Mathematical Modeling of Pilot Scale Olive Mill Wastewater Phytoremediation Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Operation of the Phytoremediation Pilot Units
2.2. Model Development of the Phytoremediation Pilot Units
2.2.1. Model State Variables
2.2.2. Bioremediation Processes
Microbial Growth
Microbial Decomposition and Removal of Organic Compounds
Microbial Transformation and Removal of Nitrogenous Compounds
Microbial Removal and Precipitation of Phosphorus (P)
Microbial Consumption of Oxygen
2.2.3. Phytoremediation Processes
Plant Uptake of Organic and Inorganic Compounds
Accumulation of Recalcitrant Organics to the Plants’ Root Zone
Rhizodegradation of Organic Compounds
2.3. Model Inputs and Parameters
2.4. Model Calibration Methodology
3. Results and Discussion
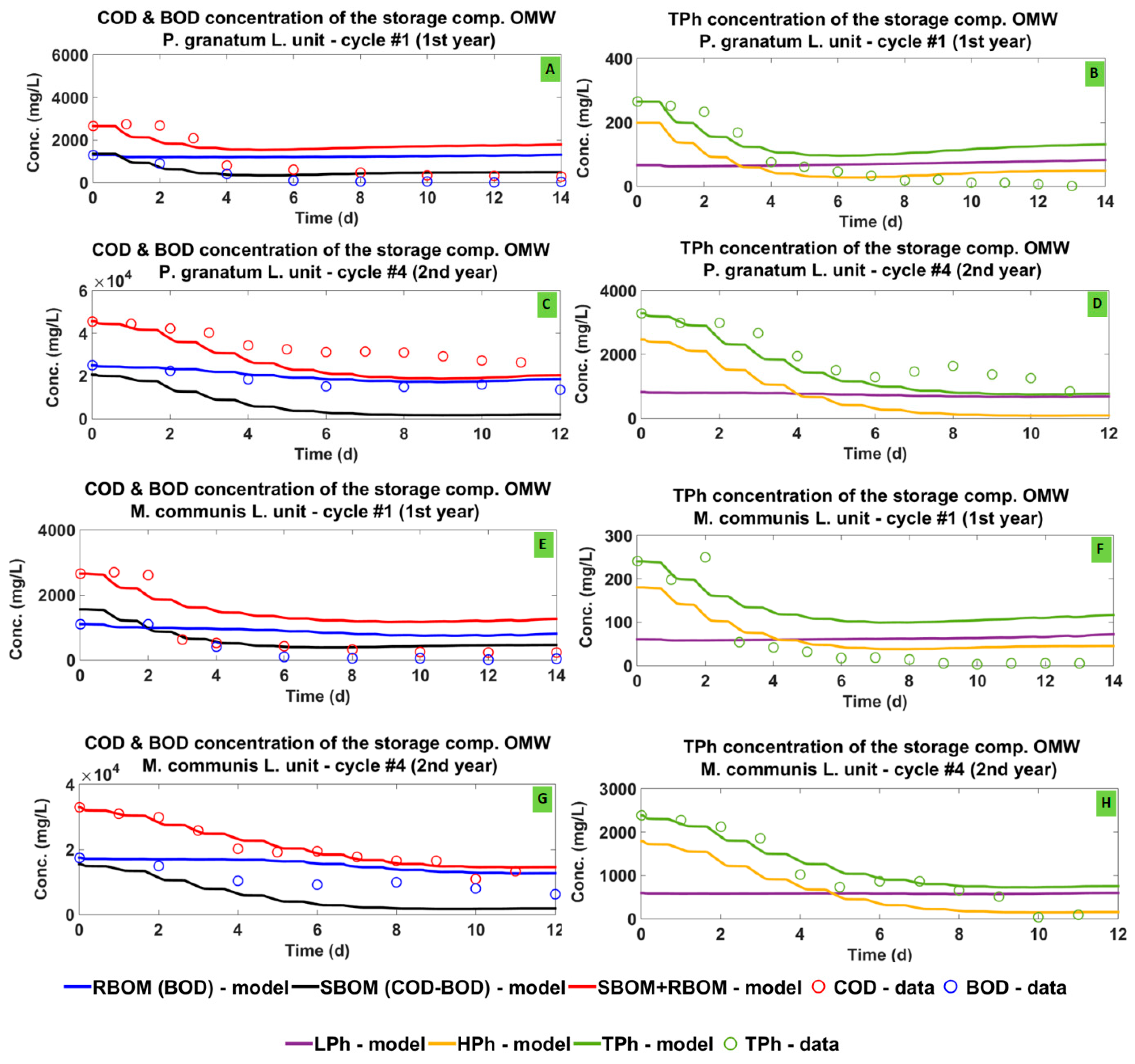
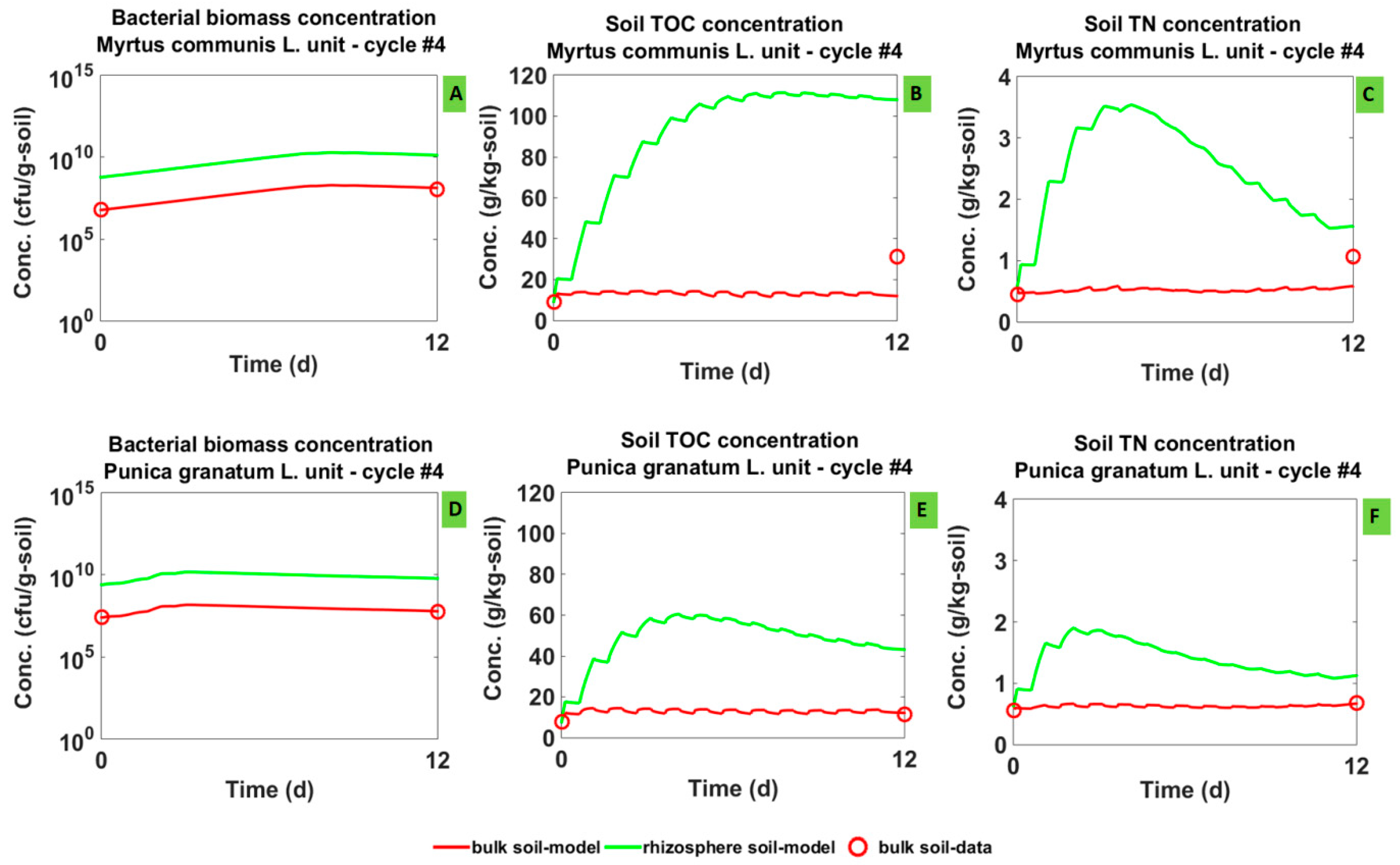
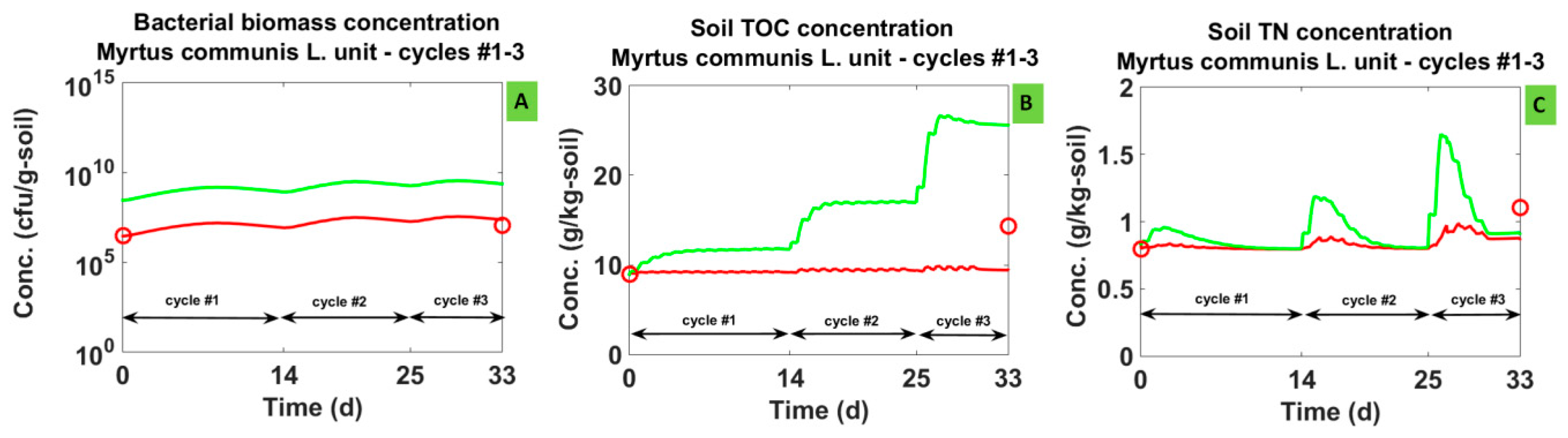
3.1. Model Calibration
3.1.1. Simulated Dynamics
3.1.2. Parameter Estimation
Kinetics of Microbial Growth
Kinetics of Organic Enzymatic Degradation
Kinetics of Phytoremediation Processes
3.2. Model Validation
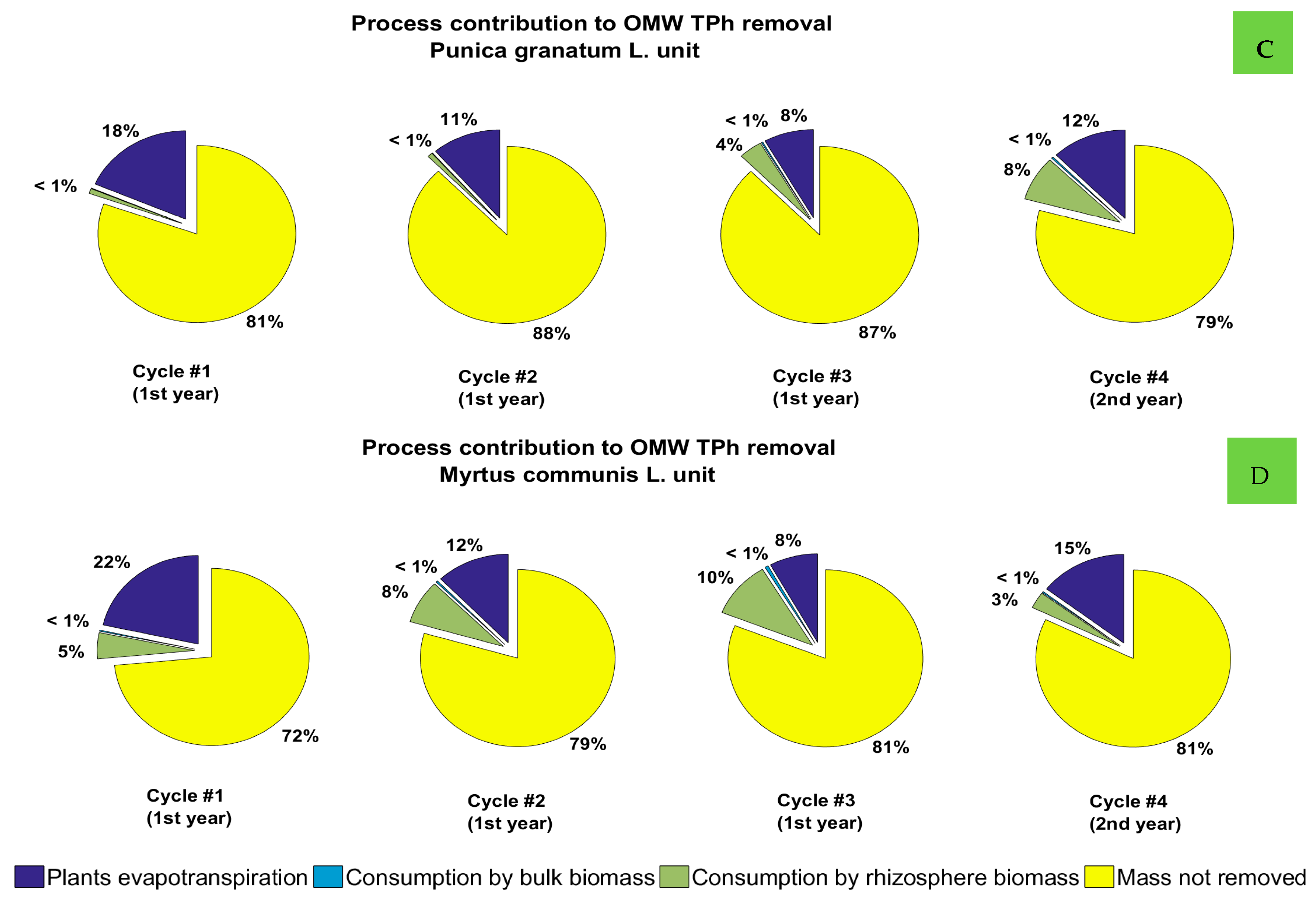
3.3. Contribution of the Phytoremediation Processes in the Removal of OMW Organics
3.4. Sensitivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Plants’ Root Biomass and Distribution in the Unit
Appendix A.2. Wastewater Flow through the Unit
Appendix A.3. Wastewater Volume Loss through Evaporation and Plant Evapotranspiration
Appendix A.4. Solutes Transport and Distribution in the Soil
Appendix A.5. Olive Mill Wastewater Oxygenation
Appendix A.6. Tables
| State Variable | Plant Uptake (Transpiration) | Adsorption on Plants’ Roots * | Enzymatic Decomposition | Ammonification | Biomass Growth | Biomass Decay | Biomass Maintenance | Phosphorus Precipitation |
|---|---|---|---|---|---|---|---|---|
| −/+ | + | |||||||
| + | − | |||||||
| −/+ | ||||||||
| + | ||||||||
| −/+ | + | |||||||
| + | ||||||||
| + | ||||||||
| and | ||||||||
| −/+ | ||||||||
| −/+ | ||||||||
| −/+ |
| Limitation Type | Expressions |
|---|---|
| Monod kinetics for non-phenolic RBOM substrate | |
| Haldane kinetics for phenolic RBOM substrate | |
| Monod kinetics for oxygen | |
| Inverse Monod kinetics for oxygen inhibition for bacterial anoxic growth | |
| Monod kinetics for NO3−N for bacterial anoxic growth | |
| Monod kinetics for inorganic nitrogen | |
| Monod kinetics for inorganic phosphorus | |
| Fraction of non-phenolic RBOM | |
| Fraction of phenolic RBOM |
| Symbol | Description | Value * | Unit | Source of Parameter Value | |
|---|---|---|---|---|---|
| Biomass stoichiometric yields | Biomass yield per unit substrate consumed | b: , f: | g-biomass/mg-substrate | Calculated on the basis of biomass chemical formulae by [15] | |
| b: , f: | |||||
| b: , f: | |||||
| b: , f: | |||||
| Conversion factors | Conversion coefficients of decayed microbial biomass to non-phenolic SBOM | g-biomass/mg-COD | |||
| g-biomass/mg-COD | |||||
| Equivalent BOD5 of gallic acid | mg-BOD5/mg-gallic acid | ||||
| Biomass processes’ kinetic constants | Biomass maintenance constant | b: 7, f: 7 | mg/g-biomass h | [92] | |
| Biomass decay specific rate | b: 5.7, f: 5.7 | 1/h | [93] | ||
| Fraction of decayed biomass contributing to SBOM pool | 0.5 | - | [16] | ||
| Saturation constant for oxygen limitation factor | b: , f: | mg/L | [94] | ||
| Saturation constant of surface limitation factor | 100 | (mg-substrate/L)/(g-biomass/kg-soil) | [42] | ||
| Assumptions on OMW and soil characteristics | Initial fraction of high MW polyphenols over TPh | 0.75 | - | [95] | |
| Initial fraction of org-N over TN | 0.85 | - | [44,81,82,96,97] | ||
| Fraction of NO3−N over in-N | 0.15 | - | [44,81,82,96,97] | ||
| Initial fraction of fungal biomass over bacterial biomass | 0.3 (both in bulk and rhizosphere soil–1st and 2nd year) | - | [98] | ||
| Initial fraction of rhizosphere biomass concentration over biomass of bulk soil (both bacteria and fungi) | 100 | - | [62] | ||
