1. Introduction
The pharmaceutical supply chain digital transformation is about establishing a vision for how digital strategies and applications can improve service, cost, quality, agility, and inventory levels and consistently improving organizational changes and processes that use these digital enablers to drive operational excellence [
1]. The digital supply chain is known as the strategic and operative exchange of information (product, design, research, and competition) between all members of the supply chain to enhance communication and build an altogether new kind of ambitious supply network that is responsive and resilient [
2,
3]. The pharmaceutical industry is diverse, complex, and regulated, and that is why digitalization started late through new digital technologies, and trends continue to drive growth with the possibilities associated with digitalization [
4]. As per Gartner’s research, sustainability has impacts spanning the entire value chain- from plan to source, make, and deliver to the service domain. Digital enablers may enhance visibility and collaborations and help achieve sustainability goals.
The conceptual framework for this study was the theory of constraints by Eliyahu Goldratt, introduced through a popular book,
The Goal. The inadequacy of traditional management and global competitiveness is why organizational managers adopt the theory of constraints (TOC) as a continuous process framework to achieve their goals [
5]. The theory suggests that every goal-oriented system does have at least one constraint, and any improvement can be achieved by addressing and mitigating the identified constraint. The supply chain is a real system composed of various functions (organizations) contributing and adding value to the products and services before reaching the intended end customers. Therefore, we can safely assume that the output of a supply chain is determined by its constraints. Researchers use the TOC to help identify, leverage, and remove constraints in its operations [
6]. Constraints can occur anywhere within manufacturing, including supply chain, logistics, or internal processes. Five different types of constraints include (a) market constraints, (b) capacity constraints, (c) logistics constraints, (d) behavioral constraints, and (e) administrative constraints [
7]. There can be managerial or policy-related constraints that are generally difficult to identify. It may require frequent involvement and interaction between internal functional groups.
Implementing any digital technology is considered sustainable if it is green and has a low impact on the three pillars of sustainability: environmental, social, and economical. However, the exponential rise in data resulting from digital technologies, such as data centers and servers, contributed significantly to digital pollution by consuming almost seven percent of global electricity. This is categorized as non-sustainable digitalization and a key discussion element towards implementation and management of digital strategies in pharmaceutical industries. Pharmaceutical companies are traditionally non-sustainable and most emission intensive compared to many other industries. They also lose significant money by potency spoilage from temperature and humidity fluctuation during storage or transportation. It is a potential hazard for patients and results in stringent compliance issues.
By implementing digital supply chain strategies, managers in organizations with high digital operations can expect 4.1% annual efficiency gains while boosting revenues by 2.9% per year [
8]. Business leaders who use emerging digital enablers such as the Internet of Things (IoT), Artificial Intelligence (AI), cloud computing (CC), and big data analytics (BDA) for business advantages could enhance business performance with improved financial performance and added value [
9].
2. Literature Review
2.1. Theory of Constraints (TOC)
The TOC suggests that every goal-oriented system has at least one constraint, and constraints limit its output. Identifying and mitigating identified constraints are required for any improvement in the system. The pharmaceutical supply chain is a real system composed of various organizations (functions) that contributes and adds value to the services and customers before reaching the final intended customers. The TOC framework helps researchers in providing the insights needed to determine why non-digitalized pharmaceutical supply chains fail to achieve organizational goals. Constraints hamper the progress or increase productivity losses within the organization. A digitalized supply chain system can have various constraints, such as constraints related to storage, constraints related to production, constraints related to flow, and constraints related to strategic partners’ information [
10]. The TOC analogy can be made to the supply chain, where weak and non-digitalized supply chain links can limit the entire supply chain’s efficiency and effectiveness. The pharmaceutical supply chain manager’s failure to manage these constraints leads to declines in its productivity. The theory of constraints plays a big role in sustainable digitalization. A limiting resource or constraint in pharmaceuticals is a resource that emits hazardous greenhouse gas, such as the transportation of drugs and vaccines. It can impact the efficiency of the end-to-end supply chain system. It will also impact any partnership with other companies. For investors, the sustainable supply chain is also the key criterion for any investment decisions.
2.2. Digitalization
Digitalization can be understood as a process of using digital technology enablers, leading to a change in value creation, business processes, and business models to provide new revenue and value-adding opportunities [
11]. Digitalization of business processes enables organizations to various innovations, including improved design and new process models, and shapes how organizations create enhanced values for their business partners, such as customers and vendors [
5]. Digital transformation is a set of strategic renewal, transformation at a different level in an organization, and leadership aspects of optimizing resources and capabilities attributes far beyond technology alone [
11].
2.3. Digitalization in the Pharmaceutical Industry
The pharmaceutical industry is defined as a system of procedures, and operational life cycles, such as drug discovery, development including testing, review, and approval, oligopolistic competition, and generic competition [
12]. Digitalization in the pharmaceutical industry can be understood as a process of using digital technology, leading to changes in value creation, business processes, and business models to provide new revenue and value-adding opportunities [
13]. The digital strategy refers to the company’s strategy applied to its strategic initiatives, including the end-to-end processes, such as requirement gathering, planning, recognizing risks and opportunities, and maintaining the digital strategy [
14]. In a survey, 73% of respondents acknowledged that digitalization helped them reach operational excellence [
15]. A digital strategy is the strategic form of companies’ digitalization intentions when digital technology and methods are applied to products, services, processes, and business models [
16]. The growth of the business has created tighter global competition, and to survive and maintain a sustainable competitive advantage, organizations must identify emerging digital technologies for developing a new business model [
10]. Digitalization in the supply chain helps build a new type of sustainable, responsive, and resilient supply network because the complex pharmaceutical supply chain involves life-saving interests and requires the mandatory participation of stakeholders [
17]. The digital strategy by pharmaceutical supply chain managers may reshape the existing non-digital supply chain model to digitalized supply chain 4.0, in which automation will boost supply chain efficiencies by automating the operational tasks.
