Determination of Heavy Metals Immobilization by Chemical Fractions in Contaminated Soil Amended with Biochar
Abstract
:1. Introduction
2. Material and Methods
2.1. Pot Experiment Description
2.2. Quality of Soil Used for Pot Experiment
2.3. Amendments Used for the Pot Experiment Characterization
2.4. Samples Digestion by CEM MARS 6
2.5. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis
2.6. Sequential Leaching
2.7. Heavy Metals Immobilization Efficiency and Potential Ecological Risk
2.8. Statistical Analysis
3. Results and Discussion
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, S.J. What Is a “Heavy Metal”? J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Adriano, D.; Wenzel, W.; Vangronsveld, J.; Bolan, N. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Bhat, S.A.; Bashir, O.; Haq, S.A.U.; Amin, T.; Rafiq, A.; Ali, M.; Américo-Pinheiro, J.H.P.; Sher, F. Phytoremediation of heavy metals in soil and water: An eco-friendly, sustainable and multidisciplinary approach. Chemosphere 2022, 303, 134788. [Google Scholar] [CrossRef]
- Li, N.; Li, H.; Su, G.; Chen, J. Heavy metal distribution profiles in soil and groundwater near pig farms in China. Chemosphere 2022, 294, 133721. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef]
- Corso, M.; An, X.; Jones, C.Y.; Gonzalez-Doblas, V.; Schvartzman, M.S.; Malkowski, E.; Willats, W.G.T.; Hanikenne, M.; Verbruggen, N. Adaptation of Arabidopsis halleri to extreme metal pollution through limited metal accumulation involves changes in cell wall composition and metal homeostasis. New Phytol. 2021, 230, 669–682. [Google Scholar] [CrossRef]
- Danilcenko, H.; Gajewski, M.; Jariene, E.; Paulauskas, V.; Mažeika, R. Effect of compost on the accumulation of heavy metals in fruit of oilseed pumpkin (Cucurbita pepo L. var. Styriaca). J. Elem. 2015, 21, 21–31. [Google Scholar] [CrossRef]
