Variations in Root Morphology and Yield among Rice Varieties in Response to Potassium under Subtropical Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Materials
2.2. Experimental Design and Crop Management
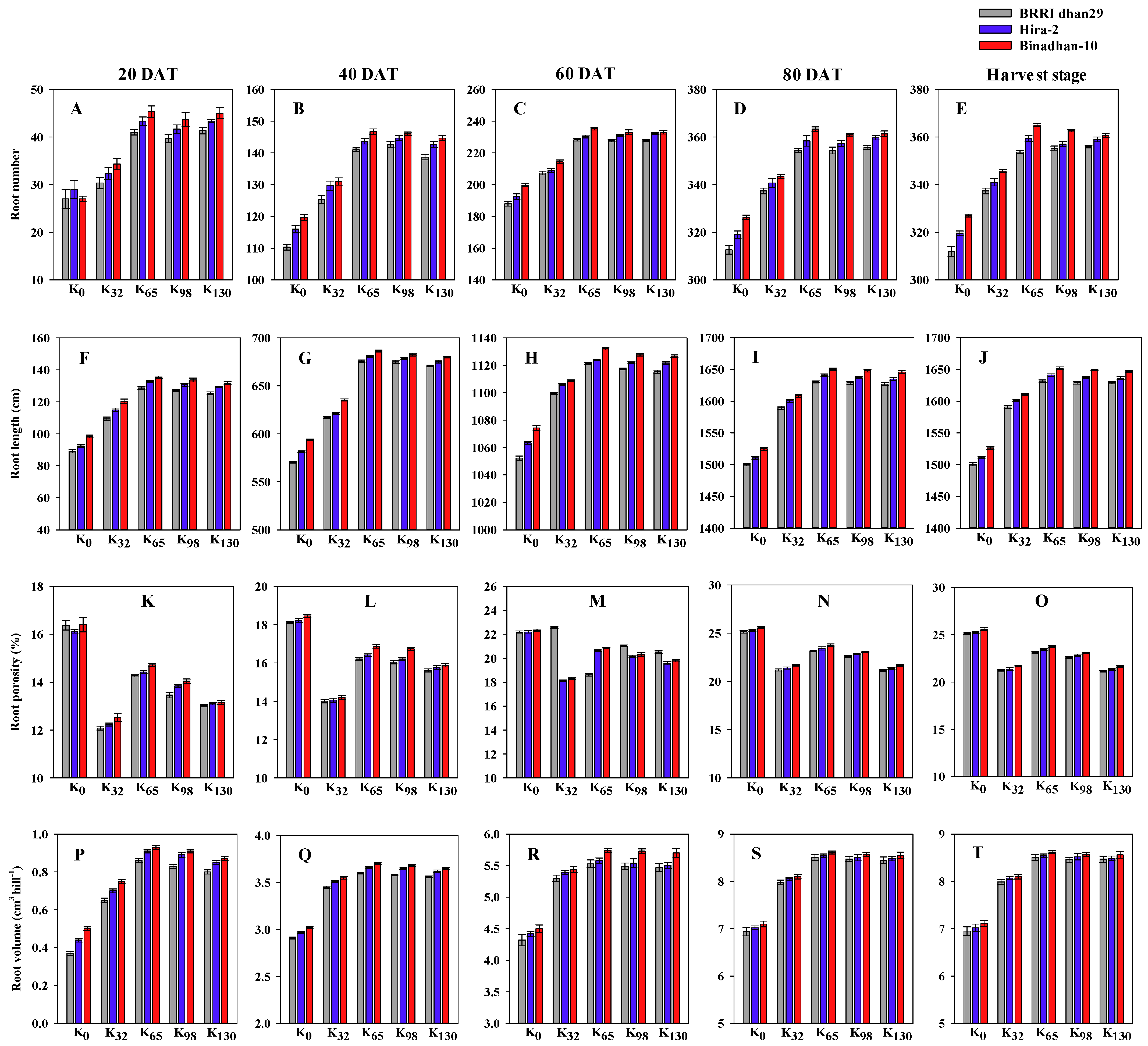
2.3. Determination of Root Morpho-Physiological Attributes
2.3.1. Root Number (RN)
2.3.2. Root Length (cm)
2.3.3. Root Volume (cm3 hill−1)
2.3.4. Root Porosity (RP, %)
2.3.5. Physiological Traits
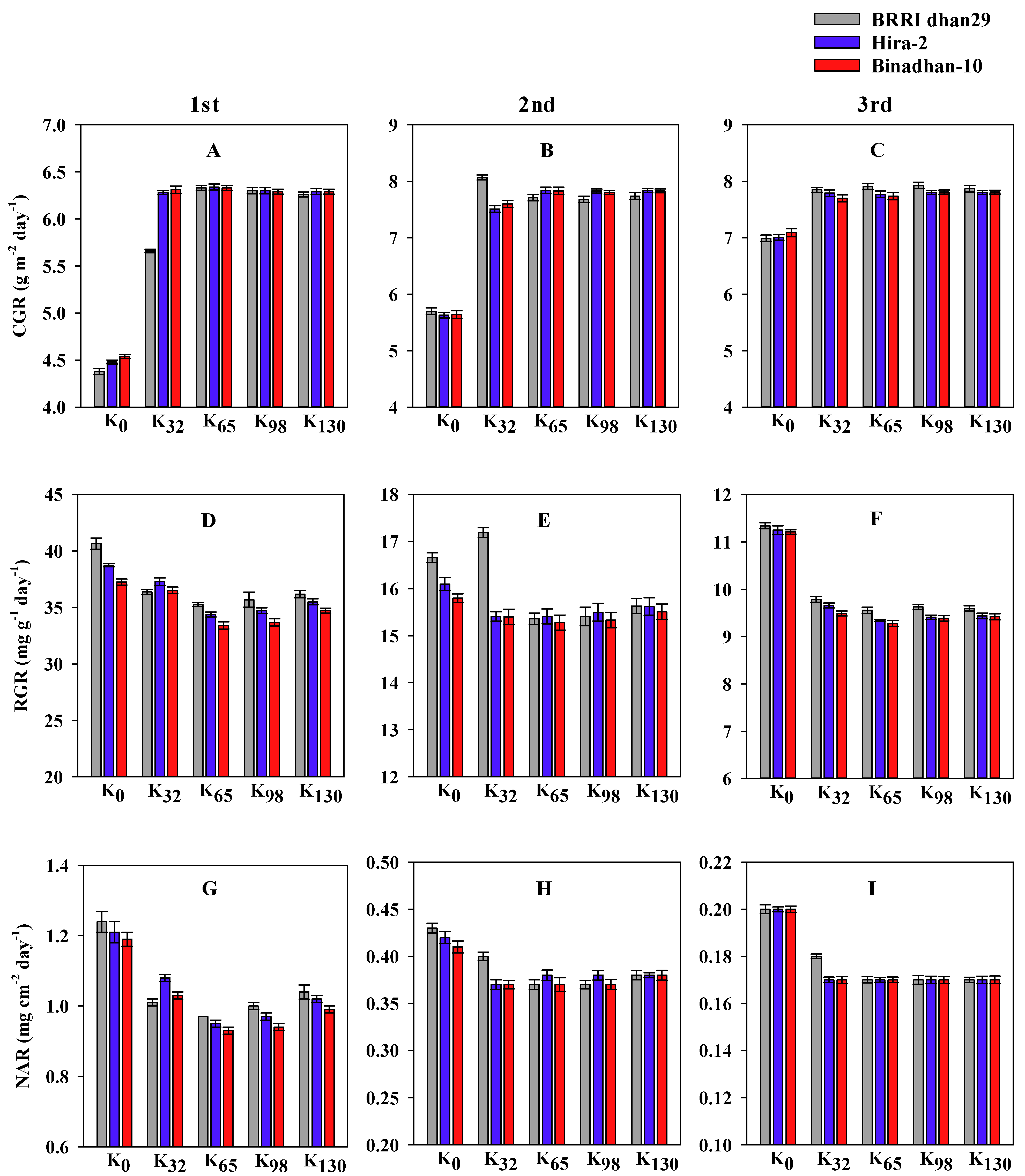
2.3.6. Crop Growth Rate (g m−2 day−1)
2.3.7. Relative Growth Rate (mg g−1 day−1)
2.3.8. Net Assimilation Rate (mg m−2 day−1)
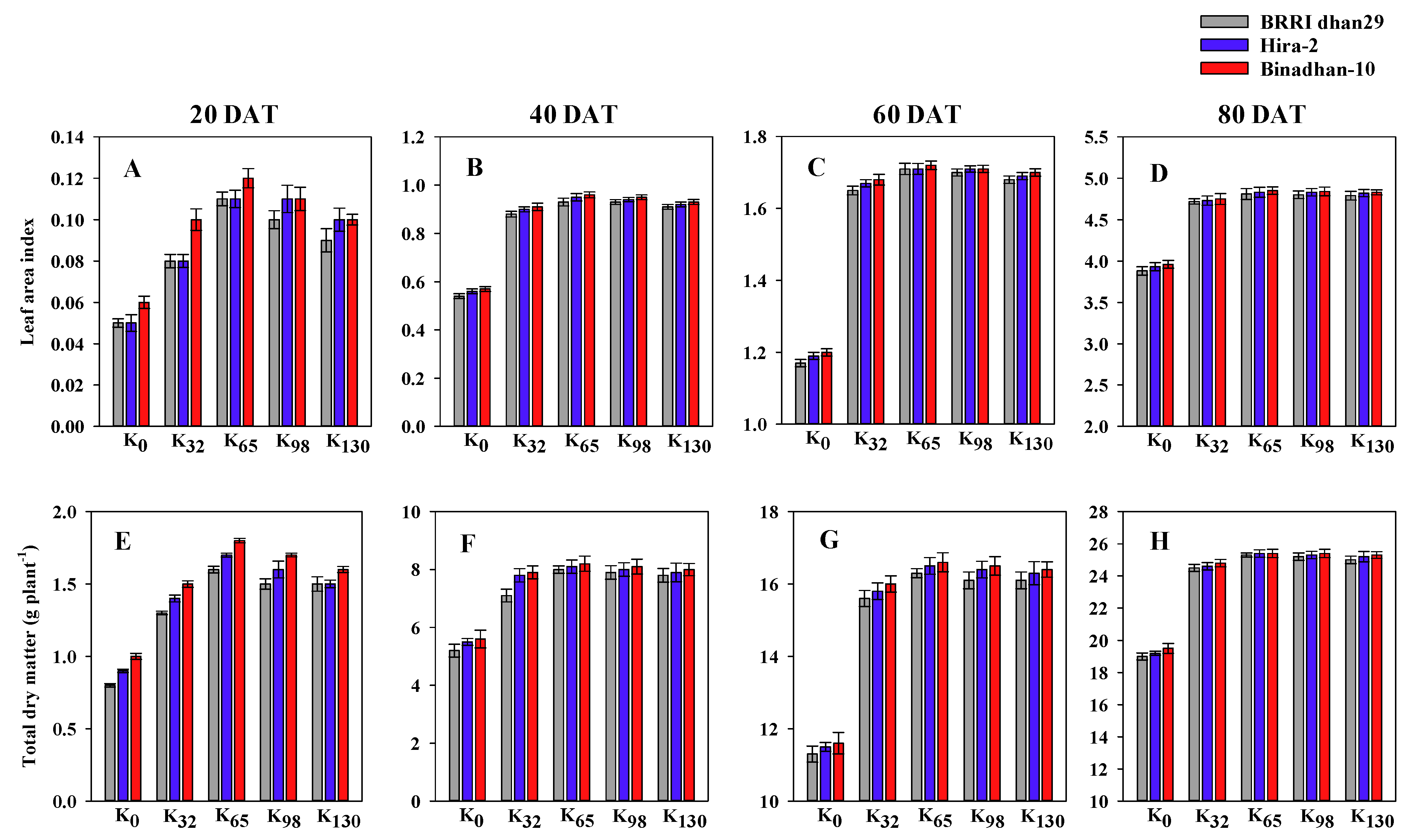
2.3.9. Total Dry Matter (TDM)
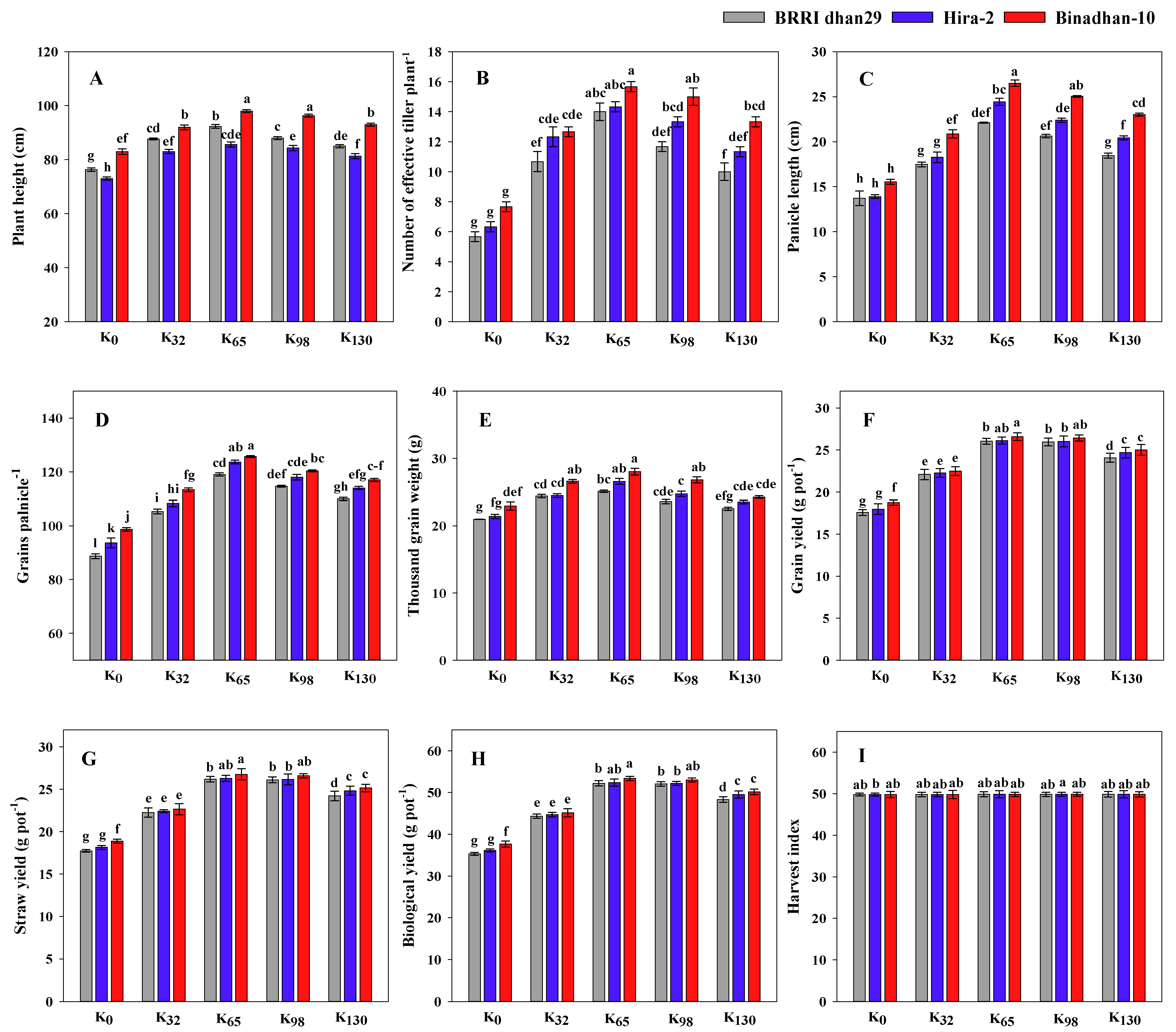
2.3.10. Yield and Yield Components
2.4. Statistical Analysis
3. Results
3.1. Root Morphological Traits, Total Dry Matter and Leaf Area Index
3.2. Growth Parameters
3.3. Grain Yield and Yield Component of the Rice Varieties