| Fraction of rhizosphere soil over unit’s total soil | 1st year: 0.1 2nd year: 0.2 | - | Calculatedon the basis of [1,63,64] | ||
| Solutes Transportation and hydrodynamic- related constants | Partition coefficient for constituent i | COD: 0.509; TPh: 0.508; TN: 0.443; TP: 6.199 | L/kg-soil | Experimentally determinedby [4] | |
| Saturated hydraulic conductivity | 15.21 | m/d | [81] | ||
| Residual volumetric soil content | 0.02 | [99] | |||
| Saturated volumetric soil content | 0.43 | [81] | |||
| Empirical coefficient | 13 | - | [81] | ||
| Soil density | 1.14 | kg/L | Experimentally determined by [4] | ||
| Distribution and biomass of plants roots | Total root tissue mass of plants | P. granatum: 0.9927 | kg/tree | Calculatedon the basis of [63] | |
| M. communis: 0.9927 | |||||
| Percentage of root tissue massat the th layer | P. granatum: = 22.96; = 24.77; = 25.50; = 26.77 M. communis L.: = 78.73; = 9.47; = 5.59; = 6.21 | % | Calculatedon the basis of [1,63,80] | ||
| Matric potential thresholds of Feddes piecewise linear reduction function | = 0 = −25 = −400 = −8000 | cm | [7,85,100] [7,85,100,101,102,103] |
| Related Process | Parameter | Unit | Pilot Unit | |
|---|---|---|---|---|
| P. granatum | M. communis | |||
| Plant uptake | - | 1.00 | 1.00 | |
| 0.95 | 1.00 | |||
| 7.02 | 11.80 | |||
| 0.67 | 0.29 | |||
| 0.02 | 2.83 | |||
| Adsorption on plant roots | * | 207 | 270 | |
| 374 | 329 | |||
| 49 | 61 | |||
| 1/h | 19 | 57 | ||
| Microbial growth *** | 1/h | 0.216 | 0.027 | |
| 0.164 | 0.236 | |||
| mg/L | 32.26 | 36.12 | ||
| 101.19 | 34.60 | |||
| 56.63 | 78.97 | |||
| 82.01 | 75.31 | |||
| 23.12 | 69.32 | |||
| 129.87 | 106.28 | |||
| 5.54 | 0.07 | |||
| 0.01 | 33.67 | |||
| 0.49 | 0.55 | |||
| 13.35 | 0.10 | |||
| 22.61 | 0.60 | |||
| Enzymatic decomposition *** | - | 1.90 | 1.79 | |
| ** | 10.75 | 18.04 | ||
| 72.73 | 25.57 | |||
| 1.02 | 2.11 | |||
| 12.56 | 18.25 | |||
| 65.80 | 203.21 | |||
| Phosphorus precipitation | 1/h | 0.377 | 0.595 | |
| Related Process | Parameter | Pilot Unit | |
|---|---|---|---|
| P. granatum | M. communis | ||
| Plant uptake | 0.0067 | 0.0063 | |
| 0.0037 | 0.0019 | ||
| 0.0127 | 0.0139 | ||
| 0.0028 | 0.0008 | ||
| 0.0002 | 0.0090 | ||
| Adsorption on plant roots | 0.0036 | 0.0030 | |
| 0.0031 | 0.0027 | ||
| 0.0142 | 0.0085 | ||
| 0.0001 | 0.0001 | ||
| Microbial growth | 0.0165 | 0.0392 | |
| 0.0445 | 0.0247 | ||
| 0.0038 | 0.0043 | ||
| 0.0044 | 0.0032 | ||
| 0.0005 | 0.0006 | ||
| 0.0004 | 0.0006 | ||
| 0.0004 | 0.0002 | ||
| 0.0004 | 0.0001 | ||
| 0.0170 | 0.0005 | ||
| 0.0004 | 0.0183 | ||
| 0.0001 | 0.0001 | ||
| 0.0299 | 0.0076 | ||
| 0.0327 | 0.0121 | ||
| Enzymatic decomposition | 0.0000 | 0.0000 | |
| 0.0016 | 0.0021 | ||
| 0.0024 | 0.0032 | ||
| 0.0011 | 0.0003 | ||
| 0.0003 | 0.0019 | ||
| 0.0216 | 0.0078 | ||
| Phosphorus precipitation | 0.0587 | 0.0377 | |
| COD Removal | TPh Removal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ (Par.) (%) | Δ (Plants ET) | Δ (Consumption by RSB) | Δ (Plants ET) | Δ (Consumption by RSB) | ||||||
| P. granatum | M. communis | P. granatum | M. communis | P. granatum | M. communis | P. granatum | M. communis | |||
| Estimated parameter | +20 | 0.06 | 0.01 | −0.33 | −0.28 | 0.06 | 0.01 | −0.41 | −0.32 | |
| +20 | −0.03 | −0.09 | 0.12 | 0.82 | −0.06 | −0.06 | 0.30 | 1.28 | ||
| +20 | 0.03 | 0.01 | −0.14 | −0.10 | 0.06 | 0.01 | −0.30 | −0.15 | ||
| +20 | 0.02 | 0.00 | −0.03 | 0.01 | 0.00 | 0.00 | 0.00 | 0.03 | ||
| +20 | −0.11 | - | 0.28 | - | −0.07 | - | 0.33 | - | ||
| +20 | 0.02 | 0.00 | −0.03 | 0.00 | 0.00 | 0.00 | −0.01 | 0.00 | ||
| +20 | 0.00 | 0.06 | 0.00 | −0.61 | 0.00 | 0.03 | −0.01 | −0.91 | ||
| +20 | −0.04 | −0.02 | 0.06 | −0.01 | −0.01 | 0.01 | 0.01 | −0.64 | ||
| +20 | 0.07 | - | −0.17 | - | 0.04 | - | −0.18 | - | ||
| +20 | 0.02 | 0.04 | −0.04 | −0.37 | 0.01 | 0.02 | −0.04 | −0.54 | ||
| +20 | 0.00 | 0.01 | 0.00 | −0.18 | 0.00 | 0.01 | 0.00 | −0.21 | ||