2.4. Digital Supply Chain in the Pharmaceutical Industries
The digital supply chain has been referred to as an intelligent, customer-centric, system-integrated, globally connected, and data-driven mechanism that leverages new technologies to deliver valuable products and more accessible and affordable services [
18,
19]. The pharmaceutical supply chain is complex and regulated. Drug cost is greatly affected by the efficiency of the supply chain. In a competitive global environment, supply chain agility and reliability are the most critical elements in the success of an organization, but the current situation needs to be more assured [
20]. The digital supply chain is part of the fourth industrial revolution, also known as Industry 4.0, that helps organizations connect ecosystems within the functional area of an organization. The digital supply chain is called the smart supply chain, a new interconnected business system extending from isolated, local, and single-company applications to systematic smart implementations of the supply chain [
21].
2.5. Digital Technology Enablers in Pharmaceutical Industries
Digital technology enablers help integrate data and information from disparate sources and locations to streamline the supply chain by driving goods and services [
22]. The digital enablers provide the backbone for allowing digital transformations of industrial manufacturing [
22]. Industry 4.0 is built on eight technological pillars known as digital enablers. These enablers are the Internet of Things (IoT), cloud analytics, additive manufacturing, augmented reality, big data analytics, autonomous robots, and horizontal and vertical system integration. The concept of industry 4.0 is applied to the pharmaceutical industry, called pharma 4.0. The realization of pharma 4.0 requires a shift in how regulatory contents and data are managed, and transformation needs to start with changes in mindset and perception of the data content [
23]. In the pharma 4.0 framework, digital enablers, such as the Internet of Things (IoT), artificial intelligence (AI), cloud computing, including cloud-based ERP, big data analytics (BDA), and blockchain technology (BCT) are majorly used. The following sections discuss these enablers from pharmaceutical application contexts in detail.
2.5.1. Internet of Things (IoT)
The IoT signifies smart devices connected through sensors and the internet that perform tasks and exchange real-time data. IoT can provide an authentic and safe way to exchange information about pharmaceutical goods and services. IoT in the supply chain is viewed as a set of physical objects connected digitally for sensing, monitoring, and interacting within the organization and improving agility, visibility, sharing of information, and tracking to facilitate the plan, control, and coordination [
24]. IoT may bring tangible benefits to businesses by improving operational processes and reducing cost and risk due to its transparency, traceability, adaptability, scalability, and flexibility [
25]. The pharmaceutical industry’s initial and fundamental IoT technology was RFID technology, which allowed microchips to transform information to the operator using a wireless RF gun. Pharmaceutical products, such as specialty treatments, vaccines, drugs, and medicines, work as intended if maintained at a specific specified temperature and humidity. Temperatures of −70 °C (ultra-low temperature), −20 °C to −40 °C (negative temperature), +2 °C to +8 °C (temperature-sensitive products), and below +25 °C (controlled temperature) are some of the precise and regulated storage thresholds for various types of pharmaceutical products [
26]. Cold-chain transportation takes care of these constraints during production, transport, and storage. IoT solutions can help pharmaceutical manufacturers remotely monitor cold-chain environments in real-time by embedding sensors on tracking equipment with auto-start and shutdown mechanisms in warehouses, vehicles, or shipments using smartphones and tablets [
26]. IoT model was proposed that provides information, such as (a) vaccine carrier information, (b) continuous monitoring using sensors, (c) location tracking using GPS, and (d) regular and urgent notifications containing carrier temperatures, humidity, and location information with a timestamp [
2].
2.5.2. Artificial Intelligence (AI)
Improved computational technologies and growing data of supply chain processes are turning AI into one of the most critical technologies in supply chain areas [
27]. Demand forecasting, end-to-end visibility, predictive maintenance, smart factories, and integrity are crucial metrics in the pharmaceutical supply chain that AI technologies can help with. AI is the computer’s ability to solve problems that are not explicitly programmed to answer independently [
28]. AI technology includes machine learning, computer vision, deep learning, cognitive computing, natural language processing, speech, supervised learning, and unsupervised learning [
29]. Compared to any classic supply chain, the biopharmaceutical supply chain is complex, with many critical challenges, such as demand forecasting accuracy, cold chain transportation, long lead time, and vulnerability to counterfeiting [
30]. AI has a critical role in improving visibility for end-to-end supply chains. AI helps in demand forecasting, automation, optimizing predictive maintenance, and protecting the integrity of the pharmaceutical supply chain from counterfeit drugs [
30]. AI needs to be implemented in the supply chain roadmap to gain competitive advantages with the improvement of metrics, such as the accurate projection and forecast the customer demand, reduction in the cost of inventory quantity, overproduction, idle machine capacity, achieving quick reactions to the change in the market, and finally providing customers a better experience [
28]. AI-enabled supply chain systems help business managers optimize key performance indicators, review in real-time, cut cost, reduce waste, and speed up the time to market [
31]. A series of algorithms and datasets enables the AI system to integrate supply chain business toward (a) getting 100% accurate projection and forecast customer demand, (b) optimization of R&D by enhancing the quality and reducing costs, (c) identifying target customers and demography, and (d) providing a better customer experience [
32]. AI technologies help business managers with eliminating waste, smart manufacturing, transparency on supplier machine availability, performance, downtime, balancing the supply chain, and optimizing inventories in real-time [
33].
2.5.3. Cloud Computing
The production and delivery of products and services in a timely fashion with a short lead time and less cost are critical supply chain objectives of many organizations. Still, unfortunately, many are not able to achieve this with the traditional non-responsive traditional supply chain technology they have [
34]. Cloud computing offers on-demand computing services with high availability, reliability, and scalability. Cloud computing can give organizations significant power with computing and storage and help them deliver services at far cheaper rates than previously affordable [
35]. Many pharmaceutical organizations are now adopting cloud-based infrastructure for their supply chain processes. Organizations can achieve many benefits by implementing a cloud-based supply chain, such as real-time end-to-end supply chain visibility, the collaboration between organizations, capturing disruption risks, and creating a knowledgeable learning community for optimizing decision-making [
34].