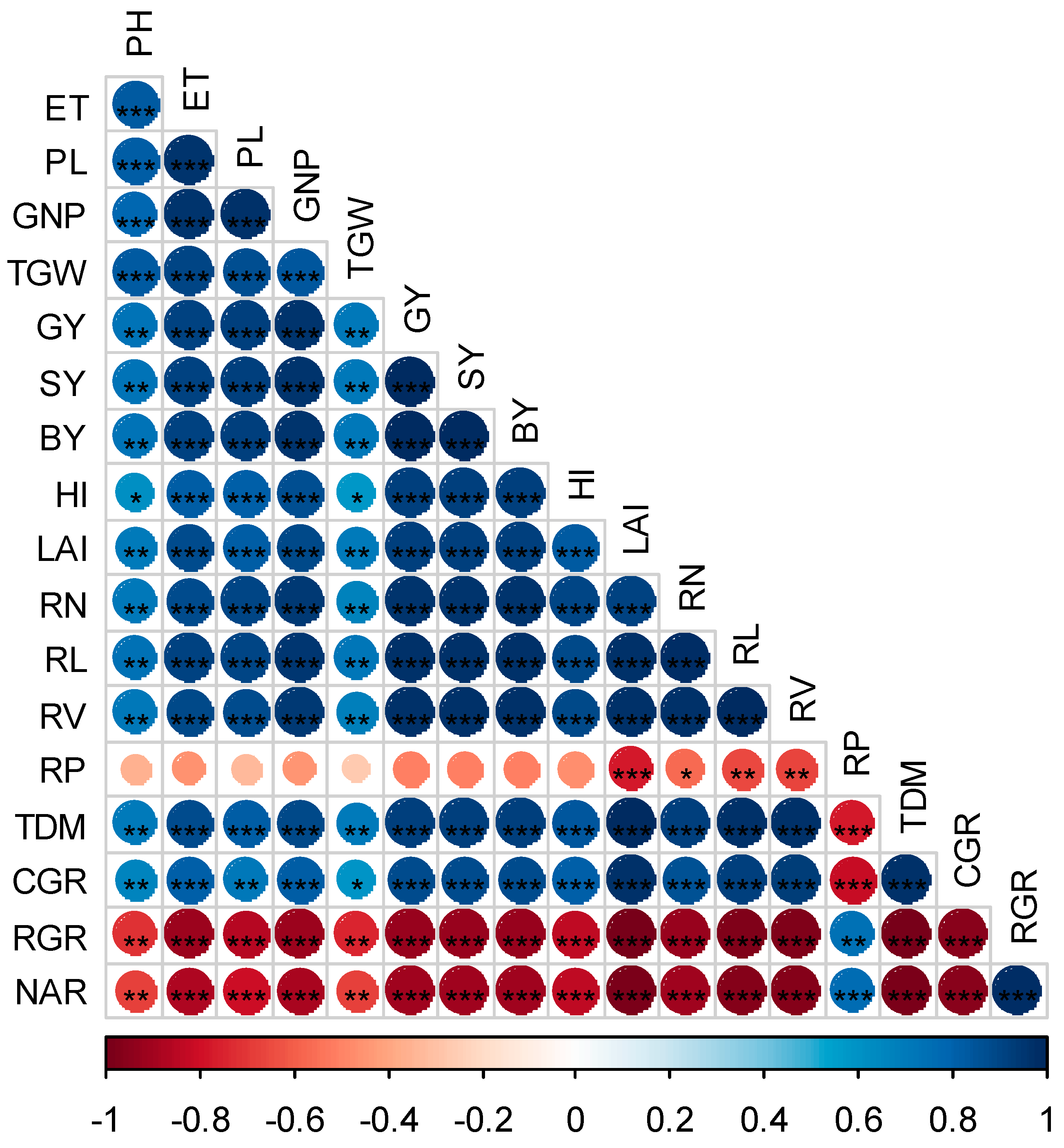
3.4. Relationship among Root Traits, Growth Indices, Yield and Yield Attributes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Chen, M.; Li, X.; Dai, Q.; Xu, K.; Guo, B.; Hu, Y.; Wang, W.; Huo, Z. Effects of soil types and irrigation modes on rice root morphophysiological traits and grain quality. Agronomy 2021, 11, 120. [Google Scholar] [CrossRef]
- Hartati, S.; Purnomo, D. Effectiveness and efficiency of potassium fertilizer application to increase the production and quality of rice in Entisols. Environ. Earth Sci. 2018, 142, 012031. [Google Scholar] [CrossRef]
- Dhillon, J.S.; Eickhoff, E.M.; Mullen, R.W.; Raun, W.R. World potassium use efficiency in cereal crops. Agron. J. 2019, 111, 889–896. [Google Scholar] [CrossRef]
- Popp, M.P.; Slaton, N.A.; Norsworthy, J.S.; Dixon, B. Rice yield response to potassium: An economic analysis. Agron. J. 2020, 113, 287–297. [Google Scholar] [CrossRef]