| Input values related to the plant and soil characteristics | +20 | −0.03 | 0.04 | −0.17 | −0.44 | −0.05 | 0.00 | −0.14 | −0.73 | |
| +100 | −0.02 | −0.01 | 0.07 | 0.12 | −0.02 | 0.00 | 0.11 | 0.07 | ||
| +20 | −0.01 | −0.01 | 0.04 | 0.12 | −0.02 | −0.01 | 0.09 | 0.18 | ||
| Input values related to the wastewater characteristics | +20 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.02 | |
| +20 | −0.01 | −0.01 | −0.01 | 0.03 | −2.70 | −2.85 | −2.26 | −2.36 | ||
| +10 | 0.12 | −0.01 | −0.19 | 0.12 | 0.02 | −0.01 | −0.06 | 0.27 | ||
| +20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||
References
- Koutsos, T.M.; Chatzistathis, T.; Balampekou, E.I. A new framework proposal, towards a common EU agricultural policy, with the best sustainable practices for the re-use of olive mill wastewater. Sci. Total Environ. 2018, 622–623, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Santori, F.; Cicalini, A.R. Process of Olive-Mill Wastewater Phytodepuration and Relative. Plant EP1216963 A, 26 June 2002. [Google Scholar]
- Bodini, S.F.; Cicalini, A.R.; Santori, F. Rhizosphere dynamics during phytoremediation of olive mill wastewater. Bioresour. Technol. 2011, 102, 4383–4389. [Google Scholar] [CrossRef] [PubMed]
- Petoussi, M.A.; Kalogerakis, N. Olive mill wastewater phytoremediation employing economically important woody plants. J. Environ. Manag. 2022, 302, 114076. [Google Scholar] [CrossRef] [PubMed]
- Garrido Hoyos, S.E.; Martinez Nieto, L.; Camacho Rubio, F.; Ramos Cormenzana, A. Kinetics of aerobic treatment of olive-mill wastewater (OMW) with Aspergillus terreus. Process. Biochem. 2002, 37, 1169–1176. [Google Scholar] [CrossRef]
- Beltran, J.; Gonzalez, T.; Garcia, J. Kinetics of the biodegradation of green table olive wastewaters by aerobic and anaerobic treatments. J. Hazard. Mater. 2008, 154, 839–845. [Google Scholar] [CrossRef]
- Achak, M.; Mandi, L.; Ouazzani, N. Removal of organic pollutants and nutrients from olive mill wastewater by a sand filter. J. Environ. Manag. 2009, 90, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Günay, A.; Çetin, M. Determination of aerobic biodegradation kinetics of olive oil mill wastewater. Int. Biodeterior. Biodegrad. 2013, 85, 237–242. [Google Scholar] [CrossRef]
- Chiavola, A.; Farabegoli, G.; Antonetti, F. Biological treatment of olive mill wastewater in a sequencing batch reactor. Biochem. Eng. J. 2014, 85, 71–78. [Google Scholar] [CrossRef]
- Lissaneddine, A.; Mandi, L.; El Achaby, M.; Mousset, E.; Rene, E.R.; Ouazzani, N.; Pons, M.-N.; Aziz, F. Performance and dynamic modeling of a continuously operated pomace olive packed bed for olive mill wastewater treatment and phenol recovery. Chemosphere 2021, 280, 130797. [Google Scholar] [CrossRef]
- Allaoui, S.; Bennani, M.N.; Ziyat, H.; Qabaqous, O.; Tijani, N.; Ittobane, N.; Hodaifa, G. Valorization of crude olive stone in the removing of polyphenols from crude olive mill wastewater: Kinetic, isotherm and mechanism study. Heliyon 2021, 7, e07525. [Google Scholar] [CrossRef]
- Elayadi, F.; Boumya, W.; Achak, M.; Chhiti, Y.; Ezzahrae, F.; Alaoui, M.; Barka, N.; El Adlouni, C. Experimental and modeling studies of the removal of phenolic compounds from olive mill wastewater by adsorption on sugarcane bagasse. Environ. Chall. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Vuppala, S.; Bavasso, I.; Stoller, M.; Di Palma, L.; Vilardi, G. Olive mill wastewater integrated purification through pre-treatments using coagulants and biological methods: Experimental, modelling and scale-up. J. Clean. Prod. 2019, 236, 117622. [Google Scholar] [CrossRef]
- Ciggin, A.S.; Iravanian, A.; Dogruel, S.; Orhon, D. Co-metabolism of olive mill wastewater in sequencing batch reactor under aerobic conditions after Fenton-based oxidation. J. Water Process. Eng. 2021, 43, 102277. [Google Scholar] [CrossRef]