- Valiukenaite, R.; Stankeviciene, M.; Stankevicius, H.; Skibniewska, K.A. Lead and Cadmium Levels in Raw Cow’ S Milk in Lithuania Determined By Inductively Coupled Plasma Sector Field Mass Spectrometry. Pol. J. Food Nutr. Sci. 2006, 15, 243–246. [Google Scholar]
- Zhang, H.; Guo, Q.; Yang, J.; Shen, J.; Chen, T.; Zhu, G.; Chen, H.; Shao, C. Subcellular cadmium distribution and antioxidant enzymatic activities in the leaves of two castor (Ricinus communis L.) cultivars exhibit differences in Cd accumulation. Ecotoxicol. Environ. Saf. 2015, 120, 184–192. [Google Scholar] [CrossRef]
- Abdu, N.; Abdullahi, A.A.; Abdulkadir, A. Heavy metals and soil microbes. Environ. Chem. Lett. 2016, 15, 65–84. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Rezania, S.; Jung, M.C.; Al-Ghamdi, A.A.; Mustafa, A.E.-Z.M.; Elshikh, M.S. Effects of fine fractions of soil organic, semi-organic, and inorganic amendments on the mitigation of heavy metal(loid)s leaching and bioavailability in a post-mining area. Chemosphere 2021, 271, 129538. [Google Scholar] [CrossRef]
- Ayaz, M.; Feizienė, D.; Tilvikienė, V.; Akhtar, K.; Stulpinaitė, U.; Iqbal, R. Biochar Role in the Sustainability of Agriculture and Environment. Sustainability 2021, 13, 1330. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Yu, O.-Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental Benefits of Biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Xu, W.; Liao, Q.; Zhang, H.; Hao, S.; Chen, S. Pyrolysis of various phytoremediation residues for biochars: Chemical forms and environmental risk of Cd in biochar. Bioresour. Technol. 2019, 299, 122581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-C.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-H.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Domańska, J.; Leszczyńska, D.; Badora, A. The Possibilities of Using Common Buckwheat in Phytoremediation of Mineral and Organic Soils Contaminated with Cd or Pb. Agriculture 2021, 11, 562. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Application of white mustard and oats in the phytostabilisation of soil contaminated with cadmium with the addition of cellulose and urea. J. Soils Sediments 2019, 20, 931–942. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Mažeika, R. Chemical composition and risk assessment of spring barley grown in artificially contaminated soil. Environ. Sci. Pollut. Res. 2021, 28, 21684–21695. [Google Scholar] [CrossRef]
- Wang, R.; Shafi, M.; Ma, J.; Zhong, B.; Guo, J.; Hu, X.; Xu, W.; Yang, Y.; Ruan, Z.; Wang, Y.; et al. Effect of amendments on contaminated soil of multiple heavy metals and accumulation of heavy metals in plants. Environ. Sci. Pollut. Res. 2018, 25, 28695–28704. [Google Scholar] [CrossRef]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Žydelis, R.; Mažeika, R. Screening of chemical composition and risk index of different origin composts produced in Lithuania. Environ. Sci. Pollut. Res. 2020, 27, 24480–24494. [Google Scholar] [CrossRef]
- Sun, S.; Huang, X.; Lin, J.; Ma, R.; Fang, L.; Zhang, P.; Qu, J.; Zhang, X.; Liu, Y. Study on the effects of catalysts on the immobilization efficiency and mechanism of heavy metals during the microwave pyrolysis of sludge. Waste Manag. 2018, 77, 131–139. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Liu, W.; Zhang, R.; Cui, M.; Liu, J.; Zhang, W. The evaluation of immobilization behavior and potential ecological risk of heavy metals in bio-char with different alkaline activation. Environ. Sci. Pollut. Res. 2021, 28, 21396–21410. [Google Scholar] [CrossRef]
- Zhu, H.-N.; Yuan, X.-Z.; Zeng, G.-M.; Jiang, M.; Liang, J.; Zhang, C.; Yin, J.; Huang, H.-J.; Liu, Z.-F.; Jiang, H.-W. Ecological risk assessment of heavy metals in sediments of Xiawan Port based on modified potential ecological risk index. Trans. Nonferrous Met. Soc. China 2012, 22, 1470–1477. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Nema, A.K. Heavy Metals in Water, Sediments, and Aquatic Macrophytes: River Hindon, India. J. Hazard. Toxic Radioact. Waste 2012, 16, 273–281. [Google Scholar] [CrossRef]