Cloud-based enterprise resource planning (cloud ERP) systems help to integrate digital technologies to improve the connectivity chain within the supply chain environment [
36]. Application of ERP in the supply chain may help pharmaceutical managers in the area, such as streamlining end-to-end planning, supporting transportation and inventory keeping in warehouses, and providing more visibility on analytics, using which better decisions can be made. ERP applications are integrated information system packages that integrate the business functions of an organization into one core application and enable the organization to operate seamlessly by sharing the same information [
37].
2.5.4. Big Data Analytics (BDA)
Business managers from pharmaceutical companies face stringent quality standards globally, inorganic growth regarding mergers and acquisitions, and massive information coming from stakeholders, and they must manage these efficiently. Digitalization introduced new challenges in capturing, collecting, analyzing, archiving, sharing, transferring, and processing large data sets in organizations [
35]. Big data is defined as continuously generated in diverse data formats (structured and unstructured) from multiple sources. BDA may help business managers by providing a new perspective and adding value to improving modeling practices and predictive analysis [
38]. Analyzing the vast amount of data from various sources can help organizations reduce costs, understand the customer better, and better manage their supply chain uncertainties [
39]. Mobile applications and social media are now the significant catalysts for digital transformation, and leveraging analytics helps increase customer satisfaction and loyalty by delivering personal experiences for pharmaceutical industries.
2.5.5. Blockchain Technology (BCT)
BCT has been recognized as a critical digital technology enabler that helps improve supply chain processes’ operational efficiency. BCT in the pharmaceutical space is a promising solution as it may help immutability, trust, traceability, transparency, security, and fault tolerance [
40]. The supply chain digital transformation is about establishing a vision for how digital strategies and applications can improve service, cost, quality, agility, and inventory levels and consistently improving organizational changes and processes that use these digital technologies to drive operational excellence BCT uses distributed ledger technology, unlike the traditional system, which holds the ledger centrally, protected, and managed by a trusted party. BCT can be applied in some of the critical pain points in the pharmaceutical cycle, such as (i) integrity, transparency, and end-to-end supply chain visibility, (ii) adverse events and efficient recall management, (iii) better cold chain shipping process, (iv) better clinical trials, and (v) managing counterfeit products. Manufacturers, LSP (logistics service providers), distributors, retailers (hospitals, pharmacies), and end customers (patients) are essential stakeholders to be involved for blockchain-enabled pharmaceutical supply chain solutions to work [
41]. Electronic bill of lading (BOL) is also one proper implementation of BCT and is helpful to all stakeholders in the pharmaceutical supply chain distributions, such as retailers, carriers, and distributors.
2.5.6. Sustainability and Digitalization
Sustainability is protecting stakeholders’ interests by focusing on non-financial environments, such as environmental, social, ethical, and governmental environments, by accomplishing financial performance and creating stockholders’ value [
42]. Sustainability is meeting the present’s needs without compromising environmental generations to meet their own needs [
43]. As defined by the triple bottom-down sustainability theory, supply chain managers must consider social, economic, and ecological objectives while deciding to make their business profitable [
44]. Digitalization has prompted organizations to build highly interconnected and complex supply chains. As a result, companies adopt the outsourcing model to outsource non-core activities to overseas suppliers with little consideration. The shift toward outsourcing and waste and emission caused by production processes throughout the global supply chain is the primary source of environmental issues [
45]. The efficiency of the pharmaceutical supply chain is impacted by agility and resilience, but it also depends on sustainability.
3. Problem Statement
The growing complexity of managing a supply chain influences an organization’s performance [
46]. Managers in an organization need to improve efficiency, agility, visibility, and sustainability in the supply chain system because 27% of business owners have suffered damage to their reputations, 58% have reported lost productivity, and 38% have reported reduced revenue from supply chain disruptions [
47]. The general business problem is that some pharmaceutical managers lose supply chain efficiency, visibility, profitability, and business by not digitalizing the supply chain system and failing to meet sustainability goals. The specific business problem is that some pharmaceutical managers need more sustainable strategies to digitalize the integrated supply chain system.
4. Purpose Statement
The purpose of this qualitative multiple-case study was to explore the strategies used by pharmaceutical supply chain managers to digitalize their integrated supply chain ecosystem to improve business practices, sustainability, and profitability.
5. Research Question
The primary research question for this study was: What strategies do pharmaceutical managers use to digitalize their integrated supply chain system to meet sustainability goals to increase profitability?
6. Research Methodology
This qualitative multiple-case study explored the strategies supply chain managers use in pharmaceutical industries to digitalize the integrated supply chain system to increase profitability. The target populations included supply chain managers from various pharmaceutical companies in the United States, which have successfully developed strategies to digitalize the integrated supply chain system to improve their business practices and profitability. The purposive sample technique was used to select supply chain managers working in a pharmaceutical company in the United States who have used successful strategies to digitalize the integrated supply chain ecosystem. Data were collected from semistructured interviews, conducting an interview protocol until data saturation was reached. The theory of constraints was used as the conceptual framework to guide research to identify the strategies. ATLAS.ti 9 software was used to organize and analyze the data collected from the interviews. Participants’ real identities were protected by providing pseudonyms to participants, such as P1. The documents were collected from the participants for review. The word cloud was used for the initial analysis to visualize word frequency to get the first look and summarize the interview transcripts. The word was then mapped to the themes and strategies identified during the detailed analysis.