- Kaysar, M.S.; Sarker, U.K.; Monira, S.; Hossain, M.A.; Haque, M.S.; Somaddar, U.; Saha, G.; Chaki, A.K.; Uddin, M.R. Dissecting the relationship between root morphological traits and yield attributes in diverse rice cultivars under subtropical condition. Life 2022, 12, 1519. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar] [CrossRef]
- Slaton, N.; Golden, B.R.; Norman, R.J.; Wilson, C.E.; Delong, R.E. Correlation and calibration of soil potassium availability with rice yield and nutritional status. Soil Sci. Soc. Am. J. 2009, 73, 1192–1201. [Google Scholar] [CrossRef]
- Fageria, N.K.; Dos Santos, A.B.; Coelho, A.M. Growth, yield and yield components of lowland rice as influenced by ammonium sulfate and urea fertilization. J. Plant Nutr. 2011, 34, 371–386. [Google Scholar] [CrossRef]
- Farinelli, R. Agronomic characteristics of upland rice under no-tillage and nitrogen and potassium fertilization. Braz. J. Soil Sci. 2004, 28, 447–454. [Google Scholar]
- Jia, Y.; Yang, X.; Feng, Y.; Jilani, G. Differential response of root morphology to potassium deficient stress among rice genotypes varying in potassium efficiency. J. Zhejiang Univ. Sci. 2008, 9, 427–434. [Google Scholar] [CrossRef]
- Hogh-Jensen, H.H.; Pedersen, M.B. Morphological plasticity by crop plants and their potassium use efficiency. J. Plant Nutr. 2003, 26, 969–984. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Guimaraes, C.M. Root system of upland rice under water stress. Trop. Agric. Res. 2011, 41, 126–134. [Google Scholar]
- Costa, S.E.V.G.A.; Souza, E.D.; Anghinoni, I.; Flores, J.P.; Andriguetti, M.H. Potassium and root distribution in soil and corn growth in long-term soil management and fertilization systems. Braz. J. Soil Sci. 2009, 33, 1291–1301. [Google Scholar] [CrossRef]
- Zaratin, C. Effects of four doses of potassium on six sprinkler-irrigated upland rice cultivars. I. Production and productivity components. Cientifica 2004, 32, 115–120. [Google Scholar]
- Patel, D.S.; Kirti, B.; Dhiraji, P.P.; Vipulkumar, P.; Suchismita, J.; Ajay, V.N.; Harshadkumar, N.C. Does plant root architecture respond to potassium under water stress? A case from rice seedling root responses. Curr. Sci. 2021, 120, 1050–1056. [Google Scholar] [CrossRef]
- Klinsawang, S.; Sumranwanich, T.; Wannaro, A.; Saengwilai, P. Effects of root hair length on potassium acquisition in rice (Oryza sativa L.). Appl. Ecol. Environ. Res. 2018, 16, 1609–1620. [Google Scholar] [CrossRef]
- United Nations Development Program; Food and Agriculture Organization. Land Resources Appraisal of Bangladesh for Agricultural Development Report 2. Agro-Ecological Region of Bangladesh; United Nations Development Program and Food and Agricultural Organization: Dhaka, Bangladesh, 1988; pp. 212–221. [Google Scholar]
- FRG. Fertilizer Recommendation Guide; Bangladesh Agricultural Research Council (BARC): Farmgate, Dhaka, 2018; p. 274.