- Zhu, W.; Bian, B.; Li, L. Heavy metal contamination of road-deposited sediments in a medium size city of China. Environ. Monit. Assess. 2007, 147, 171–181. [Google Scholar] [CrossRef]
- Pituello, C.; Francioso, O.; Simonetti, G.; Pisi, A.; Torreggiani, A.; Berti, A.; Morari, F. Characterization of chemical–physical, structural and morphological properties of biochars from biowastes produced at different temperatures. J. Soils Sediments 2014, 15, 792–804. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Meier, S.; Curaqueo, G.; Khan, N.; Bolan, N.; Cea, M.; Eugenia, G.M.; Cornejo, P.; Ok, Y.S.; Borie, F. Chicken-manure-derived biochar reduced bioavailability of copper in a contaminated soil. J. Soils Sediments 2017, 17, 741–750. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.-L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2018, 216, 463–471. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Shi, Z.; Li, L.; Li, Y.; Mao, Z.; Liao, L.; Zhang, H.; Wu, Y. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Environ. Pollut. Bioavailab. 2021, 33, 55–65. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of Biochar and Modified Biochar in Heavy Metal Contaminated Soil: A Descriptive Review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Lu, H.-L.; Li, K.-W.; Nkoh, J.N.; Shi, Y.-X.; He, X.; Hong, Z.-N.; Xu, R.-K. Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 2022, 301, 134674. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, P.; Quilliam, R.; DeLuca, T.; Vamerali, T.; Jones, D. Does biochar application alter heavy metal dynamics in agricultural soil? Agric. Ecosyst. Environ. 2014, 184, 149–157. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Q.; Liu, X.; Wang, Y. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 2018, 216, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Kiikkilä, O. Heavy-metal pollution and remediation of forest soil around the Harjavalta Cu-Ni smelter, in SW Finland. Silva Fenn. 2003, 37, 399–415. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Basta, N.; Gradwohl, R.; Snethen, K.; Schroder, J. Chemical Immobilization of Lead, Zinc, and Cadmium in Smelter-Contaminated Soils Using Biosolids and Rock Phosphate. J. Environ. Qual. 2001, 30, 1222–1230. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Pantsar-Kallio, M.; Reinikainen, S.-P.; Oksanen, M. Interactions of soil components and their effects on speciation of chromium in soils. Anal. Chim. Acta 2001, 439, 9–17. [Google Scholar] [CrossRef]
- Seaman, J.C.; Arey, J.; Bertsch, P.M. Immobilization of Nickel and Other Metals in Contaminated Sediments by Hydroxyapatite Addition. J. Environ. Qual. 2001, 30, 460–469. [Google Scholar] [CrossRef]
- Ullah, S.; Naeem, A.; Calkaite, I.; Hosney, A.; Depar, N.; Barcauskaite, K. Zinc (Zn) mitigates copper (Cu) toxicity and retrieves yield and quality of lettuce irrigated with Cu and Zn-contaminated simulated wastewater. Environ. Sci. Pollut. Res. 2023, 30, 54800–54812. [Google Scholar] [CrossRef]
- Buneviciene, K.; Drapanauskaite, D.; Mažeika, R.; Tilvikiene, V. Biofuel ash granules as a source of soil and plant nutrients. Zemdirb. Agric. 2021, 108, 19–26. [Google Scholar] [CrossRef]
- Buneviciene, K.; Drapanauskaite, D.; Mazeika, R.; Baltrusaitis, J. A Mixture of Green Waste Compost and Biomass Combustion Ash for Recycled Nutrient Delivery to Soil. Agronomy 2021, 11, 641. [Google Scholar] [CrossRef]
- Praspaliauskas, M.; Pedišius, N. A review of sludge characteristics in Lithuania’s wastewater treatment plants and perspectives of its usage in thermal processes. Renew. Sustain. Energy Rev. 2017, 67, 899–907. [Google Scholar] [CrossRef]
