhang, T.; Zhao, J.; et al. Silver contamination and its toxicity and risk management in terrestrial and aquatic ecosystems. Sci. Total Environ. 2023, 871, 161926. [Google Scholar] [CrossRef]
- Tretyakova, M.; Vardavas, A.; Vardavas, C.; Iatrou, E.; Stivaktakis, P.; Burykina, T.; Mezhuev, Y.; Tsatsakis, A.; Golokhvast, K. Effects of coal microparticles on marine organisms: A review. Toxicol. Rep. 2021, 8, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Neckel, A.; Oliveira, M.L.; Castro Bolaño, L.J.; Maculan, L.S.; Moro, L.D.; Bodah, E.T.; Moreno-Ríos, A.L.; Bodah, B.W.; Silva, L.F. Biophysical matter in a marine estuary identified by the Sentinel-3B OLCI satellite and the presence of terrestrial iron (Fe) nanoparticles. Mar. Pollut. Bull. 2021, 173, 112925. [Google Scholar] [CrossRef]
- Hower, J.C.; Finkelman, R.B.; Eble, C.F.; Arnold, B.J. Understanding coal quality and the critical importance of comprehensive coal analyses. Int. J. Coal Geol. 2022, 263, 104120. [Google Scholar] [CrossRef]
- Hodson, M.E.; Islam, M.; Metcalf, M.; Wright, A.C. Amendments of waste ochre from former coal mines can potentially be used to increase soil carbon persistence. Appl. Geochem. 2023, 151, 105618. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal as a promising source of critical elements: Progress and future prospects. Int. J. Coal Geol. 2018, 186, 155–164. [Google Scholar] [CrossRef]
- Harris, P.; McManus, P.; Sainsbury, P.; Viliani, F.; Riley, E. The institutional dynamics behind limited human health considerations in environmental assessments of coal mining projects in New South Wales, Australia. Environ. Impact Assess. Rev. 2021, 86, 106473. [Google Scholar] [CrossRef]
- Hossen, M.A.; Chowdhury, A.I.H.; Mullick, M.R.A.; Hoque, A. Heavy metal pollution status and health risk assessment vicinity to Barapukuria coal mine area of Bangladesh. Nanotechnol. Monit. Manag. 2021, 16, 100469. [Google Scholar] [CrossRef]
- Cardoso, A.; Turhan, E. Examining new geographies of coal: Dissenting energyscapes in Colombia and Turkey. Appl. Energy 2018, 224, 398–408. [Google Scholar] [CrossRef]
- Huang, Q.; Talan, D.; Restrepo, J.H.; Baena, O.J.R.; Kecojevic, V.; Noble, A. Characterization study of rare earths, yttrium, and scandium from various Colombian coal samples and non-coal lithologies. Int. J. Coal Geol. 2019, 209, 14–26. [Google Scholar] [CrossRef]
- López, I.C.; Ward, C.R. Composition and mode of occurrence of mineral matter in some Colombian coals. Int. J. Coal Geol. 2008, 73, 3–18. [Google Scholar] [CrossRef]
- Blandón, A.; Gorin, G. Combining palynofacies and petrography in the study of sub-bituminous tropical coals: A case history from Lower Tertiary coals in Colombia. Int. J. Coal Geol. 2013, 108, 65–82. [Google Scholar] [CrossRef]
- Moore, T.A.; Dai, S.; Huguet, C.; Pearse, J.; Liu, J.; Esterle, J.S.; Jia, R. Petrographic and geochemical characteristics of selected coal seams from the Late Cretaceous-Paleocene Guaduas Formation, Eastern Cordillera Basin, Colombia. Int. J. Coal Geol. 2022, 259, 104042. [Google Scholar] [CrossRef]
- Silva, L.F.; Crissien, T.J.; Sampaio, C.H.; Hower, J.C.; Dai, S. Occurrence of carbon nanotubes and implication for the siting of elements in selected anthracites. Fuel 2020, 263, 116740. [Google Scholar] [CrossRef]
- Dane. National Administrative Department of Statistics. Results National Census of Población of Vivienda. Santa Marta, Magdalena. 2019; pp. 1–46. Available online: https://www.dane.gov.co/files/censo2018/informacion-tecnica/presentaciones-territorio/191004-CNPV-presentacion-Magdalena.pdf (accessed on 28 January 2023).