7. Results of the Findings
Three themes with nine strategies emerged during data analysis. The themes were (a) constraints or barriers in the current supply chain system, (b) digital technology enablers, and (c) sustainable, resilient, and agile supply chain systems. On initial analysis, the word cloud showed SAP, visibility, data, vision, cloud, integrity, regulatory, digitalization, agile, sustainability, analytics, and constraints as the key and most frequently used words by participants in the interviews shown in
Figure 1.
On detailed analysis, three main themes and nine key strategies emerged from the interviews with participants. The strategies were those used to address the problems in their earlier supply chain systems. The references coded refers to the number of times the data references were coded to the strategies.
Table 1 shows the summary of themes, strategies, and coding.
These themes and strategies that emerged from the interviews with participants are discussed in detail in the following section.
7.1. Identifying Constraints or Barriers in the Current Supply Chain System
The first theme that emerged during the interviews was the constraints or barriers in the current supply chain system. Related to the first theme of constraints, the word cloud from ATLAS.ti includes participants’ most frequently used words in the interview, as shown in
Figure 2. Frequent words used by participants are training, communication, change management, compliances, data integrity, regulatory, batch restrictions in different countries, demand sensing or forecasting, temperature sensitiveness, cold chain, third-party providers, restrictions, shelf life, expiry, and auditable planning, and operational barriers in pharmaceutical industries by supply chain extension include difficulties in coordination between multiple stakeholders, quality control problems, difficulties in managing flows and lack of inflexibilities, procurement, and storage problems, and logistics inefficiencies by the low shelf life of medicines [
21]. Cost and price barriers by third-party logistics, counterfeiting, drug diversion, and difficulties in monitoring the supply chain are the fundamental nature of the extension and diversity of items [
44]. Compared to other industries, managers in the pharmaceutical industry suffer from many constraints, such as counterfeit issues, unfavorable reactions to the patients regarding efficacy, if the temperature is not maintained during product life cycles, manufacturing and labeling issues, transportation and shipment issues, and storing and warehouse issues [
3]. The participants from different pharmaceutical organizations mentioned the constraints or barriers they faced at the start of their digital implementations, such as batch restrictions, good practice, quality guidelines and regulations in countries of business, multiple stakeholders, and not having proper change management to interact with internal and external stakeholders. Other constraints were needing an innovative automated system of records for end-to-end visibility, cold chain, missing digital maturity, and missing a cost-effective model in the supply chain. These constraints relate to the first theme of identifying constraints or barriers in the current supply chain system as per the TOC conceptual framework. The following four primary strategies provide a detailed overview of the strategy used by the participants in the first theme of constraints or barriers in the current supply chain system. To address the problem of constraints or barriers as the initial theme, managers need strategies to eliminate or minimize these problems. These strategies are discussed in detail in the following section.
7.1.1. Develop a Deep Understanding of All the Constraints of the Current Supply Chain System
All participants identified multiple constraints toward successfully implementing digital strategies in the pharmaceutical supply chain. P1 identified conditions such as data integrity, controlled process, certified documents, regulatory compliance from good practice, quality guidelines, regulations, and other local regulatory agencies, systems lacking for tracking adverse events, and change management processes. P2 identified constraints such as multiple stakeholders, being certifiable and auditable at the highest level, regulatory compliance from good practice, quality guidelines, regulations, and other local regulatory agencies, cold chain processes, master data integrity, system lacking for tracking the adverse events, and change management processes. P3 identified constraints such as regulatory compliance from good practice, quality guidelines, regulations, and other local regulatory agencies, master data integrity, a system lacking for tracking adverse events, and change management processes. P4 identified constraints such as demand sensing, cost and pricing in different geographies, capacity, and yield, and a system lacking for tracking adverse events. P5 identified constraints such as systems lacking for monitoring adverse events. P5 also identified regulatory compliance from good practice, quality guidelines, regulations, and other local regulatory agencies, restricted batches, temperature-sensitive, third-party logistics and multiple stakeholders, and cold chain. Each participant discussed the importance of identifying all constraints and barriers to their current supply chain system. The goal of each manager was to work toward a digital solution that recognizes the presence of these constraints and then look for a solution that addresses and fits the purposes. Collaboration with vendors and businesses reengineering their processes helped pharmaceutical managers efficiently deal with their present constraints.
7.1.2. Develop a Deep Understanding of and Possibilities of a Digital Supply Chain Integrated System
Each participant identified the gap of needing to fully understand the self-thinking supply chain integrated ecosystem when they started the digital supply chain initiatives. P1, P3, and P4 also discussed needing more relevant in-house expertise. P2 and P5 suggested a need for a strategic alliance with external third parties to understand digitalization possibilities in their existing supply chain system. The self-thinking supply chain helps managers monitor supply chain performances by analyzing a massive volume of available data, forecasting and identifying risks, and automatically acting before the risk occurs [
27]. The self-thinking supply chain is driven by new digital technologies designed to be self-aware and requires minimum human intervention to mitigate risk. The participants recommended exploring more understanding of the self-thinking supply chain for them to make accurate decisions in real-time and mitigate any risk from disruptions and changes in demand across the cycle. Understanding digital tools such as IoT, AI, cloud, and big data analytical tools that facilitate a self-thinking supply chain will help organizations decide and prepare for digital transformations.
7.1.3. Define a Vision for the Supply Chain Digital Strategy Aligned with the Organization’s Overall Strategy for Digitalized Operations
The participants discussed the need for a vision to implement a supply chain digital strategy successfully. Everyone suggested that supply chain digital strategy should be either wholly aligned or part of a corporate strategy rather than wholly detached from corporate strategy. An ideal scenario is when the digital strategy is a corporate vision, and supply chain digitalization is part of that vision. Senior leadership from non-information technology (IT) can adopt three strategies to achieve digital transformation: create and procure endorsement for an IT-enabled business transformation vision, develop a robust non-IT business leadership team, and develop a change management function for the transformation [
48]. Senior leadership in a pharmaceutical company can include the strategies identified in this study while defining their digital vision and objectives.