- Kovács-Bokor, É.; Domokos, E.; Biró, B. Toxic metal phytoextraction potential and health-risk parameters of some cultivated plants when grown in metal-contaminated river sediment of Danube, near an industrial town. Environ. Geochem. Health 2021, 43, 2317–2330. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, S.; Chen, Y.; Wang, F.; Yang, D.; Ren, J.; Lu, H.; Ping, L.; Chai, Y. Biochar reduces cadmium accumulation in rice grains in a tungsten mining area-field experiment: Effects of biochar type and dosage, rice variety, and pollution level. Environ. Geochem. Health 2018, 41, 43–52. [Google Scholar] [CrossRef]
- Li, Z.; Deng, H.; Yang, L.; Zhang, G.; Li, Y.; Ren, Y. Influence of potassium hydroxide activation on characteristics and environmental risk of heavy metals in chars derived from municipal sewage sludge. Bioresour. Technol. 2018, 256, 216–223. [Google Scholar] [CrossRef]
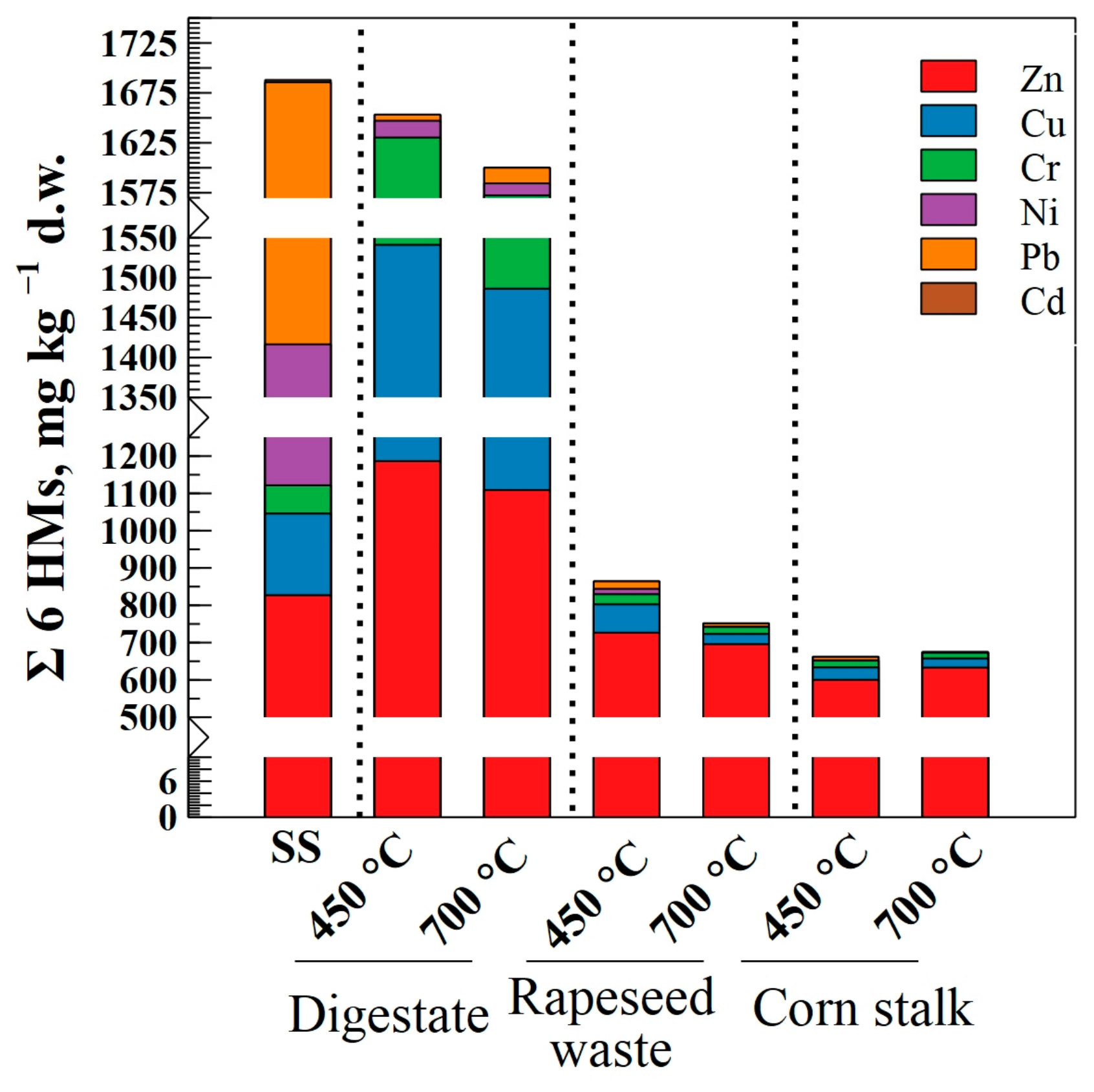
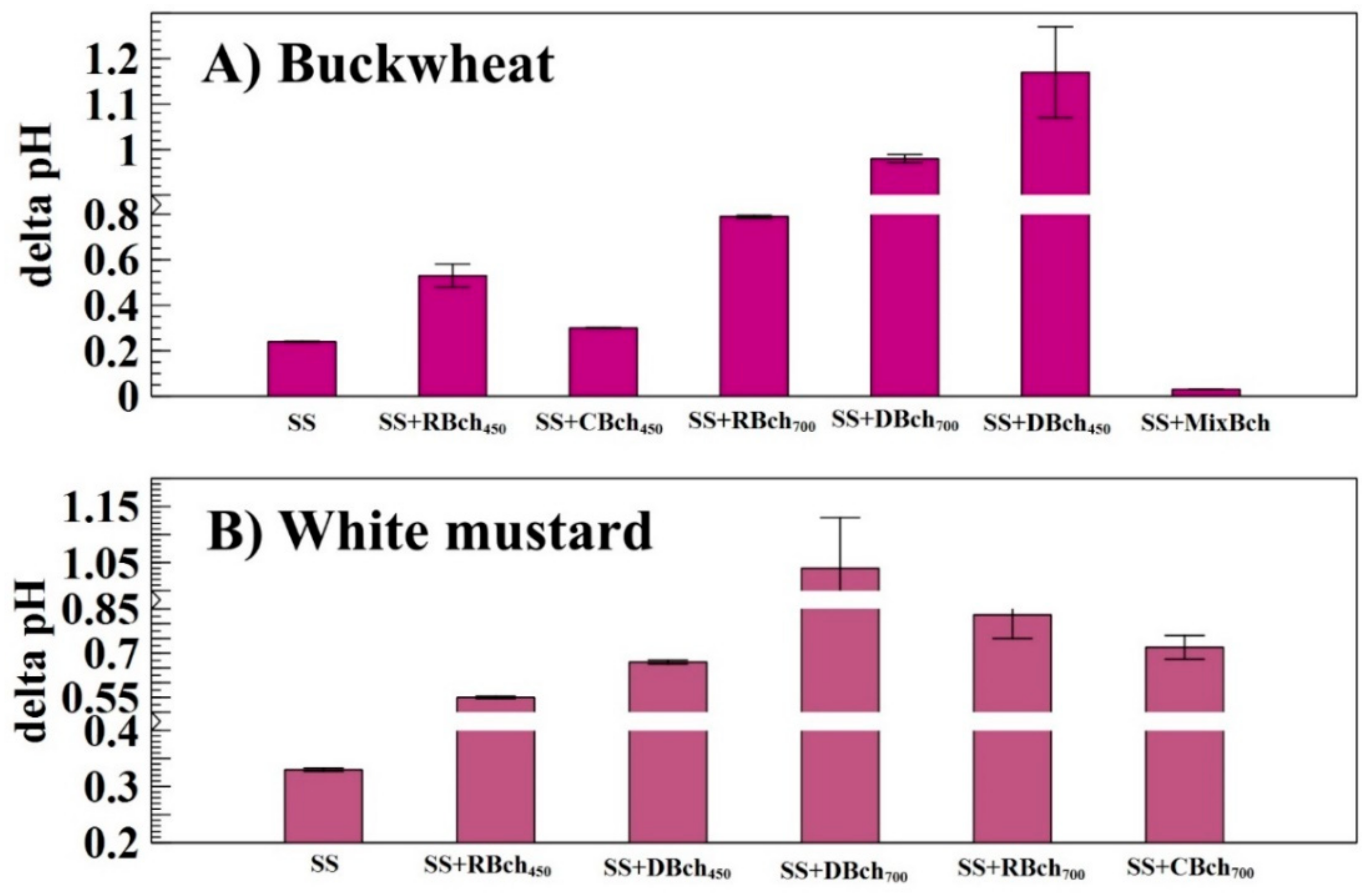
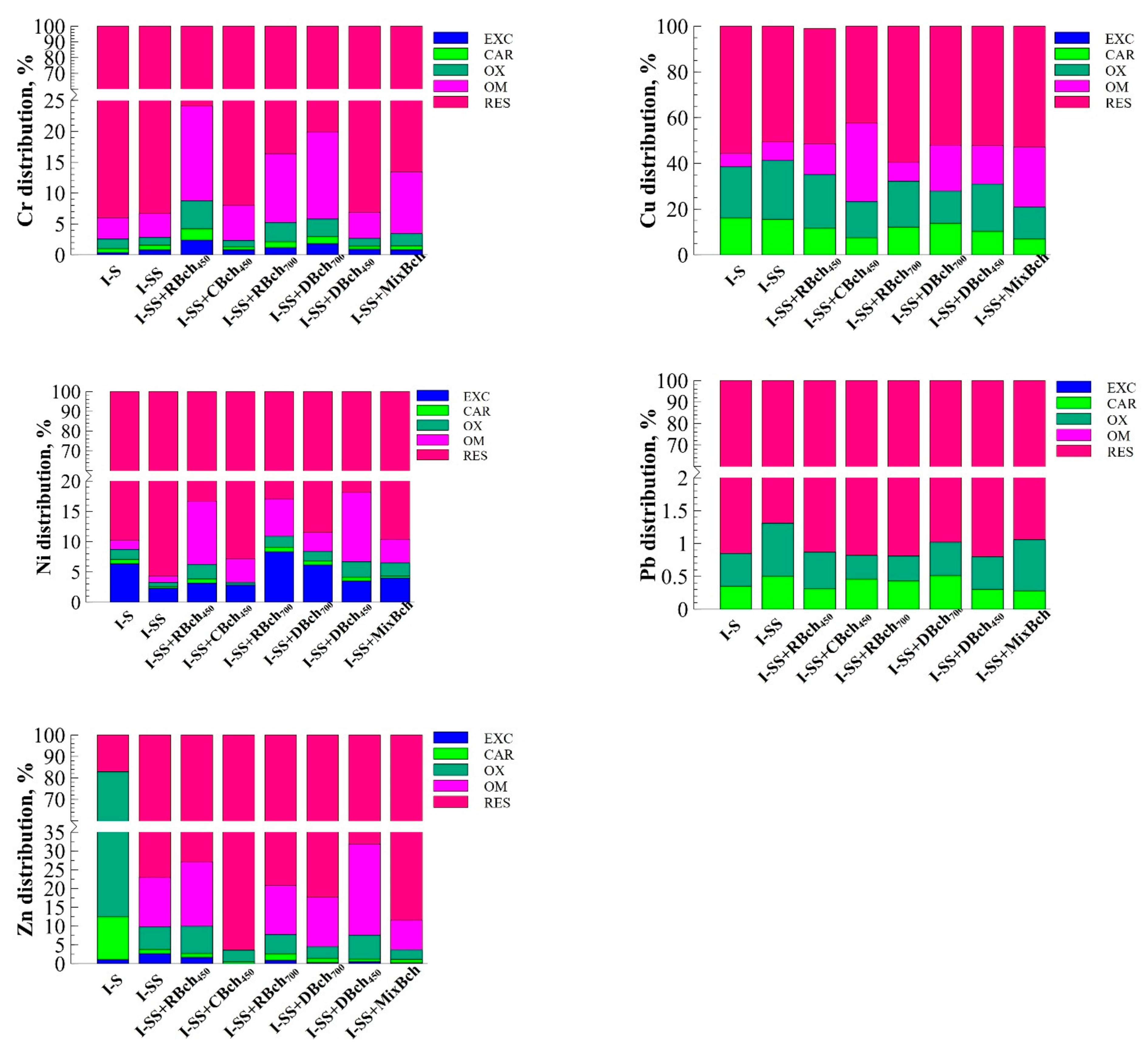

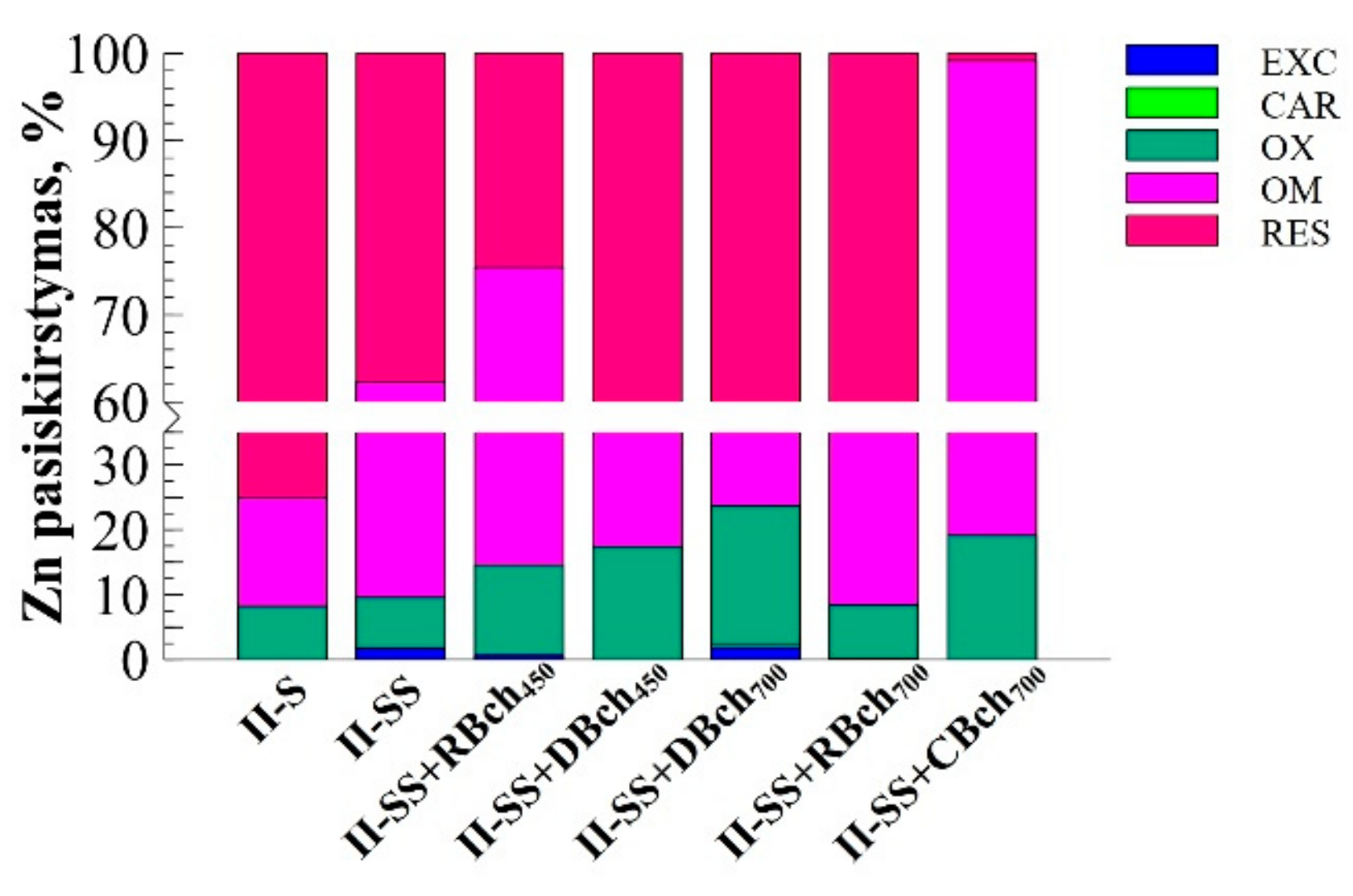
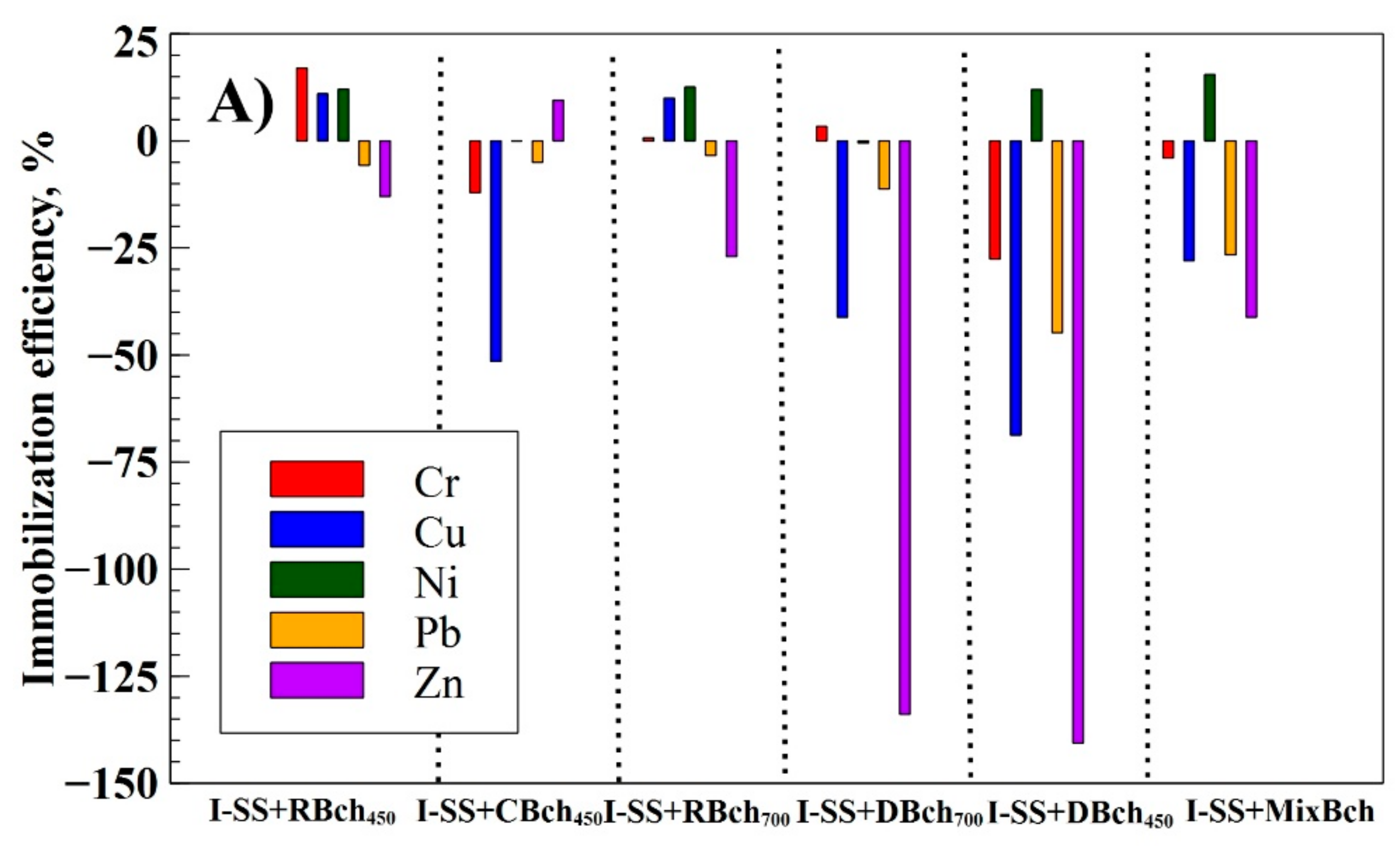
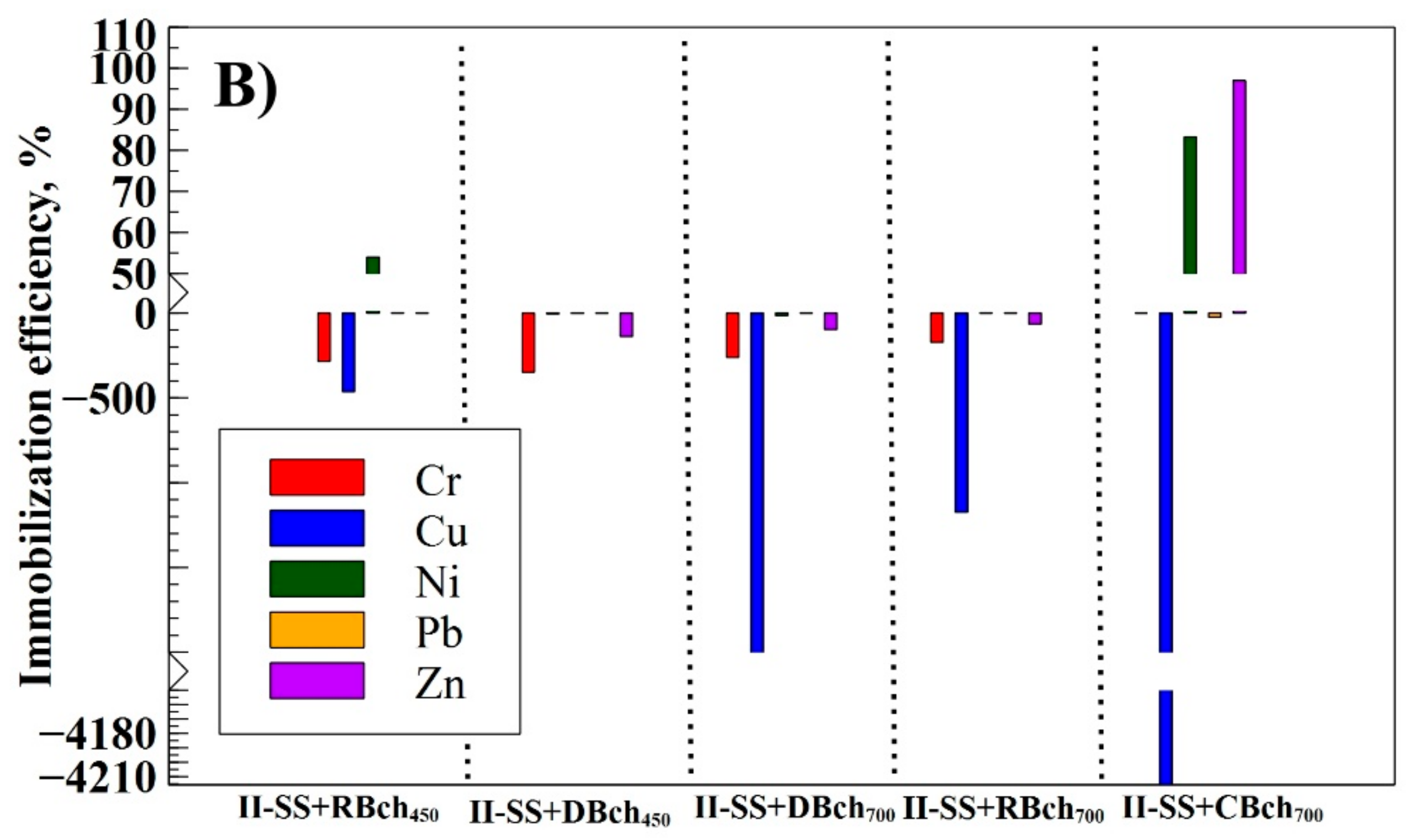
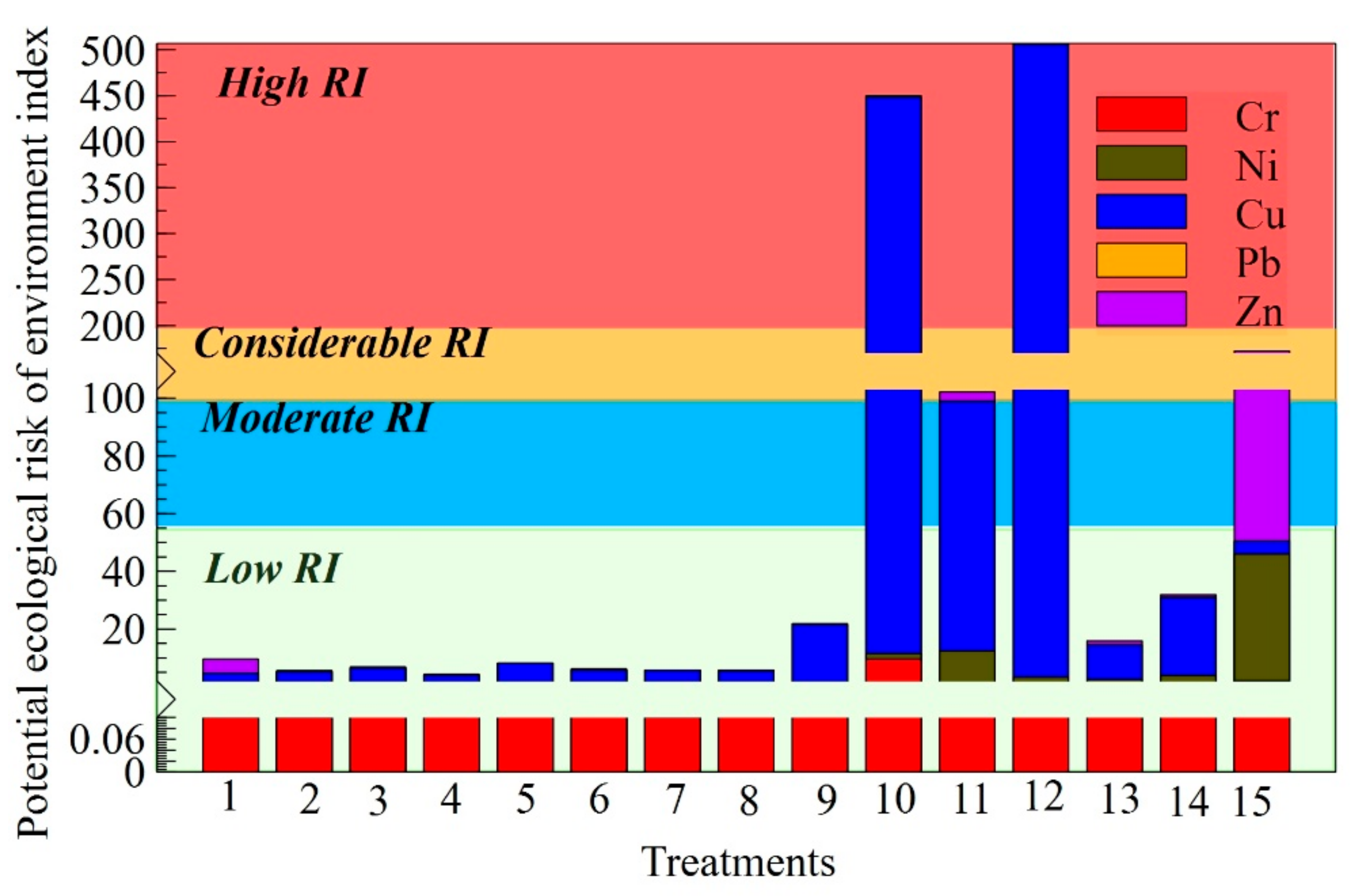
| Num. | Treatment Abbreviation | Amount of Soil, g | Amount of Sewage Sludge, g | Amount of Biochar, g | Cultivated Plants |
|---|---|---|---|---|---|
| 1. | I-S | 7000 | 0 | 0 | Buckwheat |
| 2. | I-SS | 6882.4 | 117.6 | 0 | Buckwheat |
| 3. | I-SS+RBch450 | 6672.4 | 117.6 | 210 | Buckwheat |
| 4. | I-SS+CBch450 | 6672.4 | 117.6 | 210 | Buckwheat |
| 5. | I-SS+RBch700 | 6672.4 | 117.6 | 210 | Buckwheat |
| 6. | I-SS+DBch700 | 6672.4 | 117.6 | 210 | Buckwheat |
| 7. | I-SS+DBch450 | 6672.4 | 117.6 | 210 | Buckwheat |
| 8. | I-SS+MixBch | 6777.4 | 117.6 | 105 | Buckwheat |
| 9. | II-S | 7000 | 0 | 0 | White mustard |
| 10. | II-SS | 6882.4 | 117.6 | 0 | White mustard |