- Patricia, G.R.O.; Blandón, A.; Perea, C.; Mastalerz, M. Petrographic characterization, variations in chemistry, and paleoenvironmental interpretation of Colombian coals. Int. J. Coal Geol. 2020, 227, 103516. [Google Scholar] [CrossRef]
- Akinyemi, S.A.; Bohórquez, F.; Islam, N.; Saikia, B.K.; Sampaio, C.H.; Crissien, T.J.; Silva, L.F. Petrography and geochemistry of exported Colombian coals: Implications from correlation and regression analyses. Energy Geosci. 2021, 2, 201–210. [Google Scholar] [CrossRef]
- Hdx. Subnational Administrative Boundaries. This dataset is part of the Colombia. Data Grid Colombia. 2023. Available online: https://data.humdata.org/dataset/cod-ab-col? (accessed on 18 January 2023).
- Qgis. Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2023. Available online: http://qgis.osgeo.org (accessed on 10 January 2023).
- Higgitt, D.L.; Warburton, J. Applications of differential GPS in upland fluvial geomorphology. Geomorphology 1999, 29, 121–134. [Google Scholar] [CrossRef]
- Luedeling, E.; Siebert, S.; Buerkert, A. Filling the voids in the SRTM elevation model-A TIN-based delta surface approach. ISPRS J. Photogramm. Remote Sens. 2007, 62, 283–294. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Q.; Su, Y.; Xue, B. Improved progressive TIN densification filtering algorithm for airborne LiDAR data in forested areas. ISPRS J. Photogramm. Remote Sens. 2016, 117, 79–91. [Google Scholar] [CrossRef]
- Dutta, M.; Saikia, J.; Taffarel, S.R.; Waanders, F.B.; Medeiros, D.d.; Cutruneo, C.M.; Silva, L.F.; Saikia, B.K. Environmental assessment and nano-mineralogical characterization of coal, overburden and sediment from Indian coal mining acid drainage. Geosci. Front. 2017, 8, 1285–1297. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Izquierdo, M.; Querol, X.; Finkelman, R.B.; Oliveira, M.L.S.; Wollenschlager, M.; Towler, M.; Pérez-López, R.; Macias, F. Leaching of potential hazardous elements of coal cleaning rejects. Environ. Monit. Assess. 2010, 175, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, A.B.; Faiia, A.M.; Szynkiewicz, A. Eight years after the coal ash spill—Fate of trace metals in the contaminated river sediments near Kingston, eastern Tennessee. Appl. Geochem. 2019, 104, 158–167. [Google Scholar] [CrossRef]
- Timpano, A.J.; Taylor, Z.; Jones, J.W. Contaminated interstitial sediment is a reservoir of trace elements with exposure potential for freshwater mussels. Environ. Adv. 2023, 12, 100357. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B.; French, D.; Hower, J.C.; Graham, I.T.; Zhao, F. Modes of occurrence of elements in coal: A critical evaluation. Earth-Sci. Rev. 2021, 222, 103815. [Google Scholar] [CrossRef]
- Oliveira, M.L.; Pinto, D.; Tutikian, B.F.; Boit, K.d.; Saikia, B.K.; Silva, L.F. Pollution from uncontrolled coal fires: Continuous gaseous emissions and nanoparticles from coal mines. J. Clean. Prod. 2019, 215, 1140–1148. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Hou, D.; Wang, Z.; Zhang, S. Quantitative investigation on the structural characteristics and evolution of high-rank coals from Xinhua, Hunan Province, China. Fuel 2021, 289, 119945. [Google Scholar] [CrossRef]