- Vlyssides, A.; Mai, S.; Barampouti, E.M. An integrated mathematical model for co-composting of agricultural solid wastes with industrial wastewater. Bioresour. Technol. 2009, 100, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Vasiliadou, I.A.; Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Mathematical modeling of olive mill waste composting process. Waste Manag. 2015, 43, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Fezzani, B.; Ben Cheikh, R. Extension of the anaerobic digestion model No. 1 (ADM1) to include phenol compounds biodegradation processes for simulating the anaerobic co-digestion of olive mill wastes at mesophilic temperature. J. Hazard. Mater. 2009, 162, 1563–1570. [Google Scholar] [CrossRef]
- Kumar, J.L.G.; Zhao, Y.Q. A review on numerous modeling approaches for effective, economical and ecological treatment wetlands. J. Environ. Manag. 2011, 92, 400–406. [Google Scholar] [CrossRef]
- Emerman, S.H. The tipping bucket equations as a model for macropore flow. J. Hydrol. 1995, 171, 23–47. [Google Scholar] [CrossRef]
- Brown, H.; Carrick, S.; Müller, K.; Thomas, S.; Sharp, J.; Rogerio, C.; Holzworth, D.; Clothier, B. Modelling soil-water dynamics in the rootzone of structured and water-repellent soils. Comput. Geosci. 2018, 113, 33–42. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Removal of organics in constructed wetlands with horizontal sub-surface flow: A review of the field experience. Sci. Total Environ. 2009, 407, 3911–3922. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting: A review. Int. Biodeterior. Biodegrad. 2013, 85, 108–119. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Chapter 8—Olive Mill Wastewater Treatment. In Utilization of by-Products and Treatment of Waste in the Food Industry, 1st ed.; Oreopoulou, V., Winfried, R., Eds.; Springer: New York, NY, USA, 2007; pp. 133–157. [Google Scholar]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.J.; Anastasiou, C.C.; O’Flaherty, V.; Mitchell, R. Bioremediation of olive mill wastewater. Int. Biodeterior. Biodegrad. 2008, 61, 127–134. [Google Scholar] [CrossRef]
- Torrecilla, J.S. Chapter 40—Phenolic Compounds in Olive Oil Mill Wastewater. In Olives and Olive Oil Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 357–365. [Google Scholar]
- Regni, L.; Gigliotti, G.; Nasini, L.; Agrafioti, E.; Galanakis, C.M.; Proietti, P. Chapter 5—Reuse of Olive Mill Waste as Soil Amendment. In Olive Mill Waste-Recent Advances for Sustainable Management, 1st ed.; Galanakis, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 97–117. [Google Scholar]
- Piotrowska, A.; Rao, M.A.; Scotti, R.; Gianfreda, L. Changes in soil chemical and biochemical properties following amendment with crude and dephenolized olive mill waste water (OMW). Geoderma 2011, 161, 8–17. [Google Scholar] [CrossRef]
- Mechri, B.; Cheheb, H.; Boussadia, O.; Attia, F.; Ben Mariem, F.; Braham, M.; Hammami, M. Effects of agronomic application of olive mill wastewater in a field of olive trees on carbohydrate profiles, chlorophyll a fluorescence and mineral nutrient content. Environ. Exp. Bot. 2011, 71, 184–191. [Google Scholar] [CrossRef]
- Víctor-Ortega, M.D.; Ochando-Pulido, J.M.; Hodaifa, G.; Martinez-Ferez, A. Final purification of synthetic olive oil mill wastewater treated by chemical oxidation using ion exchange: Study of operating parameters. Chem. Eng. Process. 2014, 85, 241–247. [Google Scholar] [CrossRef]
- Kotsou, M.; Mari, I.; Lasaridi, K.; Chatzipavlidis, I.; Balis, C.; Kyriacou, A. The effect of olive oil mill wastewater (OMW) on soil microbial communities and suppressiveness against Rhizoctonia solani. Appl. Soil Ecol. 2004, 26, 113–121. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Belaqziz, M.; Tan, S.P.; El-Abbassi, A.; Kiai, H.; Hafidi, A.; O’Donovan, O.; McLoughlin, P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE 2017, 12, e0182622. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Asses, N.; Ayed, L.; Bouallagui, H.; Sayadi, S.; Hamdi, M. Biodegradation of different molecular-mass polyphenols derived from olive mill wastewaters by Geotrichum candidum. Int. Biodeterior. Biodegrad. 2009, 63, 407–413. [Google Scholar] [CrossRef]