| 11. | II-SS+RBch450 | 6672.4 | 117.6 | 210 | White mustard |
| 12. | II-SS+DBch450 | 6672.4 | 117.6 | 210 | White mustard |
| 13. | II-SS+DBch700 | 6672.4 | 117.6 | 210 | White mustard |
| 14. | II-SS+RBch700 | 6672.4 | 117.6 | 210 | White mustard |
| 15. | II-SS+CBch700 | 6672.4 | 117.6 | 210 | White mustard |
| pHKCl | P2O5, mg/kg | Ntotal, mg/kg | K2O, mg/kg | Ctotal, g/kg |
|---|---|---|---|---|
| 4.50 | 88 | 1.26 | 191 | 12.5 |
| Raw Material | Combustion Temp., ° C | Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| pH | Bulk Density, g/cm3 | N, % | C, % | Corg, % | H, % | P, % | ||
| Digestate | 450 | 8.49 | 0.574 | 1.30 | 38.96 | 31.24 | 0.75 | 1.31 |
| 700 | 9.27 | 0.529 | 2.11 | 57.55 | 52.60 | 0.83 | 1.77 | |
| Waste of biodiesel production from rapeseed | 450 | 9.25 | 0.744 | 0.08 | 36.81 | 32.76 | 0.28 | 0.80 |
| 700 | 10.23 | 0.735 | 0.13 | 43.38 | 40.12 | 0.39 | 0.76 | |
| Corn stalk | 450 | 9.53 | 0.197 | 1.07 | 42.77 | 38.95 | 1.26 | 0.053 |
| 700 | 9.75 | 0.186 | 1.16 | 58.96 | 53.05 | 1.62 | 0.050 | |
| Parameters | ||||
|---|---|---|---|---|
| pH | Organic Matter, % | Dry Matter, % | N, % | P, % |
| 7.10 | 66.10 | 94.20 | 5.52 | 2.61 |
| Fraction Number | Fraction Name | Extraction Conditions | Extraction Solution |
|---|---|---|---|
| I | Exchangeable (EXC) | Oscillation for 2 h at 25 °C, pH = 7 | 10 mL 1 mol/L MgCl2 |
| II | Bound to carbonates (CAR) | Oscillation for 2 h at 25 °C, pH = 5 | 10 mL 1 mol/L CH3COONa |
| III | Bound to Fe-Mn oxides (OX) | Oscillation for 6 h in the oven at 96 °C, pH = 2 | 10 mL 0.04 mol/L NH2OH-HCl and 25% CH3COOH |
| IV | Bound to organic matter (OM) |
|
|
| V | Residual (RES) | Digestion using an automated CEM MARS 6® (Matthews, NC, USA) | HNO3-HCl (5:1) |
| Pollution Degree | RI Value | Grade of Potential Ecological Risk of Environment | ||
|---|---|---|---|---|
| < 1 | Clean | ≤15 | ≥50 RI | Low risk |
| 1 ≤ < 3 | Low | 15 ≤ < 30 | 50 ≤ RI < 100 | Moderate risk |
