Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains and Cellulolytic Activity
2.2. Identification of Cellulase-Producing Bacteria
2.3. Solid State Fermentation
2.3.1. Inoculum Preparation
2.3.2. Solid-State Fermentation
2.3.3. Enhanced Production of Cellulase and High Biomass of Bacillus amyloliquefaciens (D1B3) through Process Optimization
2.3.4. Statistical Analysis and Mathematic Models
2.4. Analysis
2.4.1. Extraction of Crude Enzyme and Quantification of Total Cellulase Activity
2.4.2. Viable Cells Enumeration
2.5. Screening of Probiotic Properties of Cellulolytic Bacteria
2.5.1. Antibacterial and Antifungal Activities
2.5.2. Hydrophobicity Test
2.5.3. Acid Tolerance
2.5.4. Bile Tolerance
2.5.5. Coaggregation with Saccharomyces Cerevisiae
2.6. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Cellulolytic Bacillus
3.2. Morphologic and Genotypic Identification of Performed Cellulolytic Bacillus Isolate
3.3. Screening of Probiotic Properties
3.3.1. Antimicrobial Activities
3.3.2. Acid Tolerance
3.3.3. Bile Salt Tolerance
3.3.4. Cell Surface Hydrophobicity and Co-Aggregation Ability
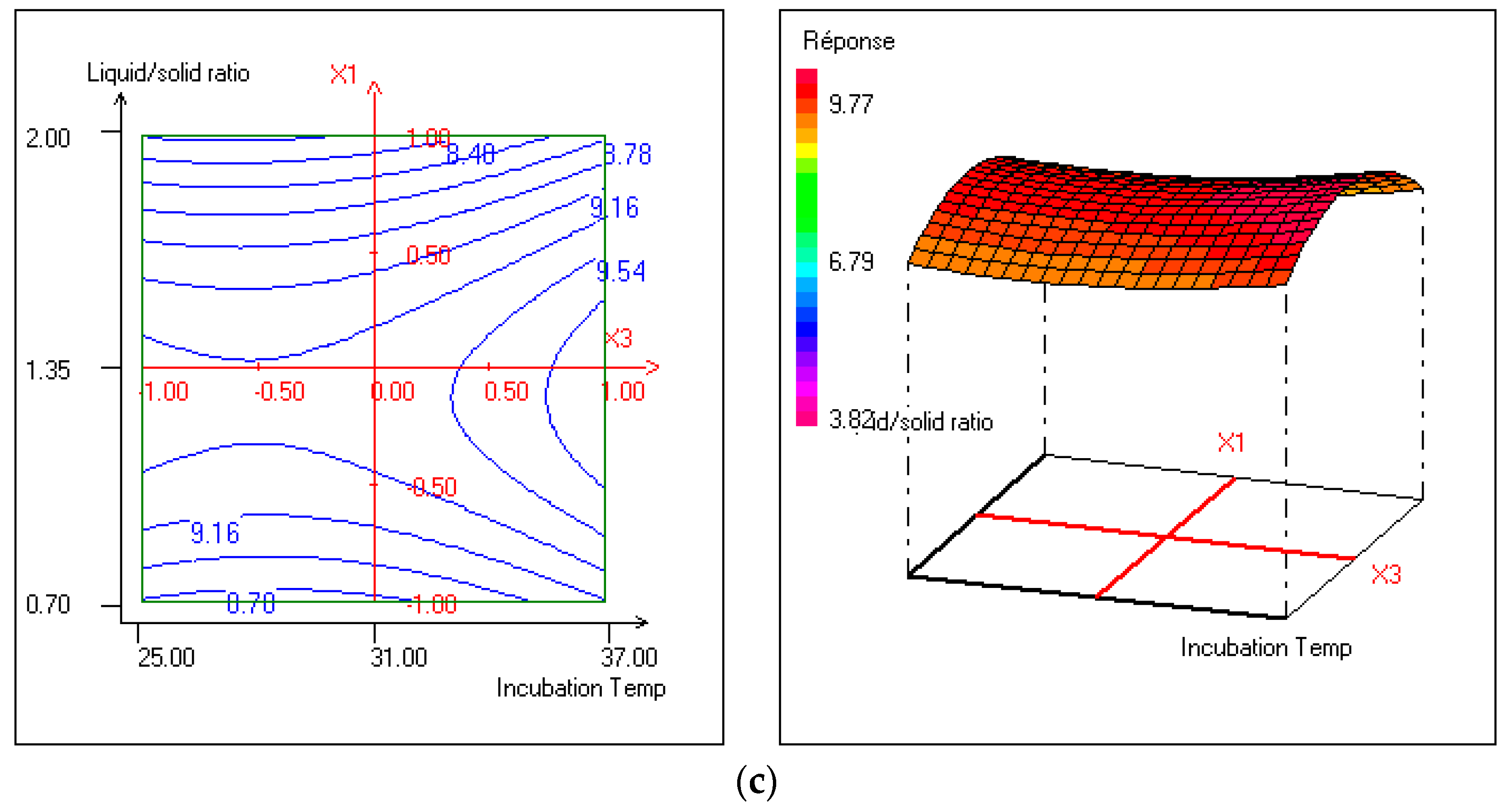
3.3.5. Optimization Production of Cellulase and High Biomass of Bacillus Amyloliquefaciens (D1B3) in the Wheat Brain using SSF and through Process Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markets and markets.com. Feed Enzymes Market by Type (Phytase, Carbohydrase and Protease), Livestock (Poultry Swine, Ruminants, and Aquatic Animals), Source (Microrganism, Plant and Animal), form (Dry and Liquid) and Region-Global Forecast to 2025. 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/feed-enzyme-market-1157.html (accessed on 23 June 2020).
- Adeola, O.; Cowieson, A.J. Board-invited review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Bimrew, A. Effect of common feed enzymes on nutrient utilization of monogastric animals. Int. J. Biotechnololgy Mol. Biol. Res. 2014, 5, 27–34. [Google Scholar] [CrossRef]
- Thapa, S.; Mishra, J.; Arora, N.; Mishra, P.; Li, H.; O’Hair, J.; Bhatti, S.; Zhou, S. Microbial cellulolytic enzymes: Diversity and biotechnology with reference to lignocellulosic biomass degradation. Rev. Environ. Sci. Biotechnol. 2020, 19, 621–648. [Google Scholar] [CrossRef]
- Nandy, G.; Chakraborti, M.; Shee, A.; Aditya, G.; Acharya, K. Gut Microbiota from Lower Groups of Animals: An Upcoming Source for Cellulolytic Enzymes with Industrial Potentials Bio-interface. Res. Appl. Chem. 2021, 11, 13614–13637. [Google Scholar]
- Budihal, S.R.; Agsar, D.; Patil, S.R. Enhanced production and application of acidothermophilic Streptomyces cellulose. Bioresour. Technol. 2016, 200, 706–712. [Google Scholar] [CrossRef]