- Sime, F.M.; Jin, J.; Wang, X.; Wick, C.D.; Miller, J.D. Characterization and simulation of graphite edge surfaces for the analysis of carbonaceous material separation from sulfide ores by flotation. Miner. Eng. 2022, 182, 107590. [Google Scholar] [CrossRef]
- Rehan, I.; Gondal, M.; Almessiere, M.; Dakheel, R.; Rehan, K.; Sultana, S.; Dastageer, M. Nutritional and toxic elemental analysis of dry fruits using laser induced breakdown spectroscopy (LIBS) and inductively coupled plasma atomic emission spectrometry (ICP-AES). Saudi J. Biol. Sci. 2021, 28, 408–416. [Google Scholar] [CrossRef]
- Galhardi, J.A.; García-Tenorio, R.; Bonotto, D.M.; Díaz Francés, I.; Motta, J.G. Natural radionuclides in plants, soils and sediments affected by U-rich coal mining activities in Brazil. J. Environ. Radioact. 2017, 177, 37–47. [Google Scholar] [CrossRef]
- Montross, S.N.; Verba, C.A.; Chan, H.L.; Lopano, C. Advanced characterization of rare earth element minerals in coal utilization byproducts using multimodal image analysis. Int. J. Coal Geol. 2018, 195, 362–372. [Google Scholar] [CrossRef]
- Kwiecińska, B.; Pusz, S.; Valentine, B.J. Application of electron microscopy TEM and SEM for analysis of coals, organic-rich shales and carbonaceous matter. Int. J. Coal Geol. 2019, 211, 103203. [Google Scholar] [CrossRef]
- Roelandts, I.; Deblond, A. Rare-earth element composition of Devonian sediments from southern Belgium: Application of an inductively coupled plasma-atomic emission spectrometry method. Chem. Geol. 1992, 95, 167–176. [Google Scholar] [CrossRef]
- Wei, W.J.; Yang, Y.; Li, X.Y.; Huang, P.; Wang, Q.; Yang, P.J. Cloud point extraction (CPE) combined with single particle-inductively coupled plasma-mass spectrometry (SP-ICP-MS) to analyze and characterize nano-silver sulfide in water environment. Talanta 2022, 239, 123117. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Bu, W.; Ni, Y.; Liu, X.; Zheng, J.; Aono, T.; Yang, C.; Hu, S. Rapid monitoring of 241Am in small amount of sediment samples by combining extraction chromatography for highly efficient separation of interfering and matrix elements and ICP-MS/MS measurement. Microchem. J. 2023, 190, 108581. [Google Scholar] [CrossRef]
- Sanchís, J.; Božović, D.; Al-Harbi, N.A.; Farré, M.; Barceló, D. Quantitative trace analysis of fullerenes in river sediment from Spain and soils from Saudi Arabia. Anal. Bioanal. Chem. 2013, 405, 5915–5923. [Google Scholar] [CrossRef]
- Farag, S.; Konyashin, I.; Ries, B. The influence of grain growth inhibitors on the microstructure and properties of submicron, ultrafine and nano-structured hardmetals—A review. Int. J Refract. Hard. Met. 2018, 77, 12–30. [Google Scholar] [CrossRef]
- Pandey, B.; Mukherjee, A.; Agrawal, M.; Singh, S. Assessment of Seasonal and Site-Specific Variations in Soil Physical, Chemical and Biological Properties Around Opencast Coal Mines. Pedosphere 2019, 29, 642–655. [Google Scholar] [CrossRef]