7.1.4. Develop a Robust Change Management System by Collaborating with Internal and External Stakeholders
Each participant discussed the need for a robust change management system and identified it as the most fundamental need for any digital transformation. As suggested by the participants, effective communication and training throughout the implementation life cycle played a crucial role in the successful change management process. P1, P4, and P5 highlighted the need to thoroughly understand the self-thinking supply chain integrated ecosystem before starting digital initiatives. Employees of an organization must be trained while preparing for digital transformation [
49]. The relationship between humans and technology will only be effective when there is collaboration, team interaction, and training [
49]. The change management process, followed by participants, included not end-users but external stakeholders, including digital tools and solutions vendors. P1, P4, and P5 indicated that communication was the critical factor, and end-users of their organization very well-received supply chain digital initiative. The training was crucial, and participants emphasized the need for multiple-user training throughout the life cycle of digital transformations.
7.2. Digital Technology Enablers
The second theme that emerged during the interviews was the digital technology enablers. We were able to relate the theme to the research question as managers who understand and work through digital technology enablers and their implications can successfully implement digital strategies and thus increase profitability. Related to the second theme of digital enablers, the word cloud from ATLAS.ti includes participants’ most frequently used words in the interview. Participants used frequent words: cloud, vision, sap, data, integration, analytics, metrics, KPI (key performance indices), quality, prototyping, piloting, yield, cost, and integration.
A digitalized organization uses digital enablers to carry out operational activities, including purchasing and selling products and services, interactions with customers, collaborating with internal and external stakeholders, and executing transactions within and outside the organization [
50]. The supply chain is a series of interconnected activities that involve coordinating, planning, and controlling products and services between suppliers, manufacturers, and customers [
8]. Digital technologies in the supply chain, compared to conventional technologies, altered the way people in an organization collaborate with others compared to the traditional supply chain consisting of physical facilities scattered geographically with linear collaboration.
Participants from different pharmaceutical organizations mentioned the implementation of digital enablers to digitalize the supply chain in their organizations. Participants also discussed the criteria for overcoming the constraint of selecting those digital tools and technologies. Most of the participants chose cloud-based ERP systems, such as SAP, as a system of record, a system of engagement, and a system of innovation. Cloud-based SaaS system also fits into the criteria for their system-related needs. Data integrity is one of the critical criteria in the pharmaceutical industry. The participants emphasized the need for big data analytical tools for end-to-end visibility in supply chain systems. These relate to the second theme of digital enablers.
The following two primary strategies provide a detailed overview of the strategy used by the participants in the second theme, digital technology enablers. To address the problem of digital technology enablers, managers need strategies to eliminate or minimize these problems.
7.2.1. Develop a Business Case by Evaluating Risk from Specific Digital Enablers, Digital System Integrators, and Application Technologies in Order
Each participant suggested a system of record, engagements, and innovation during their supply chain digital transformation journey. The document generated by the system must be certifiable and auditable at the highest level and accepted by regulatory bodies. SAP 6.0, an ERP software, was an automatic choice for P1, P2, P3, P4, and P5. P2 further explained that SAP as a system of record helped him in the digital supply chain functionality, such as logistics, planning, scheduling, procurement, manufacturing, transportation, billing, and finances. Tracking the adverse event is one of the most critical parameters in any pharmaceutical industry. Each participant explained that the track and trace system built and customized in the SAP system helped the participants do business efficiently.
P1 suggested using cloud-based services and cloud-based SaaS (software as a service) systems for the supply chain digital journey of quality-related needs. P1 further explained that the LIMS system, a cloud-based quality system that helped him convert manual analog processes to digitally recorded systems, is necessary for good practice, quality guidelines, and regulations procedures. P2 also suggested using HANA, which is cloud-based, to manage its supply chain processes. P3 said about using the service cloud to maintain everything from a customer-centric perspective.
Each participant strongly suggested the need for BDA. P2 said that analytics combined with AI, machine learning, and robotic process automation helped them innovate immensely. P2 has implemented most of the data analytics functionality from SAP. They have also implemented Qlikview, which provided a data analytics platform on the SaaS model. P3 said about using SAP data analytics tools for their transactional needs for end-to-end visibility of processes. P3 also used Tableau and AERA data analytics tools for their supply chain need. P4 said that data analytics helped them in incremental innovation.
Each participant discussed the need for a strong business case for steering investment for supply chain digitalization. For ensuring a solid business case, these factors are critical to driving a strong business case: to pursue a digital initiative that drives rapid business growth, leverage digital supply chain initiatives to support cost optimization, and enhance supply chain agility through digitalization [
51]. P1, P3, and P5 suggested working closely with senior management about digital initiatives by preparing a business case. P2 and P4 discussed the need to prepare metrics for benefit projection for digital initiatives and submit business cases.
7.2.2. Prioritize Those Enablers, Launch Pilot Projects to Design Solutions, and then Scale Up by Rolling Out a Full-Scale Digital Model
Each participant suggested prioritizing those digital enablers and launching pilot projects on a smaller scale. P1 said they carry out digital projects in various sprints, and prototyping is a prerequisite for any sprint. P3 noted that the pilot decides if it is a green light to go or a red light. P3 further said that in the case of large-scale deployment with significant investment in technologies, piloting is to work out the bugs and ensure that they have taken the right approach before they go live globally.
7.3. Sustainable, Resilient, and Agile Supply Chain System
The third theme from the interviews is continuing a sustainable, resilient, and agile supply chain. We were able to relate the theme to the research question as managers who keep improving with sustainability, resilience, and agility of the current system after the successful implementation of digital strategies can increase profitability. Related to the second theme of digital enablers, the word cloud from ATLAS.ti includes participants’ most frequently used words in the interview. Frequent words used by participants are sustainability, yield, agile, efficient, resilient, disruption, accuracy, maturity, and capacity.