- Kato, Y.; Kamoshita, A.; Yamagishi, J. Growth of Three Rice Cultivars (Oryza sativa L.) under Upland Conditions with Different Levels of Water Supply. Plant Prod. Sci. 2006, 9, 435–445. [Google Scholar] [CrossRef]
- Jensen, C.R.; Luxmoore, R.J.; Van Gundy, S.D.; Stolzy, L.H. Root air space measurements by a pycnometer method. Agron. J. 1969, 61, 474–475. [Google Scholar] [CrossRef]
- Evans, G.C. Quantitative Analysis of Growth; Blackwell Scientific Publication: Oxford, UK, 1972. [Google Scholar]
- Hunt, R. Plant Growth Analysis; Edward Arnold: London, UK, 1978; pp. 26–38. [Google Scholar]
- Peng, S.B.; Huang, J.L.; Sheehy John, E.; Laza Rebecca, C.; Viseras Romeo, M.; Zhong, X.H.; Centeno Grace, S.; Khush Gurdev, S.; Cassman Kenneth, G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computation: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 30 March 2023).
- James, D.W.; Tindall, T.A.; Turst, C.J.; Hussein, A.N. Alfalfa cultivar responses to phosphorus and potassium deficiency: Biomass. J. Plant Nutr. 1995, 18, 2431–2445. [Google Scholar] [CrossRef]
- Chen, J.J.; Gabelman, W.H. Isolation of tomato strains varying in potassium acquisition using a sand-zeolite culture system. Plant Soil. 1995, 176, 65–70. [Google Scholar] [CrossRef]
- Liu, F.H.; Liang, X.N.; Zhang, S.W. Accumulation and utilization efficiency of potassium in ramie varieties. J. Plant Nutr. 2000, 23, 785–792. [Google Scholar] [CrossRef]
- Zhang, G.P.; Chen, J.X.; Tirore, E.A. Genotypic variation for potassium uptake and utilization efficiency in wheat. Nutr. Cycl. Agroecosys. 1999, 54, 41–48. [Google Scholar]
- Pettersson, S.; Jensén, P. Variation among species and varieties in uptake and utilization of potassium. Plant Soil. 1983, 72, 231–237. [Google Scholar] [CrossRef]
- Yang, X.E.; Liu, J.X.; Wang, W.M. Potassium internal use efficiency relative to growth vigor, potassium distribution, and carbohydrate allocation in rice genotypes. J. Plant Nutr. 2004, 27, 837–852. [Google Scholar] [CrossRef]
- Saha, P.K.; Akter, M.; Miah, M.A.M.; Zaman, S.K. Effects of organic and inorganic sources of K on rice yield and soil K balance in the rice-rice cropping system. Bangladesh J. Agric. Res. 2011, 36, 305–311. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A.; Coelho, A.M. Yield and yield components of upland rice as influenced by nitrogen sources. J. Plant Nutr. 2011, 34, 361–370. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef]
- Amtmann, A.; Hammond, J.P.; Armengaud, P.; White, P.J. Nutrient sensing and signaling in plants: Potassium and phosphorus. Adv. Bot. Res. 2006, 43, 209–257. [Google Scholar] [CrossRef]
- White, P.J.; Karley, A.J. Potassium. Plant Cell Monographs 17, Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Thorne, G.N. Effects of age and environment on net assimilation rate of barley. Ann. Bot. 1961, 25, 29–38. [Google Scholar] [CrossRef]
- Yang, X.E.; Wang, W.M.; He, Z.L. Physiological and Genetic Characteristics of High Nutrient Efficiency of Plants in Acid Soils. In The Red Soils of China: Their Nature, Management and Utilization; Wilson, M.J., He, Z.L., Yang, X.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 78–83. [Google Scholar]
- Raju, G.S.N.; Mukhopadhyay, A.K. Each of the sequence of addition of K and NH4 and pre-absorbed cations on fixation of applied NH4 ions in soils. J. Indian Soc. Soil Sci. 1975, 23, 172–176. [Google Scholar]
- Badhe, M.N.; Begum, M.; Varade, S.B. Effect of potassic fertilizers on ammonium fixation in soils of Marathwada. In Bulletin 10; Indian Soil Science Society: New Delhi, India, 1976; pp. 103–106. [Google Scholar]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Horie, T.; Shiraiwa, T.; Homma, K.; Katsura, K.; Maeda, Y.; Yoshida, H. Can yields of lowland rice resume the increases that showed in the 1980s? Plant Prod. Sci. 2005, 8, 259–274. [Google Scholar] [CrossRef]