- Tang, Q.; Sheng, W.; Li, L.; Zheng, L.; Miao, C.; Sun, R. Alteration behavior of mineral structure and hazardous elements during combustion of coal from a power plant at Huainan, Anhui, China. Environ. Pollut. 2018, 239, 768–776. [Google Scholar] [CrossRef]
- Park, H.; Wang, L.; Yun, J.H. Coal beneficiation technology to reduce hazardous heavy metals in fly ash. J. Hazard. Mater. 2021, 416, 125853. [Google Scholar] [CrossRef]
- Han, J.; Yu, D.; Wang, Q.; Yu, N.; Wu, J.; Liu, Y.; Luo, L.; Pan, H. Beneficiation of coal ash from ash silos of six Chinese power plants and its risk assessment of hazardous elements for land application. Process Saf. Environ. Prot. 2022, 160, 641–649. [Google Scholar] [CrossRef]
- Eskanazy, G.; Finkelman, R.B.; Chattarjee, S. Some considerations concerning the use of correlation coefficients and cluster analysis in interpreting coal geochemistry data. Int. J. Coal Geol. 2010, 83, 491–493. [Google Scholar] [CrossRef]
- Silva, L.F.; Crissien, T.J.; Schneider, I.L.; Blanco, R.P.; Sampaio, C.H. Nanometric particles of high economic value in coal fire region: Opportunities for social improvement. J. Clean. Prod. 2020, 256, 120480. [Google Scholar] [CrossRef]
- Liu, Y.; Molinari, S.; Dalconi, M.C.; Valentini, L.; Ricci, G.; Carrer, C.; Ferrari, G.; Artioli, G. The leaching behaviors of lead, zinc, and sulfate in pyrite ash contaminated soil: Mineralogical assessments and environmental implications. J. Environ. Chem. Eng. 2023, 11, 109687. [Google Scholar] [CrossRef]
- Salih, N.; Mansurbeg, H.; Muchez, P.; Axel, G.; Préat, A. Hydrothermal Fluids and Cold Meteoric Waters along Tectonic-Controlled Open Spaces in Upper Cretaceous Carbonate Rocks, NE-Iraq: Scanning Data from In Situ U-Pb Geochronology and Microthermometry. Water 2021, 13, 3559. [Google Scholar] [CrossRef]
- Salih, N. The Impact of Hydrothermal Fluids on Porosity Enhancement and Hydrocarbon Migration in Qamchuqa Formation, Lower Cretaceous, Kirkuk Oil Company. Minerals 2023, 13, 377. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Chen, Y.; Zhang, L.; Sheng, X. A trace amount of MXene@PDA nanosheets for low-temperature zinc phosphating coatings with superb corrosion resistance. Appl. Surf. Sci. 2022, 603, 154455. [Google Scholar] [CrossRef]
- Jiang, Z.; Nie, K.; Arinzechi, C.; Li, J.; Liao, Q.; Si, M.; Yang, Z.; Li, Q.; Yang, W. Cooperative effect of slow-release ferrous and phosphate for simultaneous stabilization of As, Cd and Pb in soil. J. Hazard. Mater. 2023, 452, 131232. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Chen, X.; Huang, C.; Yang, S. Biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles for phosphate adsorption. J. Hazard. Mater. 2020, 396, 122626. [Google Scholar] [CrossRef]
- Moumen, E.; Bazzi, L.; El Hankari, S. Metal-organic frameworks and their composites for the adsorption and sensing of phosphate. Coord. Chem. Rev. 2022, 455, 214376. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M. Metal organic frameworks and their composites as effective tools for sensing environmental hazards: An up to date tale of mechanism, current trends and future prospects. Coord. Chem. Rev. 2023, 474, 214859. [Google Scholar] [CrossRef]