- Chakraborty, D.; Sarkar, N.; Biswas, I.; Jacob, S. Molecular Aspect of Prokaryote and Eukaryote Cellulase and Thieir Modulation for Potential Application in Biofuel Production Genetic and Metabolic Engineering for Improved Buofuel Production from Lignocellulosic, Biomass; Elsevier: Amsterdam, The Netherlands, 2020; pp. 81–95. [Google Scholar]
- Doi, R.H. Cellulases of mesophilic microorganisms: Cellulosome and noncellulosome producers. Ann. N. Y. Acad. Sci. 2008, 1125, 267–279. [Google Scholar] [CrossRef]
- Islam, F.; Roy, N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res. Notes 2018, 11, 445. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Design aspect of solid state fermentation as applied to Microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 1–25. [Google Scholar]
- Bentil, J.A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A.S. Cellulase production by white-rot basidiomycetous fungi: Solid-state versus submerged cultivation. Appl. Microbiol. Biotechnol. 2018, 102, 5827–5839. [Google Scholar] [CrossRef]
- Croos, A.M.B.; Sarathadevi, R.; Kapilan, R. Isolation of cellulose producing Bacillus Cereus from Cow dung and determination of Kinetic properties of the crude enzyme. J. Nat. Sci. Found. Sri Lanka 2019, 47, 261–267. [Google Scholar] [CrossRef]
- Li, F.; Xie, Y.; Gao, X.; Shan, M.; Sun, C.; Niu, Y.D.; Shan, A. Screening of cellulose degradation bacteria from Min pigs and optimization of its cellulase production. Electron. J. Biotechnol. 2020, 48, 29–35. [Google Scholar] [CrossRef]
- Maravi, P.; Kumar, A. Optimization and statistical modeling of microbial cellulase production using submerged culture. J. Appl. Biol. Biotechnol. 2021, 9, 142–152. [Google Scholar]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Simon, M. Cutting Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar]
- Khalid, F.; Khalid, A.; Fu, Y.; Hu, Q.; Zheng, Y.; Khan, S.; Wang, Z. Potential of Bacillus velezensis as a probiotic in animal feed: A review. J. Microbiol. 2021, 59, 627–633. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult. Sci. 2015, 94, 1504–1511. [Google Scholar] [CrossRef]
- Sellami, A. Sélection et Caractérisation de Bactéries du genre Bacillus à Activité Cellulolytique. Master’s Thesis, University of Carthage, Tunis, Tunisia, 2011. [Google Scholar]
- Ariffin, H.; Abdullah, N.; UmKalsom, M.S.; Shirai, Y.; Hassan, M.A. Production and characterisation of cellulase by Bacilluspumilus EB3. Int. J. Eng. Technol. 2006, 3, 47–53. [Google Scholar]
- Wilson, K. Preparation of genomic DNA from bacteria. Curr. Protoc. Mol. Biol. 1989, 56, 241–245. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities, Pure and Applied Chemistry. Biochem. Eng. Cent. 1987, 59, 257–268. [Google Scholar]