The correlation between sustainability and the entire supply chain is well established, and the organizations require consideration and tracking [
52]. Supply chain disruptions represent the most prominent risk in the pharmaceutical industry. Participants from different pharmaceutical organizations mentioned the need for sustainable, agile, and resilient supply chain systems to improve their supply chain systems continuously. Resilience was a critical factor for the participants in a difficult situation like COVID. Sustainability is equally important to maintain a green supply chain from an environmental and social perspective. Each participant discussed the importance of maintaining KPIs and metrics to gauge the health of the current system. These relate to the third theme of a sustainable, agile, and resilient supply chain. Maintaining supply chain systems up and running by tapping full potential also matches the continuous improvement of the conceptual framework, TOC.
The following two strategies provide a detailed overview of the strategy used by the participants in the third theme, a sustainable, resilient, and agile supply chain system. To address the problem of a sustainable, resilient, and agile supply chain system as the initial theme, managers need strategies to eliminate or minimize these problems:
7.3.1. Establishing and Measuring Key Performance Indices (KPI) to Measure and Improve Supply Chain Effectiveness
Each participant discussed the need to build key performance indices (KPI) to measure the digital solution’s effectiveness during and after the implementation. Documents they provided also proved that KPIs were the primary strategies of the plan for digital milestones and business process improvements. P1 said they are generating plenty of metrics from their current digitalized system, and that is helping them to monitor the current state of their system. P2 said they are generating 50 to 60 metrics across the processes to measure the system’s effectiveness and whether they ended up with a better digital ecosystem. P3 mentioned generating key performance indices using analytics to check the current system. P3 further mentioned generating harmonized key performance indices at some level in the organization with an agreement with everyone to meet the criteria necessary for success. P4 said key performance indices are essential for measuring the success of digital maturity. P5 implemented key performance indices in critical areas, such as transportation, working with logistics partners, and measuring the efficacy of pharmaceutical drugs.
7.3.2. Maintain Sustainability and Continue Resiliency to Prepare for Uncertain Times
Participants discussed creating a sustainable system. They emphasized the necessity to maintain resiliency during an uncertain time, such as COVID. Managers must adopt a self-thinking system approach and focus on processes and measures to build an organization that is sustainable, reliable, and resilient [
53].
8. Application of Research to the Professional Practice
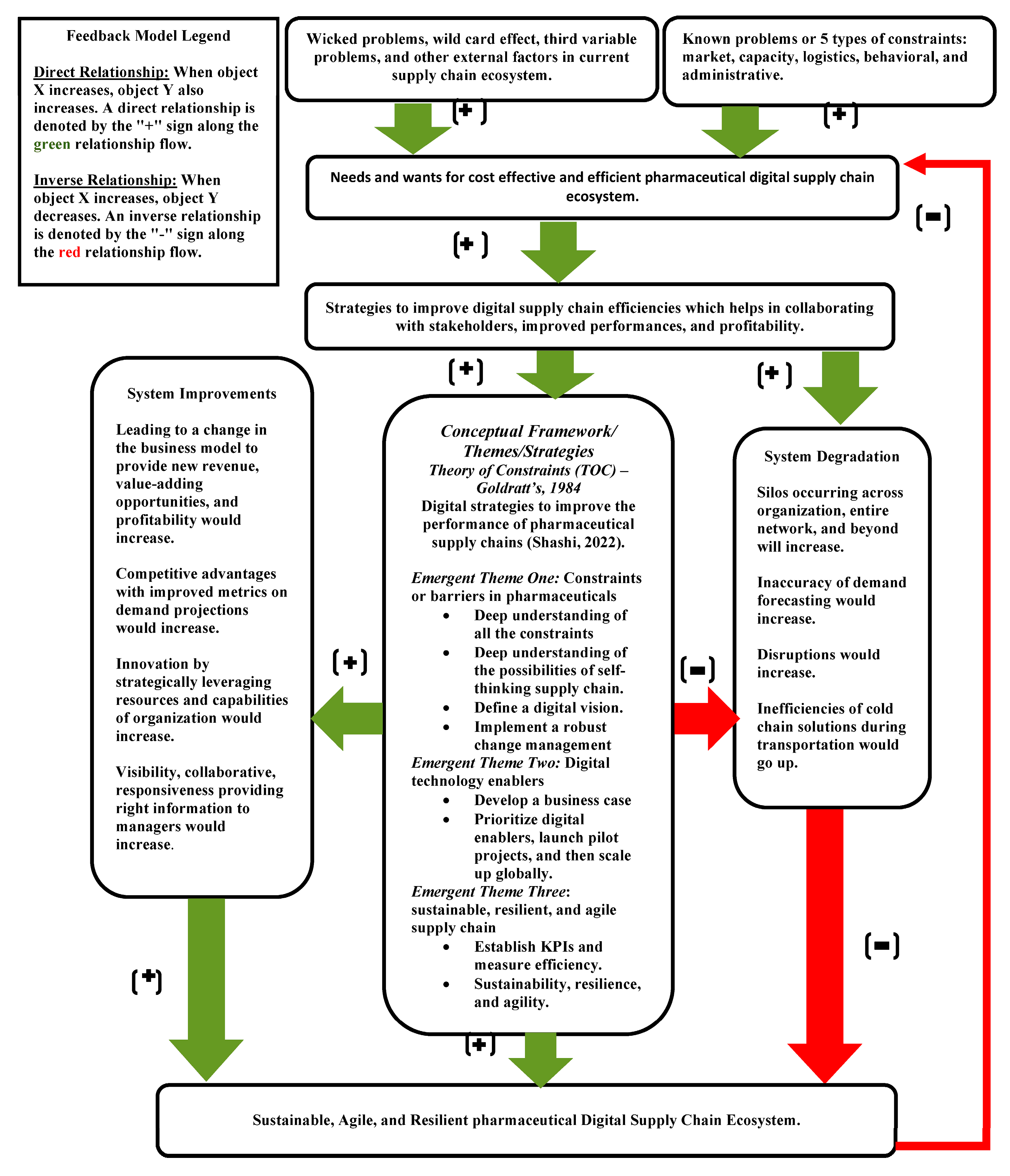
A system model was developed as an outcome of this research (see
Figure 2). Direct relationship variables will help improve the efficiency, and inversely related variables will negatively impact the efficiency of pharmaceutical supply chain systems. There can be some wicked problems, wild card effects, third variable problems, and external factors which are impacting the performance of the current supply chain system. Conventional processes cannot solve wicked problems, and standardized digitalization techniques must be applied. Fulfilling sales and operation planning (S&OP) objectives and maturity levels in pharmaceutical companies is one of the areas in supply chain operation which face wicked problems. Organizations need to be able to connect with customer demand, manufacturing capacity, transportation, and distribution capability, including third-party logistics and raw materials sourcing, and ultimately fail to achieve S&OP maturity levels. Sustainability in a supply chain system also falls under wicked problems. Wicked problems may not be solved permanently but can be managed efficiently by mitigating the risk of dynamic characteristics of these events.