- Aresta, M.; Acquaviva, M.I.; Baruzzi, F.; Lo Noce, R.M.; Matarante, A.; Narracci, M.; Stabili, L.; Cavallo, R.A. Isolation and characterization of polyphenols-degrading bacteria from olive-mill wastewaters polluted soil. World J. Microbiol. Biotechnol. 2010, 26, 639–647. [Google Scholar] [CrossRef]
- Karakaya, A.; Laleli, Y.; Takaç, S. Development of process conditions for biodegradation of raw olive mill wastewater by Rhodotorula glutinis. Int. Biodeterior. Biodegrad. 2012, 75, 75–82. [Google Scholar] [CrossRef]
- Aquilanti, L.; Taccari, M.; Bruglieri, D.; Osimani, A.; Clementi, F.; Comitini, F.; Ciani, M. Integrated biological approaches for olive mill wastewater treatment and agricultural exploitation. Int. Biodeterior. Biodegrad. 2014, 88, 162–168. [Google Scholar] [CrossRef]
- Daâssi, D.; Lozano-Sánchez, J.; Borrás-Linares, I.; Belbahri, L.; Woodward, S.; Zouari-Mechichi, H.; Mechichi, T.; Nasri, M.; Segura-Carretero, A. Olive oil mill wastewaters: Phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 2014, 113, 62–70. [Google Scholar] [CrossRef] [PubMed]
- García García, I.; Jiménez Peña, P.R.; Bonilla Venceslada, J.L.; Martín Martín, A.; Martín Santos, M.A.; Ramos Gómez, E. Removal of phenol compounds from olive mill wastewater using Phanerochaete chrysosporium, Aspergillus niger, Aspergillus terreus and Geotrichum candidum. Process. Biochem. 2000, 35, 751–758. [Google Scholar] [CrossRef]
- Salgado, J.M.; Abrunhosa, L.; Venâncio, A.; Domínguez, J.M.; Belo, I. Combined bioremediation and enzyme production by Aspergillus sp. in olive mill and winery wastewaters. Int. Biodeterior. Biodegrad. 2016, 110, 16–23. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; van Loosdrecht, M. ActivatedSludge Models ASM1, ASM2, ASM2d, and ASM3, 1st ed.; IWA Publishing: London, UK, 2000. [Google Scholar]
- Zhao, Q.; Thompson, A.M.; Callister, S.J.; Tfaily, M.M.; Bell, S.L.; Hobbie, S.E.; Hofmockel, K.S. Dynamics of organic matter molecular composition under aerobic decomposition and their response to the nitrogen addition in grassland soils. Sci. Total Environ. 2022, 806, 150514. [Google Scholar] [CrossRef]
- Kapellakis, I.; Tzanakakis, V.A.; Angelakis, A.N. Land application-based olive mill wastewater management. Water 2015, 7, 362–376. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Polyphenols dynamics and phytotoxicity in a soil amended by olive mill wastewaters. J. Environ. Manag. 2007, 84, 134–140. [Google Scholar] [CrossRef]
- Cardinali, A.; Cicco, N.; Linsalata, V.; Minervini, F.; Pati, S.; Pieralice, M.; Tursi, N.; Lattanzio, V. Biological activity of high molecular weight phenolics from olive mill wastewater. J. Agric. Food Chem. 2010, 58, 8585–8590. [Google Scholar] [CrossRef] [PubMed]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the main phenolic compounds of olive mill wastewater (OMW) on the kinetics of acetoclastic methanogenesis. Process. Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Pirt, S.J. Maintenance energy: A general model for energy-limited and energy-sufficient growth. Arch. Microbiol. 1982, 133, 300–302. [Google Scholar] [CrossRef]
- Karpouzas, D.G.; Ntougias, S.; Iskidou, E.; Rousidou, C.; Papadopoulou, K.K.; Zervakis, G.I.; Ehaliotis, C. Olive mill wastewater affects the structure of soil bacterial communities. Appl. Soil Ecol. 2010, 45, 101–111. [Google Scholar] [CrossRef]
- Mekki, A.; Dhouib, A.; Sayadi, S. Changes in microbial and soil properties following amendment with treated and untreated olive mill wastewater. Microbiol. Res. 2006, 161, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, M.G.; Lanza, B.; Mucciarella, M.R.; Russi, F.; Iannucci, E.; Marfisi, P.; Madeo, A. Effects of olive mill wastewater spreading on the physico-chemical and microbiological characteristics of soil. Int. Biodeterior. Biodegrad. 2008, 62, 403–407. [Google Scholar] [CrossRef]