- Ma, W.; Liu, S.; Li, Z.; Lv, J.; Yang, L. Release and transformation mechanisms of hazardous trace elements in the ash and slag during underground coal gasification. Fuel 2020, 281, 118774. [Google Scholar] [CrossRef]
- Marove, C.A.; Tangviroon, P.; Tabelin, C.B.; Igarashi, T. Leaching of hazardous elements from Mozambican coal and coal ash. J. Afr. Earth Sci. 2020, 168, 103861. [Google Scholar] [CrossRef]
- Ali, M.U.; Liu, Y.; Yousaf, B.; Wong, M.H.; Li, P.; Liu, G.; Wang, R.; Wei, Y.; Lu, M. Morphochemical investigation on the enrichment and transformation of hazardous elements in ash from waste incineration plants. Sci. Total Environ. 2022, 828, 154490. [Google Scholar] [CrossRef] [PubMed]
- Lahrouch, F.; Baptiste, B.; Dardenne, K.; Rothe, J.; Elkaim, E.; Descostes, M.; Gerard, M. Uranium speciation control by uranyl sulfate and phosphate in tailings subject to a Sahelian climate, Cominak, Niger. Chemosphere 2022, 287, 132139. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, L.; Sun, R.; Hu, Y.; Tang, G.; Chen, W.; Du, Y.; Che, D. Effects of silicon-aluminum additives on ash mineralogy, morphology, and transformation of sodium, calcium, and iron during oxy-fuel combustion of zhundong high-alkali coal. Int. J. Greenh. Gas Control. 2019, 91, 102832. [Google Scholar] [CrossRef]
- Arbuzov, S.; Chekryzhov, I.; Spears, D.; Ilenok, S.; Soktoev, B.; Popov, N. Geology, geochemistry, mineralogy and genesis of the Spetsugli high-germanium coal deposit in the Pavlovsk coalfield, Russian Far East. Ore Geol. Rev. 2021, 139, 104537. [Google Scholar] [CrossRef]
- Yuan, Z.; Jia, G.; Cui, X.; Song, X.; Wang, C.; Zhao, P.; Ragauskas, A.J. Effects of temperature and time on supercritical methanol Co-Liquefaction of rice straw and linear low-density polyethylene wastes. Energy 2022, 245, 123315. [Google Scholar] [CrossRef]
- Li, B.; Zhuang, X.; Querol, X.; Moreno, N.; Córdoba, P.; Shangguan, Y.; Yang, L.; Li, J.; Zhang, F. Geological controls on the distribution of REY-Zr (Hf)-Nb (Ta) enrichment horizons in late Permian coals from the Qiandongbei Coalfield, Guizhou Province, SW China. Int. J. Coal Geol. 2022, 231, 103604. [Google Scholar] [CrossRef]
- Ribeiro, J.; Flores, D.; Ward, C.R.; Silva, L.F. Identification of nanominerals and nanoparticles in burning coal waste piles from Portugal. Sci. Total Environ. 2010, 408, 6032–6041. [Google Scholar] [CrossRef]
- Silva, L.F.; Oliveira, M.L.; Neace, E.R.; O’Keefe, J.M.; Henke, K.R.; Hower, J.C. Nanominerals and ultrafine particles in sublimates from the Ruth Mullins coal fire, Perry County, Eastern Kentucky, USA. Int. J. Coal Geol. 2011, 85, 237–245. [Google Scholar] [CrossRef]
- Silva, L.F.; Oliveira, M.L.; Philippi, V.; Serra, C.C.; Hower, J.C.; Xue, W.; Chen, W.; O’Keefe, J.M.; Romanek, C.S.; Hopps, S.D.; et al. Geochemistry of carbon nanotube assemblages in coal fire soot, Ruth Mullins fire, Perry County, Kentucky. Int. J. Coal Geol. 2012, 94, 206–213. [Google Scholar] [CrossRef]