There can be various constraints in the current supply chain system, such as capacity, market, logistics, behavioral, and administrative constraints. These constraints can be known or unknown to the managers managing their current supply chain systems. Capacity constraints are related to bottlenecks that restrict the flow or throughput in the supply chain processes. Supply chain managers must know their bottleneck because it helps increase flow by improving one process instead of working through a complete value stream. If demand is less than the capability of the slowest process, the bottom or capacity constraints may be unknown to management. Supply chain managers must use their bottleneck to determine if they can meet customer demands. Marketing constraints are probably the most difficult thing to break. It can happen by margin squeeze that affects profits or even a lack of a sale and losing business to competitors. The theory of constraints approach elevates potential customers’ perceived value of our product or services to remain loyal to us or win new business. This approach should be distinct from asking customers what they want and simply supplying. The offering must have unique characteristics that will take time for competitors to counter immediately.
Logistics constraints in a supply chain may include supplier constraints pertaining to capacity and minimum or maximum order supply. It may also include storage, loading or unloading, and transportation constraints. Behavioral constraints limit the benefits of system integration by hindering collaborative initiatives. Buying and supplying firms are conceptualized as different firms rather than an integrated supply chain ecosystem. The challenges arising when managers merge technical systems across firms pertain to system integration.
A pharmaceutical supply chain aims to meet and exceed customer requirements by minimizing end-to-end supply chain costs. Organizations must consider the efficient supply chain system as an integrated system and strive toward optimizing that. Wicked problems and various constraints must be analyzed in detail, and proper strategies must be formulated to improve the efficiency of the supply chain. Digitalization can be understood as using digital technology, leading to changes in value creation, business processes, and business models to provide new revenue and value-adding opportunities.
As shown in the model, supply chain efficiencies can be impacted positively (system improvement) or negatively (system degradation) by some significant variables. The critical variables influencing supply chain efficiency are demand forecasting, disruptions, end-to-end visibility, collaboration, responsiveness, and cold chain systems. Demand forecasting majorly impacts inventory and can lead to either shortage, oversupply from vendors, or overstock. The bullwhip is also an essential factor, and lack of collaboration may result in excessive inventory built-up or shortage. Organizations can have competitive advantages over others by improving metrics on demand projections.
The traditional supply chain is linear, and some organizations operate in silos by maintaining the broken non-digitalized disconnected system. This results in a non-responsive system without visibility or collaboration among internal or external supply chain partners. The likelihood of disruption increases by the global nature of the pharmaceutical business, such as outsourcing manufacturing operations, sourcing from multiple vendors, political and economic factors, and natural disasters. Disruptions have adverse effects on supply chain performance and profitability. It is challenging to avoid disruptions altogether, but mitigation strategies must be formulated to reduce the likelihood and damage caused.
Collaboration between supply chain partners is a critical capability pharmaceutical supply chain managers use to improve supply chain efficiency and overall performance. A strong line of communication is required between the sourcing team, logistics team, manufacturing and operation team, regulatory teams, and all the teams who are part of the supply chain. Pharmaceutical managers recognize the ever-changing environment and driving forces in case of disruptions. They recognize the value of collaboration as a strategic response to a volatile global environment such as the present corona epidemic situation.
Pharmaceutical products are imported and exported globally on a larger scale. China and India contribute significantly to the supply of active pharmaceutical ingredients (API) and raw materials around the globe. One of the critically important challenges for pharmaceutical companies is to control environmental parameters, such as temperature changes, humidity, and sunlight, to maintain the desired quality of the product. Cold chain management comes into the picture to effectively handle both pharmaceutical and biological products.
Pertain to the first theme; pharmaceutical managers must develop a strategy to understand all the constraints in the current supply chain system, develop an understanding of all the possibilities of a self-thinking supply chain, develop a digital vision, and establish a change management system. The self-thinking supply chain will help pharmaceutical managers continuously monitor supply chain performance through ever-evolving data and technology. It can help anticipate risks and disruption and automatically take preventive action to mitigate them. The self-thinking supply chain will be self-aware, so less human intervention will be required to manage the risk in the end-to-end system.
Pertain to the second theme, pharmaceutical managers must develop a business case first. Prioritizing the digital enablers, launching pilot projects, and finally rolling out to all the sites globally are part of the strategy toward digitalization of the pharmaceutical supply chain ecosystem. The business case is one of the pivotal documents for pharmaceutical supply chain transformations. It initially establishes the project’s goal of keeping the stakeholders’ expectations. It also helps resolve any issue towards understanding goals in the beginning. Later during the project, the business case becomes the guiding document and may help the project team to monitor the project and its environment. The business case also plays a critical role in comparing the project’s outcome with the plan once the project is completed.