- Fageria, N.K. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.H.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Pre anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chu, G.; Liu, L.J.; Wang, Z.Q.; Wang, X.M.; Zhang, H.; Yang, J.C.; Zhang, J.H. Mid-season nitrogen application strategies for rice varieties differing in panicle size. Field Crops Res. 2013, 150, 9–18. [Google Scholar] [CrossRef]
- Tabatabaei, A.S.; Shakeri, S.S.; Mirjalili, M.R. Effect of different levels of potassium Sulphate on yield, yield components and protein content of wheat cultivars. Appl. Math. Eng. Manag. Technol. 2014, 2, 119–123. [Google Scholar]
- Ma, L.; Ren, G.X.; Shi, Y. Effects of potassium fertilizer on diurnal change of photosynthesis in Stevia Rebaudiana Bertoni. Adv. Mat. Res. 2012, 542–543, 1087–1090. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, F.; Tian, X. Coronatine-induced lateral formation in cotton (Gossypium hirsutum) seedlings under potassium-sufficient and-Deficient conditions in relation to auxin. J. Plant Nutr. Soil Sci. 2009, 172, 435–444. [Google Scholar] [CrossRef]
- Dolan, L.; Davies, J. Cell expansion in roots. Curr. Opin. Plant Biol. 2004, 7, 33–39. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Pellerin, S. Shoot and root growth of hydroponic maize (Zea mays L.) as influenced by K deficiency. Plant Soil. 2008, 304, 157–168. [Google Scholar] [CrossRef]
- Walker, D.J.; Black, C.R.; Miller, A.J. The role of cytosolic potassium and pH in the growth of barley roots. Plant Physiol. 1998, 118, 957–964. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1995. [Google Scholar] [CrossRef]
- Wang, X.G.; Cao, M.J.; Wang, W. Effect of potassium concentration in the soil on the morphological and physiological characteristics of soybean root. Soybean Sci. 2005, 24, 126–130. [Google Scholar]
- Zou, C.Q.; Li, Z.S.; Li, J.Y. Study on difference in morphological and physiological characters of wheat varieties to potassium. Plant Nutr. Fert. Sci. 2001, 7, 36–43. [Google Scholar]
- Mengel, K.; Steffens, D. Potassium uptake of ryegrass (Lolium perenne) and red clover (Trifolium pratense) as related to root parameters. Biol. Fert. Soils 1985, 1, 53–58. [Google Scholar] [CrossRef]
- Armstrong, W.; Drew, M.C. Root Growth and Metabolism under Oxygen Deficiency. In Plant Roots: The Hidden Half, 3rd ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 729–761. [Google Scholar]
- Brown, K.M.; Zhang, Y.J.; Kim, H.J.; Lynch, J.P. The ethylene underground. Acta Hortic. 2003, 618, 193–198. [Google Scholar] [CrossRef]
- Drew, M.C.; He, C.J.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Zayed, B.A.; Elkhoby, W.M.; Shehata, S.M.; Ammar, M.H. Role of potassium application on the productivity of some inbred and hybrid rice varieties under newly reclaimed saline soils. Afr. Crop Sci. J. 2007, 8, 53–60. [Google Scholar]
- Mengel, K. Factors and process affecting potassium requirement of crops. Potash Rev. 1982, 16, 1–12. [Google Scholar]
- Niazi, K.; Mehdi, S.M.; Mahmood, T.; Iqbal, J. Potassium fertilizer use efficiency in sodic soils. In 4th National Congress of Soil Science Abstract; Soil Salinity Research Institute: Pindi Bhattian, Pakistan, 1992; p. 73. [Google Scholar]
- Ramazanpour, M.R.; Dastfal, M.; Malakouti, M.J. The effect of potassium in reducing drought stress in wheat in darab region of fars. J. Plant Soil Sci. 2008, 22, 127–135. [Google Scholar]