- Hower, J.C.; O’Keefe, J.M.; Henke, K.R.; Wagner, N.; Copley, G.C.; Blake, D.R.; Garrison, T.M.; Oliveira, M.L.; Kautzmann, R.M.; Silva, L.F. Gaseous emissions and sublimates from the Truman Shepherd coal fire, Floyd County, Kentucky: A re-investigation following attempted mitigation of the fire. Int. J. Coal Geol. 2013, 116–117, 63–74. [Google Scholar] [CrossRef]
- Wilcox, J.; Wang, B.; Rupp, E.C.; Taggart, R.K.; Hsu-Kim, H.; Oliveira, M.L.; Cutruneo, C.M.; Taffarel, S.R.; Silva, L.F.; Hopps, S.D.; et al. Observations and Assessment of Fly Ashes from High-Sulfur Bituminous Coals and Blends of High-Sulfur Bituminous and Subbituminous Coals: Environmental Processes Recorded at the Macro-and Nanometer Scale. Energy Fuels 2015, 29, 7168–7177. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Kang, D.; Yang, J.; Luo, J. Emission control strategies of hazardous trace elements from coal-fired power plants in China. J. Environ. Sci. 2020, 93, 66–90. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Suárez-Ruiz, I.; Flores, D. Coal related fires in Portugal: New occurrences and new insights on the characterization of thermally affected and non-affected coal waste piles. Int. J. Coal Geol. 2022, 252, 103941. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth-Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Lieberman, N.R.; Izquierdo, M.; Muñoz-Quirós, C.; Cohen, H.; Chenery, S.R. Geochemical signature of superhigh organic sulphur Raša coals and the mobility of toxic trace elements from combustion products and polluted soils near the Plomin coal-fired power station in Croatia. Appl. Geochem. 2020, 114, 104472. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, X.; Yu, I.K.; Wang, L.; Zhou, J.; Sun, X.; Rinklebe, J.; Shaheen, S.M.; Ok, Y.S.; Lin, Z.; et al. Potentially toxic elements in solid waste streams: Fate and management approaches. Environ. Pollut. 2019, 253, 680–707. [Google Scholar] [CrossRef]
- Dill, H.G.; Jolanta, K.; Andrei, B.; Sorin-Ionut, B.; Stephan, K.; Borrego, A.G. Organic debris and allochthonous coal in Quaternary landforms within a periglacial setting (Longyearbyen Mining District, Norway)—A multi-disciplinary study (coal geology-geomorphology-sedimentology). Int. J. Coal Geol. 2021, 233, 103625. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, Z.; Sun, J.; Zou, H.; He, K.; Wang, X.; Li, J.; Cui, S.; Zhang, N.; Cao, J. Emission characteristics and formation mechanisms of PM2.5 and gases from different geological maturities coals combustion. Fuel 2022, 315, 123240. [Google Scholar] [CrossRef]
- Vo, T.L.; Nash, W.; Del Galdo, M.; Rezania, M.; Crane, R.; Mousavi Nezhad, M.; Ferrara, L. Coal mining wastes valorization as raw geomaterials in construction: A review with new perspectives. J. Clean. Prod. 2022, 336, 130213. [Google Scholar] [CrossRef]


| Identified Elements | La Guajíra 2017 | La Guajíra 2022 | Cesar 2017 | Cesar 2022 |
|---|---|---|---|---|
| Fullerene s (C60, C70, C80) | ▼ | ▲ | ▼ | ▲ |
| Graphenes | ▲ | ▲ | ▼ | ▲ |
| Carbon nanotubes | ▼ | ▲ | ▼ | ▲ |
| Amorphous organic nanophases | ▲ | ▲ | ▲ | ▲ |