Digital enablers are tools and technologies used to digitalize the various aspects of the pharmaceutical supply chain system. The business managers in pharmaceutical organizations face significant threat levels by not embracing digital transformation in supply chain business processes. Digitalization is about implementing cutting-edge technologies and not just changes in business processes. Digitalizing business processes means developing digital strategies to react to and apply emerging digital solutions such as the IoT, Artificial Intelligence (AI), Cloud Computing (CC), and blockchain to introduce innovation, improve, enhance, and create new business processes. Digital transformation is a set of strategic renewal, a transformation at a different level in an organization, and the leadership aspects of optimizing resources and capabilities attribute far beyond technology alone [
13].
Pilot projects can help pharmaceutical organizations to test digital innovation in a less critical context. This needs to be conducted on a single smaller site. Pilot projects prove viability and aim to deliver only some of the organization’s goals, as mentioned in the business case. Piloting can also help confirm scalability and adapt or reject digitalization concepts early without involving all sites globally. Piloting also helps to define the project budget and get buy-in from all stakeholders before rolling it out on a larger scale.
Pertaining to the third emergent theme, a robust strategy must be developed to maintain a sustainable, resilient, and agile supply chain system. Establishing key performance indices (KPIs) to measure supply chain efficiency is part of the strategy. The sustainable supply chain must synchronize organizational commitment, environmental considerations, ethical values, and culture. Maintaining sustainability is not the responsibility of any single partner in the supply chain ecosystem. A long-term commitment is needed from all partners, including raw material sourcing, manufacturing, packaging, logistics, distribution, and recycling. Resilience in the pharmaceutical context is the ability of the organization to bounce back from unpredicted disruption by restoring operations to the same original capacity. Resiliency in the pharmaceutical supply chain system can be enhanced by collaboration, information sharing between all the partners, and a mutually creating knowledge base. Resiliency in the pharmaceutical supply chain reduces the vulnerability of supply chain disruptions and helps mitigate disruptions’ economic impact.
Due to the growing uncertainty in the global supply chain environments, flexibility has been proven highly critical in meeting customer changing demand efficiently at optimal cost and utilizing all the resources. Flexibility is characterized by agility and demonstrates the firm’s capability to adapt to unpredictable global business environments. The agile supply chain helps pharmaceutical organizations make a more responsive and uninterrupted flow of products and services through the supply chain. Agility also helps deliver predictability by reducing overall lead time by sensing and rapidly responding to changes.
9. Application of Research for the Social Change
Improving the supply chain system in pharmaceutical industries may help improve the quality of care patients receive by potentially reducing healthcare costs resulting from decreased costs, which could benefit the community by providing community members with more affordable, higher quality, and reliable healthcare services that can augment an individual’s self-worth and dignity. Managers at pharmaceutical companies could pass the cost savings to community members by providing patients with more affordable medical services. Monitoring counterfeit products and protecting consumers from adverse effects are critical elements in the pharmaceutical industry. An effective supply chain strategy in a pharmaceutical company must ensure that end consumers are protected. The green supply chain is also essential to protect stakeholders and the environment, and an integrated digital supply chain process helps organizations maintain this.
10. Recommendations for Future Research
The purpose of this qualitative multiple-case study was to explore the strategies used by pharmaceutical managers to digitalize the integrated supply chain system to increase their profitability. Since the sample size included participants who worked at various pharmaceutical companies in the US, a recommendation for future research is to incorporate a larger sample size. For example, future research on this topic could use supply chain managers globally in various regions of different countries. This study focused on qualitative research, so future research may utilize mixed-method research to compile and analyze data regarding digital strategies. Mixed-method research may allow researchers to reach a large population. Due to ever-increasing contemporary technologies in other industries, pharmaceutical companies are building their vision for digitalizing by incorporating more digital enablers. These new digital enablers also need to be analyzed in the context of pharmaceutical supply chain requirements.
11. Conclusions
The results of this study may help business leaders understand why some pharmaceutical organization operates in silos by the non-sustainable, broken, and non-digitized disconnected system. Profitability in a pharmaceutical company starts with a sustainable supply chain. The key to a successful business is longevity, and sustainability measures make it profitable. Cost-effective digital and sustainable supply chain strategies support flexibility, better use resources, and enhance shareholder values and profitability. The findings of this study can positively influence social change by improving the supply chain system in pharmaceutical industries by reducing associated costs and helping the community with affordable healthcare costs. The findings could positively influence supply chain managers, researchers, and practitioners by providing them with key sustainable digital maturity strategies. The chosen conceptual framework for the study was the theory of constraints to understand and identify the constraints that limit a system from achieving higher performance. With the help of the TOC conceptual framework, I explored the strategies, digital tools, and technologies the participating pharmaceutical managers used to successfully digitalize their supply chain system. All the identified themes aligned with the conceptual framework and professional and academic literature review.
A detailed literature review concluded that digital enablers under the framework of industry 4.0 and pharma 4.0 provide a backbone allowing digital transformation in pharmaceutical industries. Enablers, such as the Internet of Things, artificial intelligence, cloud computing, big data analytics, and blockchains, could result in a new operating model of the digitalized supply chain in the pharmaceutical industry. However, defining a vision for digitalization and understanding constraints in existing supply chain systems are some of the initial critical steps for developing a digital road map. While implementing digital enablers, steps, such as validation of internal competency for the right skill set, feasibility analysis, prototype, and scaling up, should be the process for a practical digital road map.
The supply chain digitalization initiative can be successful, sustainable, and resilient with diligent planning and execution. Sustainability reflects commitment toward the environment and socio-economic factors and plays a big part in corporate culture, future potential investment, and profitability. However, in many cases, strategies related to digitalization and sustainability operate decentralized and independently of each other. Integrating both strategies may help build brand equity and positive supply chain performance, help the circular economy, and drive revenue.