- Zuthi, M.F.R.; Guo, W.S.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I. Enhanced biological phosphorus removal and its modeling for the activated sludge and membrane bioreactor processes. Bioresour. Technol. 2013, 139, 363–374. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Reichenauer, T.G.; Germida, J.J. Phytoremediation of organic contaminants in soil and groundwater. ChemSusChem 2008, 1, 708–717. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.; Chang, H.; Li, Z.; Xue, J. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus Uptake by Plants: From Soil to Cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Hayes, J. Mechanisms and control of nutrient uptake in plants. Int. Rev. Cytol. 2003, 229, 73–114. [Google Scholar] [PubMed]
- Manzoni, S.; Molini, A.; Porporato, A. Stochastic modelling of phytoremediation. Proc. R. Soc. A 2011, 467, 3188–3205. [Google Scholar] [CrossRef]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationships Between Lipophilicity and Root Uptake and Translocation of Non-ionised Chemicals by Barley. Pestic. Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Hopmans, J.W.; Bristow, K.L. Current Capabilities and Future Needs of Root Water and Nutrient Uptake Modeling. Adv. Agron. 2002, 77, 103–183. [Google Scholar]
- Hussain, I.; Aleti, G.; Naidu, R.; Puschenreiter, M.; Mahmood, Q.; Rahman, M.M.; Wang, F.; Shaheen, S.; Syed, J.H.; Reichenauer, T.G. Microbe and plant assisted-remediation of organic xenobiotics and its enhancement by genetically modified organisms and recombinant technology: A review. Sci. Total Environ. 2018, 628–629, 1582–1599. [Google Scholar] [CrossRef] [PubMed]
- U.S. Enviromental Protection Agency. Introduction to Phytoremediation; EPA/600/R-99/107; U.S. Environmental Protection Agency: Washington, DC, USA, 2000; pp. 1–72. [Google Scholar]
- Marathe, R.A.; Chaudhary, D.T.; Shinde, Y.R. Roots density and activity of pomegranate grown in light textured soil of semi-arid region. Vegetos 2017, 30, 48–50. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N. Applied Parameter Estimation for Chemical Engineers, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 79–81. [Google Scholar]
- Tziotzios, G.; Lyberatos, G.; Pavlou, S.; Vayenas, D.V. Modelling of biological phenol removal in draw-fill reactors using suspended and attached growth olive pulp bacteria. Int. Biodeterior. Biodegrad. 2008, 61, 142–150. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mofarrah, E.; Mehranian, M. Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. J. Hazard. Mater. 2005, B123, 187–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Lashermes, G.; Houot, S.; Doublet, J.; Steyer, J.P.; Zhu, Y.G.; Barriuso, E.; Garnier, P. Modelling of organic matter dynamics during the composting process. Waste Manag. 2012, 32, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Haudin, C.S.; Zhang, Y.; Lashermes, G.; Houot, S.; Garnier, P. Modeling the release of organic contaminants during compost decomposition in soil. Chemosphere 2015, 119, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Dettenmaier, E.M.; Doucette, W.J.; Bugbee, B. Chemical hydrophobicity and uptake by plant roots. Environ. Sci. Technol. 2009, 43, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Doucette, W.J.; Shunthirasingham, C.; Dettenmaier, E.M.; Zaleski, R.; Fantke, P.; Arnot, J.A. A review of measured bioaccumulation data on terrestrial plants for organic chemicals: Metrics, variability, and the need for standardized measurement protocols. Environ. Toxicol. Chem. 2018, 37, 21–33. [Google Scholar] [CrossRef]
- Kamath, R.; Rentz, J.A.; Schnoor, J.L.; Alvarez, P.J.J. Phytoremediation of hydrocarbon-contaminated soils: Principles and applications. Stud. Surf. Sci. Catal. 2004, 151, 447–478. [Google Scholar]
- Lamshoeft, M.; Gao, Z.; Resseler, H.; Schriever, C.; Sur, R.; Sweeney, P.; Webb, S.; Zillgens, B.; Reitz, M.U. Evaluation of a novel test design to determine uptake of chemicals by plant roots. Sci. Total Environ. 2018, 613–614, 10–19. [Google Scholar] [CrossRef]