- Tagg, J.R.; Daani, A.S.; Wannamaker, L.W. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756. [Google Scholar] [CrossRef]
- Dalie, D.K.D.; Deschamps, A.M.; Atanasova-Penichon, V.; Richard-Forget, F. Potential of Pediococcus pentosaceus (L006) Isolated from Maize Leaf to Suppress Fumonisin-Producing Fungal Growth. J. Food Prot. 2010, 73, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, J.; Yan, L.; Chen, W.; Liu, X.M.; Zhang, H.P. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Sci. Technol. 2009, 42, 1640–1646. [Google Scholar] [CrossRef]
- Du Toit, M.; Franz, C.M.A.P.; Dicks, L.M.T.; Schillinger, U.; Haberer, P.; Warlies, B.; Ahrens, F.; Holzapfel, W.H. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serumcholesterol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol. 1998, 1–2, 93–104. [Google Scholar] [CrossRef]
- Lee, A.; Cheng, K.C.; Liu, J.R. Isolation and characterization of a Bacillus amyloliquefaciens strain with zearalenone removal ability and its probiotic potential. PLoS ONE 2017, 12, e0182220. [Google Scholar] [CrossRef]
- Manhar, A.K.; Saikia, D.; Bashir, Y.; Mech, R.K.; Nath, D.; Konwar, B.K.; Mandal, M. In vitro evaluation of celluloytic Bacillus amyloliquefaciens AMS1 isolated from traditional fermented soybean (Churpi) as an animal probiotic. Res. Vet. Sci. 2015, 99, 149–156. [Google Scholar] [CrossRef]
- Chateau, N.; Deschamps, A.M.; Sassi, A.H. Heterogeneity of bile salts resistance in the Lactobacillus isolates of a probiotic consortium. Lett. Appl. Microbiol. 1994, 18, 42–44. [Google Scholar] [CrossRef]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Britsh J. Nutr. 2012, 109, S35–S50. [Google Scholar] [CrossRef]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Chincholkar, S.B. Comparative accounts of probiotic characteristics of Bacillus spp. Isolated from food wastes. Food Res. Int. 2009, 42, 505–510. [Google Scholar] [CrossRef]
- Barbosa, T.M.; Serra, C.R.; La Ragione, R.M.; Woodward, M.J.; Henriques, A.O. Screening for Bacillus Isolates in the Broiler. Appl. Environ. Microbiol. 2005, 71, 968–978. [Google Scholar] [CrossRef]
- Rosenberg, M. Microbialadhesion to hydrocarbons: Twenty-five years of doing MATH. FEMS Microbiol. Lett. 2006, 262, 129–134. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Reimhult, E. Biofilm formation at oil-water interfaces is not a simple function of bacterial hydrophobicity. Colloids Surf. Bionterfaces 2020, 194, 1163. [Google Scholar] [CrossRef]
- Pelletier, C.; Bouley, C.; Cayuela, C.; Bouttier, S.; Bourlioux, P.; Bellon-Fontaine, M.N. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Rumen Appl. Environ. Microbiol. 1997, 63, 1725–1731. [Google Scholar] [CrossRef]