- Beringer, H. Functions of Potassium in Plant Metabolism with Particular Reference to Yield. In Potassium in Soils and Crops; Sekhon, G.S., Ed.; Potash Research Institute of India: New Delhi, India, 1978; pp. 185–202. [Google Scholar]
- Thakur, A.C.; Borah, R.; Bhagwati, P.C. Performance of summer rice varieties under different dates of transplanting in rice (winter)-rice (summer) system. J. Agric. Sci. Soc. Northeast India 2002, 15, 79–81. [Google Scholar]
- Garnett, T.; Conn, V.; Kaiser, B.N. Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ. 2009, 32, 1272–1283. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, H.; Zhang, J.H. Root morphology and physiology in relation to the yield performance of rice. J. Integr. Agric. 2012, 11, 920–926. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Chu, G.; Wang, Z.Q.; Zhang, H.; Yang, J.C.; Zhang, J.H. Agronomic and physiological performance of rice under integrative crop management. Agron. J. 2016, 108, 117–128. [Google Scholar] [CrossRef]

| Sl. No | Variety | Genetic Source | Parental Origin/Accession Number | Source |
|---|---|---|---|---|
| 1. | BRRI dhan29 | Inbred | BG90-2 × BR51-46-5 | BRRI |
| 2. | Binadhan-10 | Inbred | IR42598-B-B-B-B-12 × Nona Bokra | BINA |
| 3. | Hira-2 | Hybrid | - | Local market |
| Soil Properties | Values |
|---|---|
| Textural class | Clay loam |
| Soil reaction | 6.12 |
| Electrical conductance (μs/cm) | 649 |
| Organic Carbon (%) | 1.028 |
| Total Nitrogen (N) (%) | 0.121 |
| Available Phosphorus (ppm) | 29.1 |
| Available Potassium (ppm) | 84.61 |
| Available Sulphur (ppm) | 24.89 |
| Variety | PH (cm) | ET (no.) | PL (cm) | GNP | TGW(g) | GY (g pot−1) | SY (g pot−1) | HI (%) |
|---|---|---|---|---|---|---|---|---|
| V1 | 85.87b | 10.40b | 18.48b | 107.53c | 23.33b | 23.15c | 23.30c | 49.84 |
| V2 | 81.47c | 11.53ab | 19.89ab | 111.53b | 24.15b | 23.42b | 23.56b | 49.84 |
| V3 | 92.47a | 12.87a | 22.19a | 115.00a | 25.73a | 23.86a | 24.01a | 49.84 |
| Potassium | ||||||||
| K0 | 77.44c | 6.56d | 14.38d | 93.67e | 21.77d | 18.11d | 18.26d | 49.80c |
| K32 | 87.56ab | 11.89c | 18.87c | 109.00d | 25.16b | 22.28c | 22.44c | 49.83b |
| K65 | 92.00a | 14.67a | 24.35a | 122.78a | 26.58a | 26.26a | 26.40a | 49.86a |
| K98 | 89.56ab | 13.33b | 22.69a | 117.67b | 25.05b | 26.13a | 26.28a | 49.86a |
| K130 | 86.44b | 11.56c | 20.64b | 113.67c | 23.44c | 24.59b | 24.74b | 49.85ab |
| Variety | ** | ** | ** | ** | ** | ** | ** | ns |
| K | ** | ** | ** | ** | ** | ** | ** | ** |
| CV (%) | 1.12 | 6.68 | 3.07 | 1.30 | 2.42 | 0.74 | 0.72 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaysar, M.S.; Sarker, U.K.; Monira, S.; Hossain, M.A.; Somaddar, U.; Saha, G.; Chaki, A.K.; Hashem, A.; Abd_Allah, E.F.; Uddin, M.R. Variations in Root Morphology and Yield among Rice Varieties in Response to Potassium under Subtropical Conditions. Sustainability 2023, 15, 8589. https://doi.org/10.3390/su15118589
Kaysar MS, Sarker UK, Monira S, Hossain MA, Somaddar U, Saha G, Chaki AK, Hashem A, Abd_Allah EF, Uddin MR. Variations in Root Morphology and Yield among Rice Varieties in Response to Potassium under Subtropical Conditions. Sustainability. 2023; 15(11):8589. https://doi.org/10.3390/su15118589
Chicago/Turabian StyleKaysar, Md. Salahuddin, Uttam Kumer Sarker, Sirajam Monira, Md. Alamgir Hossain, Uzzal Somaddar, Gopal Saha, Apurbo Kumar Chaki, Abeer Hashem, Elsayed Fathi Abd_Allah, and Md. Romij Uddin. 2023. "Variations in Root Morphology and Yield among Rice Varieties in Response to Potassium under Subtropical Conditions" Sustainability 15, no. 11: 8589. https://doi.org/10.3390/su15118589