| Silicates | ||||
| Quartz, SiO2 | ▲ | ▲ | ▲ | ▲ |
| Chlorite, Na0.5Al6(Si,Al)8O20(OH)10.H2O | ▲ | ▲ | ▲ | ▲ |
| Illite, K1.5Al4(Si6.5Al1.5)O20(OH)4 | ▲ | ▲ | ▲ | ▲ |
| Kaolilite, Al2Si2O5(OH)4 | ▲ | ▲ | ▲ | ▲ |
| Microcline, KAlSi3O8 | ▲ | ▲ | ▲ | ▲ |
| Montmorillonite, (Na,Ca)(Al,Mg)2Si4O10(OH)2.n(H2O) | ▲ | ▲ | ▼ | ▲ |
| Mullite, Al6Si2O13 | ▲ | ▲ | ▼ | ▲ |
| Muscovite, KAl2(AlSi3)O10(OH)2 | ▲ | ▲ | ▲ | ▲ |
| Zircon, ZrSiO4 | ▼ | ▼ | ▼ | ▼ |
| Sulphides | ||||
| Bismuthinite, Bi2S3 | ▼ | N.D. | N.D. | N.D. |
| Cattierite, CoS2 | ▼ | N.D. | N.D. | N.D. |
| Chalcopyrite, CuFeS2 | ▼ | N.D. | ▼ | ▼ |
| Galena, PbS | ▼ | N.D. | ▼ | N.D. |
| Marcasite, FeS2 | ▼ | N.D. | ▼ | N.D. |
| Molybdenite, MoS2 | ▼ | ▼ | ▼ | ▼ |
| Millerite, NiS | ▼ | ▼ | ▼ | ▼ |
| Pyrite, FeS2 | ▼ | ▼ | ▼ | ▼ |
| Pyrrhotite Fe(1 − x)S | ▼ | ▼ | ▼ | ▼ |
| Sphalerite, ZnS | ▼ | ▼ | ▼ | ▼ |
| Carbonates | ||||
| Calcite, CaCO3 | ▼ | N.D. | ▼ | N.D. |
| Dolomite, CaMg(CO3)2 | ▼ | N.D. | ▼ | ▼ |
| Siderite, FeCO3 | ▼ | N.D. | ▼ | N.D. |
| Phosphates | ||||
| Brushite, CaPO3(OH).2H2O | ▼ | ▼ | ▼ | N.D. |
| Monazite, (Ce, La, Th, Nd, Y)PO4 | ▼ | ▼ | ▼ | ▼ |
| Xenotime, (Y, Er)PO4 | ▼ | ▼ | ▼ | ▼ |
| Sulfates | ||||
| Ammoniojarosite, [(NH4)Fe3(SO4)2(OH)6] | ▼ | N.D. | ▼ | N.D. |
| Anhydrite, CaSO4 | ▼ | N.D. | ▼ | N.D. |
| Alunogen, Al2(SO4)3.17H2O | N.D. | N.D. | ▼ | ▼ |
| Barite, BaSO4 | ▼ | ▼ | ▼ | ▼ |
| Bassanite, CaSO4·1/2H2O | N.D. | N.D. | N.D. | ▼ |
| Copiapite, MgFe4(SO4)6(OH2) 18H2O | N.D. | N.D. | ▼ | N.D. |
| Coquimbite, Fe2(SO4)3·9H2O | N.D. | N.D. | ▼ | N.D. |
| Epsomite, MgSO4 7H2O | N.D. | N.D. | N.D. | ▼ |
| Ferrohexahydrite, FeSO4 6H2O | N.D. | N.D. | N.D. | ▼ |
| Halotrichite, FeAl2 (SO4)4.22H2O | N.D. | N.D. | N.D. | ▼ |
| Hexahydrite, MgSO4 6H2O | N.D. | N.D. | ▼ | ▼ |
| Gypsum, Ca[SO4]·2H2O | N.D. | N.D. | ▼ | ▼ |
| Jarosite, KFe3+3(SO4)2(OH) 6 | N.D. | N.D. | ▼ | ▼ |
| Melanterite, FeSO4 7H2O | N.D. | N.D. | ▼ | ▼ |
| Natrojarosite, NaFe3(SO4)2(OH)6 | N.D. | N.D. | N.D. | N.D. |
| Pickeringite, MgAl2(SO4)4·22H2O | N.D. | N.D. | ▼ | ▼ |
| Rozenite, FeSO4 4H2O | N.D. | N.D. | ▼ | N.D. |
| Schwertmannite, Fe3+16O16(OH)12(SO4)2 | N.D. | N.D. | ▼ | N.D. |
| Oxides and hydroxides | ||||
| Anatase, TiO2 | ▼ | ▼ | ▼ | ▼ |
| Brookite, TiO2 | ▼ | ▼ | ▼ | ▼ |
| Chromite, (Fe,Mg)Cr2O4 | ▼ | ▼ | ▼ | ▼ |
| Hematite, Fe2O3 | ▲ | ▲ | ▼ | ▲ |
| Goethite, Fe(OH)3 | ▼ | ▼ | ▼ | ▼ |
| Gibbsite, Al(OH)3 | ▼ | ▼ | ▼ | ▼ |
| Maghemite, Fe2O3 | ▼ | ▼ | ▼ | ▼ |
| Magnetite, Fe3O4 | ▼ | ▼ | ▼ | ▼ |
| Rutile, TiO2 | ▼ | ▼ | ▼ | ▼ |
| Amorphous inorganic phases | ▲ | ▲ | ▲ | ▲ |
| Regions Studied | Elements |
|---|---|
| La Guajíra—2017 | As, Be, Br, Cl, Cd, Co, Cu, Cr, F, Hg, La, Mn, Mo, Nb, Nd, Ni, Pb, S, Sb, Se, Sn, V, Y, Zn |
| La Guajíra—2022 | As, Cd, Co, Cu, Cr, La, Mn, Mo, Nb, Nd, Ni, Pb, S, Sb, Se, Sn, V, Y, Zn |
| Cesar—2017 | As, Be, Cl, Cd, Co, Cu, Cr, F, Hg, La, Mn, Mo, Nb, Nd, Ni, Pb, S, Sb, Se, Sn, V, Y, Zn |
| Cesar—2022 | Cd, Co, Cu, Cr, Hg, La, Mn, Nb, Nd, Ni, Pb, S, Se, V, Y, Zn |
| Element | Mean | Min | Max |
|---|---|---|---|