- Valeriano, V.D.; Parungao-Balolong, M.M.; Kang, D.-K. In vitro evaluation of the mucin-adhesion ability and probiotic potential of Lactobacillus mucosae LM1. J. Appl. Microbiol. 2014, 117, 485–497. [Google Scholar] [CrossRef]
- Furukawa, S.; Nojima, N.; Yoshida, K.; Hirayama, S.; Ogihara, H.; Morinaga, Y. The Importance of Inter-Species Cell-Cell Co-Aggregation between Lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae BY4741 in Mixed-Species Biofilm Formation. Biosci. Biotechnol. Biochem. 2011, 75, 1430–1434. [Google Scholar] [CrossRef]
- Hirayama, S.; Nojima, N.; Furukawa, S.; Ogihara, H.; Morinaga, Y. Steric microstructure of mixed-species biofilm formed by interaction between Lactobacillus plantarum ML11-11 and Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2019, 83, 2386–2389. [Google Scholar] [CrossRef]




| Isolate Code | Diameter of Clear Zone of Hydrolysis (cm) |
|---|---|
| D1B1 | 1.1 ± 0.2 |
| D1B3 | 2.4 ± 0.0 |
| D1B4 | 2.2 ± 0.1 |
| D1B7 | 1.8 ± 0.3 |
| D1B10 | 1.1 ± 0.2 |
| D1B12 | 1.6 ± 0.0 |
| COL7B2 | 1.4 ± 0.4 |
| COL7B5 | 2.1 ± 0.0 |
| COL7B6 | 1.1 ± 0.0 |
| COL7B7 | 2 ± 0.1 |
| COL7B8 | 1.1 ± 0.0 |
| COLB11 | 1.5 ± 0.2 |
| C3-1B8 | 1.9 ± 0.2 |
| Indicator Strains | Zone of Inhibition(mm) |
|---|---|
| Aspergillus spp. | 24 ± (0.0) |
| Klebsiella pneumoniae 10031 | 24 ± (2.0) |
| Escherichia coli 10536 | 24 ± (0.0) |
| Pseudomonas aeruginosa 9027 | 27 ± (1.0) |
| Streptococcus faecalis 10541 | - |
| Staphylococcus aureus 6538 | - |
| Staphylococcus epidermidis 12228 | - |
| Bacillus cereus 11778 | - |
| Strains | pH 2.5 | pH 3 | pH 4 | |
|---|---|---|---|---|
| 1 h | Bacillus amyloliquefaciens | 90.50 ± 0.15 b | 91.56 ± 0.09 b | 93.38 ± 0.1 b |
| Lactobacillus plantarum 299 | 91.58 ± 0.33 a | 95.14 ± 0.07 a | 96.72 ± 0.03 a | |
| 2 h | Bacillus amyloliquefaciens | 81.44 ± 0.15 d | 87.46 ± 0.1 d | 89.73 ± 0.06 d |
| Lactobacillus plantarum 299 | 84.92 ± 0.05 c | 89.87 ± 0.21 c | 91.26 ± 0.04 c | |
| 3 h | Bacillus amyloliquefaciens | 72.64 ± 0.08 f | 82.03 ± 0.14 f | 87.06 ± 0.04 e |
| Lactobacillus plantarum 299 | 81.02 ± 0.06 e | 84.85 ± 0.07 e | 89.65 ± 0.02 d |
| Strain | % Hydrophobicity | % Coaggregation |
|---|---|---|
| D1B3 | 1.70 ± 0.2 | 69.79 ± 0.1 |
| TEST | Factors | Responses | |||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| Liquid-to-Solid Ratio (%) | pH Buffer | Temperature (°C) | Biomass (logcfu/g) | Cellulase Activity (g/L.min) | |
| 1 | 0.70 | 6.0 | 31 | 9.00 | 0.0113 |
| 2 | 2.00 | 6.0 | 31 | 7.64 | 0.0126 |
| 3 | 0.70 | 7.0 | 31 | 7.74 | 0.0106 |
| 4 | 2.00 | 7.0 | 31 | 8.30 | 0.0092 |