- Shone, M.G.T.; Wood, A.V. A comparison of the uptake and translocation of some organic herbicides and a systemic fungicide by barley: I. Absorption in relation to physico-chemical properties. J. Exp. Bot. 1974, 25, 390–400. [Google Scholar] [CrossRef]
- Li, Y.; Chiou, C.T.; Li, H.; Schnoor, J.L. Improved prediction of the bioconcentration factors of organic contaminants from soils into plant/crop roots by related physicochemical parameters. Environ. Int. 2019, 126, 46–53. [Google Scholar] [CrossRef]
- Namiki, S.; Otani, T.; Motoki, Y.; Seike, N.; Iwafune, T. Differential uptake and translocation of organic chemicals by several plant species from soil. J. Pestic. Sci. 2018, 43, 96–107. [Google Scholar] [CrossRef]
- Magdich, S.; Ben Ahmed, C.; Jarboui, R.; Ben Rouina, B.; Boukhris, M.; Ammar, E. Dose and frequency dependent effects of olive mill wastewater treatment on the chemical and microbial properties of soil. Chemosphere 2013, 93, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Arous, F.; Aloui, F.; Sayadi, S. Nitrogen dynamics in soil amended by Olive Mill Waste Waters and poultry manure codigestate Nitrogen dynamics in soil amended by olive mill waste waters and poultry manure codigestate. J. Biotech. Phyto. 2018, 2, 1–8. [Google Scholar]
- Silva, J.S.; Rego, F.C.; Martins-Loução, M.A. Root distribution of mediterranean woody plants. Introducing a new empirical model. Plant Biosyst. 2003, 137, 63–72. [Google Scholar] [CrossRef]
- Teh, C.B.S. Introduction to Mathematical Modeling of Crop Growth: How the Equations are Derived and Assembled into a Computer Program, 1st ed.; Brown Walker Press: Boca Raton, FL, USA, 2006; pp. 111–114. [Google Scholar]
- Kendy, E.; Gerard-Marchant, P.; Walter, M.T.; Zhang, Y.; Liu, C.; Steenhuis, T.S. A soil-water-balance approach to quantify groundwater recharge from irrigated cropland in the North China Plain. Hydrol. Process. 2003, 17, 2011–2031. [Google Scholar] [CrossRef]
- Fredlund, D.G.; Rahardjo, H.; Fredlund, M.D. Unsaturated Soil Mechanics in Engineering Practice, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 274–277. [Google Scholar]
- Green, S.R.; Kirkham, M.B.; Clothier, B.E. Root uptake and transpiration: From measurements and models to sustainable irrigation. Agric. Water Manag. 2006, 86, 165–176. [Google Scholar] [CrossRef]
- Peters, A. Modified conceptual model for compensated root water uptake—A simulation study. J. Hydrol. 2016, 534, 1–10. [Google Scholar] [CrossRef]
- Fredlund, D.G.; Xing, A. Equations for the soil-water characteristic curve. Can. Geotech. J. 1994, 31, 521–532. [Google Scholar] [CrossRef]
- Corapcioglu, M.Y. Advances in Porous Media; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; Volume 3, pp. 52–62. [Google Scholar]
- Hu, Q.; Wang, J.S.Y. Aqueous-phase diffusion in unsaturated geologic media: A review. Crit. Rev. Environ. Sci. Technol. 2003, 33, 275–297. [Google Scholar] [CrossRef]
- Lugli, F.; Mahler, C.F. A soil-plant model applied to phytoremediation of metals. Int. J. Phytoremediation 2016, 18, 295–307. [Google Scholar] [CrossRef]
- Garcia, H.E.; Gordon, L.I. Oxygen solubility in seawater: Better fitting equations. Limnol. Oceanogr. 1992, 37, 1307–1312. [Google Scholar] [CrossRef]
- Benson, B.B.; Krause, D., Jr. The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol. Oceanogr. 1984, 29, 620–632. [Google Scholar] [CrossRef]
- Van De Werf, H.; Verstraete, W. Estimation of active soil microbial biomass by mathematical analysis of respiration curves: Calibration of the test procedure. Soil Biol. Biochem. 1987, 19, 253–260. [Google Scholar] [CrossRef]
- Smith, O.L. An analytical model of the decomposition of soil organic matter. Soil Biol. Biochem. 1979, 11, 585–606. [Google Scholar] [CrossRef]
- Sole-Mauri, F.; Illa, J.; Magrí, A.; Prenafeta-Boldú, F.X.; Flotats, X. An integrated biochemical and physical model for the composting process. Bioresour. Technol. 2007, 98, 3278–3293. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Moraetis, D.; Stamati, F.E.; Nikolaidis, N.P.; Kalogerakis, N. Olive mill wastewater irrigation of maize: Impacts on soil and groundwater. Agric. Water Manag. 2011, 98, 1125–1132. [Google Scholar] [CrossRef]