| (%) | (%) | (%) | |
| Al | 9.7 | 2.31 | 11.9 |
| Ca | 0.3 | 0.05 | 0.9 |
| Fe | 4.2 | 0.9 | 11.7 |
| K | 2.8 | 0.2 | 3.1 |
| Mg | 0.2 | 0.1 | 0.5 |
| Na | 0.3 | 0.1 | 0.5 |
| Na | 0.5 | 0.1 | 0.6 |
| P | 0.3 | 0.01 | 0.3 |
| S | 0.3 | 0.07 | 1 |
| Ti | 0.3 | 0.1 | 0.5 |
| (ppm) | (ppm) | (ppm) | |
| As | 37.8 | 19.2 | 108 |
| Ba | 501 | 132 | 843 |
| Bi | 0.5 | 0.3 | 0.9 |
| Cd | 0.3 | 0.1 | 0.6 |
| Co | 5.1 | 0.5 | 13.1 |
| Cr | 103 | 18.3 | 227 |
| Cs | 20.1 | 7.2 | 50.1 |
| Cu | 30.1 | 9.5 | 103.5 |
| Ga | 27.3 | 3.4 | 40.1 |
| Li | 153 | 9.1 | 303 |
| Mn | 119 | 7.3 | 1212 |
| Mo | 3.1 | 2.7 | 10.1 |
| Nd | 19.2 | 6.1 | 45.1 |
| Ni | 30.2 | 3.9 | 77.9 |
| Pb | 40.1 | 21.2 | 201 |
| Sb | 7.9 | 1.2 | 80.1 |
| Sn | 3.2 | 0.8 | 18.9 |
| Sr | 102 | 40.6 | 361 |
| Ta | 0.4 | 0.1 | 2.4 |
| Th | 19.6 | 8.6 | 20.3 |
| U | 3.1 | 1.1 | 6.8 |
| V | 101 | 42.5 | 191 |
| W | 3.9 | 0.9 | 8.1 |
| Y | 11.3 | 1.2 | 20.3 |
| Zn | 47.3 | 16.1 | 117 |
| Zr | 101 | 21.1 | 203 |
| Element | Mean | Min | Max |
|---|---|---|---|
| (%) | (%) | (%) | |
| Al | 11.9 | 2.38 | 13.3 |
| Ca | 0.4 | 0.09 | 0.8 |
| Fe | 4.3 | 0.7 | 13.9 |
| K | 3.1 | 0.3 | 3.8 |
| Mg | 0.3 | 0.1 | 1.4 |
| Na | 0.4 | 0.1 | 0.9 |
| Na | 0.3 | 0.1 | 0.7 |
| P | 0.2 | 0.06 | 0.4 |
| S | 0.5 | 0.09 | 1.1 |
| Ti | 0.4 | 0.1 | 0.7 |
| (ppm) | (ppm) | (ppm) | |
| As | 41.9 | 21.3 | 117 |
| Ba | 533 | 172 | 943 |
| Bi | 0.8 | 0.3 | 1.9 |
| Cd | 0.4 | 0.1 | 0.7 |
| Co | 6.8 | 0.9 | 18.5 |
| Cr | 133 | 21.2 | 292 |
| Cs | 23.1 | 8.2 | 58.2 |
| Cu | 38.4 | 12.7 | 122.2 |
| Ga | 30.7 | 4.4 | 44.7 |
| Li | 168 | 11.5 | 392 |
| Mn | 147 | 8.3 | 1811 |
| Mo | 4.7 | 3.3 | 13.9 |
| Nd | 20.3 | 7.1 | 49.8 |
| Ni | 33.8 | 4.9 | 80.7 |
| Pb | 43.1 | 19.2 | 299 |
| Sb | 9.8 | 1.9 | 81.3 |
| Sn | 4.5 | 0.9 | 21.3 |
| Sr | 111 | 47.1 | 347 |
| Ta | 1.4 | 0.8 | 6.3 |
| Th | 21.2 | 8.1 | 25.7 |
| U | 5.3 | 2 | 9.9 |
| V | 131 | 40.1 | 205 |
| W | 4.1 | 1.8 | 7.7 |
| Y | 14.9 | 6.8 | 28.1 |
| Zn | 51.2 | 17 | 141 |
| Zr | 118 | 23.1 | 219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.L.S.; Valença, G.O.; Pinto, D.; Moro, L.D.; Bodah, B.W.; de Vargas Mores, G.; Grub, J.; Adelodun, B.; Neckel, A. Hazardous Elements in Sediments Detected in Former Decommissioned Coal Mining Areas in Colombia: A Need for Environmental Recovery. Sustainability 2023, 15, 8361. https://doi.org/10.3390/su15108361
Oliveira MLS, Valença GO, Pinto D, Moro LD, Bodah BW, de Vargas Mores G, Grub J, Adelodun B, Neckel A. Hazardous Elements in Sediments Detected in Former Decommissioned Coal Mining Areas in Colombia: A Need for Environmental Recovery. Sustainability. 2023; 15(10):8361. https://doi.org/10.3390/su15108361
Chicago/Turabian StyleOliveira, Marcos L. S., Gabriela Oliveira Valença, Diana Pinto, Leila Dal Moro, Brian William Bodah, Giana de Vargas Mores, Julian Grub, Bashir Adelodun, and Alcindo Neckel. 2023. "Hazardous Elements in Sediments Detected in Former Decommissioned Coal Mining Areas in Colombia: A Need for Environmental Recovery" Sustainability 15, no. 10: 8361. https://doi.org/10.3390/su15108361