| 5 | 0.70 | 6.5 | 25 | 9.00 | 0.0133 |
| 6 | 2.00 | 6.5 | 25 | 8.34 | 0.0160 |
| 7 | 0.70 | 6.5 | 37 | 9.00 | 0.0134 |
| 8 | 2.00 | 6.5 | 37 | 8.50 | 0.0116 |
| 9 | 1.35 | 6.0 | 25 | 9.22 | 0.0141 |
| 10 | 1.35 | 7.0 | 25 | 8.57 | 0.0138 |
| 11 | 1.35 | 6.0 | 37 | 9.77 | 0.0164 |
| 12 | 1.35 | 7.0 | 37 | 9.72 | 0.0102 |
| 13 | 1.35 | 6.5 | 31 | 10.68 | 0.0100 |
| 14 | 1.35 | 6.5 | 31 | 9.54 | 0.0113 |
| 15 | 1.35 | 6.5 | 31 | 9.47 | 0.0106 |
| 16 | 1.35 | 6.5 | 31 | 9.24 | 0.0108 |
| 17 | 1.35 | 6.5 | 31 | 9.44 | 0.0109 |
| Model | Y1 | Y2 | ||
|---|---|---|---|---|
| Model Parameters | Coefficient | p-Value | Coefficient | p-Value |
| b0 | 9.422 | *** | 0.01098 | *** |
| b1 | −0.245 | * | 0.00025 | n.s |
| b2 | −0.162 | n.s | −0.00125 | *** |
| b3 | 0.232 | * | −0.00075 | ** |
| b11 | −0.931 | *** | 0.00001 | n.s |
| b22 | −0.321 | * | 0.00001 | n.s |
| b33 | 0.219 | n.s | 0.00251 | *** |
| b12 | 0.480 | ** | −0.00100 | ** |
| b13 | 0.040 | n.s | −0.00100 | ** |
| b23 | 0.150 | n.s | −0.00150 | ** |
| Model Validation | ||||
| Significance level (%) | ** | *** | ||
| Df | 6 | 6 | ||
| Sum of squares | 6.215 | 5.98 × 10−5 | ||
| Mean square | 0.6906 | 6.64 × 10−6 | ||
| R2 | 0.945 | 0.975 | ||
| R2A | 0.862 | 0.938 | ||
| Variable | Value | Factor | Value |
|---|---|---|---|
| X1 | −0.243642 | Liquid-to-solid ratio | 1.19 |
| X2 | −0.606961 | pH buffer | 6.20 |
| X3 | 0.998078 | Temperature | 36.99 |
| Response | Name | Value | d (i) % | Weight | di min % | di max % |
|---|---|---|---|---|---|---|
| Y1 | Biomass | 9.828 | 100 | 1 | 100 | 100 |
| Y2 | Enzyme activity | 0.0144 | 100 | 1 | 100 | 100 |
| Desirability | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzaiene, T.; Ziadi, M.; Enneifer, M.; Sellami, A.; Aydi, A.; Cherif, A.; Hamdi, M. Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential. Sustainability 2023, 15, 8394. https://doi.org/10.3390/su15108394
Bouzaiene T, Ziadi M, Enneifer M, Sellami A, Aydi A, Cherif A, Hamdi M. Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential. Sustainability. 2023; 15(10):8394. https://doi.org/10.3390/su15108394
Chicago/Turabian StyleBouzaiene, Taroub, Manel Ziadi, Malek Enneifer, Abir Sellami, Abdelkarim Aydi, Ameur Cherif, and Moktar Hamdi. 2023. "Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential" Sustainability 15, no. 10: 8394. https://doi.org/10.3390/su15108394
APA StyleBouzaiene, T., Ziadi, M., Enneifer, M., Sellami, A., Aydi, A., Cherif, A., & Hamdi, M. (2023). Cellulolytic Bacillus Strain: Production Optimization Using Wheat Bran under Solid-State Fermentation and Investigation of Its Probiotic Potential. Sustainability, 15(10), 8394. https://doi.org/10.3390/su15108394