- Tsiknia, M.; Tzanakakis, V.A.; Oikonomidis, D.; Paranychianakis, N.V.; Nikolaidis, N.P. Effects of olive mill wastewater on soil carbon and nitrogen cycling. Appl. Microbiol. Biotechnol. 2014, 98, 2739–2749. [Google Scholar] [CrossRef]
- Kaczmarek, W. A comparison of bacterial and fungal biomass in several cultivated soils. Acta Microbiol. Pol. 1984, 33, 239–247. [Google Scholar]
- Rawls, W.J.; Brakensiek, D.L.; Saxton, K.E. Estimation of soil water properties. Trans. ASAE 1982, 25, 1316–1320, 1328. [Google Scholar] [CrossRef]
- Šimůnek, J.; Hopmans, J.W. Modeling compensated root water and nutrient uptake. Ecol. Modell. 2009, 220, 505–521. [Google Scholar] [CrossRef]
- Albasha, R.; Mailhol, J.C.; Cheviron, B. Compensatory uptake functions in empirical macroscopic root water uptake models—Experimental and numerical analysis. Agric. Water Manag. 2015, 155, 22–39. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, T.; Zhang, K.; Greenwood, D.J.; Hammond, J.P.; White, P.J. An easily implemented agro-hydrological procedure with dynamic root simulation for water transfer in the crop-soil system: Validation and application. J. Hydrol. 2009, 370, 177–190. [Google Scholar] [CrossRef]
- Heinen, M. Compensation in Root Water Uptake Models Combined with Three-Dimensional Root Length Density Distribution. Vadose Zone J. 2014, 13, 1–9. [Google Scholar] [CrossRef]




| Variable | Unit | Description | Compartments |
|---|---|---|---|
| mg-O2/L | Dissolved oxygen concentration | Soil solution of the wetted region of soil compartment (4 soil compartments) and stored OMW (storage compartment) | |
| mg/L | Readily biodegradable non-phenolic organic matter concentration (BOD5) | ||
| mg/L | Slowly biodegradable non-phenolic organic matter concentration (COD) | ||
| mg/L | High MW phenolic compound concentration (gallic acid) | ||
| mg/L | Low MW phenolic compound concentration (gallic acid) | ||
| mg-N/L | Concentration of nitrogen in organic form | ||
| mg-N/L | Concentration of nitrogen in inorganic form | ||
| mg-P/L | Concentration of phosphorus | ||
| mg-COD/kg-root tissue | Concentration of adsorbed mass of non-phenolic SBOM on plants’ root tissue | Plants’ root tissue (4 soil compartments) | |
| mg-gallic acid/kg-root tissue | Concentration of adsorbed mass of high MW phenolic compounds on plants’ root tissue | ||
| mg-N/kg-root tissue | Concentration of adsorbed mass of nitrogen in organic form on plants’ root tissue | ||
| g-biomass/kg-soil | Bulk soil bacterial biomass concentration | Bulk soil of the wetted region (4 soil compartments) | |
| g-biomass/kg-soil | Bulk soil fungal biomass concentration | ||
| g-biomass/kg-soil | Rhizosphere soil bacterial biomass concentration | Rhizosphere soil of the wetted region (4 soil compartments) | |
| g-biomass/kg-soil | Rhizosphere soil fungal biomass concentration | ||
| L | Liquid phase volume for the kth compartment or OMW storage compartment | Soil solution of the wetted region (4 soil compartments) and stored OMW (storage compartment) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petoussi, M.A.; Kalogerakis, N. Mathematical Modeling of Pilot Scale Olive Mill Wastewater Phytoremediation Units. Sustainability 2023, 15, 8630. https://doi.org/10.3390/su15118630
Petoussi MA, Kalogerakis N. Mathematical Modeling of Pilot Scale Olive Mill Wastewater Phytoremediation Units. Sustainability. 2023; 15(11):8630. https://doi.org/10.3390/su15118630
Chicago/Turabian StylePetoussi, Margarita A., and Nicolas Kalogerakis. 2023. "Mathematical Modeling of Pilot Scale Olive Mill Wastewater Phytoremediation Units" Sustainability 15, no. 11: 8630. https://doi.org/10.3390/su15118630
APA StylePetoussi, M. A., & Kalogerakis, N. (2023). Mathematical Modeling of Pilot Scale Olive Mill Wastewater Phytoremediation Units. Sustainability, 15(11), 8630. https://doi.org/10.3390/su15118630