| 3 ≤ < 6 | Moderate | 30 ≤ < 60 | 100 ≤ RI < 200 | Considerable risk |
| 6 ≤ < 9 | Considerable | 60 ≤ < 120 | RI > 200 | High risk |
| > 9 | High | > 120 | Very high risk |
| Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|
| I-S | 0.06 | 0.8 | 0.11 | 0.01 | 4.86 |
| I-SS | 0.07 | 0.98 | 0.04 | 0.01 | 0.30 |
| I-SS+RBch450 | 0.32 | 0.98 | 0.2 | 0.01 | 0.37 |
| I-SS+CBch450 | 0.09 | 0.68 | 0.08 | 0.01 | 0.26 |
| I-SS+RBch700 | 0.20 | 1.36 | 0.21 | 0.01 | 0.04 |
| I-SS+DBch700 | 0.25 | 0.91 | 0.13 | 0.01 | 0.32 |
| I-SS+DBch450 | 0.07 | 0.89 | 0.22 | 0.01 | 0.13 |
| I-SS+MixBch | 0.16 | 0.92 | 0.12 | 0.01 | 0.22 |
| II-S | 0.22 | 4.10 | 0.14 | 0.00 | 0.33 |
| II-SS | 4.82 | 87.42 | 0.38 | 0.01 | 1.65 |
| II-SS+RBch450 | 0.52 | 17.32 | 2.29 | 0.01 | 3.07 |
| II-SS+DBch450 | 0.37 | 100.50 | 0.53 | 0.01 | 0.89 |
| II-SS+DBch700 | 0.32 | 2.41 | 0.38 | 0.01 | 1.34 |
| II-SS+RBch700 | 0.61 | 5.40 | 0.55 | 0.02 | 0.87 |
| II-SS+CBch700 | 1.05 | 0.89 | 8.78 | 0.01 | 121.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barčauskaitė, K.; Anne, O.; Mockevičienė, I.; Repšienė, R.; Šiaudinis, G.; Karčauskienė, D. Determination of Heavy Metals Immobilization by Chemical Fractions in Contaminated Soil Amended with Biochar. Sustainability 2023, 15, 8677. https://doi.org/10.3390/su15118677
Barčauskaitė K, Anne O, Mockevičienė I, Repšienė R, Šiaudinis G, Karčauskienė D. Determination of Heavy Metals Immobilization by Chemical Fractions in Contaminated Soil Amended with Biochar. Sustainability. 2023; 15(11):8677. https://doi.org/10.3390/su15118677
Chicago/Turabian StyleBarčauskaitė, Karolina, Olga Anne, Ieva Mockevičienė, Regina Repšienė, Gintaras Šiaudinis, and Danutė Karčauskienė. 2023. "Determination of Heavy Metals Immobilization by Chemical Fractions in Contaminated Soil Amended with Biochar" Sustainability 15, no. 11: 8677. https://doi.org/10.3390/su15118677





